Abstract
Worldwide, over 4 million premature deaths each year are attributed to the burning of biomass fuels for cooking and heating. Epidemiological studies associate household air pollution with lung diseases, including chronic obstructive pulmonary disease, lung cancer, and respiratory infections. Animal dung, a biomass fuel used by economically vulnerable populations, generates more toxic compounds per mass burned than other biomass fuels. The type of animal dung used varies widely depending on local agro-geography. There are currently neither standardized experimental systems for dung biomass smoke research nor studies assessing the health impacts of different types of dung smoke. Here, we used a novel reproducible exposure system to assess outcomes related to inflammation and respiratory infections in human airway cells exposed to six different types of dung biomass smoke. We report that dung biomass smoke, regardless of species, is pro-inflammatory and activates the aryl hydrocarbon receptor and JNK transcription factors; however, dung smoke also suppresses interferon responses after a challenge with a viral mimetic. These effects are consistent with epidemiological data, and suggest a mechanism by which the combustion of animal dung can directly cause lung diseases, promote increased susceptibility to infection, and contribute to the global health problem of household air pollution.
Keywords: biomass smoke, household air pollution, respiratory toxicology
Introduction
Biomass smoke, generated from the burning of solid fuels, such as animal dung, wood, and crop residues, for cooking and household heat, is the leading environmental risk factor for all-cause mortality (1). According to the Global Alliance for Cleaner Cookstoves, one person dies every eight seconds due to biomass smoke inhalation (2). Yet, 3 billion people worldwide, especially individuals with a low socioeconomic status, are exposed to biomass smoke (3). Women and children often breathe high levels of biomass smoke since they usually spend the most time near household fires based on cultural practices (2, 4). The burning of biomass fuels is a global health disparity issue.
The respiratory tract, particularly the airway epithelium, is the primary target for inhaled biomass smoke. Epidemiological evidence associates biomass smoke exposure with lung diseases, including Chronic Obstructive Pulmonary Disease (COPD), lung cancer, and respiratory infections (1, 4, 5). Similarly, in vitro studies have shown that human lung cells exposed to certain types of biomass smoke have heightened inflammatory responses and impaired immune defenses (6–8). Biomass smoke exerts toxic effects on the lung, leading to human health problems.
Animal dung is a biomass fuel that is widely burned by people living in low-income countries, since it is cheap, easy to collect and prepare for burning, and available in areas with limited vegetation. However, the inhalation of dung biomass smoke is of particular concern to human health. Cow dung biomass smoke was found to contain a greater oxidative capacity, more particulates per mass of fuel burned, and higher levels of microbial products compared to other combustion products, including wood smoke and diesel exhaust (4, 9, 10).
We and others have reported the pro-inflammatory effects of dung biomass smoke in vitro and in vivo, which was generated by burning selected types of animal dung (6, 8–11). However, the type of animal dung burned in households varies depending on local geography, climate, and agricultural practices. While cow dung is the main biomass fuel source in some parts of India and East Asia, it is rarely used in other areas. People living in Africa, the Middle East, the Himalayas, and South America often burn dung from goats, horses, elephants, yaks, camels, llamas, and other local animals (12–18). As a result, there is a critical knowledge gap concerning which type of animal dung should be used in the laboratory setting and whether different types of dung have different biological effects and health impacts. Currently, there are no studies examining the toxicological effects of different types of dung biomass smoke on lung cells. Here, we investigate the inflammatory effects and immune responses of human airway epithelial cells exposed to biomass smoke from six different types of animal dung, specifically horse (Equus caballus), U.S. domestic cow (Bos taurus taurus), Indian domestic cow (Bos taurus indicus), African elephant (Loxodonta africana), goat (Capra hircus), and white rhinoceros (Ceratotherium simum). These sources encompass both foregut fermenters (cattle and goats) and hindgut fermenters (horses, elephants, and rhinos), and represent common biomass fuels in different parts of the world (12–16). Perhaps surprisingly, we report that dung biomass smoke has similar biological effects on lung epithelial cells regardless of source.
Materials and Methods
Generation of dung biomass smoke
Horse dung and U.S.A. cow dung were collected from local farms from animals fed grass and hay forage. Dried cow dung from dairy cows living in Shivpuri Ashram, India that are fed an organic diet of grass and grains was purchased from KGP Products LLC (Shivapuri, India). African elephant, domestic goat, and white rhinoceros dung were collected from animals exhibited at Rochester’s Seneca Park Zoo fed timothy hay and commercial grain. The dung was dried overnight at 50°C, ground up, and packed into cigarettes as previously described by McCarthy et al. (8). Briefly, dried dung was broken down using a food processor, passed through a sieve to obtain pieces between 850 μm and 420 μm, mixed with 2% glycerol, and rolled into cigarette tubes (Zen) using a cigarette packing machine. Dung cigarettes had a length of 87 mm, diameter of 8 mm, and a filter length of 25 mm. The mass (mean ± standard deviation; n = 8 individual cigarettes) of different types of dung cigarettes was 0.74 ± 0.03 g (horse), 0.75 ± 0.02 g (U.S. cow), 0.76 ± 0.05 g (India cow), 0.65 ± 0.04 g (elephant), 0.86 ± 0.05 g (goat), and 0.72 ± 0.06 g (rhinoceros). For smoke generation, the dung “cigarettes” were placed in a Baumgartner-Jaeger CSM2072i cigarette smoking machine (CH Technologies, Westwood, NJ) that was programmed to puff the cigarettes once per minute for 2 seconds at a volume of 35-ml volume per puff. Dung cigarettes were replaced after burning to within 0.5cm of the filter or after 8 puffs, whichever was sooner. The dung smoke was mixed with filtered dilution air to achieve the desired concentration, and was then directed into a plastic exposure chamber containing the cell cultures to be exposed. The number of cigarettes and amount of dilution air used were adjusted to obtain similar total particulate matter (TPM) and carbon monoxide (CO) levels at the site of exposure across the different types of dung. The TPM of the smoke in the exposure chamber was determined by gravimetric sampling to be 55 ± 20 mg/m3 (mean ± S.D. across all experiments reported) and CO was measured with a Lascar USB CO Data Logger at a mean concentration of 82 ± 14 ppm (mean ± S.D. across all experiments reported). The estimated rate of particulate deposition onto cell culture inserts was 40 ng*cm−2*min−1.
Dung smoke extract (DSE) was made using a method similar to that described previously (19–21). Animal dung (5 g) was burned in a Buchner funnel connected to a vacuum flask and pulled through 20 ml of cell culture media using a vacuum. DSE, similar to tobacco smoke extract, was found to have a peak absorbance at 320 nm. Each preparation of dung smoke extract was normalized by measuring its optical density at 320 nm. An absorbance of 1.000 was defined as 1,000 absorbance units/ml.
Cell culture
Primary human small airway epithelial cells (SAECs; Lonza, Walkersville, MD) and SAECs with an NF-κB reporter (SABiosciences, Valencia, CA) were cultured as previously described (8, 21, 22). For dung smoke exposures, SAECs were plated onto culture inserts (1 μm pore size; Millipore), moved to the air-liquid interface (ALI) for 24 hours, and exposed to air or whole dung smoke for 10–30 minutes. For dose response studies, since dose or exposure = concentration × time, we chose to vary the exposure time rather than the concentration of dung smoke in the chamber. After dung smoke exposure, cells remained in contact with the smoke-exposed culture media for up to 24 hours to mimic the continual exposure of airway epithelial cells to dung biomass smoke. In some experiments, cells were subsequently treated with 0.5 μM polyinosine-polycytidylic acid (poly I:C) (Invivogen, San Diego, CA). Cytotoxicity was assessed using Alamar Blue reagent (Invitrogen - Thermo Fisher Scientific, Rockford, IL) according to the manufacturer’s protocol.
For reporter assays, 16-human bronchial epithelial cells (16-HBE), the H1L1.1c2 cell line with an aryl hydrocarbon receptor (AhR) reporter (a gift from M.S. Denison, Ph.D., University of California, Davis) and the human embryonic kidney (HEK)-293 cell line with an interferon-sensitive response element (ISRE)-driven luciferase construct (a gift from L. Martinez-Sobrido) were cultured as previously described (23–25).
Luciferase Assays
To screen for the presence of AhR ligands, H1L1.1c2 reporter cells were incubated with 8–32 units/ml of DSE for 24 hours and lysed using 5X Luciferase Cell Culture Lysis Reagent (Promega, Madison WI). For activator protein (AP)-1 reporter assays, 16-HBE were transfected with a 3XAP-1 plasmid (a gift from Alexander Dent, Addgene, Cambridge, MA) using Lipofectamine LTX with PLUS Reagent (Thermo Fisher Scientific, Rockford, IL) 24 hours before dung biomass smoke extract treatment. To measure interferon production by SAECs, supernatants from SAECs were added to HEK-293 ISRE reporter cells (1:1 with media) for 24 hours. Cells were lysed and luciferase assays were performed with the Luciferase Assay System (Promega, Madison, WI) according to the manufacturer’s instructions.
