Abstract
Physical activity is associated with a lower risk of disease recurrence among colon cancer survivors. The pathways through which physical activity may alter disease outcomes are unknown, but may include changes in metabolic growth factors, such as insulin.
Methods
Between January 2015 and August 2015, 39 stage I–III colon cancer survivors were randomized to one of three groups: usual-care control, 150 min·wk−1 of aerobic exercise (low-dose), and 300 min·wk−1 of aerobic exercise (high-dose) for six months. The pre-specified key metabolic growth factor outcome was fasting insulin. Insulin resistance was quantified using the homeostatic model assessment.
Results
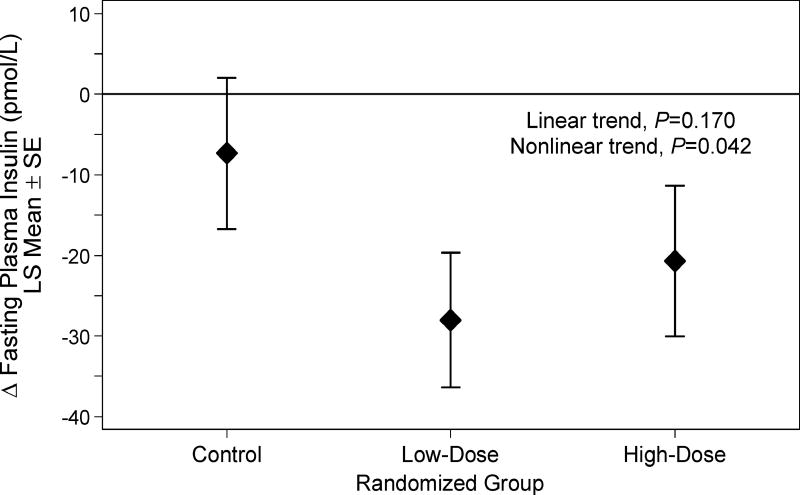
Mean age was 56.5±10.0 years, 51% had stage III disease, 72% were treated with chemotherapy, and the mean time since finishing treatment was 10.9±6.1 months. Over six months, the low-dose group completed 141.5±9.9 min·wk−1 of aerobic exercise, and the high-dose group completed 247.2±10.7 min·wk−1 of aerobic exercise. Fasting insulin concentrations decreased 7.4±9.4 pmol/L in the control group, 28.0±8.3 pmol/L in the low-dose group, and 20.7±9.3 pmol/L in the high-dose group (nonlinear Ptrend=0.042). Insulin resistance decreased 0.11±0.20 in the control group, 0.63±0.17 in the low-dose group, and 0.43±0.19 in the high-dose group (nonlinear Ptrend=0.012).
Discussion
Aerobic exercise reduces insulin concentrations and insulin resistance among patients with stage I–III colon cancer. Prescribing 150 min·wk−1 of aerobic exercise may be sufficient for reducing insulin concentrations and insulin resistance, which may partially mediate the relationship between physical activity and colon cancer prognosis.
Keywords: body composition, metabolism, physical activity, prevention, randomized trial, recurrence
INTRODUCTION
Each year more than 103,000 people are diagnosed with colon cancer in the United States (Siegel, et al. 2016). Three-quarters of patients will be diagnosed with disease that is localized to the colon (stage I–II) or spread to regional lymph nodes (stage III). Despite surgical resection, either alone or in combination with chemotherapy, up to one-half of patients with stage I–III colon cancer will experience disease recurrence (Siegel, et al. 2014). Consequently, there exists a need to identify additional therapies that reduce the risk of recurrent disease in this population.
The prescription of physical activity or exercise is a potential therapy that has been reported in observational studies to be associated with a lower risk of recurrence and death among colon cancer survivors (Je, et al. 2013). The relationship between physical activity and disease outcomes is independent of known prognostic factors, and occurs in a dose-response fashion, such that higher volumes of physical activity or exercise, up to 300 minutes per week (min·wk−1), are associated with more favorable disease outcomes (Schmid and Leitzmann 2014).
The biologic or biobehavioral pathways through which exercise may favorably alter colon cancer outcomes have not been elucidated, but may include exercise-induced alterations in metabolic growth factors, such as insulin, C-peptide, insulin-like growth factor-1 (IGF-1), and insulin-like growth factor-binding protein-3 (IGFBP-3). Colon cancer cells have insulin/IGF-1 receptors on their surface (Belfiore and Malaguarnera 2011), and insulin/IGF-1 promote colon cancer cell proliferation and inhibit apoptosis (Koenuma, et al. 1989). In vitro studies demonstrate that states of hyperinsulinemia increase colon cancer cell resistance to 5-fluorouracil (Chen, et al. 2011b) and oxaliplatin chemotherapy (Chen, et al. 2011a; Volkova, et al. 2014). Preclinical models demonstrate that exposure to insulin promotes colonic tumor multiplicity (Tran, et al. 1996), and IGF-1 promotes a pro-metastatic hepatic microenvironment for colon cancer cells (Fernandez, et al. 2016). In the general population, hyperinsulinemia is associated with an increased risk of cancer-specific mortality (Wargny, et al. 2017). Among men and women with stage I–III colon cancer, elevated concentrations of C-peptide and lower concentrations of IGFBP-3 are associated with a higher risk of mortality (Haydon, et al. 2006; Wolpin, et al. 2009). Genetic variants within insulin-related genes are associated with colon cancer risk, recurrence, and survival (Fu, et al. 2016; Simons, et al. 2015). Together, this evidence supports the hypothesis that insulin/IGF-1 may be important mediators of the relationship between exercise and disease outcomes among colon cancer survivors.
