Abstract
Group 2 innate lymphoid cells (ILC2s) reside in multiple organs in the body, where they play roles in immunity, tissue homeostasis, and metabolic regulation. However, little is known about the regulatory mechanisms of ILC2s in different organs. Here, we identified ILC2s in the mouse uterus and found that they express cell surface molecules, including the IL-33 receptor ST2, that are roughly comparable to those expressed by lung ILC2s. Both in vivo and in vitro treatment with IL-33 induced type 2 cytokine production in uterine ILC2s, suggesting that they respond to IL-33 in a manner similar to ILC2s in other organs. Importantly, uterine ILC2s were nearly absent in ovariectomized mice and were increased in wild-type mice by estrogen administration, whereas lung ILC2s were unaffected by both ovariectomy and estrogen administration. Likewise, a marked reduction in uterine ILC2s was observed in mice deficient in ERα or ERβ. Furthermore, uterine ILC2s highly expressed estrogen receptor α (ERα), and in vitro culture of isolated uterine ILC2s with 17β-estradiol modified expression of a number of genes. Finally, an increased prevalence in neonatal mortality was observed in litters from dams lacking the IL-33 receptor, ST2. Taken together, our findings indicate that unlike lung IL2Cs, uterine ILC2s are regulated by female sex hormones, which may specialize them for specific physiological functions.
Introduction
Innate lymphoid cells (ILCs) are the most recently identified immune cell type and contribute to inflammation, immunity, and the maintenance of tissue integrity and homeostasis (1). Groups 2 ILCs (ILC2s) respond to innate immunological stimuli and signals from stressed or injured cells, particularly cytokines that are produced by mucosal epithelium, such as thymic stromal lymphopoietin, interleukin (IL)-25, and IL-33 (2-6). Originally, ILC2s were described in type 2 immune responses that protect against helminth infections and drive airway inflammation implicated in allergic diseases (7). ILC2s are also involved in airway epithelium repair after viral infections through the production of the epidermal growth factor, amphiregulin (8). Recently, ILC2s have been shown to regulate thermogenesis from beige fat (9, 10), and to prevent metabolic syndrome and insulin resistance (11, 12). Thus, ILC2s likely carry out pleiotropic functions beyond innate immunity that are specific to the organs in which they reside.
The female reproductive tract (FRT) is unique because it must accept a semi-allograft fetus while protecting it against pathogens. Although a shift from Th1- to Th2-type adaptive immunity in the placental environment during pregnancy is considered critical for the preservation of the fetus (13), many gaps remain in our understanding of FRT immune regulation. Innate immune cells, such as NK cells, have been implicated in the successful pregnancy by establishing an immunosuppressive environment and promoting tissue growth (14, 15). Furthermore, NK cells have been shown to induce regulatory T (Treg) cells that may promote immune tolerance in the fetal-maternal interface (16). IL-33 is present in the endothelium of the FRT, as well as in smooth muscle cells of the placenta and in CD14+ macrophages in the chorioamniotic membranes (17). IL-33 has been implicated in ovarian tissue homeostasis and folliculogenesis (18, 19), and its production by endometrial stromal cells may promote embryo implantation in mice (20).
Given the potential role of IL-33 in both the FRT and the pleiotropic functions of ILC2s, it is possible that IL-33-reponsive ILC2s are present in the uterus. The aim of this study was to examine this possibility, as well as how these cells are regulated in the organ. We found a small but distinct population of ILC2s in the mouse uterus, which are activated by IL-33 to produce type 2 cytokines. Importantly, uterine ILC2s highly expressed the estrogen receptor, ERα, and the number of uterine ILC2s, but not lung ILC2s, was controlled by female sex hormone levels. Furthermore, in vitro culture of isolated uterine ILC2s with estrogen modified expression of a number of genes. These observations suggest that sex hormones likely play critical roles in the regulation of ILC2s in an organ-specific manner, and that sex differences need to be considered when studying these cell types.
Materials and Methods
Mice
BALB/cJ (BALB/c, stock number 000651), C57BL/6J (C57BL/6, stock number 000664), Esr1-/- (αER-/-, C57BL/6 background, stock number 004744), Esr2-/- (βER-/-, C57BL/6 background, stock number 004745), and ovariectomized or sham-operated BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The IL-5-reporter C.129S4(B6)-Il5tm1Ktk (Il5Venus) mice (21) and ST2-/- mice (22) were gifts from Dr. Kiyoshi Takatsu, Toyama University, Japan and Dr. Andrew McKenzie, Medical Research Council, London, UK, respectively. The Il5Venus and ST2-/- mice (both on BALB/cJ background) were maintained in the Mayo Clinic animal facility. Male and female mice aged 8–12 weeks were used in all the experiments. All animal experiments and handling procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee and performed according to their guidelines.
Reagents
Fluorescently-labeled antibodies against CD3 (145-2C11), CD25 (PC61), CD44 (IM7), CD16/CD32 (2.4G2), CD14 (rmC5-3), CD45R/B220 (RA3-6B2), Thy1.2 (53-2.1), ICOS (7E.17G9), Sca-1 (D7), CD69 (H1.2F3), MHC II (AMS-32.1), and Siglec-F (E50-2440) were purchased from BD Biosciences (San Jose, CA). The anti-T1/ST2 (DJ8) antibody was from MD Biosciences (St. Paul, MN). The antibodies to CD127/IL-7Rα (A7R34) and Gata3 (TWAJ) were from eBioscience (San Diego, CA). Recombinant mouse IL-7 and IL-33 were purchased from eBioscience and recombinant mouse IL-25 was from R&D Systems (Minneapolis, MN). The placebo, 17β-estradiol (E2), and combination progesterone (P4)/E2 pellets were ordered from Innovative Research of America (Sarasota, FL) and E2 powder for tissue culture was purchased from Sigma-Aldrich (St. Louis, MO).
Flow cytometry and cytokine production analyses
Lungs, uteri, and femurs were harvested from naïve wild-type (WT) or gene-deficient mice, as well as from those treated with IL-33 or IL-25. To obtain single-cell suspensions, lungs or uteri were homogenized using the gentleMACS dissociator (Miltenyi Biotec, San Diego, CA) in buffer containing a cocktail of collagenases, (Liberase TM, Roche Diagnostics, Indianapolis, IN) as described previously (6). Single-cell suspensions of bone marrow were also obtained from femurs. For flow cytometry, single-cell suspensions were stained with antibodies for lineage markers (CD3, CD14, CD16/CD32, and B220), CD25 and CD44, followed by analysis (BD FACSCanto®) or sorting (BD FACSAria®). Lineage-negative, CD25+CD44hi lymphocytes were identified as ILC2s (6). Permeabilization for intranuclear staining for Gata3 was performed by using a Foxp3/transcription factor staining kit (eBioscience). CountBright™ absolute counting beads (Life Technologies, Grand Island, NY) were added to calculate the absolute cell numbers in each sample.