Enzyme-Linked Immunosorbent Assays (ELISAs)
The levels of interleukin (IL)-8, granulocyte macrophage colony and interferon-inducible gamma protein (IP)-10 in SAEC supernatants were quantified by ELISAs (R&D Systems, Minneapolis, MN).
Western blot analysis
Western blots and densitometric analysis were performed as previously described (20). Blots were probed with antibodies for cyclooxygenase (Cox)-2 (Cayman Chemical, Ann Arbor, MI), aryl hydrocarbon receptor (AhR) (Enzo Life Sciences, Farmingdale, NY), β-tubulin (Abcam, Cambridge, MA), phospho-nuclear factor κappa B (NFκB) p65 (Cell Signaling, Danvers, MA), NFκB p65 (Santa Cruz Biotechnology, Dallas, TX), phospho-c-jun N-terminal kinase (JNK) (Cell Signaling, Danvers, MA), and JNK (Cell Signaling, Danvers, MA).
Gene Expression Analysis
RNA isolation from SAECs and quantitative PCR was performed as previously described (21, 22). We used primers for 18S RNA (22), cytochrome P450 (CYP)1A1 (8), interferon (IFN)-β (forward: 5′-AACTGCAACCTTTCGAAGCC-3′; reverse: 5′-TGTCGCCTACTACCTGTTGTGC-3′) and IFN-λ (forward: 5′-GGTGACTTTGGTGCTAGGCT-3′; reverse: 5′-GGCCTTCTTGAAGCTCGCT-3′).
Statistical Analysis
Results are reported as mean ± SD or SEM. Analysis of variance (ANOVA) with multiple comparisons was performed using GraphPad Prism version 7.0 (GraphPad Software, La Jolla California USA, www.graphpad.com). A p-value <0.05 was considered significant.
Results
Dung Biomass Smoke is Pro-Inflammatory Regardless of Source
Previous studies have found that the combustion products of biomass fuels induce pro-inflammatory responses in human airway epithelial cells (6, 8, 26, 27). Mehra et al. showed that cow dung smoke extract increased the production of interleukin (IL)-8, a potent neutrophil chemoattractant, in SAECs grown in submerged cultures (6). Similarly, we reported that SAECs exposed to smoke from the combustion of horse dung at the air-liquid interface (ALI) had upregulated levels of IL-8, as well as increased production of GM-CSF, an inflammatory cytokine that activates immune cells, and cyclooxygenase (Cox)-2, a pro-inflammatory enzyme that produces prostaglandins (8). Here, we compared the effects of smoke generated from burning 6 different types of animal dung. SAECs were exposed to whole dung biomass smoke at the air-liquid interface (ALI) for 15 or 30 minutes, and cytokine production in the conditioned medium was measured 24 h later. The exposure time and smoke concentration were chosen to be similar to human exposure data from breathing zone air samples in homes that use biomass for cooking (28, 29). We found that primary human SAECs exposed to all six different types of dung biomass smoke showed a trend towards an increase in the secretion of IL-8 and GM-CSF after a 15-minute exposure, with significant upregulation of cytokines observed after a 30-minute exposure (Figure 1A–B). We confirmed the increased levels of pro-inflammatory cytokines in SAECs exposed to 30-minutes of six different types of dung in multiple independent experiments (Figure 1C–D). Additionally, the increased cytokine production was not caused by cytotoxicity of different types of dung biomass smoke after a 30-minute exposure (Figure 2). We also analyzed expression of the key pro-inflammatory response protein Cox-2 by western blot. SAECs exposed to horse, U.S. cow, Indian cow, elephant, goat, and rhino dung biomass smoke similarly upregulated the protein expression of Cox-2 (Figure 3A–B).
Figure 1.
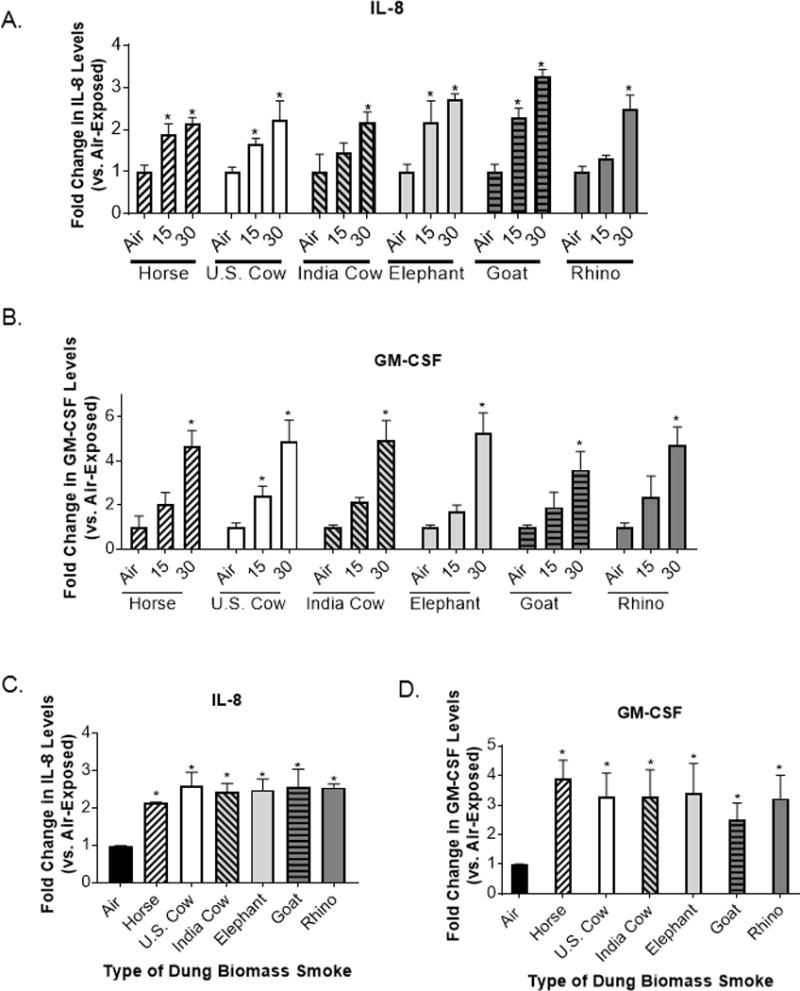
Six different types of dung biomass smoke induce pro-inflammatory cytokine production in SAECs.
SAECs were exposed to air or dung smoke (horse, U.S. cow, India cow, elephant, goat, or rhinoceros (rhino)) for 15 or 30 minutes and cell supernatants were collected 24-hours post-exposure. Dose-response effects of dung biomass smoke exposure on (A) IL-8 and (B) GM-CSF production in SAECs were determined by ELISA. Data represent mean ± SD (n = 3 – replicates per exposure group from an independent experiment), *p<0.05 by two-way ANOVA (compared to air-exposed cells using Tukey’s post-hoc analysis). (C) IL-8 and (D) GM-CSF levels were measured in SAECs exposed to 30 minutes of dung smoke in multiple experiments. Data represent mean ± SEM for n=3 independent experiments with 3 replicate cultures per experiment.*p<0.05 by one-way ANOVA (compared to air-exposed cells using a Dunnett’s post-hoc analysis).
Figure 2.
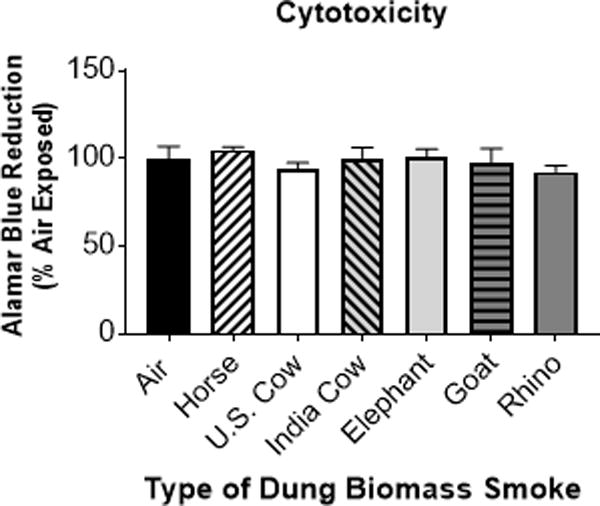
Exposure to 30 minutes of six different types of dung biomass smoke does not cause cytotoxicity in SAECs.
SAECs were exposed to air or dung smoke (horse, U.S. cow, India cow, elephant, goat, or rhinoceros (rhino)) for 30 minutes. Alamar Blue reagent was added to the cells 24-hours post-exposure. After a 4-hour incubation, cell supernatants containing Alamar Blue were collected. Reduction of Alamar Blue was determined by measuring the fluorescence of the samples (excitation = 560 nm and emission = 590 nm). Data represent mean (n = 3 replicates per exposure group from an independent experiment).
Figure 3.
