Abstract
Echogenic liposomes (ELIPs) are an excellent candidate for ultrasound activated therapeutics and imaging. Although multiple experiments have established their echogenicity, the underlying mechanism has remained unknown. However, freeze-drying in the presence of mannitol during ELIP preparation has proved critical to ensuring echogenicity. Here, the role of this key component in the preparation protocol was investigated by measuring scattering from freshly prepared freeze-dried aqueous solution of mannitol—and a number of other excipients commonly used in lyophilization—directly dispersed in water without any lipids in the experiment. Mannitol, meso-erythritol, glycine, and glucose that form a highly porous crystalline phase upon freeze-drying generated bubbles resulting in strong echoes during their dissolution. On the other hand, sucrose, trehalose, and xylitol, which become glassy while freeze-dried, did not. Freeze-dried mannitol and other crystalline substances, if thawed before being introduced into the scattering volume, did not produce echogenicity, as they lost their crystallinity in the thawed state. The echogenicity disappeared in a degassed environment. Higher amounts of sugar in the original aqueous solution before freeze-drying resulted in higher echogenicity because of the stronger supersaturation and crystallinity. The bubbles created by the freeze-dried mannitol in the ELIP formulation play a critical role in making ELIPs echogenic.
I. INTRODUCTION
Liposomes are vesicles enclosed by a lipid bilayer having an aqueous core. They are widely used as a drug delivery vehicle, which can be activated by various triggers such as temperature, pH, enzymes, ultrasound, or laser. There has been a growing interest to trigger the liposomal contents release by ultrasound for simultaneous drug delivery (Bader et al., 2016; Haworth et al., 2016; Shekhar et al., 2016; Sutton et al., 2016) and imaging. Liposomes prepared using a special protocol involving freeze-drying in the presence of mannitol rendered them echogenic (Huang et al., 2001; Huang et al., 2002; Huang and MacDonald, 2004; Kopechek et al., 2011; Nahire et al., 2012; Paul et al., 2012; Raymond et al., 2014). Although we as well others verified their echogenicity, the exact mechanism remains unknown. Previous experiments established that freeze-drying in the presence of mannitol is necessary for the liposomes to be echogenic (Huang et al., 2002; Paul et al., 2012). Furthermore, freeze-dried liposomes in the presence of other excipients or cryoprotectants were found not to be echogenic (Huang et al., 2001). Here, we investigate the role of mannitol in the echogenicity of echogenic liposomes (ELIP).
To understand the role of mannitol in echogenicity of ELIPs, we briefly note here their preparation protocol (Huang et al., 2001; Buchanan et al., 2008; Paul et al., 2012). The required lipids are dissolved in chloroform and exposed to a gentle stream of N2 to evaporate the solvent leaving a thin film, which is left overnight under vacuum to completely remove the solvent. The dried lipid film is mixed with a 0.32 M mannitol solution, and the resulting mixture is sonicated in a bath sonicator. The formed liposome suspension is then freeze-dried, and the final product is stored in the powdered form, to be rehydrated before use. Although the exact mechanism of echogenicity is far from established, it is speculated that mannitol, being a weak cryoprotectant, leaves defects in the freeze-dried lipid bilayer, which in turn trap gas pockets resulting in their oscillations when subjected to ultrasound excitation (Huang et al., 2001). In fact, freeze-drying or lyophilization was found to be a crucial step—samples prepared bypassing this procedure were not echogenic. The same study also found that lyophilization in the presence of other sugars—trehalose, sucrose or lactose that are known to be better cryoprotectant—also did not give rise to any acoustic activity (Huang et al., 2001).
Here, we report on a phenomenon that we believe has a direct bearing on the observed echogenicity of liposomes prepared by freeze-drying in the presence of mannitol. We measured scattering from freeze-dried mannitol directly dispersed in water in the absence of any lipid in an experimental setup previously used to investigate echogenicity of ELIPs (Paul et al., 2012). The freeze-dried mannitol of the same molarity as that used to prepare ELIPs, when dispersed in water even without any lipid bilayer, generated a strong scattered response during dissolution. Furthermore, several other excipients, such as meso-erythritol, glycine, and glucose, which attain crystalline state after freeze-drying, were also found to be echogenic, whereas sucrose, trehalose, and xylitol, which become glassy while freeze-dried, were not. We briefly review the properties of mannitol and other excipients used during freeze-drying in the pharmaceutical industries which, as we noted, play a critical role in echogenicity of ELIPs.
II. FREEZE-DRYING (LYOPHILIZATION) IN THE PRESENCE OF EXCIPIENTS
Due to its importance in stabilizing pharmaceutical products, freeze-drying has been widely studied resulting in an extensive literature (Franks, 1998; Tang and Pikal, 2004; Rey and May, 2010). The primary goal of the process is to ensure long-term stability, elegant cake appearance, short reconstitution time, and maintenance of biological activities upon reconstitution (Abdelwahed et al., 2006). These objectives are achieved by choosing an optimal chemical formulation—appropriate amount of excipients such as mannitol under right conditions. For freeze-dried liposomes, one wants to prevent hydrolysis of phospholipids, loss of encapsulated agents, and alteration of size by aggregation (van Winden, 2003).