Colon cancer survivors have fasting insulin concentrations that are 58% higher than healthy persons without a history of colon cancer (Jiang, et al. 2014). A study of 17 colon cancer survivors demonstrated that a three-month prescription of exercise reduced insulin and IGFBP-3 concentrations (Lee, et al. 2013). However, no studies have examined the dose-response effects of exercise on these metabolic growth factors among colon cancer survivors.
These observations provided the scientific rationale for the COURAGE trial, a randomized controlled trial investigating the safety, feasibility, and biological efficacy of 150 and 300 min·wk−1 of aerobic exercise versus usual care control over six months among men and women recently-treated for stage I–III colon cancer (Brown, et al. 2016). We have previously reported that exercise was safe, feasible, and led to reductions in serum intercellular adhesion molecule-1 (Brown, et al. 2017a) and visceral adipose tissue (Brown, et al. 2017b). Here we report metabolic growth factor outcomes. Fasting insulin was pre-specified as the key growth factor of interest. As an exploratory aim of this report, we characterize the relationship between changes in visceral adipose tissue with changes in fasting insulin. Our hypotheses were that: 1) exercise would reduce fasting insulin concentrations in a dose-response fashion; and 2) reductions in visceral adipose tissue would correlate with reductions in fasting insulin.
MATERIALS and METHODS
Participants
Study methods of the COURAGE trial were published (Brown et al. 2016). Participants were eligible if they: 1) were diagnosed with histologically-proven stage I–III colon cancer; 2) completed surgical resection and post-operative chemotherapy within 36 months of entering the study; 3) self-reported participating in ≤150 min·wk−1 of moderate or vigorous intensity physical activity using the Paffenbarger Physical Activity Questionnaire (Paffenbarger, et al. 1978); 4) were of age ≥18 years; 5) provided written physician approval; 6) had no additional surgery planned within the six-month intervention period (including colostomy reversal); and 7) had the ability to walk unassisted for six minutes. Participants were ineligible if they: 1) had a history of another primary cancer (other than non-melanoma skin-cancer); 2) had evidence of metastatic cancer; 3) were pregnant or breast feeding; 4) were unable to provide a baseline blood sample; 5) had a myocardial infarction or coronary revascularization procedure within the past three months; 6) had uncontrolled hypertension, defined as a systolic blood pressure ≥180 mm Hg or diastolic blood pressure ≥100 mm Hg; 7) had high-risk or uncontrolled heart arrhythmias (not including atrial fibrillation); 8) had clinically significant heart valve disease; 9) had decompensated heart failure; 10) had a known aortic aneurysm; or 11) had any other condition which, in the opinion of the investigator, may impede testing of the study hypothesis or make it unsafe to engage in the exercise program.
Participants were stratified by cancer stage (AJCC 7th Edition: I vs II vs III) and randomized to one of three groups: low-dose aerobic exercise (150 min·wk−1), high-dose aerobic exercise (300 min·wk−1), or usual care control. This study was approved by the University of Pennsylvania Institutional Review Board and registered on clinicaltrials.gov as NCT02250053. All participants provided written informed consent and written approval from their physician prior to participation in any study-related activities.
Intervention
Detailed methods of the exercise intervention are published (Brown et al. 2016). Participants randomized to the low-dose or high-dose exercise groups were provided with an in-home treadmill (LifeSpan Fitness, TR1200i, Salt Lake City, UT) and heart rate monitor (Polar Electro, RS400, Kempele Finland). Exercise intensity was prescribed at 50–70% of the age-predicted maximum heart rate [equivalent to 3–6 METs; (Ainsworth, et al. 2000)]. The low-dose and high-dose groups progressed towards of the goal of 150 or 300 min·wk−1 of exercise, respectively. Participants met with a certified clinical exercise physiologist to introduce the exercise prescription, and familiarize the participant with use of the treadmill, completion of exercise logs, use of a heart rate monitor, appropriate warm-up and cool-down, stretches, and proper footwear for aerobic exercise. The exercise physiologist provided ongoing behavioral and clinical support and monitored exercise adherence to the study protocol throughout the duration of the study.
Participants randomized to the usual-care control group were asked to maintain their pre-study levels of physical activity or follow the recommendations provided by their physician. After completing six month measures, control group participants were provided with an in-home treadmill and individualized exercise program, like that prescribed to the two exercise groups. Upon completion of study-related activities, all participants could keep their study-provided treadmills.
Measurements
Baseline and follow-up measurements were obtained by trained staff members who were blinded to treatment assignment. Demographic characteristics including age, sex, and race were self-reported. Daily caloric intake and the proportion of calories from carbohydrate sources were quantified using three-day diet records that were analyzed using the Nutrition Data System for Research software (v.2014) by a registered dietitian who was blinded to study group. Moderate to vigorous intensity physical activity was quantified using an accelerometer [ActiGraph GT3X+; (Troiano, et al. 2008)]. Clinical information including cancer stage and treatment with chemotherapy were obtained from the cancer registry, pathology reports, and physician records. Body mass index (BMI; kg/m2) was calculated using standard anthropometric measures [weight (kg) and height (m)], and dual-energy x-ray absorptiometry was used to quantify visceral adipose tissue (Brown et al. 2017b). Comorbid health conditions were self-reported.