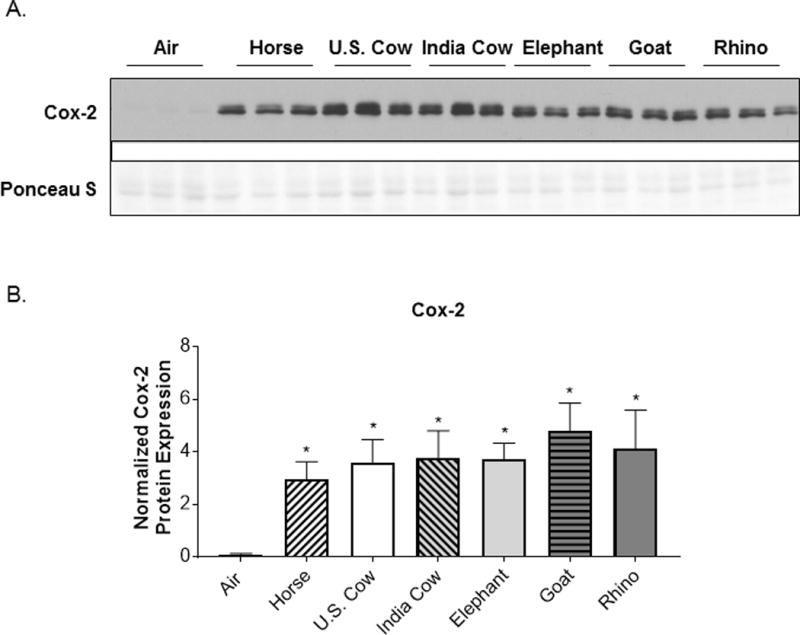
Cox-2 protein expression is upregulated by six different types of dung biomass smoke.
SAECs were exposed to air or dung smoke (horse, U.S. cow, India cow, elephant, goat, or rhinoceros (rhino)) for 30 minutes and cell lysates were collected 24-hours post-exposure. (A) Representative Western blot showing 3 replicates per condition of Cox-2 protein expression and Ponceau S staining in SAECs exposed to air or different types of dung smoke. (B) Densitometry of Cox-2 protein levels was performed. Data represent mean ± SD (n = 3 replicates per exposure group from 1 of 3 independent experiments), *p<0.05 by one-way ANOVA (compared to air-exposed cells using Tukey’s post-hoc analysis).
Different Types of Dung Biomass Smoke Activate the Aryl Hydrocarbon Receptor
The aryl hydrocarbon receptor (AhR) is a key transcription factor involved in pulmonary immune responses to urban combustion products, including diesel exhaust and tobacco smoke (19, 30, 31). To investigate whether different types of animal dung biomass smoke contain ligands of the AhR, we used a well-characterized AhR activity assay utilizing mouse liver cells with a luciferase reporter (23, 25, 32, 33). We found that AhR reporter cells treated with dung smoke extracts generated from the combustion of horse, U.S. cow, Indian cow, elephant, goat and rhino dung displayed a dose-dependent increase in luciferase activity (Figure 4A). All six different types of dung smoke extract displayed statistically significant increases in AhR reporter activity at a concentration of 32 absorbance units/ml (Figure 4A). This concentration of extract is similar to the amount of smoke absorbed by SAEC culture medium during a 30 minute exposure to whole dung smoke (Figure 4E). Multiple studies show that AhR protein is degraded after activation (31, 34). To confirm AhR activation by dung smoke in human airway cells, we assessed AhR protein levels by Western blot. We found that total AhR protein is reduced in SAECs exposed to 6 different types of dung biomass smoke (Figure 4B–C). Additionally, horse, U.S. cow, and Indian cow dung smoke upregulated the mRNA expression of cytochrome P450 (CYP)1A1, a downstream target gene of the AhR (Figure 4D). Overall, our data shows that dung smoke from all 6 species contains similar levels of AhR ligands, and that human airway cells respond to these AhR ligands by upregulating and AhR target gene and by activation-mediated degradation of the AhR protein.
Figure 4.
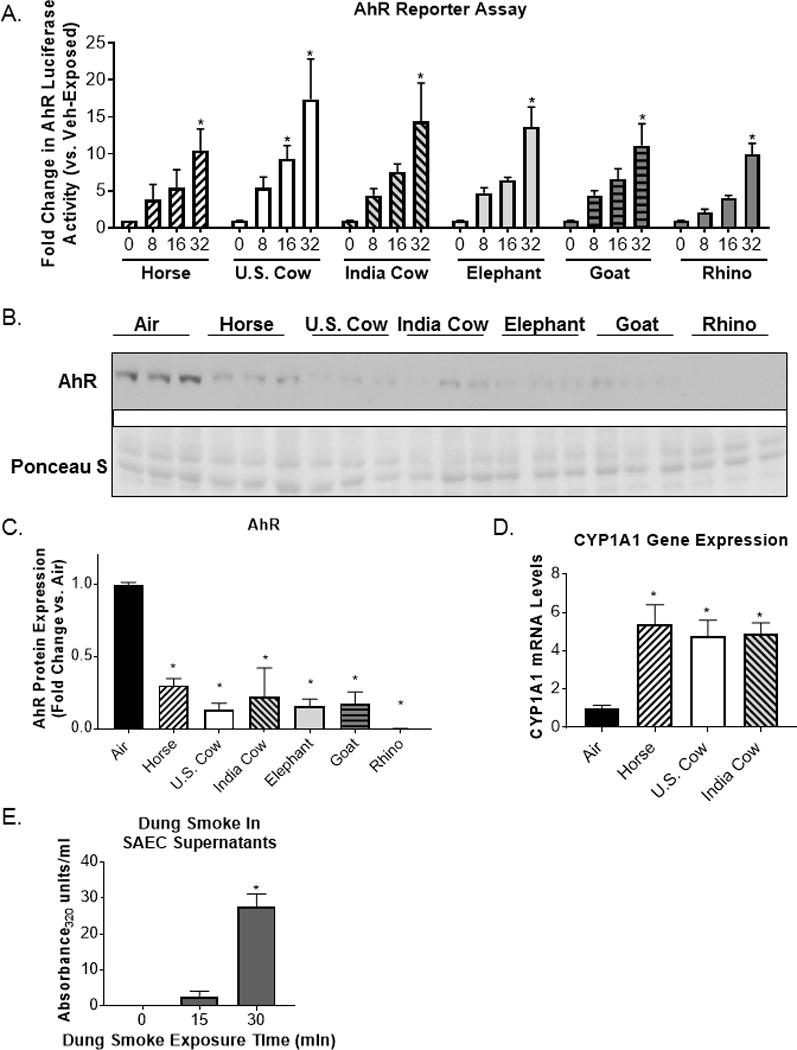
Dung smoke contains AhR ligands and activates the AhR.
(A) A cell line containing an AhR luciferase reporter was treated with the indicated concentrations of dung biomass smoke extract (horse U.S. cow, India cow, elephant, goat, or rhinoceros (rhino)) for 24 hours. Data represent mean ± SEM for 3 independent experiments with 3 replicate cultures per experiment, *p<0.05 by two-way ANOVA (compared to vehicle using Tukey’s post-hoc analysis). (B) SAECs were exposed to dung biomass smoke for 30 minutes and cell lysates were collected 24 hours post-exposure. AhR was detected by western blot. (C) Densitometry of AhR protein expression was performed. Data represent mean ± SD (n = 3 replicates per exposure group from 1 of 3 independent experiments), *p<0.05 by one-way ANOVA (compared to air-exposed cells using Tukey’s post-hoc analysis). (D) SAECs were exposed to horse, U.S. cow, or Indian cow dung smoke for 30 minutes. RNA was isolated 6 hours post-exposure. (D) CYP1A1 gene expression was measured by qPCR and normalized to 18S mRNA levels. Data represent mean ± SD (n = 3 replicates per exposure group from an independent experiment), *p<0.05 by one-way ANOVA (compared to air-exposed cells using Tukey’s post-hoc analysis). (E) SAECs were exposed to dung biomass smoke for up to 30 minutes. Cell supernatants were collected 24-hours post-exposure and their optical density at 320 nm was measured. Data represent mean + SEM (n=3 – mean of 3 independent experiments), *p<0.05 by one-way ANOVA (compared to air-exposed cells using Tukey’s post-hoc analysis).
Different Types of Animal Dung Biomass Smoke Stimulate Activator Protein-1 but Not Nuclear Factor Kappa B in Human Airway Epithelial Cells
Inflammatory responses in the respiratory epithelium are often regulated by AP-1 and nuclear factor kappa B (NF-κB) (35). These transcription factors lead to the production of pro-inflammatory mediators, including IL-8, GM-CSF, and Cox-2. However, the smoke from all six types of animal dung did not induce NFκB activity in SAECs containing an NFκB reporter (Figure 5A). We also found that there was either no change, or a reduction in phosphorylated NFκB p65 protein in SAECs 30 minutes after exposure to Indian cow or elephant dung biomass smoke (Figure 5B–C) rather than an increase in phospho-p65 that would be expected with NF-κB activation (36). This is consistent with our previous report that horse dung biomass smoke exposure neither activated an NFκB reporter nor upregulated phospho-p65 in SAECs (8). Conversely, using 16-HBE cells transfected with an AP-1 luciferase reporter, we found that all six types of dung biomass smoke extract at 16 and 32 units/ml increased AP-1 transcriptional activity by ~2-3-fold (Figure 6A–B). We have previously reported similar AP-1 activity results with SAECs exposed to 30 minutes of whole horse dung biomass smoke (8). We also observed increased phospho-JNK protein expression in SAECs shortly after exposure to Indian cow or elephant dung biomass smoke (Figure 6C-D). This data supports previous work showing that SAECs exposed to horse dung biomass smoke or Indian cow dung smoke extract stimulate JNK-AP-1 signaling (6, 8). The inflammatory responses of dung biomass smoke seem to be mediated by AP-1 rather than NF-κB in airway epithelial cells.