During freezing, pure crystalline ice forms from the aqueous solution leaving behind solutes and liposomes that become more and more concentrated and increasingly more viscous, eventually reaching an amorphous glassy phase. Mannitol is widely used in lyophilization as a bulking agent because it promotes efficient freeze-drying and elegant appearance of the freeze-dried products. However the same properties of mannitol that underlie these advantages, namely, its tendency to crystallize from frozen aqueous solution and the high eutectic temperature of mannitol/ice mixture (about −1.5 °C) (thereby shorter freeze-drying cycle), also make it a weak cryoprotectant (Kim et al., 1998). The crystallization of mannitol generates mechanical stresses leading to destabilization of the bilayers (Izutsu et al., 1994; Kim et al., 1998; Pyne et al., 2002). In contrast, a good cryoprotectant such as trehalose (arguably the best cryoprotectant), sucrose or lactose protects the lipid bilayer from collapsing under the compressive stresses during freezing by forming a glassy state inside and outside the liposomes and by hydrogen bonding with the lipid head groups (van Winden, 2003). Freezing and subsequent primary drying (sublimation of ice under vacuum), must be carried out below the glass transition temperature of the maximal freeze-concentrate of the material to avoid a collapse of the freeze-dried structure. Therefore, a high leads to substantial economic benefits. The primary drying is followed by a secondary drying step to remove unfrozen water absorbed in the product by a gradual increase of temperature (Abdelwahed et al., 2006). Similar to mannitol, glycine is also a common bulking agent with a high eutectic melting point with ice (−3 °C) enabling high drying temperature that crystallizes upon freeze-drying. Note that the degree of mannitol crystallization during freeze-drying can be controlled by the chemical composition of the solute and the processing conditions (Liao et al., 2005), e.g., by altering the pH (Akers et al., 1995) or increasing the concentration of the buffer solution (Telang et al., 2003). By choosing an appropriate concentration mannitol can even be freeze-dried in an amorphous state (Izutsu et al., 1994). Furthermore, Even though when used alone glycine and mannitol each crystallizes during freeze-drying, in combination they inhibit crystallization and give rise to stable freeze-dried products (Pyne et al., 2003).
In view of the above, one concludes that there are two different classes of excipients. The bulking agents such as mannitol are typically crystalline after freeze-drying and provide an elegant appearance of the final products and shorter drying time. The stabilizing agents such as trehalose are glassy after freeze-drying; they protect the bilayer structure but result in a longer process. Table I provides a list of common excipients and their final states. We also note their solubility—a sugar whose solubility is lower in water tends to crystallize, e.g., mannitol with solubility 20% w/w crystallizes during freezing whereas sucrose with a solubility of 67% w/w attains a glassy state. Crystallization is determined by supersaturation (driving force) and molecular mobility. For sugars with higher solubility, the high concentration of solute gives rise to a higher viscosity of the aqueous matrix. At high viscosity, the mobility contribution to the free energy of crystallization dominates, and it prevents crystallization forming a glassy state.
TABLE I.
Solubility of excipients in water and their state after freeze-drying.
| Excipient | Solubility (w/w) at 20 oC (%) | Reference | State after freeze drying | Reference |
|---|---|---|---|---|
| Sucrose | 66.5 | (Hartel, 2001) | Glassy | (Rey and May, 2010), (Ohkuma et al., 2008), (Hartel et al., 2011) |
| Glucose | 47.8 | Crystalline | (Thanatuksorn et al., 2008), (Rahman and Roos, 2017) | |
| Fructose | 78.9 | Glassy | (Rahman and Roos, 2017) | |
| Mannitol | 20 | Crystalline | (Rey and May, 2010), (Izutsu et al., 1994; Cavatur et al., 2002; Pyne et al., 2002; Telang et al., 2003), (Kim et al., 1998) | |
| Xylitol | 63 | Glassy | (Roos, 1997) | |
| Meso-Erythritol | 37 | Crystalline | (Chen et al., 2015; Fujii et al., 2015) | |
| Glycine | 25 | (Budavari, 1996) | Crystalline | (Rey and May, 2010), (Akers et al., 1995; Chongprasert et al., 2001; Pyne and Suryanarayanan, 2001; Pyne et al., 2003) |
| Trehalose | 68.9 | (Higashiyama, 2002) | Glassy | (Rey and May, 2010), (Ohkuma et al., 2008) |
While role of mannitol in ensuring echogenicity has been firmly established, the purported mechanism remains weakly linked with its poor cryoprotective capabilities. Here, we find that freeze-dried mannitol dissolving in the water is echogenic even in the absence of lipids or their bilayer structure. We claim that the echogenicity of the dissolving substance is predicated on the crystallinity of the freeze-dried highly porous products. We investigate scattering from freeze-dried aqueous solutions of all excipients from the Table I along with mannitol, some adopting crystalline state upon freeze-drying and others becoming glassy. For comparison, we also investigate scattering from mannitol without freeze-drying.
III. METHODS AND MATERIALS
The excipients—mannitol, D-glucose, sucrose, trehalose, xylitol, meso-erythritol, and glycine—were procured from Sigma-Aldrich (St. Louis, MO). The required concentration of excipient was dissolved in water, and the sample was frozen at −80 °C. After it was frozen for 5 h, the sample vial was thawed for 3 min in 50 °C water and again it was put in the freezer (VWR SignalTM, Radnor, PA) at −80 °C for 5 h. This cycle of freeze–thaw was performed three times, then frozen again for 5 h. The final frozen sample was dried in a lyophilizer (Freezone 2.5, Labconco, Kansas City, MO) at −86 °C and 0.01 mBar for 72 h. The excipients were used in both raw forms (the state of the excipient when it arrived from the supplier in powder/granular form) as well as in freeze-dried form for scattering studies for comparison purposes. The scattering experiments were also performed in DI (deionized) water as well as in 0.5% wt./vol BSA (bovine serum albumin)-water solution.