For cultures, uterine single cells or sorted ILC2s (i.e., Lin-CD25+CD44hi cells) were resuspended in RPMI 1640 medium supplemented with 50 μM β-mercaptoethanol, 100 units/mL penicillin, 100 μg/mL streptomycin, and 10% FBS (RPMI-10). Cells were then cultured at a concentration of 0.5–4×105 cells/mL, either with untreated medium or with an IL-33 (10 ng/mL) and IL-7 (10 ng/mL) treated medium, for seven days at 37°C and 5% CO2. Supernatants were analyzed for IL-5 and IL-13 using DuoSet ELISAs (R&D Systems, Minneapolis, MN). In some experiments, uterine single-cell suspensions were obtained from Il5Venus mice, cultured with either PBS or IL-33 and IL-7-treated PBS for 48 hours, and examined via flow cytometry.
In vivo administration of cytokines and hormones
To examine the effects of IL-25 or IL-33 on ILC2s in vivo, IL-33 (400 ng), IL-25 (400 ng), or vehicle (PBS) was administered intraperitoneally (i.p.) three times (on days 1, 2 and 3) to naïve Il5Venus mice. Lungs and uteri were collected 24 hours after the final administration, and ILC2s were identified via flow cytometry as described above. To determine the effect of exogenous E2 on ILC2s in the lungs and uteri, timed-release E2 pellets (0.25 mg released over 60 days) were inserted between the ear and shoulder of naïve BALB/c female mice. Additionally, to investigate the effect of loss and reconstitution of female sex hormones, timed-release pellets (40 mg P4 and 0.1 mg E2, released over 60 days) were inserted in ovariectomized (OVX) mice. Placebo pellets containing only matrix components were used as a control. Lungs and uteri were collected 2-3 weeks after pellet placement and ILC2 numbers were determined via flow cytometry or homogenized in 0.5 mL PBS for cytokine analyses. The homogenates were centrifuged at 10,000 × g at 4°C for 15 minutes, and protein concentrations in the homogenates were quantified using a Bio-Rad DC Protein Assay kit. Subsequently, IL-33 and IL-5 levels were quantified by DuoSet or Quantikine ELISA kits, respectively (R & D Systems).
Gene microarray analysis and quantitative PCR
For gene microarray analysis, ILC2s were sorted from single-cell suspensions of pooled lungs and uteri from 5 and 20 mice, respectively. ILC2s were cultured for 16 hours in RPMI medium with 10% charcoal/dextran treated calf serum (HyClone, GE Healthcare Life Sciences, Logan, UT) with or without 100 ng/mL E2. Total RNA was purified from lung and uterine ILC2s with TRIzol and PureLink RNA Mini Kit columns (Thermo Fisher Scientific, Waltham, MA). mRNA was amplified using the GeneChip™ 3′ IVT Pico Kit (Thermo Fisher Scientific, Waltham, MA) and probed via the GeneChip™ Mouse Genome 430 2.0 Array (Thermo Fisher Scientific). Microarray data have been deposited in the ArrayExpress database at EMBL-EBI (www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-5789 (direct link https://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-5789).
For quantitative PCR, total RNA was purified from lung and uterine ILC2s with TRIzol and PureLink RNA Mini Kit columns as described above, and cDNA was reverse transcribed with iScript (Bio-Rad Laboratories, Hercules, Calif). mRNA transcripts for Esr1, Esr2, Bik and Il1f9 were quantified by real-time PCR using TaqMan Gene Expression Arrays and TaqMan Gene Expression Master Mix (Thermo Fisher Scientific), per the manufacturer's instructions. Data was normalized to 18S transcript levels.
Mating of ST2-/- mice
Wild-type BALB/c and ST2-/- female mice were mated with BALB/c and C57BL/6 male mice. The litter sizes and conditions at birth and 24 hours post-partum were recorded.
Statistics
Data are presented as means ± SEMs for either the number of mice or experiments, as indicated. In box-and-whisker plots, the ends of each box designate the first and third quartiles, the center line represents the median, and the whisker shows the tenth and ninetieth percentiles. Statistics were performed using the Mann-Whitney or Fisher's exact test, as appropriate for each set of experimental conditions. A value of p<0.05 was considered significant.
Results
ILC2s are present in the uterus
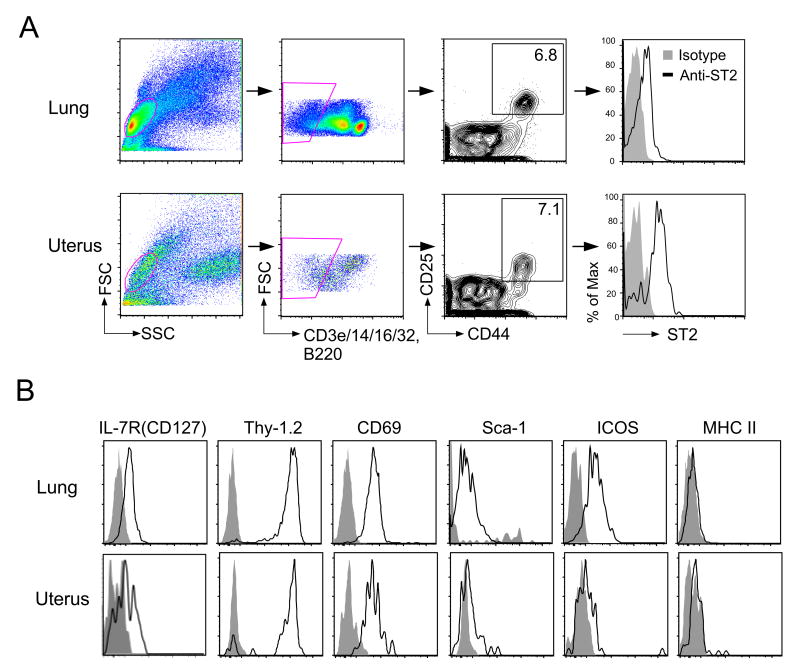
Although the presence of ILC2s has been demonstrated in multiple organs (1), it is unclear whether ILC2s are localized to the uterus. To address this question, we obtained single-cell suspensions of lung and uterine tissues from naïve BALB/c female mice, and stained them for ILC2s. We found that cells taken from the lung contained a distinct population of Lin-CD25+CD44hi lymphocytes (Figure 1A, top row), which has previously been identified as ILC2s (6). A similar population of lymphocytes was identified in the uterus (Figure 1A, bottom row); ILC2s constituted approximately 0.3% of the total uterine cells. Similar to lung ILC2s, uterine ILC2s expressed the IL-33 receptor ST2 and the canonical transcription factor Gata3 (Figure 1A and Supplemental Figure 1). A panel of other surface molecules were also expressed by both uterine and lung ILC2s, including CD127 (IL-7R), Thy-1.2, and CD69, but not MHC II (Figure 1B). In contrast, uterine ILC2s showed minimal expression of Sca-1 and ICOS, whereas both were highly expressed in lung ILC2s, suggesting that the tissue microenvironment may affect ILC2 phenotypes.