Figure 5.
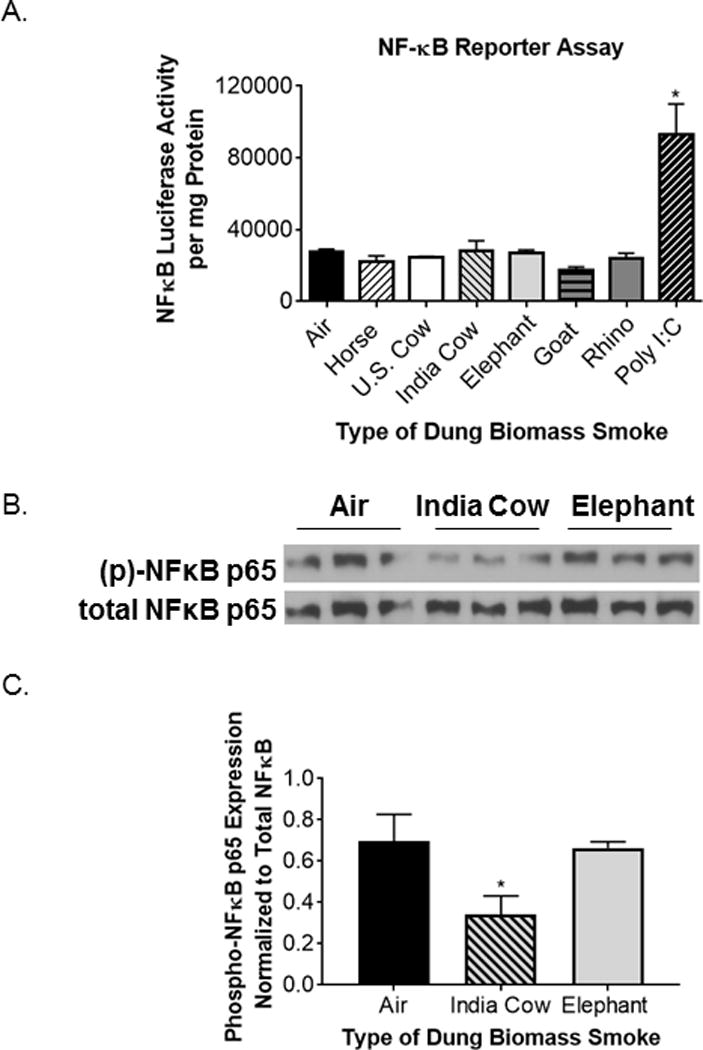
Six types of dung biomass smoke do not activate NFκB in human SAECs.
(A) Luciferase activity was measured in SAECs transduced with a NFκB luciferase reporter 24 hours post-dung biomass smoke (horse, U.S. cow, India cow, elephant, goat, or rhino) exposure. Based on a previous study, polyinosinic:polycytidylic acid (poly I:C, 0.5 μg/ml) was used as a positive control. Data represent mean ± SD (n= 3 replicates per exposure group from an independent experiment), *p<0.05 by one-way ANOVA (compared to air-exposed cells). (B) SAECs were exposed to air, Indian cow dung smoke, or elephant dung smoke for 30 minutes and cell lysates were collected 30 minutes post-exposure. A representative Western blot showing 3 technical replicates per exposure group is shown. (C) Densitometry of phospho-NFκB p65 and total NFκB p65 expression was performed on 3 replicates per exposure group. Data represent mean ± SD (n = 3 replicates per exposure group from a representative experiment), *p<0.05 by one-way ANOVA (compared to air-exposed cells).
Figure 6.
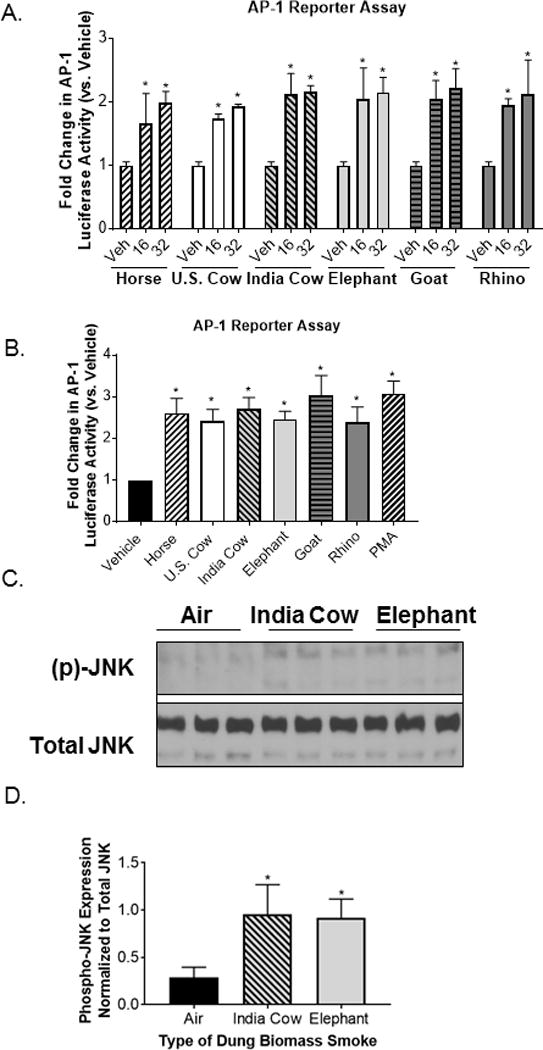
Different types of dung biomass smoke activate JNK-AP-1 in airway epithelial cells.
(A) 16-HBEs were transfected with an AP-1 luciferase reporter and treated with 16 or 32 units/ml of DSE (horse, U.S. cow, India cow, elephant, goat, or rhino) for 24 hours. AP-1 luciferase activity was measured in cell lysates. Data represent mean ± SD (n = 3 replicates per exposure group from an independent experiment), *p<0.05 by two-way ANOVA (compared to vehicle (Veh)-treated cells using Tukey’s multiple comparison post-hoc analysis). (B) AP-1 luciferase activity in 16-HBEs treated with 32 units/ml of DSE. Phorbol myristate acetate (PMA, 50 nM) was included as a positive control. Data represent mean ± SEM (n = 3 – mean of 3 independent experiments), *p<0.05 by one-way ANOVA (compared to Veh-treated cells using Dunnett’s post-hoc analysis). (C) SAECs were exposed to air, Indian cow dung smoke, or elephant dung smoke (30 minutes) and cell lysates were collected 30 minutes post-exposure. A representative Western blot showing 3 technical replicates per exposure group is shown. (C) Densitometry of phospho-JNK and total JNK expression was performed on 3 replicates per exposure group. Data represent mean ± SD (n = 3 replicates per exposure group from an independent experiment), *p<0.05 by one-way ANOVA (compared to air-exposed cells using Tukey’s post-hoc analysis).
Dung Biomass Smoke Impairs the Immune Responses of SAECs to a Viral Mimetic
Along with inflammatory responses, epidemiological evidence correlates biomass smoke exposure with an increased risk for acute respiratory infections (1, 4). To investigate the effects of dung biomass smoke on immune responses to an infectious agent, we treated SAECs with polyinosinic:polycytidylic acid (poly(I:C)), an analog of viral double-stranded RNA, after exposure to air or dung biomass smoke. In response to viral RNA, SAECs produce high levels of type I and type III interferons (IFNs), which induce an anti-viral state that prevents viral replication (37). Using an interferon sensitive response element (ISRE)-luciferase reporter to measure IFN bioactivity in SAEC supernatants, we found that all six types of dung biomass smoke impair poly(I:C)-induced IFN protein production (Figure 7A-B). Exposure-dependence of the suppression of IFN production was confirmed in SAECs exposed to horse, U.S. cow, and India cow dung biomass smoke and subsequently challenged with poly(I:C) (Figure 7C-E). Additionally, we measured the mRNA levels of IFN-β, a type I IFN, and IFN-λ, a type III IFN that is the main IFN produced by respiratory epithelial cells after viral infection (37). We found that SAECs treated with poly(I:C) increased the gene expression of IFN-β and IFN-λ by about 100-fold after 6 hours (Figure 8A-D). Yet, horse, U.S. cow, Indian cow, elephant, goat, and rhino dung biomass smoke exposure dramatically impaired the upregulation of IFN-β and IFN-λ mRNA after poly(I:C) treatment (Figure 8A-D).
Figure 7.