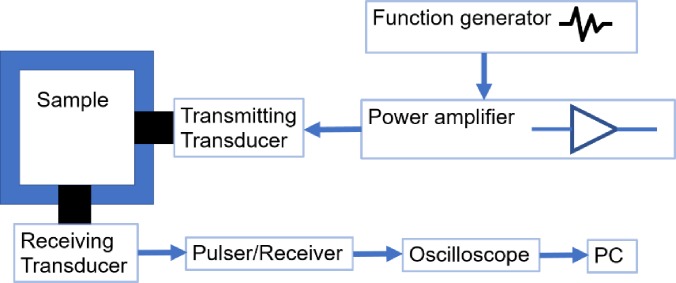
Figure 1 shows a schematic representation of the experimental setup for scattering study. A chamber (50 mm × 50 mm × 45 mm) made of polycarbonate was filled with DI water. We employed two spherically focused immersion transducers (Panametrics Transducer, Olympus NDT Corporation, Waltham, MA), each having an element diameter of 1.6 cm and a focal length of 3.05 cm. The transmitting and receiving transducers were confocally positioned at right angles. Two transducers with central frequencies of 2.25 MHz (−6 dB: 1.48–2.90 MHz) were used in the experiments for transmitting and receiving the signal. All transducers were calibrated using a needle hydrophone (HNC400, ONDA Corporation). Before each scattering measurement, 100 mg of the freeze-dried/raw excipient was added to the acoustic chamber, near the confocal volume of the transducers. Scattering measurements were performed 30 s after the introduction of the excipient. An arbitrary/function generator (Agilent, 33250A, Santa Clara, CA) was utilized to generate a 32-cycle sinusoidal pulse of 2.25 MHz frequency at a pulse repetition frequency (PRF) of 100 Hz. This signal was then amplified using a 55 dB power amplifier (A-150, ENI, Rochester, NY) and used to excite the transmitting transducer. All scattering experiments were performed at a single typical excitation setting—500 kPa, 2.25 MHz, 32 cycle. Note that previously we have examined the acoustic response of ELIPs as a function of frequency and excitation amplitude (Paul et al., 2012). But for the purpose of demonstrating the echogenicity of dispersed mannitol, scattering measurement at the single setting is sufficient. The scattered signal was passively received by the receiving transducer utilizing a pulser/receiver (5800, Panametrics-NDT, Waltham, MA) in receiving mode with a 20 dB gain (HP filter: 1 MHz, LP filter 35 MHz). The amplified signals were then fed into the oscilloscope (TDS 2012, Tektronix, Beaverton, OR) to view them in real time. Signals were acquired from the oscilloscope using LabView (National Instruments, Austin, TX) software. Thirty voltage-time RF traces were acquired in an average mode (64 samples) and stored for further processing. A Matlab FFT program was used to get the average response in the frequency domain (30 averaged voltage-time acquisitions are used). The scattered response at the fundamental frequency component was converted into a dB scale by taking the reference voltage to be unity. For experiments performed in degassed water, degassing is achieved by boiling the water, cooling to room temperature and then vacuum degassing for 48 h. All the scattering experiments were repeated five times. Data are presented as mean ± standard deviation and analyzed by the Student's t-test. Scattered response from echogenic excipients, significantly different from control values are indicated by * for p < 0.001 in the figures.
FIG. 1.
(Color online) Schematic of the experimental setup to measure acoustic scattering.
IV. RESULTS AND DISCUSSION
A. Scattering from freeze dried mannitol solution
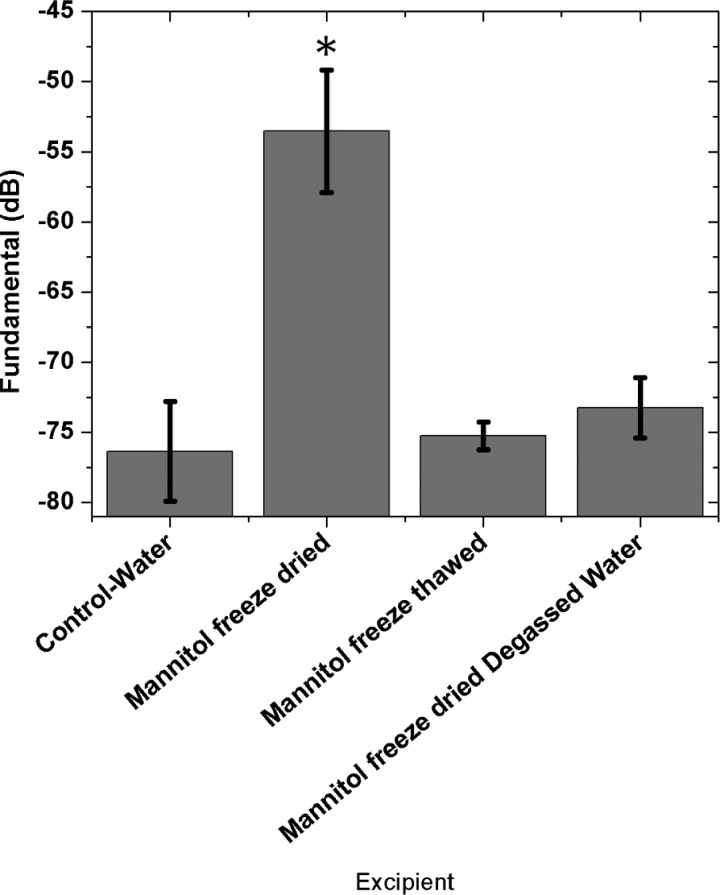
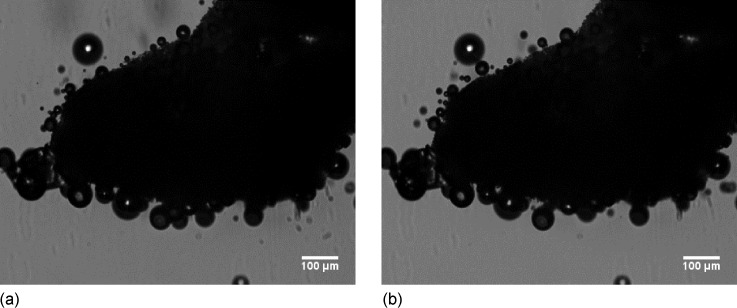
We measured scattering in the water-filled acoustic chamber after mannitol, both in freeze-dried and freeze–thawed forms, were dispersed in it. The freeze-dried mannitol during dissolution generated strong signals (Fig. 2), whereas scattering from freeze–thawed (frozen sample thawed to an aqueous solution at room temperature) mannitol sample is comparable to the control. Upon freeze–thaw mannitol loses crystallinity. Figure 2 also shows that freeze-dried mannitol in degassed water is not echogenic demonstrating that crystallinity is not sufficient, and the presence of dissolved air in the liquid is necessary for echogenicity. During dissolution, the freeze-dried aqueous solution gives rise to bubbles that generate echogenicity. In Fig. 3, we show two images 3 s apart of a small amount of freeze-dried mannitol powder dispersed in water on a glass slide under microscope (AmScope FMA050, MA) at 10× magnification (the frame rate of the integrated camera was 6 frames/s). We can see bubbles around the surface of the freeze-dried granules of mannitol being generated during dissolution. Note that there were no bubbles in the water before freeze-dried mannitol was introduced on the slide.