Figure 1. ILC2s are present in the uterine tissue of naïve mice.
(A) Single-cell suspensions were prepared from the lungs (top panels) and uteri (lower panels) of naïve BALB/c mice. ILC2s are identified as lineage (CD3, CD14, CD16/32 and B220)-negative (Lin-) and CD25+CD44hi lymphocytes, as described by Bartemes et al. (6). Right panel: The expression of the IL-33 receptor, ST2 is presented. Data are representative of >10 experiments showing similar results. (B) Lin-CD25+CD44hi cells identified in (A) were examined for the expression of selected cell surface molecules via flow cytometry. Grey filled histograms, isotype control; solid line, indicated target.
Uterine ILC2s respond to IL-33
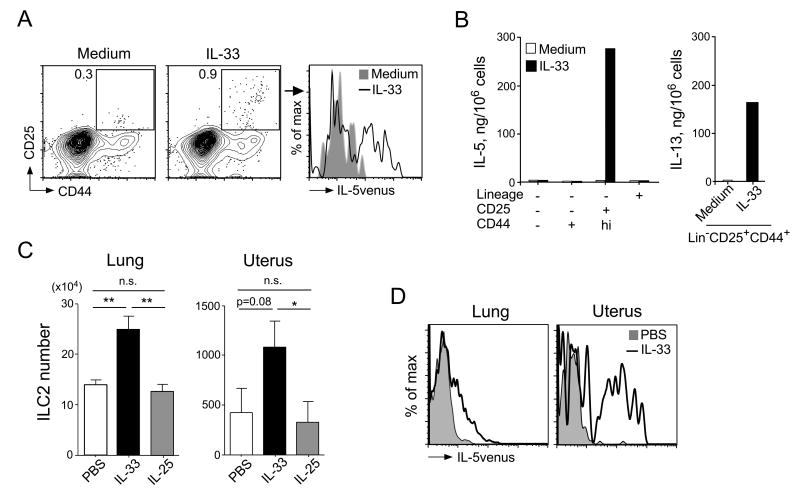
A robust production of IL-5 and IL-13 in response to stimulation with IL-33 is a hallmark of ILC2s (6, 23). To examine whether uterine ILC2s share this capacity, we obtained uterine single-cell suspensions from naïve IL-5 reporter Il5Venus mice and stimulated the cells with IL-33 for seven days in vitro. We found that the prevalence of ILC2s (i.e., Lin-CD25+CD44hi cells) was higher in samples treated with IL-33 than in those cultured in untreated medium (Figure 2A). Furthermore, approximately 50% of the uterine ILC2s cultured with IL-33 tested positive for IL-5venus, whereas none of the ILC2s cultured with untreated medium were positive. Additionally, all other uterine cell populations treated with IL-33 were negative for IL-5 (data not shown).
Figure 2. Uterine ILC2s are activated by IL-33 in vitro and in vivo.
(A) Uterine single-cell suspensions from naïve IL-5-reporter C.129S4(B6)-Il5tm1Ktk (Il5Venus) mice were cultured for seven days with untreated medium or with IL-33 and IL-7 (each at 10 ng/mL). ILC2s were identified using the gating strategy outlined in Figure 1A, and IL-5venus expression was examined by flow cytometry. (B) Three populations of Lin- cells, including CD25-CD44-, CD25-CD44+, and CD25+CD44hi, and Lin+ cells were sorted from uterine, single-cell suspensions taken from naïve BALB/c mice. Cells were cultured with medium alone or with IL-33 and IL-7 for seven days. The levels of IL-5 (left panel) and IL-13 (right panel) in the supernatants were analyzed by ELISA. Data are representative of two experiments. (C) Naïve Il5Venus mice were injected i.p. with PBS, IL-33, or IL-25 (400 ng/dose), once daily for three days. Twenty-four hours after the last injection, lungs and uteri were harvested and analyzed by flow cytometry. Data are presented as the mean ± SEM. n = 5–6 mice/group. *, p<0.05 and **, p<0.01 (Mann-Whitney U) between the groups indicated by horizontal lines. (D) Naïve Il5Venus mice were treated as described in (C), and IL-5venus expression was examined after gating on ILC2s. Grey filled histogram, PBS; solid line, IL-33. n = 5–6 mice/group.
To verify this observation, we isolated ILC2s from naïve BALB/c uterine single-cell suspensions by cell sorting. We observed that treatment with IL-33 in vitro resulted in robust production of IL-5 and IL-13 (>100 ng/106 cells) by uterine ILC2s (Figure 2B). Conversely, no other Lin- populations, including Lin-CD25-CD44- and Lin-CD25-CD44+, or Lin+ cells, produced IL-5 in response to IL-33. The IL-5 levels produced by uterine ILC2s were comparable to, if not larger than, those produced by lung ILC2s (6).
We next examined whether uterine ILC2s respond to IL-33 in vivo. To this end, Il5Venus mice were administered a 400-ng daily dose of IL-33 or IL-25 in PBS, or untreated PBS, for three days. IL-33 treatment significantly increased the number of lung ILC2s by approximately 75% (Figure 2C, p<0.01). Similarly, significantly more ILC2s were present in the uteri of IL-33-treated mice than in IL-25-treated mice (p<0.05, Figure 2C, right panel). Furthermore, IL-33 treatment resulted in expression of IL-5venus in approximately 25% and 50% of lung and uterine ILC2s, respectively (Figure 2D). In contrast, IL-25 treatment did not affect the number of ILC2s (Figure 2C) or IL-5venus expression (data not shown). Additionally, while IL-5 was below the level of detection in uterine tissue lysates from untreated mice, and was only minimally induced by IL-25 (3.9 ± 2.4 pg/10 mg protein), IL-33 increased IL-5 levels to 535.3 ± 213.5 pg/10 mg protein (mean±SEM, p<0.05, n=5). Taken together, this series of in vitro and in vivo experiments shows that ILC2s in the uterus are responsive to IL-33 and produce large quantities of IL-5 and IL-13, similar to those in the lungs.