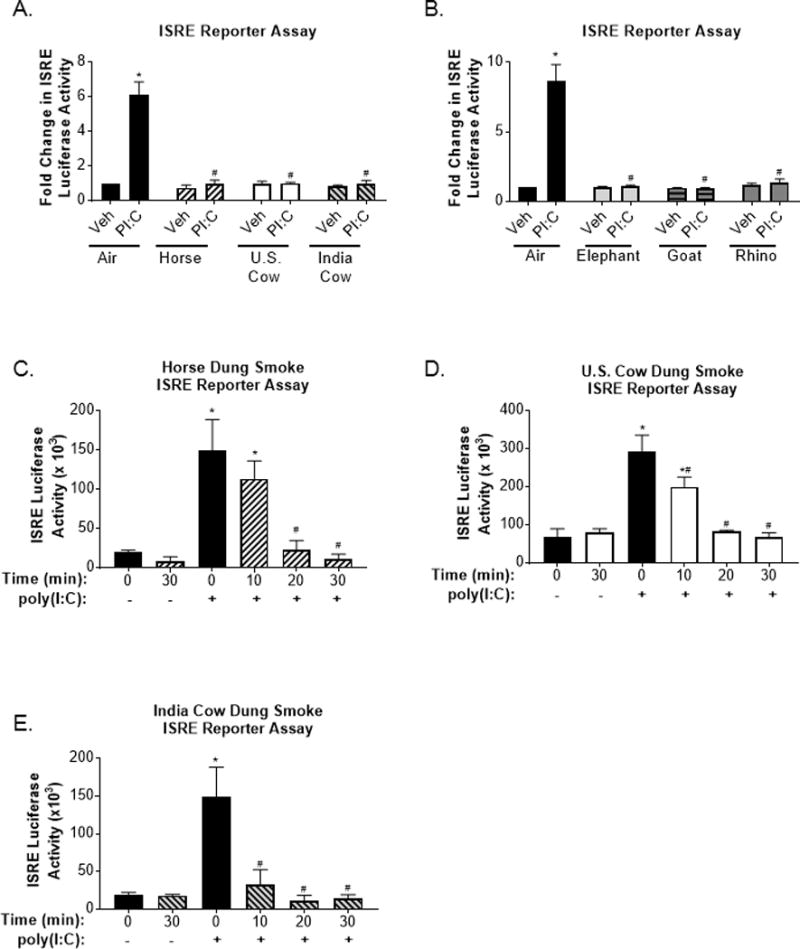
Interferon production in response to a viral-like challenge is attenuated in SAECs exposed to various types of dung biomass smoke.
SAECs were exposed to (A) horse, U.S. cow, or India cow dung biomass smoke or (B) elephant, goat, or rhino dung biomass smoke for 30 minutes and subsequently treated with poly(I:C) (PI:C; 0.5 μg/ml) or a vehicle control (Veh) of SAGM. Cell supernatants were collected 24 hours post-PI:C treatment. HEK cells containing an ISRE reporter were incubated with the SAEC supernatants and media (1:1 v/v) for 24 hours to assess interferon levels. Data are expressed as mean ± SEM (n=3 – mean of independent experiments),*p<0.05 (Veh compared to PI:C) and #p<0.05 (Air compared to Dung Smoke) by two-way ANOVA using Sidak’s post-hoc analysis. Exposure-response effects on poly(I:C)-induced IFN production in SAECs exposed to whole (C) horse, (D) U.S. cow, or (E) Indian cow dung biomass smoke for 10, 20, or 30 minutes were also assessed. Data are expressed as mean ± SD (n=3 replicates per group from an independent experiment), *p<0.05 (Veh compared to PI:C) and #p<0.05 (Air compared to Dung Smoke) by two-way ANOVA using using Sidak’s and Tukey’s post-hoc analyses.
Figure 8.
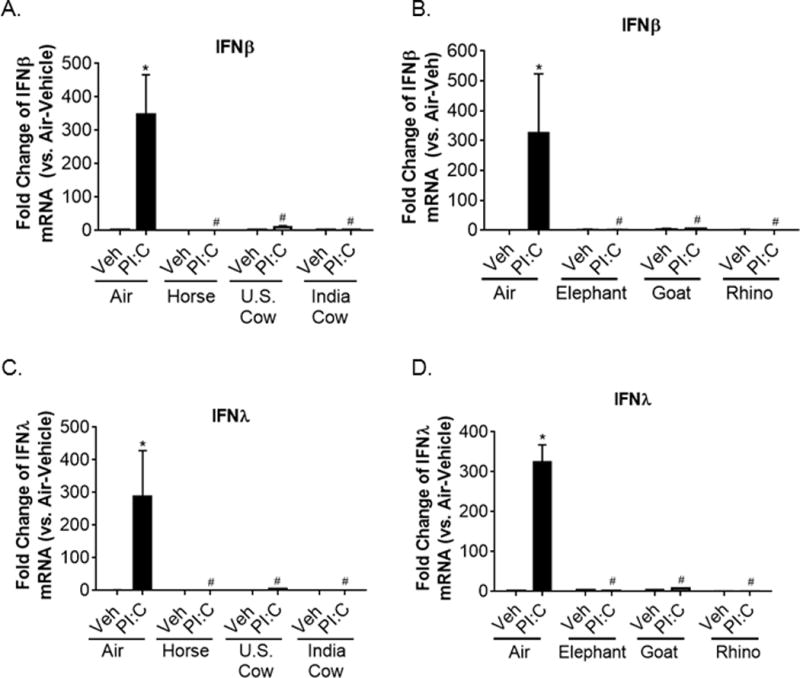
Poly(I:C)-induced type I and type III interferon gene expression is suppressed in SAECs exposed to multiple types of dung biomass smoke. SAECs were exposed to the indicated type of dung biomass smoke for 30 minutes and challenged with PI:C. RNA was isolated 6 hours post-PI:C treatment. (A-B) Interferon-β, (C-D) interferon-λ, and 18S RNA gene expression were assessed by qPCR. Data are expressed as mean ± SD (n=3 replicates per group from an independent experiment),*p<0.05 (Veh compared to PI:C) and #p<0.05 (Air compared to Dung Smoke) by two-way ANOVA using Sidak’s and Tukey’s post-hoc analyses.
The production of IFNs also leads to the upregulation of interferon-gamma inducible protein (IP)-10, a cytokine that recruits immune cells to the lung and promotes their activation during innate immune responses to viral infections (38, 39). Similar to the IFN reporter assay, SAECs treated with poly(I:C) had high supernatant levels of IP-10 (Figure 9A-E). However, prior exposure to horse, U.S. cow, Indian cow, African elephant, goat, or white rhinoceros dung biomass smoke attenuated IP-10 production to levels observed in air-exposed-vehicle treated cells (Figure 9A-B). In SAECs exposed to horse, U.S. cow, and Indian cow whole dung smoke for increasing amounts of time, an exposure-dependent attenuation of poly(I:C)-induced IP-10 production was observed (Figure 9C-E). This data indicates that many different types of dung biomass smoke dampen innate immune responses of airway epithelial cells.
Figure 9.
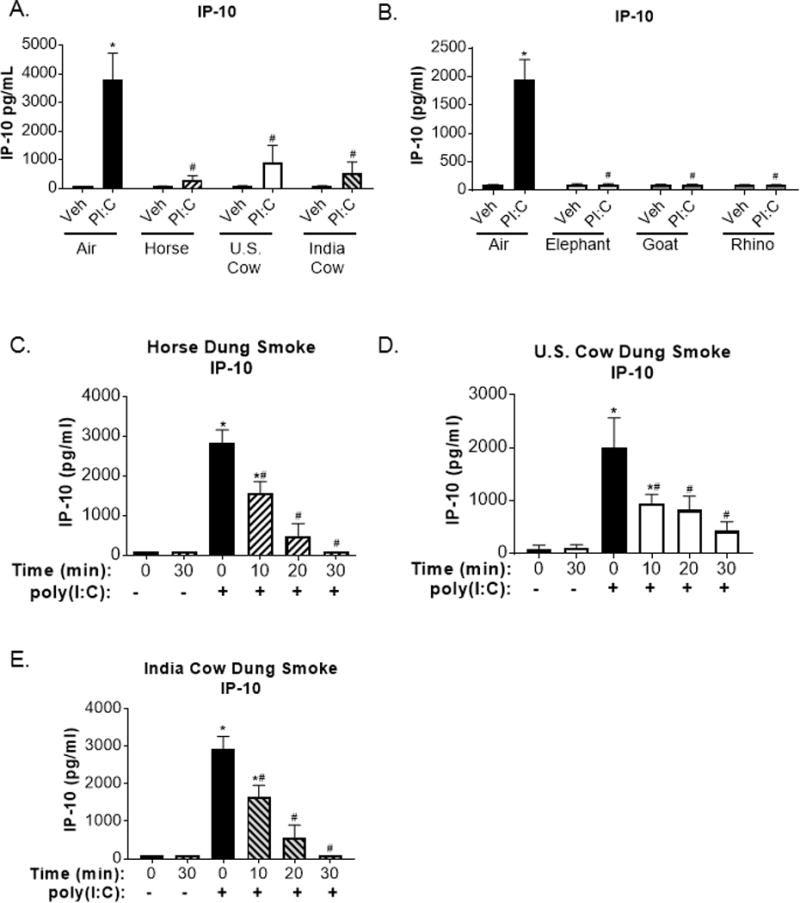
Poly(I:C)-induced IP-10 expression is suppressed in SAECs exposed to multiple types of dung biomass smoke.