FIG. 2.
Scattering from a freeze-dried as well as a freeze–thawed sample of mannitol in normal and degassed water. (*) Indicates significant difference from the control (p < 0.001).
FIG. 3.
Bubble formation from the surface of a freeze-dried mannitol granule when dispersed in water under microscope at 10× magnification; two frames (a) and (b) 3 s apart.
B. Scattering from other freeze-dried excipients
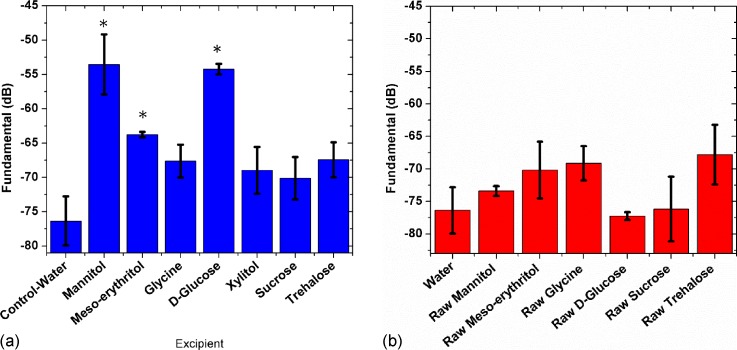
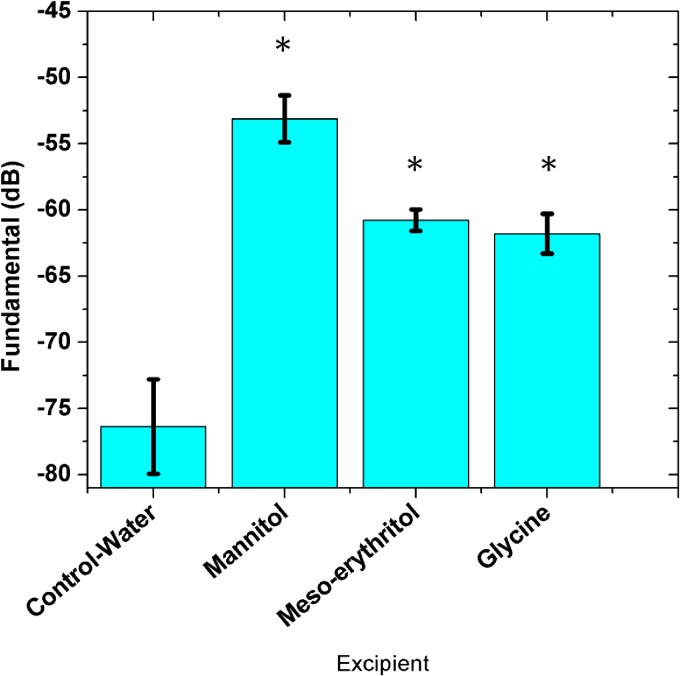
Experiments were performed to check the echogenicity from a freeze-dried aqueous solution of other excipients which form crystalline (glycine, glucose, and meso-erythritol) and glassy (sucrose, trehalose, xylitol) states after freeze-drying. For consistency, a 0.32 M solution of each of these excipients was freeze-dried. Figure 4(a) compares the scattered responses from various freeze-dried excipients dispersed in water. Mannitol and glucose generated strong signals whereas sucrose, trehalose signals were weak. Meso-erythritol signal was significant compared to the control signal, but not as strong as mannitol and glucose signal. The scattered signals from glycine (it crystallizes) as well as the other three (they assume amorphous form) were weak. The scattered signals from freeze-dried glucose were most consistent (very low standard deviation). For comparison, we also show scattering from the raw form (not freeze-dried) of these excipients dispersed in the solution in Fig. 4(b). It shows that the freeze-drying which results in crystallinity is critical for generating bubbles during dissolution and thereby obtaining a strong scattered response.
FIG. 4.
(Color online) Scattering from (a) freeze-dried excipients (b) raw (without freeze-drying) excipients dispersed in water. (*) indicates significant difference from the control (p < 0.001).