Estrogen increases ILC2s in the uterus, not in the lung
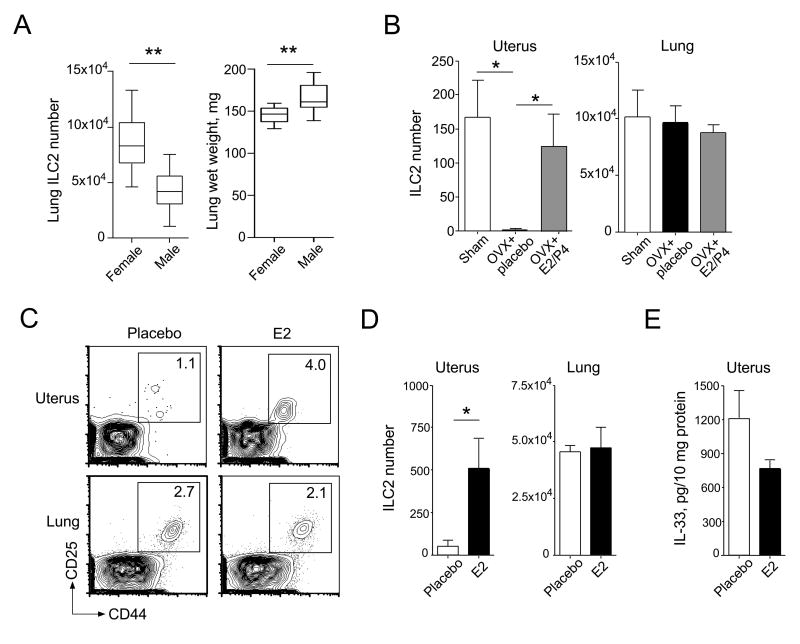
In our previous work (6), we observed sex differences in the prevalence of lung ILC2s. For example, lungs from naïve female BALB/c mice contained an average of 8.5±0.6×104 ILC2s while lungs from their male counterparts averaged 4.3±0.4×104 ILC2s (Figure 3A, p<0.01, n=16). Male lungs were significantly larger (166±5 mg) compared to female lungs (145±3 mg, p<0.01), suggesting that lung volume does not account for this difference. This observation, along with an emerging body of literature suggesting that IL-33 and ST2 play roles in the FRT (17-20), led us to hypothesize that female sex hormones, such as estrogen (17β-estradiol or E2) and progesterone (P4), might affect ILC2 abundance in the lungs and uteri.
Figure 3. Female sex hormones regulate ILC2 numbers in the uterus.
(A) Lungs were harvested from naïve BALB/c female and male mice (n = 16 mice/each group), weighed, and examined for ILC2s by flow cytometry, as described in Figure 1A. The results are shown as box-and-whisker plots. **, p<0.01 (Mann-Whitney U) between the groups indicated by horizontal lines. (B) Naïve BALB/c mice were sham-operated or ovariectomized (OVX). OVX mice were implanted with placebo pellets (OVX + placebo) or a combination of E2 and P4 pellets (OVX + E2/P4). ILC2s in the uteri and lungs were examined three weeks after pellet implantation. Data are shown as the mean ± SEM (n = 4 mice/group). *, p<0.05 and **, p<0.01 (Mann-Whitney U) between the groups indicated by horizontal lines. (C and D) Naïve BALB/c mice were implanted with placebo or estrogen (E2, 0.25 mg released over 60 days) pellets, and uterine and lung ILC2 numbers were determined two weeks later. Representative FACS plots (C) and the summarized data from 4 mice per group (D) are shown. Data are shown as the mean ± SEM. *, p<0.05 (Mann-Whitney U) between the groups indicated by horizontal lines. (E) The levels of IL-33 in uterine homogenates from (C) were analyzed by ELISA. Data are shown as the mean ± SEM of 4 mice per group.
In support of this hypothesis, we found that loss of female sex hormones in OVX adult female BALB/c mice significantly decreased uterine ILC2 numbers, compared to female mice subjected to sham surgery (Figure 3B). Reconstitution of E2 and P4 for three weeks after OVX restored uterine ILC2 numbers. Interestingly, lung ILC2 numbers were not affected by OVX, consistent with recent observations by other investigators (24). Additionally, because OVX may affect hormones other than E2 and P4, we examined whether an increase in E2 alone was sufficient to affect ILC2s in otherwise normal mice. To this end, we placed timed-release E2 pellets subcutaneously in naïve WT female mice. After 14 days of E2 exposure, the number of uterine ILC2s significantly increased by approximately 5-fold, compared to mice exposed to placebo pellets (Figure 3C and 3D). Lung ILC2 numbers were again not affected by E2 administration. Furthermore, no differences in uterine IL-33 levels were observed in mice treated with placebo or E2 (Figure 3E). Taken together, these findings suggest that ILC2 numbers in the uterus are increased by female sex hormones, particularly E2, while those in the lungs remain unchanged.