SAECs were exposed to (A) horse, U.S. cow, or India cow dung biomass smoke or (B) elephant, goat, or rhino dung biomass smoke for 30 minutes and subsequently treated with poly(I:C) (PI:C; 0.5 μg/ml) or vehicle (Veh). Cell supernatants were collected 24 hours post-PI:C treatment and IP-10 levels were measured by ELISA. Data are expressed as mean ± SEM (n=3 – mean of independent experiments),*p<0.05 (Veh compared to PI:C) and #p<0.05 (Air compared to Dung Smoke) by two-way ANOVA using using Sidak’s and Tukey’s post-hoc analyses. Exposure-response effects on poly(I:C)-induced IP-10 production in SAECs exposed to whole (C) horse, (D) U.S. cow, or (E) Indian cow dung biomass smoke for 10, 20, or 30 minutes were determined by ELISA. Data are expressed as mean ± SD (n=3 replicates per group from an independent experiment), *p<0.05 (Veh compared to PI:C) and #p<0.05 (Air compared to Dung Smoke) by two-way ANOVA using Sidak’s and Tukey’s post-hoc analyses.
Discussion
According to Dr. Francis S. Collins, the Director of the National Institutes of Health, “indoor air pollution caused by cookstoves is one of the top health risks in developing countries” and causes an estimated 4.3 million premature deaths every year (1, 4, 5, 40). Exposure to biomass smoke is epidemiologically associated with non-communicable lung diseases and respiratory infections, and the respiratory epithelium plays a major role in the toxicological response to biomass smoke since it is the initial site of contact of inhaled air pollutants (1, 4, 5). One type of biomass fuel of particular concern in regard to pulmonary health is animal dung. People with a low socioeconomic status, who cannot afford or geographically access more efficient fuels, often burn animal dung for cooking and heating their homes. However, the combustion of cow dung produces more toxic by-products, including particulates, reactive oxidative species, and microbial products, compared to the burning of other biomass fuels, such as wood (9, 10). Further, the type of animal dung used as a biomass fuel widely varies depending on location, climate, and local agriculture. Yet, there are currently no studies examining the toxicological effects of different types of dung biomass smoke on the lung. Most reported studies of dung biomass smoke have focused on the combustion of cow dung, which is often stereotypically viewed by people living in developed countries as the main source of dung biomass fuel in low-income countries. Yet, many different types of animal dung are used for fuel across the globe. Horse dung is burned for household energy in Mongolia, elephant dung is a fuel source in Africa and nature reserves in Southeast Asia, and goat dung is often relied on by nomadic people in the Middle East, Himalayas, and parts of Africa, and white rhinoceros dung is found in Africa where people often rely on local animal dung for fuel (13–16). Thus, we compared the functional inflammatory responses and immune defenses of airway epithelial cells exposed to smoke from the combustion of horse, U.S. cow, India cow, African elephant, goat, and white rhinoceros dung.
A majority of studies to date investigating the toxicological impact of biomass smoke have used aqueous smoke extracts or smoke particulate-containing condensates to treat submerged cell cultures, while a few have used particulates collected from homes that use dung biomass for cooking and heating (6, 7, 41–45). Here, we primarily exposed lung airway cells to whole dung biomass smoke at the air-liquid interface, and only used extracts when using non-airway reporter cell lines. In order to establish a reproducible system for smoke generation, we took advantage of existing technology in the form of an automated cigarette smoking machine. Our use of dung cigarettes represents a compromise between fidelity to field conditions and the need for a consistent and reproducible system for generating dung biomass smoke in the laboratory (given that a traditional 3 stone fire is both inherently uncontrolled, as well as unwelcome in a laboratory setting). Nevertheless, the smoke produced in this system is very similar to dung biomass smoke in field studies (8, 46). We exposed airway cells to particulate levels of smoke that have been measured in the breathing zone around biomass cooking fires during peak cooking times, which can reach up to 50 mg/m3 (28, 29). Since we did not change the medium after the dung smoke exposures, any particulates that have settled on the SAECs and any smoke components that have dissolved in the medium remain present during the following incubation periods, which we believe reflects human inhalation exposures where particles remain in the lung for hours to days after inhalation.
We found that dung from 5 different animal species, including both hindgut and foregut fermenters, induced a pro-inflammatory response in primary human SAECs. Based on previous data with horse dung biomass smoke, we measured the production of IL-8 and GM-CSF in response to the combustion of various sources of dung (6, 8). Similar to results previously reported by us and others from in vitro studies examining smoke from a single source of dung, we found increased levels of IL-8 and GM-CSF in SAEC supernatants after cells were exposed to cow, goat, elephant, and rhinoceros dung biomass smoke (Figure 1) (6, 8). This is consistent with the increased levels of IL-8 and GM-CSF measured in people and mice exposed to biomass smoke (6, 10, 47). We also found that different types of dung biomass smoke upregulated the expression of Cox-2 (Figure 3), which plays an important role in airway inflammation (48). Additionally, these three mediators have been found to play a role in the development of biomass smoke-associated lung diseases, specifically COPD and lung cancer. IL-8 and GM-CSF recruit immune cells to the lung, promote an inflammatory microenvironment, and contribute to cancer cell proliferation (49–51). Cox-2 produces lipid mediators that promote vasodilation, bronchoconstriction, mucus production, angiogenesis, and other actions that cause pulmonary inflammation and tumorigenesis (48, 52). Overall, various types of dung biomass smoke cause inflammatory responses in airway epithelial cells and may contribute to the pathogenesis of inflammatory lung diseases.
Important regulators of airway inflammatory responses to inhaled pollutants that we assessed with dung biomass smoke exposure were the AhR and AP-1 (19, 30, 31). Similar to previous work examining the effects of dung smoke by us and others, we found that six different types of dung biomass smoke can activate the AhR (Figure 5) and AP-1 (Figure 6) in human airway epithelial cells (6, 8). AhR and JNK-AP-1 activation has also been reported in submerged cultures of lung cells exposed to other types of biomass smoke. Migliaccio et al. showed that mouse monocytes treated with particulate matter from wood smoke had increased expression of an AhR-regulated gene, and Martey et al. found that human lung fibroblasts exposed to tobacco smoke extract displayed AhR nuclear translocation (31, 53). Additionally, increased levels of phosphorylated JNK were observed in human bronchial epithelial cells treated with wood smoke extract (26). Various biomass fuel combustion products, including dung biomass smoke from multiple species, activate transcription factors that play a role in lung inflammation.
Surprisingly, we found that combustion of six different types of dung did not increase NFκB transcriptional activity in SAECs (Figure 5). This result corresponds with our previous work showing that SAECs exposed to horse dung biomass smoke did not induce classical NFκB signaling. Yet, airway epithelial cells treated with cigarette smoke extract show increased NFκB activation (27, 54, 55). This difference may be related to differences in the composition of tobacco and dung biomass smoke. For example, tobacco-specific components of cigarette smoke, including nicotine and nicotine-derived nitrosamines, have been found to promote NFκB activation in airway epithelial cells (56, 57). Overall, both dung biomass smoke and tobacco smoke cause inflammatory mediator production in airway epithelial cells; however, the biological effects are not exactly the same.
In addition to inflammatory lung diseases, we examined the impact of dung biomass smoke exposure on anti-viral defenses. Respiratory infections are a leading cause of morbidity and mortality in countries where solid biomass fuels are the primary source of energy (4). Additionally, household air pollution is epidemiologically associated with an increased risk for respiratory infections and human macrophages exposed to wood biomass smoke have impaired immunological functions (7). We show, for the first time, that human SAECs exposed to six different types of dung biomass have attenuated poly(I:C)-induced interferon and IP-10 production (Figures 7–9). These antiviral mediators play an important role in mucosal immunity of the lung by inhibiting viral replication, promoting the death of infected cells, and recruiting and activating immune cells involved in adaptive responses (38, 39, 58). This in vitro evidence has important implications for future studies investigating how biomass smoke exposure by vulnerable populations causes increased number and severity of respiratory infections, and indicates that dung biomass smoke impairs some key innate immune responses to viruses. While other researchers have used cow dung smoke extract to examine inflammatory responses, we are the first to examine biomass smoke from different types of animal dung and provide the first evidence that dung biomass smoke suppresses anti-viral defenses (6).
In conclusion, we have shown that airway epithelial cells exposed to six different types of dung biomass smoke upregulate inflammatory mediators, activate pro-inflammatory transcription factors, and attenuate innate immune mediator production in response to a viral mimetic. The similar, in vitro biological effects observed with acute exposure to different types of dung biomass smoke suggests that human inhalation of biomass smoke from the combustion of various types of animal dung causes similar toxicological responses in the lung and biomass smoke-related respiratory diseases.