Even though meso-erythritol and glycine form crystalline states upon freeze-drying, the scattered signals from them at 0.32 M were not very strong. To explain this we note that at 0.32 M solution, different excipients, due to their difference in molecular weights, result in different solute concentration (wt./vol%), viz., D-glucose (5.8), mannitol (5.8), sucrose (11), trehalose (11), xylitol (4.9), meso-erythritol (3.9), glycine (2.4). Specifically, meso-erythritol and glycine (wt./vol%) concentrations are low compared to others at 0.32 M. We, therefore, measure scattered responses from a higher (wt./vol%) concentration—10% wt./vol samples of freeze-dried mannitol, glycine, and meso-erythritol in Fig. 5. The scattered responses from glycine and meso-erythritol increased [compared to those in Fig. 4(a)] considerably at this higher concentration but not from mannitol, which was already high at 5.8% [Fig. 4(a)]. In any event, the freeze-dried excipients that are crystalline displayed high echogenicity when in sufficient concentration: at 10% wt./vol the enhancements compared to control were more than 20 dB for mannitol, and more than 15 dB for meso-erythritol and glycine.
FIG. 5.
(Color online) Scattering from freeze-dried excipients prepared with a 10% wt./vol solution. (*) indicates significant difference from the control (p < 0.001).
We noted before that altering the freeze-drying condition can inhibit crystallization of mannitol, e.g., addition of sodium phosphate to mannitol solution before freeze-drying inhibits crystallization of mannitol during freeze drying (Izutsu et al., 1994). Consequently, Huang et al. (2002) observed decreased echogenicity in liposomes formulated with mannitol in the presence of increasing concentration of sodium phosphate. A similar reduction in echogenicity of liposomes was also observed by Huang et al. (2002) when europium chloride was added to the solution before lyophilization, once again, we believe, due to decreased crystallinity in the presence of europium chloride.
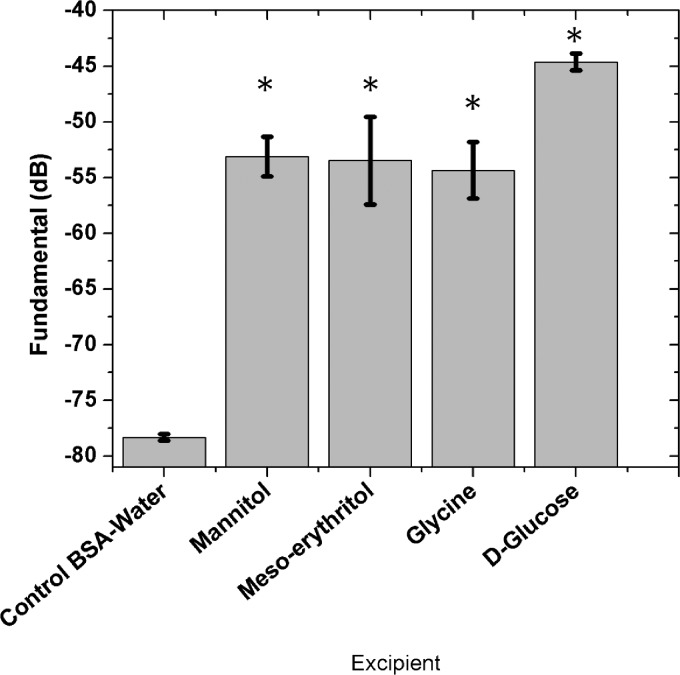
C. Scattering study from excipients in BSA-water solution
ELIPs are to be used inside the human body in the presence of many naturally occurring proteins. Therefore, we measure scattering in a BSA-solution of 0.5% wt./vol. Note that past experiments (Kopechek et al., 2011; Paul et al., 2012; Raymond et al., 2014) on ELIPs were also performed in such a BSA solution. Figure 6 shows the scattering from mannitol (10% wt./vol), meso-erythritol (10% wt./vol), glycine (10% wt./vol), and glucose (0.32 M or 5% wt./vol) in BSA-water solution along with control (BSA-water). The scattered response for each of these excipients is more in BSA solution compared to that in water solution [Fig. 4(a) and Fig. 5]. We believe that the free bubble generated by the excipients get coated by a layer of BSA acting as a surfactant. Their stability is consequently enhanced, and they generate stronger signals for a longer duration. Note that the same can happen in ELIP suspension.
FIG. 6.
Scattering from excipients in BSA-water solution. (*) indicates significant difference from the control (p < 0.001).
V. DISCUSSION AND CONCLUSION
It has been well established in the literature that liposomes prepared following a careful multi-step procedure can be made echogenic. However, the mechanism for the echogenicity of these ELIPs has so far remained elusive. We as well as others experimentally demonstrated that freeze-drying in the presence of mannitol is a crucial component to ensure echogenicity of ELIPs. However, the link between this step and resulting echogenicity has at best been tenuous. It has been speculated that mannitol is only weakly cryoprotective, and therefore leaves air trapped in freeze-dried mannitol which leads to the echogenicity. Indeed, it is well known that mannitol fails to provide adequate stability to the bilayers during freeze-drying as it crystallizes, while other sugars such as sucrose, trehalose, xylitol protect the bilayer stability.
Here, we showed that freeze-dried aqueous solutions of excipients such as mannitol, meso-erythritol, glycine, and glucose that assume a crystalline state upon freeze-drying, when dispersed in water are echogenic even without any liposomes. On the other hand, sucrose, trehalose, and xylitol that form a glassy state upon freeze-drying are not echogenic under similar conditions. Also, note that freeze-dried aqueous solutions of the former class of excipients are not echogenic in degassed solution. If the freeze-dried samples were first freeze–thawed before being introduced into water, they lost their echogenicity due to loss of crystallinity. Increasing the wt./vol % of glycine and meso-erythritol in the freeze-dried sample enhanced echogenicity due to the presence of higher amount of crystalline substance. The freeze-dried mannitol was optically seen to generate bubbles upon dispersion in water. The phenomenon of freeze-dried mannitol creating bubbles in aqueous dispersion and their consequent echogenicity have not been reported before in the literature.