Estrogen regulates ILC2s in uterus
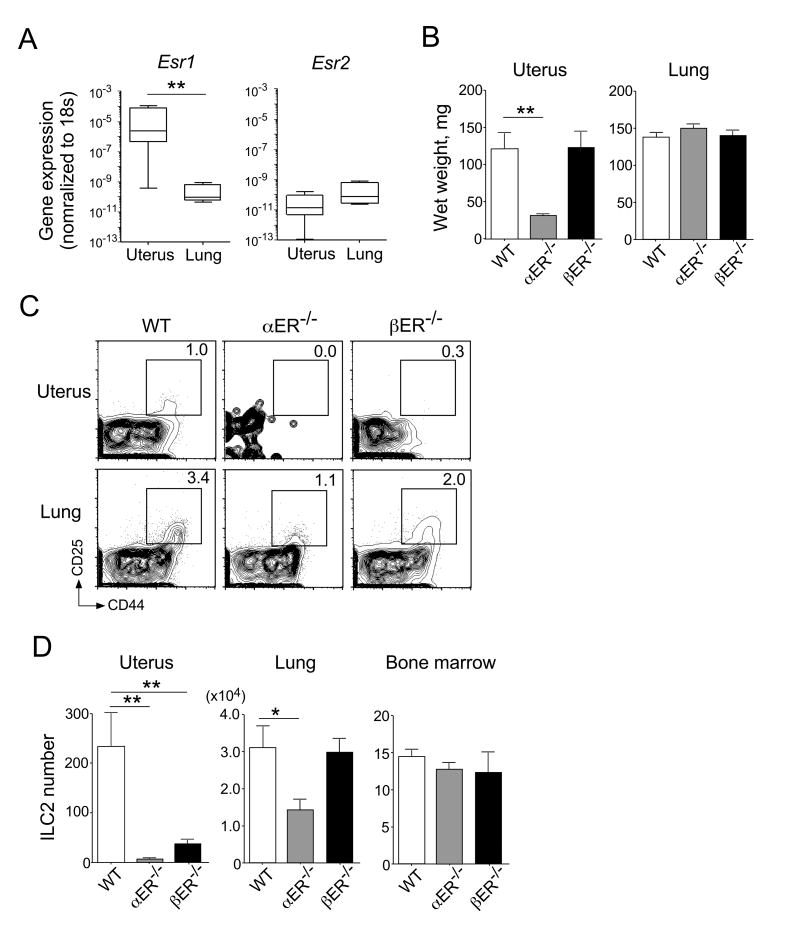
E2 signaling is mediated predominantly by estrogen receptor α (ERα) and estrogen receptor β (ERβ) (25). Following E2 ligation and subsequent nuclear translocation, they function primarily as transcription factors and regulate expression of a number of genes (26). These receptors are differentially distributed throughout the body (26) and their downstream effects are likely determined by the tissue they are localized to and the binding of co-factors (26). We characterized the expression of estrogen receptors by ILC2s in uterine and lung tissues of WT female mice. Following FACS sorting and real-time qRT-PCR, we found that Esr1, which encodes for ERα, was more highly expressed by uterine ILC2s than lung ILC2s (Figure 4A). In contrast, no apparent difference in the expression of Esr2, which encodes ERβ, was observed between uterine and lung ILC2 populations. Moreover, ERα-deficient mice (αER-/-) showed hypoplastic uteri, whereas ERβ-deficient mice (βER-/-) retained fully developed uteri (Figure 4B), consistent with previous findings (27). No difference in lung size was observed between αER-/- and βER-/- mice (Figure 4B).
Figure 4. Expression of the estrogen receptors is necessary for the accumulation of ILC2s in the uterus.
(A) ILC2s were isolated from the uteri and lungs of BALB/c mice injected i.p. daily with IL-33 (400 ng/dose) for three days. The expression levels of Esr1 and Esr2 were examined by real-time qRT-PCR. Box-and-whisker plots summarize the results from five (uterus) or eight (lungs) mice. **, p<0.01 (Mann-Whitney U) between the groups indicated by horizontal lines. (B) The wet weight of the uteri and lungs of WT C57BL/6, αER-/-, or βER-/- mice. Data are shown as the mean ± SEM from six mice. **, p<0.01 (Mann-Whitney U) between the groups indicated by the horizontal line. (C and D) ILC2 numbers in the uteri, lungs, and bone marrow were examined in naïve WT C57BL/6, αER-/-, or βER-/- mice by flow cytometry. Representative FACS plots (C) and the summarized data from six (uteri, lungs) or three (bone marrow) mice per group (D) is shown. Data are shown as the mean ± SEM. *, p<0.05 and **, p<0.01 (Mann-Whitney U) between the groups indicated by the horizontal line.
By FACS analyses, we found that ILC2 numbers were significantly decreased in the uteri of αER-/- and βER-/- mice, as compared to WT C57BL/6 age-matched controls (Figure 4C and 4D, p<0.01). Additionally, the number of ILC2s in the lungs was decreased in αER-/- mice compared to WT mice. In contrast, no significant change in the number of ILC2s was observed in the lungs and bone marrow of βER-/- mice, suggesting that ERβ signaling is likely critical for uterine ILC2s, but not for ILC2s in other organs. Similarly, ERα signaling may affect uterine ILC2s more strongly than those in the lungs.
To examine whether estrogen directly affects uterine ILC2s, we isolated ILC2s from both the lungs and uteri of naïve BALB/c mice, cultured them for 16 hours with E2, and analyzed their gene expression by microarray. At the baseline (i.e. without E2), over 14,000 genes were detectable in both uterine and lung ILC2s. Of those genes, 11,032 were common to ILC2s from both compartments, while 3,407 or 3,791 genes were expressed only in uterine or lung ILC2s, respectively. As expected, the genes for prototypic ILC2s, such as Gata3, Rora, Il5, and Il7r, were highly expressed by both uterine and lung ILC2s. Genes that represent other ILC subsets, such as Tbx21 and Rorc, were minimally expressed by uterine or lung ILC2s (data not shown). Furthermore, consistent with real-time qRT-PCR (Figure 4A), Esr1 was highly expressed by uterine ILC2s as compared to lung ILC2s (933 vs. 13 units) whereas no apparent differences were observed in Esr2 (47 vs. 48 units).
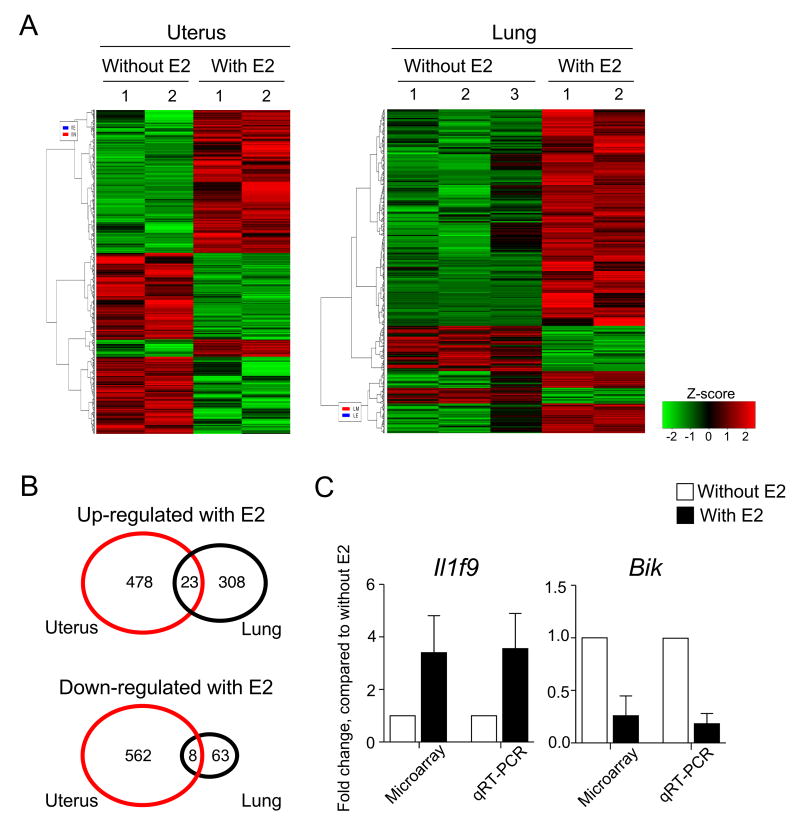
When cultured with E2, both lung and uterine ILC2s altered expression of a number of genes (Figure 5A). Overall, 2.5 times more genes were affected by E2 treatment in uterine ILC2s than in lung ILC2s (Figure 5B). Few genes were regulated commonly by both uterine and lung ILC2s. Pathways analysis revealed E2-induced changes in the molecules involved in cellular assembly and organization and cell death and survival in both uterine and lung ILC2s while the molecules involved in gene expression and cell cycle were relatively unique to uterine ILC2s and lung ILC2s, respectively (Table 1). Furthermore, increased expression of the IL-1 family member Il1f9 (IL-36g) and decreased expression of the pro-apoptotic gene Bik in E2-treated uterine ILC2s were confirmed by real-time qRT-PCR (Figure 5C). Taken together, these findings suggest that estrogen and the estrogen receptor play a key role in steady-state accumulation of ILC2s in the uterus.