Acknowledgments
We would like to acknowledge the local farms, Seneca Park Zoo, and Dr. Jeff Wyatt for their assistance in obtaining different types of animal dung. We would also like to thank Dr. Luis Martinez-Sobrido for his assistance with the ISRE reporter assay and Stephen Pollock for his technical help with the large Western blots.
Funding Information
This work was supported by the National Institutes of Health Grants [HL120908, T32ES007026, P30ES001247, and T32HL066988].
Abbreviations
- 16-HBE
16-human bronchial epithelial cells
- AP-1
activator protein-1
- ALI
air-liquid interface
- AhR
aryl hydrocarbon receptor
- CO
carbon monoxide
- COPD
Chronic Obstructive Pulmonary Disease
- Cox-2
cyclooxygenase-2
- DSE
dung smoke extract
- ELISAs
enzyme-linked immunosorbent assays
- GM-CSF
granulocyte macrophage colony-stimulating factor
- HEK
human embryonic kidney cells
- IP-10
interferon-inducible gamma protein-10
- ISRE
interferon-sensitive response element
- IL-8
interleukin-8
- JNK
c-jun N-terminal kinase
- NFκB
nuclear factor κappa B
- poly IC
polyinosine-polycytidylic acid
- SAECs
small airway epithelial cells
- TPM
total particulate matter
References
- 1.Martin WJ, 2nd, Glass RI, Balbus JM, Collins FS. Public health. A major environmental cause of death. Science (New York, NY) 2011;334(6053):180–181. doi: 10.1126/science.1213088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yadama GN. Fires, fuel, and the fate of 3 billion: The state of the energy impoverished. Oxford: Oxford University Press; 2013. [Google Scholar]
- 3.Martin WJ, II, Glass RI, Araj H, Balbus J, Collins FS, Curtis S, Diette GB, Elwood WN, Falk H, Hibberd PL, et al. Household air pollution in low- and middle-income countries: Health risks and research priorities. PLoS Med. 2013;10(6):e1001455. doi: 10.1371/journal.pmed.1001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon SB, Bruce NG, Grigg J, Hibberd PL, Kurmi OP, Lam K-bH, Mortimer K, Asante KP, Balakrishnan K, Balmes J, et al. Respiratory risks from household air pollution in low and middle income countries. The Lancet Respiratory Medicine. 2014;2(10):823–860. doi: 10.1016/S2213-2600(14)70168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Burden of disease from household air pollution for 2012. 2014 [Google Scholar]
- 6.Mehra D, Geraghty PM, Hardigan AA, Foronjy R. A comparison of the inflammatory and proteolytic effects of dung biomass and cigarette smoke exposure in the lung. PloS one. 2012;7(12):e52889. doi: 10.1371/journal.pone.0052889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rylance J, Fullerton DG, Scriven J, Aljurayyan AN, Mzinza D, Barrett S, Wright AK, Wootton DG, Glennie SJ, Baple K, et al. Household air pollution causes dose-dependent inflammation and altered phagocytosis in human macrophages. American journal of respiratory cell and molecular biology. 2015;52(5):584–593. doi: 10.1165/rcmb.2014-0188OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarthy CE, Duffney PF, Gelein R, Thatcher TH, Elder A, Phipps RP, Sime PJ. Dung biomass smoke activates inflammatory signaling pathways in human small airway epithelial cells. American journal of physiology Lung cellular and molecular physiology. 2016;311(6):L1222–L1233. doi: 10.1152/ajplung.00183.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mudway IS, Duggan ST, Venkataraman C, Habib G, Kelly FJ, Grigg J. Combustion of dried animal dung as biofuel results in the generation of highly redox active fine particulates. Particle and fibre toxicology. 2005;2:6. doi: 10.1186/1743-8977-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sussan TE, Ingole V, Kim JH, McCormick S, Negherbon J, Fallica J, Akulian J, Yarmus L, Feller-Kopman D, Wills-Karp M, et al. Source of biomass cooking fuel determines pulmonary response to household air pollution. American journal of respiratory cell and molecular biology. 2014;50(3):538–548. doi: 10.1165/rcmb.2013-0201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Air pollution and cancer. Geneva: International Agency for Research on Cancer; 2013. [Google Scholar]
- 12.Bruun O. Precious steppe: Mongolian nomadic pastoralists in pursuit of the market. Lanham: Lexington Books; 2013. [Google Scholar]
- 13.de Carle D, Linder W, Houle J, Broderick LG. Burn baby burn sh*t fuel inferno: Ethnographic fuel use in the west mongolian altai. 36th Annual Association for Environmental Archaeology Conference; University of York, UK. 2015. [Google Scholar]
- 14.Udzungwa Elephant Project. Two more uses of elephant dung: Fuel & mossie repellent. 2010 Apr 27; [cited 2016. Available from: http://udzungwa.wildlifedirect.org/2010/04/27/two-more-uses-of-elephant-dung-fuel-mossie-repellent/
- 15.Blakemore E. India’s newest internet sensation: Cow dung patties. Smithonian Washington D.C.: Smithonian Institution; 2015. Dec 28, [Google Scholar]
- 16.Carroll M. Masai mara: A tribal quest. The Independent; London, UK: 2008. Sep 12, [Google Scholar]
- 17.Xiao Q, Saikawa E, Yokelson RJ, Chen P, Li C, Kang S. Indoor air pollution from burning yak dung as a household fuel in tibet. Atmospheric Environment. 2015;102:406–412. [Google Scholar]
- 18.Barnes J. Camels and llamas at work. Milwaukee, MI: Gareth Stevens Publishing; 2006. [Google Scholar]
- 19.Baglole CJ, Maggirwar SB, Gasiewicz TA, Thatcher TH, Phipps RP, Sime PJ. The aryl hydrocarbon receptor attenuates tobacco smoke-induced cyclooxygenase-2 and prostaglandin production in lung fibroblasts through regulation of the nf-kappab family member relb. The Journal of biological chemistry. 2008;283(43):28944–28957. doi: 10.1074/jbc.M800685200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baglole CJ, Sime PJ, Phipps RP. Cigarette smoke-induced expression of heme oxygenase-1 in human lung fibroblasts is regulated by intracellular glutathione. American journal of physiology Lung cellular and molecular physiology. 2008;295(4):L624–636. doi: 10.1152/ajplung.90215.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsiao HM, Sapinoro RE, Thatcher TH, Croasdell A, Levy EP, Fulton RA, Olsen KC, Pollock SJ, Serhan CN, Phipps RP, et al. A novel anti-inflammatory and pro-resolving role for resolvin d1 in acute cigarette smoke-induced lung inflammation. PloS one. 2013;8(3):e58258. doi: 10.1371/journal.pone.0058258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsiao H-M, Thatcher TH, Levy EP, Fulton RA, Owens KM, Phipps RP, Sime PJ. Resolvin d1 attenuates poly(i:C)-induced inflammatory signaling in human airway epithelial cells via tak1. Journal of immunology (Baltimore, Md : 1950) 2014;193(10):4980–4987. doi: 10.4049/jimmunol.1400313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garrison PM, Tullis K, Aarts JM, Brouwer A, Giesy JP, Denison MS. Species-specific recombinant cell lines as bioassay systems for the detection of 2,3,7,8-tetrachlorodibenzo-p-dioxin-like chemicals. Fundamental and applied toxicology : official journal of the Society of Toxicology. 1996;30(2):194–203. doi: 10.1006/faat.1996.0056. [DOI] [PubMed] [Google Scholar]
- 24.Nogales A, Baker SF, Ortiz-Riano E, Dewhurst S, Topham DJ, Martinez-Sobrido L. Influenza a virus attenuation by codon deoptimization of the ns gene for vaccine development. Journal of virology. 2014;88(18):10525–10540. doi: 10.1128/JVI.01565-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song J, Clagett-Dame M, Peterson RE, Hahn ME, Westler WM, Sicinski RR, DeLuca HF. A ligand for the aryl hydrocarbon receptor isolated from lung. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(23):14694–14699. doi: 10.1073/pnas.232562899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perng DW, Chang TM, Wang JY, Lee CC, Lu SH, Shyue SK, Lee TS, Kou YR. Inflammatory role of amp-activated protein kinase signaling in an experimental model of toxic smoke inhalation injury. Critical care medicine. 2013;41(1):120–132. doi: 10.1097/CCM.0b013e318265f653. [DOI] [PubMed] [Google Scholar]