Metabolic Growth Factor Outcomes
All study participants underwent a fasting blood draw at baseline and follow-up. EDTA-preserved plasma was stored at −80°C. Insulin and C-peptide concentrations were quantified using a radioimmunoassay (EMD Millipore, Billerica, MA). IGF-1, IGFBP-3, and fructosamine concentrations were quantified using an enzyme-linked immunosorbent assay (DSL, Webster, TX). Glucose concentrations were quantified spectrophotometrically (Roche, Indianapolis, IN). Baseline and follow-up plasma samples were assayed simultaneously and in duplicate at the end of the study. Coefficients of variation for all samples were ≤10%. The homeostatic model assessment (HOMA) was used to quantify insulin resistance (Levy, et al. 1998).
Statistical Analysis
Descriptive statistics presented for baseline variables include counts and proportions for categorical variables and medians with interquartile [25–75%] ranges for continuous variables. Categorical baseline characteristics were compared among the three groups using Fisher’s exact test, and continuous baseline characteristics were compared among the three study groups using the Kruskal-Wallis test. This trial was statistically powered to detect changes in the co-primary study endpoints soluble intercellular adhesion molecule-1 and soluble vascular adhesion molecule-1 (Brown et al. 2016; Brown et al. 2017a). However, the study had adequate statistical power to examine changes in fasting insulin concentrations. Based on prior research (Houmard, et al. 2004), over six months we estimated a mean change in fasting insulin concentrations of +6.6 pmol/L in the control group, −3.3 pmol/L in the low-dose group, and −5.9 pmol/L in the high-dose group with a pooled standard deviation of ±4.6 pmol/L. Against the hypothesis of a dose-response relationship, 39 participants provided 80% power with a type I error rate of 5% (α=0.05). All inferential analyses were conducted on an intention-to-treat basis. Dependent variables were log transformed in the inferential analysis to improve distributional normality and back transformed to facilitate interpretation. Changes were evaluated from baseline to follow-up in the three groups using repeated-measures mixed-effects regression models. This statistical approach includes all available data and accounts for the correlation between repeated measures. The baseline value of the dependent variable and cancer stage (randomization stratification factor) were included as covariates in the regression models (Fitzmaurice, et al. 2012). Group-by-time interaction terms were included as fixed-effects in the regression model. Model fit was assessed using graphical techniques. Results from the repeated-measures mixed-effects regression models are presented as least-square means ± standard error or 95% confidence intervals. To evaluate the presence of a dose-response relationship across randomized groups, a test of trend was conducted by examining linear and nonlinear (quadratic) contrasts. Linear regression models were used to characterize changes in visceral adipose tissue with changes in growth factor concentrations from baseline to six months (Schousboe, et al. 2017).
RESULTS
Between January 2015 and August 2015, 39 colon cancer survivors were recruited and randomized with data collection ending in February 2016. Baseline characteristics study participants have been described (Brown et al. 2017a), and are briefly presented in Table 1. Age ranged from 35–81 years. BMI ranged from 20–43 kg/m2; 31% of participants were overweight (BMI 25.0–29.9 kg/m2) and 51% were obese (BMI ≥30 kg/m2). Visceral adipose tissue ranged from 34.2–257.9 cm2. Time since finishing cancer directed treatment ranged from 1–21 months. Five participants had type 2 diabetes mellitus at baseline, all were diagnosed ≥3 years prior to study enrollment, and all were using metformin (one as monotherapy, four as combination therapy with a sulfonylurea or dipeptidyl peptidase-4 inhibitor).
Table 1.
Baseline characteristics of the participants
| Characteristic | Total (n=39) |
Control (n=13) |
Low-Dose (n=14) |
High-Dose (n=12) |
P |
|---|---|---|---|---|---|
| Age, years | 56.5 [49.1–63.3] | 56.5 [51.0–60.9] | 59.1 [54.3–66.4] | 54.6 [45.0–62.0] | 0.493 |
| Sex, % | |||||
| Male | 15 (38%) | 4 (31%) | 7 (50%) | 4 (33%) | 0.601 |
| Female | 24 (62%) | 9 (69%) | 7 (50%) | 8 (67%) | |
| Race, % | |||||
| White | 31 (80%) | 8 (62%) | 12 (86%) | 11 (92%) | 0.332 |
| Black | 6 (15%) | 3 (23%) | 2 (14%) | 1 (8%) | |
| Other | 2 (5%) | 2 (15%) | 0 (0%) | 0 (0%) | |
| Caloric Consumption, kcal·d−1 | 1735 [1270–1962] | 1800 [1233–2110] | 1776 [1483–2111] | 1632 [1196–1739] | 0.725 |
| Calories from Carbohydrate, % | 46.8 [39.7–51.3] | 43.5 [36.9–49.4] | 48.7 [46.0–54.8] | 37.7 [45.1–51.3] | 0.261 |
| Moderate or Vigorous Physical Activity, min·d−1 | 15.7±8.7 | 12.2±8.1 | 18.8±9.6 | 15.7±7.3 | 0.174 |
| Body Mass Index, kg·m−2 | 30.3 [25.3–35.3] | 29.0 [25.0–33.5] | 30.4 [27.1–32.1] | 33.6 [25.6–37.7] | 0.408 |
| Waist Circumference, cm | 102 [91–110] | 94 [90–107] | 99 [91–107] | 109 [110–114] | 0.154 |
| Visceral Adipose Tissue, cm2 | 130.8 [82.2–168.8] | 116.7 [61.4–150.3] | 133.0 [82.2–162.1] | 138.8 [117.4–206.6] | 0.227 |
| Cancer Stage, % | |||||
| I | 5 (13%) | 1 (8%) | 2 (14%) | 2 (17%) | 0.999 |
| II | 14 (36%) | 5 (38%) | 5 (36%) | 4 (33%) | |
| III | 20 (51%) | 7 (54%) | 7 (50%) | 6 (50%) | |
| Chemotherapy, % | 28 (72%) | 10 (77%) | 10 (71%) | 8 (67%) | 0.906 |
| Time Since Treatment, Months | 10 [5–16] | 12 [8–16] | 7.5 [4–13] | 10 [6–17] | 0.417 |
| Comorbid Conditions, % | |||||
| Hypertension | 13 (33%) | 4 (31%) | 6 (43%) | 3 (25%) | 0.695 |
| Hyperlipidemia | 6 (15%) | 1 (8%) | 2 (14%) | 3 (25%) | 0.480 |
| Type 2 Diabetes | 5 (13%) | 1 (8%) | 1 (7%) | 3 (25%) | 0.409 |
| Cardiovascular Disease | 4 (10%) | 2 (15%) | 1 (7%) | 1 (8%) | 0.827 |
P values are from the overall test of group differences. Data are median [interquartile range], or counts with percentages.