Figure 5. Gene microarray analysis of ILC2s cultured with E2.
(A-C) ILC2s were isolated from the uteri and lungs of naive BALB/c mice and cultured with or without 100 ng/mL E2 for 16 hours. mRNA was analyzed by microarray. (A) A heat map generated from hierarchical clustering of differentially expressed genes shows effects of E2 treatment on uterine (left panel) and lung (right panel) ILC2s. (B) The numbers of genes that were up-regulated (upper panel) or down-regulated (lower panel) significantly (p<0.05) more than 2-fold by E2 treatment are shown for uterine and lung ILC2s. Numbers in the overlapping area indicate the genes shared by lung and uterine ILC2s. (C) Changes in expression of IL-36g (Il1f9, left panel) and Bik (right panel) mRNA by E2 treatment were determined by microarray analysis and real-type qRT-PCR. Fold change was calculated relative to the transcript levels in cells cultured without E2. Data are shown as the mean ± SEM (n=2 or 3).
Table 1. Top Molecular and Cellular Functions Regulated by E2 in Uterine and Lung ILC2s.
| Uterine ILC2s | Lung ILC2s | ||||
|---|---|---|---|---|---|
| Function | p-value | # of molecules | Function | p-value | # of molecules |
| 1. Gene Expression | 6.8 × 10-3 – 1.1 × 10-8 | 217 | 1. Cellular Assembly and Organization | 1.6 × 10-2 – 5.7 × 10-5 | 73 |
| 2. Cellular Assembly and Organization | 9.4 × 10-3 – 5.5 × 10-6 | 187 | 2. DNA Replication, Recombination, and Repair | 1.6 × 10-2 – 6.6 × 10-5 | 22 |
| 3. Cellular Function and Maintenance | 8.1 × 10-3 – 5.5 × 10-6 | 195 | 3. Cell Death and Survival | 1.6 × 10-2 – 1.1 × 10-4 | 96 |
| 4. Cell Morphology | 9.5 × 10-3 – 3.4 × 10-5 | 218 | 4. Cell Cycle | 1.6 × 10-2 – 2.0 × 10-4 | 70 |
| 5. Cell Death and Survival | 9.4 × 10-3 – 5.0 × 10-5 | 271 | 5. Post-Translational Modification | 1.6 × 10-2 – 2.3 × 10-4 | 57 |
The IL-33/ST2 axis affects reproduction
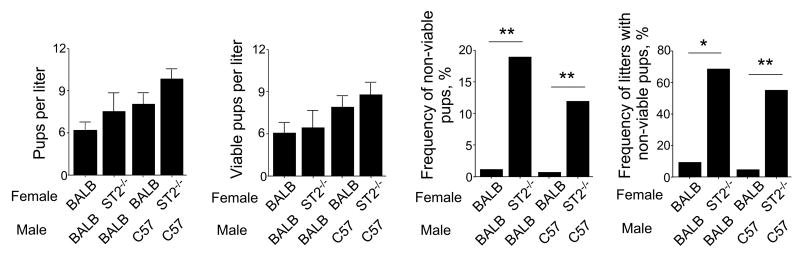
As mentioned above, uterine ILC2s, which express the IL-33 receptor, ST2, are regulated by female sex hormones. Recently, an increase in fetal resorption was observed in allogeneic matings of mice deficient in inducible Treg cells (28). Therefore, to address the potential role of uterine ILC2s in reproduction, we mated ST2-/- female mice with congenic BALB/c or allogeneic C57BL/6 males. No differences were observed in litter size between WT and ST2-/- females, irrespective of whether the males expressed the same or different MHC haplotypes (Figure 6). However, more pups were found dead within 24 hours of birth from ST2-/- mothers. Both the percentage of pups that died and the percentage of affected litters were higher in ST2-/- BALB/c dams, as compared to WT BALB/c dams (p<0.01 or p<0.05). The use of male mice with MHC haplotypes did not result in significant changes, compared to controls. These findings suggest that the IL-33/ST2 pathway plays a role in creating a uterine environment in dams that is optimal for the growth and/or well-being of pups.
Figure 6. The IL-33/ST2 pathway affects viability of pups.
WT BALB/c and ST2-/- female mice were mated with WT BALB/c and C57BL/6 male mice. For each litter, the size and condition at birth and 24 hours post-partum were recorded. The number of pups that were either dead at birth or died within 24 hours after birth were counted as non-viable pups. Data are presented as mean ± SEM from nine or more litters or a pool of more than 58 pups in each group. *, p<0.05 and **, p<0.01 (Fisher's exact test) between the groups indicated by the horizontal line.
Discussion
In this study, we provide novel insight into immune cells in the FRT and reveal that ILC2s populate the uterus. Similar to those in other mucosal organs, uterine ILC2s express the IL-33 receptor, ST2 and produce a large quantity of IL-5 and IL-13 in response to IL-33 stimulation. Furthermore, uterine ILC2s express many of the cell surface molecules found on ILC2s in the lungs, such as CD127, Thy-1.2, and CD69. However, we did note that several molecules common to lung ILC2s were missing or minimally expressed in uterine ILC2s (Figure 1). Particularly interesting was the difference in ICOS expression between uterine and lung ILC2s, as some ILC2 functions are diminished in the absence of ICOS signaling (29). These findings indicate that while uterine ILC2s share some features with ILC2s in other organs, they are likely regulated in an organ-specific manner.