- 27.Kode A, Yang SR, Rahman I. Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells. Respiratory research. 2006;7:132. doi: 10.1186/1465-9921-7-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ezzati M, Kammen DM. The health impacts of exposure to indoor air pollution from solid fuels in developing countries: Knowledge, gaps, and data needs. Environmental health perspectives. 2002;110(11):1057–1068. doi: 10.1289/ehp.021101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Vliet ED, Asante K, Jack DW, Kinney PL, Whyatt RM, Chillrud SN, Abokyi L, Zandoh C, Owusu-Agyei S. Personal exposures to fine particulate matter and black carbon in households cooking with biomass fuels in rural ghana. Environmental research. 2013;127:40–48. doi: 10.1016/j.envres.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaguin M, Fardel O, Lecureur V. Exposure to diesel exhaust particle extracts (depe) impairs some polarization markers and functions of human macrophages through activation of ahr and nrf2. PloS one. 2015;10(2):e0116560. doi: 10.1371/journal.pone.0116560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martey CA, Baglole CJ, Gasiewicz TA, Sime PJ, Phipps RP. The aryl hydrocarbon receptor is a regulator of cigarette smoke induction of the cyclooxygenase and prostaglandin pathways in human lung fibroblasts. American journal of physiology Lung cellular and molecular physiology. 2005;289(3):L391–399. doi: 10.1152/ajplung.00062.2005. [DOI] [PubMed] [Google Scholar]
- 32.Windal I, Denison MS, Birnbaum LS, Van Wouwe N, Baeyens W, Goeyens L. Chemically activated luciferase gene expression (calux) cell bioassay analysis for the estimation of dioxin-like activity: Critical parameters of the calux procedure that impact assay results. Environmental science & technology. 2005;39(19):7357–7364. doi: 10.1021/es0504993. [DOI] [PubMed] [Google Scholar]
- 33.Seidel SD, Li V, Winter GM, Rogers WJ, Martinez EI, Denison MS. Ah receptor-based chemical screening bioassays: Application and limitations for the detection of ah receptor agonists. Toxicological sciences : an official journal of the Society of Toxicology. 2000;55(1):107–115. doi: 10.1093/toxsci/55.1.107. [DOI] [PubMed] [Google Scholar]
- 34.Roberts BJ, Whitelaw ML. Degradation of the basic helix-loop-helix/per-arnt-sim homology domain dioxin receptor via the ubiquitin/proteasome pathway. The Journal of biological chemistry. 1999;274(51):36351–36356. doi: 10.1074/jbc.274.51.36351. [DOI] [PubMed] [Google Scholar]
- 35.Barnes PJ. Transcription factors in airway diseases. Laboratory investigation; a journal of technical methods and pathology. 2006;86(9):867–872. doi: 10.1038/labinvest.3700456. [DOI] [PubMed] [Google Scholar]
- 36.Hochrainer K, Racchumi G, Anrather J. Site-specific phosphorylation of the p65 protein subunit mediates selective gene expression by differential nf-κb and rna polymerase ii promoter recruitment. Journal of Biological Chemistry. 2013;288(1):285–293. doi: 10.1074/jbc.M112.385625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazear HM, Nice TJ, Diamond MS. Interferon-lambda: Immune functions at barrier surfaces and beyond. Immunity. 2015;43(1):15–28. doi: 10.1016/j.immuni.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindell DM, Lane TE, Lukacs NW. Cxcl10/cxcr3-mediated responses promote immunity to respiratory syncytial virus infection by augmenting dendritic cell and cd8(+) t cell efficacy. European journal of immunology. 2008;38(8):2168–2179. doi: 10.1002/eji.200838155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wareing MD, Lyon AB, Lu B, Gerard C, Sarawar SR. Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza a virus infection in mice. Journal of leukocyte biology. 2004;76(4):886–895. doi: 10.1189/jlb.1203644. [DOI] [PubMed] [Google Scholar]
- 40.McDaniels AK. Hopkins to use $30 million grant to study impact of cooking methods on air pollution. The Baltimore Sun; Baltimore, MD: 2016. [Google Scholar]
- 41.Ghio AJ, Soukup JM, Dailey LA, Tong H, Kesic MJ, Budinger GRS, Mutlu GM. Wood smoke particle sequesters cell iron to impact a biological effect. Chemical research in toxicology. 2015;28(11):2104–2111. doi: 10.1021/acs.chemrestox.5b00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corsini E, Budello S, Marabini L, Galbiati V, Piazzalunga A, Barbieri P, Cozzutto S, Marinovich M, Pitea D, Galli CL. Comparison of wood smoke pm2.5 obtained from the combustion of fir and beech pellets on inflammation and DNA damage in a549 and thp-1 human cell lines. Archives of toxicology. 2013;87(12):2187–2199. doi: 10.1007/s00204-013-1071-z. [DOI] [PubMed] [Google Scholar]
- 43.Kocbach A, Herseth JI, Lag M, Refsnes M, Schwarze PE. Particles from wood smoke and traffic induce differential pro-inflammatory response patterns in co-cultures. Toxicology and applied pharmacology. 2008;232(2):317–326. doi: 10.1016/j.taap.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Kocbach A, Namork E, Schwarze PE. Pro-inflammatory potential of wood smoke and traffic-derived particles in a monocytic cell line. Toxicology. 2008;247(2–3):123–132. doi: 10.1016/j.tox.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 45.Danielsen PH, Møller P, Jensen KA, Sharma AK, Wallin H, Bossi R, Autrup H, Mølhave L, Ravanat J-L, Briedé JJ, et al. Oxidative stress, DNA damage, and inflammation induced by ambient air and wood smoke particulate matter in human a549 and thp-1 cell lines. Chemical research in toxicology. 2011;24(2):168–184. doi: 10.1021/tx100407m. [DOI] [PubMed] [Google Scholar]
- 46.Venkataraman C, Rao GU. Emission factors of carbon monoxide and size-resolved aerosols from biofuel combustion. Environmental science & technology. 2001;35(10):2100–2107. doi: 10.1021/es001603d. [DOI] [PubMed] [Google Scholar]
- 47.Banerjee A, Mondal NK, Das D, Ray MR. Neutrophilic inflammatory response and oxidative stress in premenopausal women chronically exposed to indoor air pollution from biomass burning. Inflammation. 2012;35(2):671–683. doi: 10.1007/s10753-011-9360-2. [DOI] [PubMed] [Google Scholar]
- 48.Rumzhum NN, Ammit AJ. Cyclooxygenase 2: Its regulation, role and impact in airway inflammation. Clinical & Experimental Allergy. 2016;46(3):397–410. doi: 10.1111/cea.12697. [DOI] [PubMed] [Google Scholar]
- 49.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(21):6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 50.Uemura Y, Kobayashi M, Nakata H, Kubota T, Bandobashi K, Saito T, Taguchi H. Effects of gm-csf and m-csf on tumor progression of lung cancer: Roles of mek1/erk and akt/pkb pathways. International journal of molecular medicine. 2006;18(2):365–373. [PubMed] [Google Scholar]
- 51.Barnes PJ. The cytokine network in chronic obstructive pulmonary disease. American journal of respiratory cell and molecular biology. 2009;41(6):631–638. doi: 10.1165/rcmb.2009-0220TR. [DOI] [PubMed] [Google Scholar]
- 52.Martey CA, Pollock SJ, Turner CK, O’Reilly KM, Baglole CJ, Phipps RP, Sime PJ. Cigarette smoke induces cyclooxygenase-2 and microsomal prostaglandin e2 synthase in human lung fibroblasts: Implications for lung inflammation and cancer. American journal of physiology Lung cellular and molecular physiology. 2004;287(5):L981–991. doi: 10.1152/ajplung.00239.2003. [DOI] [PubMed] [Google Scholar]
- 53.Migliaccio CT, Kobos E, King QO, Porter V, Jessop F, Ward T. Adverse effects of wood smoke pm(2.5) exposure on macrophage functions. Inhalation toxicology. 2013;25(2):67–76. doi: 10.3109/08958378.2012.756086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sundar IK, Chung S, Hwang JW, Lapek JD, Jr, Bulger M, Friedman AE, Yao H, Davie JR, Rahman I. Mitogen- and stress-activated kinase 1 (msk1) regulates cigarette smoke-induced histone modifications on nf-kappab-dependent genes. PloS one. 2012;7(2):e31378. doi: 10.1371/journal.pone.0031378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X, Togo S, Al-Mugotir M, Kim H, Fang Q, Kobayashi T, Wang X, Mao L, Bitterman P, Rennard S. Nf-kappab mediates the survival of human bronchial epithelial cells exposed to cigarette smoke extract. Respiratory research. 2008;9(1):66–66. doi: 10.1186/1465-9921-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ho Y-S, Chen C-H, Wang Y-J, Pestell RG, Albanese C, Chen R-J, Chang M-C, Jeng J-H, Lin S-Y, Liang Y-C, et al. Tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (nnk) induces cell proliferation in normal human bronchial epithelial cells through nfκb activation and cyclin d1 up-regulation. Toxicology and applied pharmacology. 2005;205(2):133–148. doi: 10.1016/j.taap.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 57.Martínez-García E, Irigoyen M, Ansó E, Martínez-Irujo JJ, Rouzaut A. Recurrent exposure to nicotine differentiates human bronchial epithelial cells via epidermal growth factor receptor activation. Toxicology and applied pharmacology. 2008;228(3):334–342. doi: 10.1016/j.taap.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 58.Durbin RK, Kotenko SV, Durbin JE. Interferon induction and function at the mucosal surface. Immunological reviews. 2013;255(1):25–39. doi: 10.1111/imr.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]