This article serves two purposes. First and foremost, it establishes a direct link between freeze-dried mannitol and echogenicity of ELIPS by displaying that the former creates bubbles and generates echogenicity even in absence of lipids or liposomes. Second, it shows that the echogenicity caused by mannitol is related to the state, crystalline or amorphous, of the freeze-dried substance—only crystallinity generates echogenicity. An ELIP suspension contains large amount of freeze-dried mannitol both inside and outside the liposomes. Upon coming in contact with water they would generate bubbles. The bubbles might be generated attached to the ELIP bilayer or as independent entities. The outside bubbles without any coating would dissolve very quickly; a micron size air bubble dissolves in milliseconds (Sarkar et al., 2009). In conventional ultrasound contrast microbubbles, the coatings of lipids or proteins stabilize them (Katiyar et al., 2009; Katiyar and Sarkar, 2010). The bubbles created by freeze-dried crystalline mannitol in an ELIP suspension could also quickly acquire a lipid coating. Note that here we saw stronger echogenicity in BSA solution than in DI water, presumably due to greater stability of BSA coated microbubbles. Mannitol inside ELIPs also could generate bubbles upon coming in contact with water. Depending on the amount of mannitol available inside the vesicle these bubbles can grow and eventually burst the vesicles. However, ELIPs have been shown to retain their structural integrity and keep their encapsulated content in aqueous solution unless triggered by an external agent (Nahire et al., 2013; Nahire et al., 2014a; Nahire et al., 2014b). Therefore, bubbles produced by mannitol inside the vesicle are either too small to burst the vesicle or occurrence of such bubble-bursting events is minimal. However, note that bubble-like structures have been observed inside vesicles in TEM (Transmission electron microscopy) images (Kopechek et al., 2011; Nahire et al., 2013). It is difficult to ascertain how much of the echogenicity of an ELIP suspension arises from free mannitol without further investigation, but would be an important topic of future research. Finally, we have demonstrated the possibility of creating ELIPs not only with mannitol but with other excipients such as glycine, glucose, and meso-erythrytol, either by themselves or in combination, a topic also to be investigated in the future.
ACKNOWLEDGMENTS
K.S. and S.M. acknowledge partial support from National Institutes of Health Grant No. R01GM114080. K.S. also acknowledges partial support from National Science Foundation CBET Grant No. 1603639.
References
- 1. Abdelwahed, W. , Degobert, G. , Stainmesse, S. , and Fessi, H. (2006). “ Freeze-drying of nanoparticles: Formulation, process and storage considerations,” Adv. Drug Delivery Rev. 58, 1688–1713. 10.1016/j.addr.2006.09.017 [DOI] [PubMed] [Google Scholar]
- 2. Akers, M. J. , Milton, N. , Byrn, S. R. , and Nail, S. L. (1995). “ Glycine crystallization during freezing: The effects of salt form, pH, and ionic strength,” Pharm. Res. 12, 1457–1461. 10.1023/A:1016223101872 [DOI] [PubMed] [Google Scholar]
- 3. Bader, K. B. , Haworth, K. J. , Shekhar, H. , Maxwell, A. D. , Peng, T. , McPherson, D. D. , and Holland, C. K. (2016). “ Efficacy of histotripsy combined with rt-PA in vitro,” Phys. Med. Biol. 61, 5253–5274. 10.1088/0031-9155/61/14/5253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buchanan, K. D. , Huang, S. , Kim, H. , Macdonald, R. C. , and McPherson, D. D. (2008). “ Echogenic liposome compositions for increased retention of ultrasound reflectivity at physiologic temperature,” J. Pharm. Sci. 97, 2242–2249. 10.1002/jps.21173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Budavari, S. (1996). “ XP002191966,” in The Merck Index: Encyclopedia of Chemicals, Drugs, and Biologicals, 13th ed. ( ACS, Washington, DC: ), pp. 1–2708. [Google Scholar]
- 6. Cavatur, R. K. , Vemuri, N. M. , Pyne, A. , Chrzan, Z. , Toledo-Velasquez, D. , and Suryanarayanan, R. (2002). “ Crystallization behavior of mannitol in frozen aqueous solutions,” Pharm. Res. 19, 894–900. 10.1023/A:1016177404647 [DOI] [PubMed] [Google Scholar]
- 7. Chen, H. , Chen, S. , Chen, H. , Wu, Y. , and Shu, G. (2015). “ Effects of sugar alcohol and proteins on the survival of Lactobacillus bulgaricus LB6 during freeze drying,” Acta Sci. Pol. Technol. Aliment. 14, 117–124. 10.17306/J.AFS.2015.2.13 [DOI] [PubMed] [Google Scholar]
- 8. Chongprasert, S. , Knopp, S. A. , and Nail, S. L. (2001). “ Characterization of frozen solutions of glycine,” J. Pharm. Sci. 90, 1720–1728. 10.1002/jps.1121 [DOI] [PubMed] [Google Scholar]