Exercise prescription program variables have been described in detail (Brown et al. 2017a). Over six months, the average exercise volumes in the low-dose and high-dose groups were 141.5±9.92 min·wk−1 (92.8±2.44% of prescribed dose) and 247.2±10.71 min·wk−1 (89.0±2.64% of prescribed dose), respectively. Daily caloric intake (group-by-time interaction P=0.743) and the proportion of daily calories from carbohydrate sources (group-by-time interaction P=0.645) were not significantly different from baseline to six months in any of the groups.
Metabolic growth factor concentrations are presented in Table 2. Fasting insulin concentration, the pre-specified key growth factor outcome, decreased 7.4±9.4 pmol/L in the control group, 28.0±8.3 pmol/L in the low-dose group, and 20.7±9.3 pmol/L in the high-dose group (nonlinear Ptrend=0.042; Figure 1). Similarly, insulin resistance decreased 0.11±0.20 in the control group, 0.63±0.17 in the low-dose group, and 0.43±0.19 in the high-dose group (nonlinear Ptrend=0.012). Fasting glucose concentration decreased in the low-dose group, whereas no difference was observed high-dose and control groups (nonlinear Ptrend=0.004). IGF-1, IGFBP-3, fructosamine, and C-peptide did not change in any of the study groups. Adjustment for type 2 diabetes mellitus as a covariate in the regression models did not substantively alter the above-described findings. No serious (grade ≥3) adverse events occurred. Non-serious (grade 1–2) adverse events occurred at similar rates among the three groups (Brown et al. 2017a).
Table 2.
Metabolic growth factor outcomes at baseline and change during six months
| Outcome | Baseline (Mean ± SD) |
Δ Baseline to Month 6 (LS Mean ± SE) |
Δ from Control (LS Mean [95% CI]) |
|---|---|---|---|
| Insulin, pmol/L | |||
| Control | 99.2±60.5 | −7.36±9.41 | — |
| Low-Dose | 101.8±40.5 | −28.02±8.35a | −20.66 [−45.32, 3.99] |
| High-Dose | 135.1±87.1 | −20.70±9.35a | −13.34 [−39.33, 12.6] |
| Test for trend | Linear, P=0.170; Nonlinear, P=0.042 | ||
| Glucose, mmol/L | |||
| Control | 5.3±1.0 | 0.01±0.16 | — |
| Low-Dose | 5.3±0.8 | −0.39±0.15a | −0.39 [−0.83, 0.05] |
| High-Dose | 6.1±2.3 | −0.09±0.17 | −0.09 [−0.56, 0.38] |
| Test for trend | Linear, P=0.931; Nonlinear, P=0.004 | ||
| Insulin Resistance (HOMA) | |||
| Control | 2.2±1.3 | −0.11±0.20 | — |
| Low-Dose | 2.2±0.9 | −0.63±0.17a | −0.52 [−1.03, −0.01]b |
| High-Dose | 2.9±2.0 | −0.43±0.19a | −0.32 [−0.86, 0.22] |
| Test for trend | Linear, P=0.125; Nonlinear, P=0.012 | ||
| IGF-1, nmol/L | |||
| Control | 58.0±15.9 | −4.57±3.23 | — |
| Low-Dose | 59.8±13.2 | −0.94±3.21 | 3.63 [−5.29, 12.56] |
| High-Dose | 64.7±17.5 | 1.62±3.57 | 6.19 [−3.25, 15.62] |
| Test for trend | Linear, P=0.054; Nonlinear, P=0.850 | ||
| IGFBP-3, nmol/L | |||
| Control | 1765.1±446.8 | −103.71±69.15 | — |
| Low-Dose | 1925.7±449.5 | −30.01±68.04 | 73.69 [−116.96, 264.34] |
| High-Dose | 2290.0±748.2 | −154.28±71.97a | −50.58 [−246.83, 145.68] |
| Test for trend | Linear, P=0.685; Nonlinear, P=0.093 | ||
| C-Peptide, nmol/L | |||
| Control | 0.64±0.4 | 0.007±0.033 | — |
| Low-Dose | 0.58±0.3 | −0.003±0.032 | −0.010 [−0.10, 0.08] |
| High-Dose | 0.75±0.3 | −0.013±0.035 | −0.020 [−0.11, 0.07] |
| Test for trend | Linear, P=0.701; Nonlinear, P=0.934 | ||
| Fructosamine, mmol/L | |||
| Control | 201.4±20.5 | 2.60±16.82 | — |
| Low-Dose | 183.0±51.2 | 22.91±15.84 | 20.31 [−24.98, 65.60] |
| High-Dose | 204.1±28.6 | −12.65±16.96 | −15.26 [−62.08, 31.57] |
| Test for trend | Linear, P=0.380; Nonlinear, P=0.382 |
SD, standard deviation; LS Mean, least squares mean; SE, standard error; CI, confidence interval; HOMA, homeostatic model assessment.