Moreover, we found that female sex hormones, in particular estrogen, regulate the number of ILC2s in the uterus, but not lungs, as demonstrated by the studies with ovariectomized mice, as well as with normal mice implanted with slow release E2 pellets (Figure 3). In agreement with this, uterine ILC2s highly express ERα at levels 105-fold higher than that of lung ILC2s, although ERβ expression is comparable (Figure 4). In a cell, the ratio of ERα to ERβ is generally an important determinant of the response to E2: an increased expression of ERβ relative to ERα inhibits the E2 response (30). Intriguingly, Laffont et al recently reported that ILC2 numbers in the lungs are not affected by ovariectomy but are increased by orchiectomy (24). Therefore, while sex hormones modulate ILC2 homeostasis, their effects are likely to be dependent on the organs where they reside; ILC2s may be more sensitive to androgens in the lungs and to estrogen in the uterus. Indeed, we found that exposure of isolated uterine ILC2s to estrogen modulates their expression of a number of genes (Figure 5, Table 1). Nonetheless, we cannot rule out the possibility that the striking effects of estrogen on uterine ILC2s are due to the effects of estrogen on uterine tissue cells that may affect survival and/or proliferation of ILC2s by direct cell-to-cell interaction or by their products. In a pilot study, estrogen alone did not enhance survival of uterine ILC2s (data not shown). Generally, the complex interactions between epithelial cells, fibroblasts, and immune cells in response to female sex hormones likely create a unique environment optimal for both immunity and reproduction in the FRT (31). Conversely, some non-cytokine molecules, such as vitamins (e.g. vitamin A), hormones (e.g. VIP), and neuropeptides (e.g. neuromedin U), can be recognized directly by ILC2s (12, 32, 33). Recent studies also show functional plasticity of ILC2s that is regulated by cytokines, such as IL-1β, IL-4, and IL-12 (34, 35). Therefore, further studies on the mechanisms involved in tissue-specific regulation of ILC2s in the uterus and other organs will help us to understand better the biology of this unique cell type.
Another lingering question is the precise role of uterine ILC2s. Previously, ILC2s have been shown to promote protective immunity against helminth pathogens and to maintain tissue integrity and homeostasis (1). Therefore, it is possible that uterine ILC2s assist in altering the uterine immune environment to facilitate tolerance of an embryo or, in the event of an infection, help down-regulate the type 1 responses that would be detrimental to the fetus (36). Specifically, uterine ILC2s might facilitate the M1 to M2 transition in macrophages, as M2 macrophages likely play protective roles in the FRT (31), and a similar role has been described in visceral adipose tissue ILC2s (11, 37). Additionally, since ILC2s can promote tissue repair by producing amphiregulin (8, 38), uterine ILC2s may contribute to the repair and rebuilding of uterine tissues during each estrus cycle. Finally, IL-33-stimulated ILC2s have been shown to be important for maintaining metabolic homeostasis in white adipose tissue (9-11). Loss of a potential homeostatic function of uterine ILC2s could have contributed to the detrimental outcome we observed for pups from ST2 deficient dams (Figure 6). However, a significant limitation of this ST2 deficient model is that the lack of ST2 is not limited to ILC2s; other immune cells in the uterus that are responsive to IL-33, such as eosinophils, Th2 cells, and regulatory T cells (39, 40), may also contribute to the detrimental effects of ST2 deficiency. Future studies should investigate the role of uterine ILC2s in regulation and maintenance of the uterus using animals that are selectively deficient for ILC2s, when they become more widely available.
Finally, our observations with respect to the sex differences and hormonal regulation of ILC2s may be relevant to normal and pathological immune responses beyond the FRT. For example, asthma is more common in males before puberty and in females after puberty (41). Additionally, increased proportions of ILC2s were identified in the cord blood of neonatal boys compared to girls (42). These observations, in combination with the findings by others and us that lung ILC2s are more prevalent in female mice (Figure 3, 24), suggest that ILC2s could potentially explain the sex differences associated with asthma. Moreover, sex-specific differences in ILC2 responses may extend beyond the airway. For example, in a mouse model of multiple sclerosis, females exhibited a diminished protective ILC2 response in the nervous system compared to males (43). In conclusion, future studies on the interplay between sex hormones and ILCs that include multiple organs may be critical to understanding the sex differences in immune system diseases. Similarly, a more comprehensive analysis of the interaction between ILC2s, tissue parenchymal cells and the microenvironment is required to elucidate the roles of ILC2s in normal biological processes and the pathophysiology of diseases in different organs.
Supplementary Material
Acknowledgments
We thank the staff of the Gene Analysis Core (GAC) at the Mayo Clinic, Rochester, MN for carrying out the microarray analysis for this study and the Division of Biomedical Statistics and Informatics at the Department of Health Sciences Research, Mayo Clinic, Rochester, MN for providing data analysis, including Pathways Analysis. We also thank Dr. Andrew N. McKenzie for providing the Il1rl1-/- mice, and LuRaye S. Eischens for secretarial help.
This work was supported by grants from the National Institute of Health, R01 AI71106 and R01 HL117823, and the Mayo Foundation.
Abbreviations
- E2
17β-estradiol
- ERα
estrogen receptor α
- ERβ
estrogen receptor β
- ILCs
innate lymphoid cells
- ILC2s
group 2 innate lymphoid cells
- FRT
female reproductive tract
- Lin-
lineage-negative
- OVX
ovariectomized
- P4
progesterone
- Treg
regulatory T
- VAT
visceral adipose tissues
- WT
wild-type
Footnotes
Conflict of interest statement: All authors declare no conflicts of interest.
Author contributions: K.B. and H.K. designed the studies and experiments, interpreted the data, and wrote the manuscript. K.B., C-C.C, K.I. and L.D. performed the experiments and interpreted the data.