- 9. Franks, F. (1998). “ Freeze-drying of bioproducts: Putting principles into practice,” Eur. J. Pharm. Biopharm. 45, 221–229. 10.1016/S0939-6411(98)00004-6 [DOI] [PubMed] [Google Scholar]
- 10. Fujii, K. , Izutsu, K.-i. , Kume, M. , Yoshino, T. , Yoshihashi, Y. , Sugano, K. , and Terada, K. (2015). “ Physical characterization of meso-erythritol as a crystalline bulking agent for freeze-dried formulations,” Chem. Pharm. Bull. 63, 311–317. 10.1248/cpb.c14-00692 [DOI] [PubMed] [Google Scholar]
- 11. Hartel, R. W. (2001). Crystallization in Foods ( Springer, New York: ). [Google Scholar]
- 12. Hartel, R. W. , Ergun, R. , and Vogel, S. (2011). “ Phase/state transitions of confectionery sweeteners: Thermodynamic and kinetic aspects,” Compr. Rev. Food Sci. Food Saf. 10, 17–32. 10.1111/j.1541-4337.2010.00136.x [DOI] [Google Scholar]
- 13. Haworth, K. J. , Raymond, J. L. , Radhakrishnan, K. , Moody, M. R. , Huang, S.-L. , Peng, T. , Shekhar, H. , Klegerman, M. E. , Kim, H. , and McPherson, D. D. (2016). “ Trans-stent B-mode ultrasound and passive cavitation imaging,” Ultrasound Med. Biol. 42, 518–527. 10.1016/j.ultrasmedbio.2015.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higashiyama, T. (2002). “ Novel functions and applications of trehalose,” Pure Appl. Chem. 74, 1263–1269. 10.1351/pac200274071263 [DOI] [Google Scholar]
- 15. Huang, S. L. , Hamilton, A. J. , Nagaraj, A. , Tiukinhoy, S. D. , Klegerman, M. E. , McPherson, D. D. , and MacDonald, R. C. (2001). “ Improving ultrasound reflectivity and stability of echogenic liposomal dispersions for use as targeted ultrasound contrast agents,” J. Pharm. Sci. 90, 1917–1926. 10.1002/jps.1142 [DOI] [PubMed] [Google Scholar]
- 16. Huang, S. L. , Hamilton, A. J. , Pozharski, E. , Nagaraj, A. , Klegerman, M. E. , McPherson, D. D. , and MacDonald, R. C. (2002). “ Physical correlates of the ultrasonic reflectivity of lipid dispersions suitable as diagnostic contrast agents,” Ultrasound Med. Biol. 28, 339–348. 10.1016/S0301-5629(01)00512-9 [DOI] [PubMed] [Google Scholar]
- 17. Huang, S. L. , and MacDonald, R. C. (2004). “ Acoustically active liposomes for drug encapsulation and ultrasound-triggered release,” Biochim. Biophys. Acta, Biomembr. 1665, 134–141. 10.1016/j.bbamem.2004.07.003 [DOI] [PubMed] [Google Scholar]
- 18. Izutsu, K.-i. , Yoshioka, S. , and Terao, T. (1994). “ Effect of mannitol crystallinity on the stabilization of enzymes during freeze-drying,” Chem. Pharm. Bull. 42, 5–8. 10.1248/cpb.42.5 [DOI] [PubMed] [Google Scholar]
- 19. Katiyar, A. , and Sarkar, K. (2010). “ Stability analysis of an encapsulated microbubble against gas diffusion,” J. Coll. Interface Sci. 343, 42–47. 10.1016/j.jcis.2009.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Katiyar, A. , Sarkar, K. , and Jain, P. (2009). “ Effects of encapsulation elasticity on the stability of an encapsulated microbubble,” J. Colloid Interface Sci. 336, 519–525. 10.1016/j.jcis.2009.05.019 [DOI] [PubMed] [Google Scholar]
- 21. Kim, A. I. , Akers, M. J. , and Nail, S. L. (1998). “ The physical state of mannitol after freeze-drying: Effects of mannitol concentration, freezing rate, and a noncrystallizing cosolute,” J. Pharm. Sci. 87, 931–935. 10.1021/js980001d [DOI] [PubMed] [Google Scholar]
- 22. Kopechek, J. A. , Haworth, K. J. , Raymond, J. L. , Douglas Mast, T. , Perrin, S. R., Jr. , Klegerman, M. E. , Huang, S. , Porter, T. M. , McPherson, D. D. , and Holland, C. K. (2011). “ Acoustic characterization of echogenic liposomes: Frequency-dependent attenuation and backscatter,” J. Acoust. Soc. Am. 130, 3472–3481. 10.1121/1.3626124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liao, X. , Krishnamurthy, R. , and Suryanarayanan, R. (2005). “ Influence of the active pharmaceutical ingredient concentration on the physical state of mannitol—Implications in freeze-drying,” Pharm. Res. 22, 1978–1985. 10.1007/s11095-005-7625-x [DOI] [PubMed] [Google Scholar]
- 24. Nahire, R. , Haldar, M. K. , Paul, S. , Ambre, A. H. , Meghnani, V. , Layek, B. , Katti, K. S. , Gange, K. N. , Singh, J. , Sarkar, K. , and Mallik, S. (2014a). “ Multifunctional polymersomes for cytosolic delivery of gemcitabine and doxorubicin to cancer cells,” Biomaterials 35, 6482–6497. 10.1016/j.biomaterials.2014.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nahire, R. , Haldar, M. K. , Paul, S. , Mergoum, A. , Ambre, A. H. , Katti, K. S. , Gange, K. N. , Srivastava, D. K. , Sarkar, K. , and Mallik, S. (2013). “ Polymer-coated echogenic lipid nanoparticles with dual release triggers,” Biomacromolecules 14, 841–853. 