Signifcantly different from baseline (within-group), P≤0.05.
Significantly different from control, P≤0.05.
Changes in outcomes are estimated using a linear mixed-effects regression model that adjusted for the baseline value of the dependent variable and cancer stage (randomization stratification factor).
Figure 1.
Between group changes in fasting insulin concentration from baseline to six months
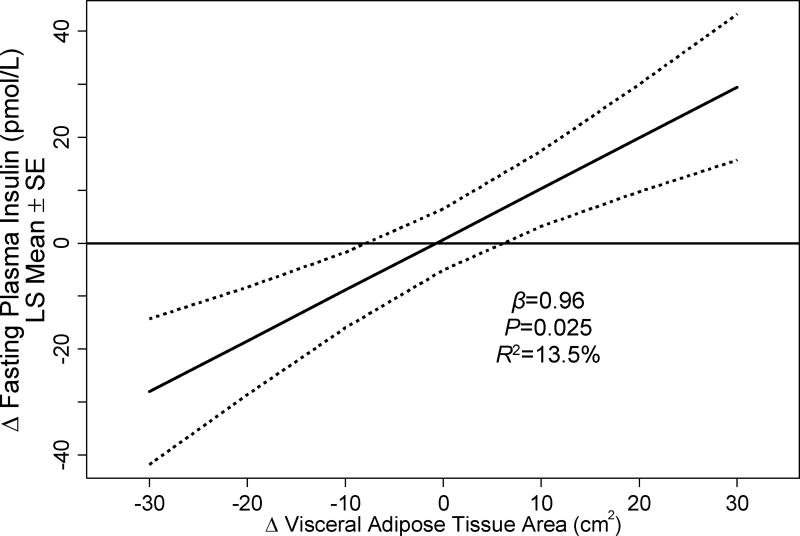
We previously reported that exercise reduced visceral adipose tissue in a dose-response fashion (linear Ptrend=0.008), such that each 60 min·wk−1 increase in exercise volume predicted a −2.7±1.4 cm2 reduction in visceral adipose tissue (Brown et al. 2017b). For each 1 cm2 reduction in visceral adipose tissue, fasting insulin concentrations were lowered by 0.96±0.41 pmol/L (P=0.025; Table 3, Figure 2); changes in visceral adipose tissue accounted for 13.5% of the shared variance of changes in fasting insulin concentrations. Changes in visceral adipose tissue were also correlated with changes in fasting glucose and insulin resistance. Adjustment for visceral adipose tissue did not alter the relationship between exercise dose and fasting insulin concentrations (nonlinear Ptrend=0.042) or insulin resistance (nonlinear Ptrend=0.010).
Table 3.
Relationship between change in visceral adipose tissue (per 1 cm2 reduction) and change in metabolic growth factor concentration during six months
| Outcome | Δ in Metabolic Growth Factor Concentration (LS Mean ± SE) |
R2 | P |
|---|---|---|---|
| Insulin, pmol/L | −0.96±0.41 | 13.5% | 0.025 |
| Glucose, mmol/L | −0.03±0.01 | 21.9% | 0.004 |
| Insulin Resistance (HOMA) | −0.024±0.009 | 16.5% | 0.013 |
| IGF-1, nmol/L | −0.22±0.13 | 7.6% | 0.098 |
| IGFBP-3, nmol/L | −1.22±3.16 | 0.4% | 0.701 |
| C-Peptide, mmol/L | 0.0007±0.001 | 0.6% | 0.650 |
| Fructosamine, mmol/L | −0.16±0.47 | 0.3% | 0.736 |
LS Mean, least squares mean; SE, standard error; R2, proportion of variability of change in metabolic growth factor concentration explained by change in visceral adipose tissue.
Figure 2.
Relationship between changes in visceral adipose tissue area and changes in fasting insulin concentration from baseline to six months
DISCUSSION
A six-month moderate-intensity aerobic exercise program among stage I–III colon cancer survivors reduced fasting insulin concentrations and insulin resistance in a predominately overweight and obese population. The findings from this randomized trial support the hypothesis that the relationship between physical activity and colon cancer prognosis may be mediated, in part, by changes in fasting insulin concentrations or insulin resistance.
The reductions in fasting insulin concentrations and insulin resistance with exercise are similar to those observed in a prior dose-response study in overweight and obese adults (Ross, et al. 2015). This prior study demonstrated that fasting insulin concentrations and insulin resistance may be lowered to a similar magnitude across distinct doses of exercise (Ross et al. 2015). However, the absolute magnitude of reduction in fasting insulin concentrations in our study was larger than that of others (Houmard et al. 2004; Ross et al. 2015). This may be the result of our sample having higher baseline fasting insulin concentrations [111 pmol/L in the current study vs 49 pmol/L (Houmard et al. 2004) and 67.5 pmol/L (Ross et al. 2015)], which is consistent with the observation that colon cancer survivors have significantly higher fasting insulin concentrations than healthy persons (Jiang et al. 2014). Our findings are similar to studies in breast cancer survivors that exercise reduces fasting insulin concentrations (Irwin, et al. 2009; Ligibel, et al. 2008).