References
- 1.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17:765–774. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 2.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang YH, Liu YJ. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin Exp Allergy. 2009;39:798–806. doi: 10.1111/j.1365-2222.2009.03241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 8.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, Artis D. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, Locksley RM. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson SA. Control of the immunological environment of the uterus. Rev Reprod. 2000;5:164–174. doi: 10.1530/ror.0.0050164. [DOI] [PubMed] [Google Scholar]
- 14.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 15.Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63:601–610. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- 16.Vacca P, Cantoni C, Vitale M, Prato C, Canegallo F, Fenoglio D, Ragni N, Moretta L, Mingari MC. Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosuppression. Proc Natl Acad Sci U S A. 2010;107:11918–11923. doi: 10.1073/pnas.1001749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topping V, Romero R, Than NG, Tarca AL, Xu Z, Kim SY, Wang B, Yeo L, Kim CJ, Hassan SS, Kim JS. Interleukin-33 in the human placenta. J Matern Fetal Neonatal Med. 2013;26:327–338. doi: 10.3109/14767058.2012.735724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlock CI, Wu J, Zhou C, Tatum K, Adams HP, Tan F, Lou Y. Unique temporal and spatial expression patterns of IL-33 in ovaries during ovulation and estrous cycle are associated with ovarian tissue homeostasis. J Immunol. 2014;193:161–169. doi: 10.4049/jimmunol.1400381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Southcombe JH, Ledee N, Perrier d'Hauterive S, Turner K, Child T, Snider JV, Redman CW, Sargent IL, Granne I. Detection of soluble ST2 in human follicular fluid and luteinized granulosa cells. PloS one. 2013;8:e74385. doi: 10.1371/journal.pone.0074385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salker MS, Nautiyal J, Steel JH, Webster Z, Sucurovic S, Nicou M, Singh Y, Lucas ES, Murakami K, Chan YW, James S, Abdallah Y, Christian M, Croy BA, Mulac-Jericevic B, Quenby S, Brosens JJ. Disordered IL-33/ST2 activation in decidualizing stromal cells prolongs uterine receptivity in women with recurrent pregnancy loss. PloS one. 2012;7:e52252. doi: 10.1371/journal.pone.0052252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, Kouro T, Itakura A, Nagai Y, Takaki S, Takatsu K. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J Immunol. 2012;188:703–713. doi: 10.4049/jimmunol.1101270. [DOI] [PubMed] [Google Scholar]
- 22.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med. 2000;191:1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 24.Laffont S, Blanquart E, Savignac M, Cenac C, Laverny G, Metzger D, Girard JP, Belz GT, Pelletier L, Seillet C, Guery JC. Androgen signaling negatively controls group 2 innate lymphoid cells. J Exp Med. 2017 doi: 10.1084/jem.20161807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsson S, Gustafsson JA. Estrogen receptors: therapies targeted to receptor subtypes. Clin Pharmacol Ther. 2011;89:44–55. doi: 10.1038/clpt.2010.226. [DOI] [PubMed] [Google Scholar]
- 26.Morani A, Warner M, Gustafsson JA. Biological functions and clinical implications of oestrogen receptors alfa and beta in epithelial tissues. J Intern Med. 2008;264:128–142. doi: 10.1111/j.1365-2796.2008.01976.x. [DOI] [PubMed] [Google Scholar]
- 27.Walker VR, Korach KS. Estrogen receptor knockout mice as a model for endocrine research. ILAR j. 2004;45:455–461. doi: 10.1093/ilar.45.4.455. [DOI] [PubMed] [Google Scholar]
- 28.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maazi H, Patel N, Sankaranarayanan I, Suzuki Y, Rigas D, Soroosh P, Freeman GJ, Sharpe AH, Akbari O. ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity. 2015;42:538–551. doi: 10.1016/j.immuni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pettersson K, Delaunay F, Gustafsson JA. Estrogen receptor beta acts as a dominant regulator of estrogen signaling. Oncogene. 2000;19:4970–4978. doi: 10.1038/sj.onc.1203828. [DOI] [PubMed] [Google Scholar]
- 31.Wira CR, Rodriguez-Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol. 2015;15:217–230. doi: 10.1038/nri3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, Nutman TB, Urban JF, Jr, Wang J, Ramalingam TR, Bhandoola A, Wynn TA, Belkaid Y. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432–437. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallrapp A, Riesenteld SJ, Burkett PR, Abdulnour REE, Nyman J, Dionne D, Hofree M, Cuoco MS, Rodman C, Farouq D, Haas BJ, Tickle TL, Trombetta JJ, Baral P, Klose CSN, Mahlakolv T, Artis D, Rozenblatt-Rosen O, Chiu IM, Levy BD, Kowalczyk MS, Regev A, Kouchroo VK. The neuropeptide NMU amplifies ILC2-deriven allergic lung inflammation. Nature. 2017 doi: 10.1038/nature24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bal SM, Bernick JH, Nagasawa M, Groot J, Shikhagaie MM, Golebski K, van Drunen CM, Lutter R, Jonkers RE, Hombrink P, Bruchard M, Villaudy J, Munneke JM, Fokkens W, Erjefalt JS, Spitz H, Ros XR. IL-1β, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol. 2016;17:636–645. doi: 10.1038/ni.3444. [DOI] [PubMed] [Google Scholar]
- 35.Lim AI, Menegatti S, Bustamante J, Bourhis LL, Allez M, Rogge L, Casanova JL, Yssel H, Di Santo JP. IL-12 drives functional plasticity of human group 2 innate lymphoid cells. J Exp Med. 2016;213:569–83. doi: 10.1084/jem.20151750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robertson SA, Care AS, Skinner RJ. Interleukin 10 regulates inflammatory cytokine synthesis to protect against lipopolysaccharide-induced abortion and fetal growth restriction in mice. Biol Reprod. 2007;76:738–748. doi: 10.1095/biolreprod.106.056143. [DOI] [PubMed] [Google Scholar]
- 37.Wu J, Carlock C, Zhou C, Nakae S, Hicks J, Adams HP, Lou Y. IL-33 is required for disposal of unnecessary cells during ovarian atresia through regulation of autophagy and macrophage migration. J Immunol. 2015;194:2140–2147. doi: 10.4049/jimmunol.1402503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in tissue homeostasis, Injury, and Inflammation. Immunity. 2015;42:1005–1019. doi: 10.1016/j.immuni.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, Harrison OJ, Owens BM, Lohning M, Belkaid Y, Fallon PG, Powrie F. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–8. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, Fagarasan S, Mielke LA, Afshar-Sterie S, Masters SL, Nakae S, Saito H, Wentworth JM, Li P, Liao W, Leonard WJ, Smith GK, Shi W, Nutt SL, Koyasu S, Kallies A. The transcriptional regulators of IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol. 2015;16:276–85. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
- 41.Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev. 2012;33:1–47. doi: 10.1210/er.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forsberg A, Bengtsson M, Eringfalt A, Ernerudh J, Mjosberg J, Jenmalm MC. GATA binding protein 3(+) group 2 innate lymphoid cells are present in cord blood and in higher proportions in male than in female neonates. J Allergy Clin Immunol. 2014;134:228–230. doi: 10.1016/j.jaci.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 43.Russi AE, Walker-Caulfield ME, Ebel ME, Brown MA. Cutting edge: c-Kit signaling differentially regulates type 2 innate lymphoid cell accumulation and susceptibility to central nervous system demyelination in male and female SJL mice. J Immunol. 2015;194:5609–5613. doi: 10.4049/jimmunol.1500068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.