10.1021/bm301894z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nahire, R. , Hossain, R. , Patel, R. , Paul, S. , Meghnani, V. , Ambre, A. H. , Gange, K. N. , Katti, K. S. , Leclerc, E. , Srivastava, D. K. , Sarkar, K. , and Mallik, S. (2014b). “ pH-Triggered echogenicity and contents release from liposomes,” Mol. Pharmaceutics 11, 4059–4068. 10.1021/mp500186a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nahire, R. , Paul, S. , Scott, M. D. , Singh, R. K. , Muhonen, W. W. , Shabb, J. , Gange, K. N. , Srivastava, D. K. , Sarkar, K. , and Mallik, S. (2012). “ Ultrasound enhanced matrix metalloproteinase-9 triggered release of contents from echogenic liposomes,” Mol. Pharmaceutics 9, 2554–2564. 10.1021/mp300165s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ohkuma, C. , Kawai, K. , Viriyarattanasak, C. , Mahawanich, T. , Tantratian, S. , Takai, R. , and Suzuki, T. (2008). “ Glass transition properties of frozen and freeze-dried surimi products: Effects of sugar and moisture on the glass transition temperature,” Food Hydrocolloids 22, 255–262. 10.1016/j.foodhyd.2006.11.011 [DOI] [Google Scholar]
- 29. Paul, S. , Russakow, D. , Nahire, R. , Nandy, T. , Ambre, A. H. , Katti, K. , Mallik, S. , and Sarkar, K. (2012). “ In vitro measurement of attenuation and nonlinear scattering from echogenic liposomes,” Ultrasonics 52, 962–969. 10.1016/j.ultras.2012.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pyne, A. , Chatterjee, K. , and Suryanarayanan, R. (2003). “ Solute crystallization in mannitol–glycine systems—implications on protein stabilization in freeze-dried formulations,” J. Pharm. Sci. 92, 2272–2283. 10.1002/jps.10487 [DOI] [PubMed] [Google Scholar]
- 31. Pyne, A. , Surana, R. , and Suryanarayanan, R. (2002). “ Crystallization of mannitol below Tg′ during freeze-drying in binary and ternary aqueous systems,” Pharm. Res. 19, 901–908. 10.1023/A:1016129521485 [DOI] [PubMed] [Google Scholar]
- 32. Pyne, A. , and Suryanarayanan, R. (2001). “ Phase transitions of glycine in frozen aqueous solutions and during freeze-drying,” Pharm. Res. 18, 1448–1454. 10.1023/A:1012209007411 [DOI] [PubMed] [Google Scholar]
- 33. Rahman, M. S. , and Roos, Y. H. (2017). Glass Transition and Phase Transitions in Food and Biological Materials ( Wiley, New York: ), p. 824. [Google Scholar]
- 34. Raymond, J. L. , Haworth, K. J. , Bader, K. B. , Radhakrishnan, K. , Griffin, J. K. , Huang, S.-L. , McPherson, D. D. , and Holland, C. K. (2014). “ Broadband attenuation measurements of phospholipid-shelled ultrasound contrast agents,” Ultrasound Med. Biol. 40, 410–421. 10.1016/j.ultrasmedbio.2013.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rey, L. , and May, J. C. (2010). Freeze-Drying/Lyophilization of Pharmaceutical and Biological Products ( CRC Press, Boca Raton, FL: ), p. 584. [Google Scholar]
- 36. Roos, Y. (1997). “ Frozen state transitions in relation to freeze drying,” J. Therm. Anal. Calorim. 48, 535–544. 10.1007/BF01979500 [DOI] [Google Scholar]
- 37. Sarkar, K. , Katiyar, A. , and Jain, P. (2009). “ Growth and dissolution of an encapsulated contrast microbubble,” Ultrasound Med. Biol. 35, 1385–1396. 10.1016/j.ultrasmedbio.2009.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shekhar, H. , Bader, K. B. , Huang, S. , Peng, T. , Huang, S. , McPherson, D. D. , and Holland, C. K. (2016). “ In vitro thrombolytic efficacy of echogenic liposomes loaded with tissue plasminogen activator and octafluoropropane gas,” Phys. Med. Biol. 62, 517–538. 10.1088/1361-6560/62/2/517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sutton, J. , Haworth, K. , Shanmukhappa, S. , Moody, M. , Klegerman, M. , Griffin, J. , Patton, D. , McPherson, D. , and Holland, C. (2016). “ Delivery of bevacizumab to atheromatous porcine carotid tissue using echogenic liposomes,” Drug Delivery 23, 3594–3605. 10.1080/10717544.2016.1212441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tang, X. C. , and Pikal, M. J. (2004). “ Design of freeze-drying processes for pharmaceuticals: Practical advice,” Pharm. Res. 21, 191–200. 10.1023/B:PHAM.0000016234.73023.75 [DOI] [PubMed] [Google Scholar]
- 41. Telang, C. , Yu, L. , and Suryanarayanan, R. (2003). “ Effective inhibition of mannitol crystallization in frozen solutions by sodium chloride,” Pharm. Res. 20, 660–667. 10.1023/A:1023263203188 [DOI] [PubMed] [Google Scholar]
- 42. Thanatuksorn, P. , Kajiwara, K. , Murase, N. , and Franks, F. (2008). “ Freeze–thaw behaviour of aqueous glucose solutions—The crystallisation of cubic ice,” Phys. Chem. Chem. Phys. 10, 5452–5458. 10.1039/b802042f [DOI] [PubMed] [Google Scholar]
- 43. van Winden, E. C. A. (2003). “ Freeze-drying of liposomes: Theory and practice,” Liposomes, Pt. A 367, 99–110. 10.1016/S0076-6879(03)67008-4 [DOI] [PubMed] [Google Scholar]