In prior cross-sectional analyses, fasting insulin concentrations were higher with larger areas of visceral adipose tissue, an effect that is attributable to increased insulin resistance (Goodpaster, et al. 2003). We demonstrated that changes in visceral adipose tissue accounted for 13.5% of the shared variance in insulin concentration, which is similar to studies in obese men that estimated the shared variance to be 16–22% (Borel, et al. 2017; Rice, et al. 1999). These data suggest that the effects of exercise to lower fasting insulin may include mechanisms beyond that of changes in visceral adipose tissue. We hypothesize that alterations in skeletal muscle insulin resistance and free fatty acid (FFA) metabolism may help to further explain this effect (Abdul-Ghani and DeFronzo 2010; DeFronzo and Tripathy 2009). Insulin resistance in skeletal muscle is associated with hyperinsulinemia (Abdul-Ghani and DeFronzo 2010; DeFronzo and Tripathy 2009) and the inability to lower FFA in the postprandial state (Jensen 2008). Skeletal muscle preferentially oxidizes FFA (Randle, et al. 1963), suppressing insulin-stimulated glucose uptake into the muscle (Boden, et al. 1994; Dresner, et al. 1999). Exercise improves the insulin suppression of FFA release (Shadid and Jensen 2006), corrects the mismatch between FFA uptake and FFA oxidation (Turcotte and Fisher 2008), and promotes insulin-stimulated glucose uptake into skeletal muscle (Hayashi, et al. 1997).
The biologic or biobehavioral pathways through which exercise may alter cancer outcomes are unknown. States of hyperinsulinemia activate the PI3K-Akt-mTOR pathway (McCurdy and Klemm 2013). In preclinical experiments, activation of the PI3K-Akt-mTOR pathway promotes the growth of colon cancer metastases (Gulhati, et al. 2011), and inhibition of this pathway induces cell-cycle arrest and apoptosis (Zhang, et al. 2009). Insulin receptor substrate 1 (IRS1) is a mediator of glucose homeostasis, and the down regulation of IRS1 is associated with insulin resistance (Karlsson and Zierath 2007). Colonic tumor expression of IRS1 and physical activity interact to influence colon cancer outcomes (Hanyuda, et al. 2016). Among patients with decreased expression of IRS1, physical activity is associated with a significantly lower risk of colon cancer-specific mortality (Ptrend=0.005), whereas no relationship is observed with IRS2. IRS1 is associated with insulin metabolism in skeletal muscle, whereas IRS2 is associated with insulin metabolism in the liver (Karlsson and Zierath 2007). These observational data provide additional support to the hypothesis that exercise may have an insulin sensitizing effect that is produced through skeletal muscle contractions, and this insulin sensitization may influence disease outcomes.
There are several limitations to this trial. The main limitation is the small sample size which limits the generalizability of our findings. We have previously reported that participants in this trial were younger than the population from which they were recruited (Brown et al. 2016). Because of the small sample size, we observed numeric differences at baseline in select metabolic growth factor concentrations. Our analyses plan pre-specified that the baseline value of the dependent variable would be included in the model to account for baseline differences, however we cannot rule out that the observed differences may be partly due to regression to the mean. The small sample size precluded our ability to undertake formal mediation analysis to explore the relationships among exercise dose, visceral adipose tissue, and insulin concentrations (Friedenreich, et al. 2011). The small sample size also limited our statistical power to examine other metabolic growth factors such as IGF-1. Additional randomized studies with larger sample sizes are needed to confirm our findings. It is not known if the relationship between exercise dose and change in insulin concentrations are linear at the population level. If a linear relationship does exist, there may be a several reasons why we did not identify such a relationship. Our study was only six months in duration. It is unknown if the observed improvements in outcomes would be sustained or improved upon over a longer time horizon. Participants were not recruited based on having hyperinsulinemia; however, 82% of our study sample was overweight or obese, consequently hyperinsulinemia was common. We examined two distinct volumes of moderate-intensity aerobic exercise, but we did not examine the effects of light- or vigorous-intensity aerobic exercise (McGarrah, et al. 2016) or resistance exercise (either as a single modality or prescribed in combination with aerobic exercise).
There are several strengths to this trial. The use of two intervention groups, each prescribed a distinct dose of exercise allowed us to examine changes in fasting insulin along the exercise dose curve. The exercise program, which emphasized home-based treadmill walking, promoted good intervention adherence that was confirmed with objective heart rate monitor measures. Most participants (97%) completed the study.
In summary, the findings from this phase II randomized dose-response trial demonstrate that moderate-intensity aerobic exercise reduces fasting insulin concentrations and insulin resistance among patients with stage I–III colon cancer. The findings from this randomized trial may be useful to help guide exercise prescriptions in this population. The relationship between physical activity and colon cancer prognosis may be mediated, in part, by changes in insulin concentrations or insulin resistance. Continued research to examine this hypothesis is warranted.
Acknowledgments
We thank the Pennsylvania Cancer Registry for their role in recruitment activities for this study. We thank Dr. Heather Collins of the University of Pennsylvania Diabetes Research Center for conducting the assays used to quantify metabolic growth factor outcomes.
Funding
This research was supported by R21-CA182767, F31-CA192560 and U54-CA155850 from the National Cancer Institute, P30-DK019525 from the National Institute of Diabetes and Digestive and Kidney Diseases, and UL1-TR000003 from the National Center for Research Resources and the National Center for Advancing Translation Science. This research was supported by discounts for treadmills from LifeSpan Fitness, LLC (Salt Lake City, UT). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Interest
The authors declare no conflicts of interest.
Author Contributions
All authors were involved in the study design. JCB, ABT, BK, and KHS collected and analyzed the data in conjunction with the authors. The manuscript was written by JCB, and was reviewed, modified, and approved in its final version by all the authors. All authors vouch for the accuracy and completeness of the data reported and the fidelity of the study to the protocol.
References
- Abdul-Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol. 2010;2010:476279. doi: 10.1155/2010/476279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer. 2011;18:R125–147. doi: 10.1530/ERC-11-0074. [DOI] [PubMed] [Google Scholar]
- Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93:2438–2446. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel AL, Nazare JA, Baillot A, Almeras N, Tremblay A, Bergeron J, Poirier P, Despres JP. Cardiometabolic risk improvement in response to a 3-yr lifestyle modification program in men: contribution of improved cardiorespiratory fitness vs. weight loss. Am J Physiol Endocrinol Metab. 2017;312:E273–E281. doi: 10.1152/ajpendo.00278.2016. [DOI] [PubMed] [Google Scholar]
- Brown JC, Troxel AB, Ky B, Damjanov N, Zemel BS, Rickels MR, Rhim AD, Rustgi AK, Courneya KS, Schmitz KH. A randomized phase II dose–response exercise trial among colon cancer survivors: Purpose, study design, methods, and recruitment results. Contemporary clinical trials. 2016;47:366–375. doi: 10.1016/j.cct.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JC, Troxel AB, Ky B, Damjanov N, Zemel BS, Rickels MR, Rhim AD, Rustgi AK, Courneya KS, Schmitz KH. Dose-Response Effects of Aerobic Exercise among Colon Cancer Survivors: A Randomized Phase II Trial. Clinical colorectal cancer. 2017a doi: 10.1016/j.clcc.2017.06.001. [ePub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JC, Zemel BS, Troxel AB, Rickels MR, Damjanov N, Ky B, Rhim AD, Rustgi AK, Courneya KS, Schmitz KH. Dose-Response Effects of Aerobic Exercise on Body Composition among Colon Cancer Survivors: A Randomized Controlled Trial. Br J Cancer. 2017b doi: 10.1038/bjc.2017.339. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Huang XF, Qiao L, Katsifis A. Insulin caused drug resistance to oxaliplatin in colon cancer cell line HT29. J Gastrointest Oncol. 2011a;2:27–33. doi: 10.3978/j.issn.2078-6891.2010.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Katsifis A, Hu C, Huang XF. Insulin decreases therapeutic efficacy in colon cancer cell line HT29 via the activation of the PI3K/Akt pathway. Curr Drug Discov Technol. 2011b;8:119–125. doi: 10.2174/157016311795563820. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes care. 2009;32:S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez MC, Rayes R, Ham B, Wang N, Bourdeau F, Milette S, Lllemann M, Bird N, Majeed A, Xu J, et al. The type I insulin-like growth factor regulates the liver stromal response to metastatic colon carcinoma cells. Oncotarget. 2016 doi: 10.18632/oncotarget.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. John Wiley & Sons; 2012. [Google Scholar]
- Friedenreich CM, Neilson HK, Woolcott CG, McTiernan A, Wang Q, Ballard-Barbash R, Jones CA, Stanczyk FZ, Brant RF, Yasui Y, et al. Changes in insulin resistance indicators, IGFs, and adipokines in a year-long trial of aerobic exercise in postmenopausal women. Endocr Relat Cancer. 2011;18:357–369. doi: 10.1530/ERC-10-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T, Pappou EP, Guzzetta AA, Calmon Mde F, Sun L, Herrera A, Li F, Wolfgang CL, Baylin SB, Iacobuzio-Donahue CA, et al. IGFBP-3 Gene Methylation in Primary Tumor Predicts Recurrence of Stage II Colorectal Cancers. Ann Surg. 2016;263:337–344. doi: 10.1097/SLA.0000000000001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, Schwartz AV, Kritchevsky S, Newman AB. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes care. 2003;26:372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, Lee EY, Weiss HL, O'Connor KL, Gao T, et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–3256. doi: 10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyuda A, Kim SA, Martinez-Fernandez A, Qian ZR, Yamauchi M, Nishihara R, Morikawa T, Liao X, Inamura K, Mima K, et al. Survival Benefit of Exercise Differs by Tumor IRS1 Expression Status in Colorectal Cancer. Ann Surg Oncol. 2016;23:908–917. doi: 10.1245/s10434-015-4967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Wojtaszewski JF, Goodyear LJ. Exercise regulation of glucose transport in skeletal muscle. Am J Physiol. 1997;273:E1039–1051. doi: 10.1152/ajpendo.1997.273.6.E1039. [DOI] [PubMed] [Google Scholar]
- Haydon AM, Macinnis RJ, English DR, Morris H, Giles GG. Physical activity, insulin-like growth factor 1, insulin-like growth factor binding protein 3, and survival from colorectal cancer. Gut. 2006;55:689–694. doi: 10.1136/gut.2005.081547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol (1985) 2004;96:101–106. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]
- Irwin ML, Varma K, Alvarez-Reeves M, Cadmus L, Wiley A, Chung GG, Dipietro L, Mayne ST, Yu H. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer Epidemiol Biomarkers Prev. 2009;18:306–313. doi: 10.1158/1055-9965.EPI-08-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Je Y, Jeon JY, Giovannucci EL, Meyerhardt JA. Association between physical activity and mortality in colorectal cancer: a meta-analysis of prospective cohort studies. International Journal of Cancer. 2013;133:1905–1913. doi: 10.1002/ijc.28208. [DOI] [PubMed] [Google Scholar]
- Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S57–63. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Zhang X, Du LL, Wang Y, Liu DB, Han CZ, Jing JX, Zhao XW, Xu XQ. Possible roles of insulin, IGF-1 and IGFBPs in initiation and progression of colorectal cancer. World J Gastroenterol. 2014;20:1608–1613. doi: 10.3748/wjg.v20.i6.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson HK, Zierath JR. Insulin signaling and glucose transport in insulin resistant human skeletal muscle. Cell Biochem Biophys. 2007;48:103–113. doi: 10.1007/s12013-007-0030-9. [DOI] [PubMed] [Google Scholar]
- Koenuma M, Yamori T, Tsuruo T. Insulin and insulin-like growth factor 1 stimulate proliferation of metastatic variants of colon carcinoma 26. Jpn J Cancer Res. 1989;80:51–58. doi: 10.1111/j.1349-7006.1989.tb02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Kim JY, Lee MK, Lee C, Min JH, Jeong DH, Lee JW, Chu SH, Meyerhardt JA, Ligibel J, et al. Effects of a 12-week home-based exercise program on the level of physical activity, insulin, and cytokines in colorectal cancer survivors: a pilot study. Support Care Cancer. 2013;21:2537–2545. doi: 10.1007/s00520-013-1822-7. [DOI] [PubMed] [Google Scholar]
- Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- Ligibel JA, Campbell N, Partridge A, Chen WY, Salinardi T, Chen H, Adloff K, Keshaviah A, Winer EP. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J Clin Oncol. 2008;26:907–912. doi: 10.1200/JCO.2007.12.7357. [DOI] [PubMed] [Google Scholar]
- McCurdy CE, Klemm DJ. Adipose tissue insulin sensitivity and macrophage recruitment: does PI3K pick the pathway? Adipocyte. 2013;2:135–142. doi: 10.4161/adip.24645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarrah RW, Slentz CA, Kraus WE. The Effect of Vigorous-Versus Moderate-Intensity Aerobic Exercise on Insulin Action. Current cardiology reports. 2016;18:117. doi: 10.1007/s11886-016-0797-7. [DOI] [PubMed] [Google Scholar]
- Paffenbarger R, Wing A, Hyde R. Paffenbarger physical activity questionnaire. Am J Epidemiol. 1978;108:161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Rice B, Janssen I, Hudson R, Ross R. Effects of aerobic or resistance exercise and/or diet on glucose tolerance and plasma insulin levels in obese men. Diabetes care. 1999;22:684–691. doi: 10.2337/diacare.22.5.684. [DOI] [PubMed] [Google Scholar]
- Ross R, Hudson R, Stotz PJ, Lam M. Effects of Exercise Amount and Intensity on Abdominal Obesity and Glucose Tolerance in Obese AdultsA Randomized TrialEffects of Exercise on Obesity and Glucose Intolerance. Annals of internal medicine. 2015;162:325–334. doi: 10.7326/M14-1189. [DOI] [PubMed] [Google Scholar]
- Schmid D, Leitzmann M. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Annals of oncology. 2014;25:1293–1311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- Schousboe JT, Langsetmo L, Schwartz AV, Taylor BC, Vo TN, Kats AM, Barrett-Connor E, Orwoll ES, Marshall LM, Miljkovic I, et al. Comparison of Associations of DXA and CT Visceral Adipose Tissue Measures With Insulin Resistance, Lipid Levels, and Inflammatory Markers. J Clin Densitom. 2017;20:256–264. doi: 10.1016/j.jocd.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadid S, Jensen MD. Pioglitazone increases non-esterified fatty acid clearance in upper body obesity. Diabetologia. 2006;49:149–157. doi: 10.1007/s00125-005-0051-0. [DOI] [PubMed] [Google Scholar]
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Simons CC, Schouten LJ, Godschalk RW, van Engeland M, van den Brandt PA, van Schooten FJ, Weijenberg MP. Genetic Variants in the Insulin-like Growth Factor Pathway and Colorectal Cancer Risk in the Netherlands Cohort Study. Sci Rep. 2015;5:14126. doi: 10.1038/srep14126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TT, Medline A, Bruce WR. Insulin promotion of colon tumors in rats. Cancer Epidemiol Biomarkers Prev. 1996;5:1013–1015. [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- Turcotte LP, Fisher JS. Skeletal muscle insulin resistance: roles of fatty acid metabolism and exercise. Phys Ther. 2008;88:1279–1296. doi: 10.2522/ptj.20080018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkova E, Robinson BA, Willis J, Currie MJ, Dachs GU. Marginal effects of glucose, insulin and insulin-like growth factor on chemotherapy response in endothelial and colorectal cancer cells. Oncology letters. 2014;7:311–320. doi: 10.3892/ol.2013.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargny M, Balkau B, Lange C, Charles M-A, Giral P, Simon D. Association of fasting serum insulin and cancer mortality in a healthy population–28-year follow-up of the French TELECOM Study. Diabetes & Metabolism. 2017 doi: 10.1016/j.diabet.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Wolpin BM, Meyerhardt JA, Chan AT, Ng K, Chan JA, Wu K, Pollak MN, Giovannucci EL, Fuchs CS. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol. 2009;27:176–185. doi: 10.1200/JCO.2008.17.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-J, Dai Q, Sun D-F, Xiong H, Tian X-Q, Gao F-H, Xu M-H, Chen G-Q, Han Z-G, Fang J-Y. mTOR signaling pathway is a target for the treatment of colorectal cancer. Annals of surgical oncology. 2009;16:2617–2628. doi: 10.1245/s10434-009-0555-9. [DOI] [PubMed] [Google Scholar]