Abstract
Background
Increased endothelial permeability is central to shock and organ dysfunction in sepsis but therapeutics targeted to known mediators of increased endothelial permeability have been unsuccessful in patient studies. We previously reported that cell-free hemoglobin (CFH) is elevated in the majority of patients with sepsis and is associated with organ dysfunction, poor clinical outcomes and elevated markers of oxidant injury. Others have shown that Vitamin C (ascorbate) may have endothelial protective effects in sepsis. In this study, we tested the hypothesis that high levels of CFH, as seen in the circulation of patients with sepsis, disrupt endothelial barrier integrity.
Methods
Human umbilical vein endothelial cells (HUVEC) were grown to confluence and treated with CFH with or without ascorbate. Monolayer permeability was measured by Electric Cell-substrate Impedance Sensing (ECIS) or transfer of 14C-inulin. Viability was measured by trypan blue exclusion. Intracellular ascorbate was measured by HPLC.
Results
CFH increased permeability in a dose- and time-dependent manner with 1 mg/ml of CFH increasing inulin transfer by 50% without affecting cell viability. CFH (1 mg/ml) also caused a dramatic reduction in intracellular ascorbate in the same time frame (1.4 mM without CFH, 0.23 mM 18 hours after 1 mg/ml CFH, p<0.05). Pre-treatment of HUVECs with ascorbate attenuated CFH induced permeability.
Conclusions
CFH increases endothelial permeability in part through depletion of intracellular ascorbate. Supplementation of ascorbate can attenuate increases in permeability mediated by CFH suggesting a possible therapeutic approach in sepsis.
Keywords: Cell-free hemoglobin, endothelial permeability, ascorbic acid, oxidative stress
INTRODUCTION
The integrity of the vascular endothelium is critical to the maintenance of homeostasis, yet is compromised in sepsis [1] leading to increased microvascular permeability, shock, and organ dysfunction [2]. A number of cellular and molecular mechanisms that regulate the endothelial barrier have been described including tight junction proteins, the actin cytoskeleton and the Rho kinase pathway [3]. However, the specific triggers of endothelial barrier breakdown of these homeostatic mechanisms in sepsis are largely unknown. Our group has identified cell-free hemoglobin (CFH), hemoglobin that has escaped the confines of the red blood cell, as one potential trigger of increased endothelial permeability in sepsis [4].
Hemoglobin consists of two alpha and two beta subunits, each containing an iron atom within a heme group. The iron atom usually exists in the ferrous (Fe2+) state, but can become oxidized to ferric (Fe3+) or ferryl (Fe4+) forms in a pro-oxidant environment. Red blood cells become fragile during sepsis leading to hemolysis and release of hemoglobin.[5,6] Failure to scavenge cell-free hemoglobin (CFH) triggers inflammation, tissue damage, and endothelial dysfunction.[7] We have previously shown that circulating levels of CFH are elevated in 80% of sepsis patients [8] and are associated with organ dysfunction and mortality [4]. In many of these patients, ferryl hemoglobin is detected [9]. Patients with sepsis also have high plasma levels of isoprostanes, a product of lipid peroxidation that serves as a biomarkers of oxidative stress [10]. While it is well recognized that hemolysis and liberation of CFH is common in sepsis, the mechanisms underlying its pathogenic effects are not well understood.
Ascorbate (vitamin C) is a potent antioxidant that is critical for endothelial barrier maintenance during inflammation [11] [12]. In endothelial cells, intracellular ascorbate can tighten the endothelial barrier by increasing intracellular nitric oxide (NO) and cyclic GMP. However, during inflammatory states including sepsis and critical illness, plasma ascorbate levels decline [13,14], potentially leading to intracellular ascorbate depletion and increased vascular permeability. Another way ascorbate protects the endothelium is by reducing pro-oxidant molecules to a less injurious form. Reduction of pro-oxidant molecules depletes ascorbate stores and may contribute to end-organ injury. One of the potential targets of ascorbate during critical illness may be CFH as it is known that CFH, a potent oxidant, can be reduced by ascorbate [15]. In this manuscript, we test the hypothesis that high levels of CFH, as seen in the circulation of patients with sepsis, cause depletion of intracellular ascorbate in endothelial cells, thereby disrupting endothelial barrier function.
MATERIALS AND METHODS
Reagents
A fresh stock of CFH was prepared before each experiment by dissolving 10 mg endotoxin-free native human hemoglobin (Cell Sciences, Canton, MA) in 10 ml sterile 0.9% NaCl. A fresh stock of ascorbate was prepared before each experiment by dissolving L-ascorbic acid (Sigma, St. Louis, MO) in sterile H2O. EDTA was purchased from Mediatech/CellGro-Corning (Manassas, VA). 14C-inulin (MW ~ 5000–5500; 2 mCi/g) was purchased from Perkin Elmer Life Sciences (Waltham, WA).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were purchased from ScienCell Research Laboratories (Carlsbad, CA) or Lonza (Basel, Switzerland) and cultured in proprietary media from the respective suppliers in the absence of antioxidant supplements at 37°C in humidified air containing 5% CO2. Cells were used between passages 1–5. Reagents were sterilized with a 0.22 μm filter prior to addition to cultured cells. For all experiments, cells were grown to confluence then incubated with CFH (0–1 mg/ml) with or without ascorbate (0–60 μM) for 18 hours.
Endothelial permeability
Electric Cell-substrate Impedance Sensing
HUVECs were cultured to confluence in 8-well arrays overlying electrodes according to the manufacturer’s protocol (Applied Biophysics, Troy, NY). Alternating current was applied to each electrode at the bottom of each well and the voltage potential on the luminal side was detected. From these two measurements, impedance was calculated according to Ohm’s Law. Resistance was then calculated and is directly correlated to barrier integrity; when the endothelial monolayer is compromised, resistance decreases.
Transfer of radiolabeled inulin
Cells were cultured to confluence as determined by light microscopy on polyethylene terephthalate cell culture inserts (6-well plates with 0.4 μm pores at a density of 2 ± 0.2 × 106 pores per cm2, Falcon BD Biosciences) with 1.7 ml antioxidant-free medium in the upper well and 2.8 ml antioxidant-free medium in the lower well. After several days of confluence to ensure development of a tight barrier, reagents were added as indicated. Transfer of 14C-inulin from luminal to abluminal chambers over 60 min at 37°C was measured in duplicate as previously described.[16] The permeability coefficient of 14C-inulin was calculated with correction for the rate of 14C-inulin transfer across filters after removal of cells with ammonium hydroxide.
Intracellular ascorbate
Ascorbate was measured in cells cultured in 6-well plates following 3 rinses with Krebs-Ringer Hepes buffer (KRH, in mM: 20 Hepes, 128 NaCl, 5.2 KCl, 1 NaH2PO4, 1.4 MgSO4, and 1.4 CaCl2) at pH 7.4. The cell monolayer was then treated with 100 μL of 25% (w/v) metaphosphoric acid, lifted from the plate with a rubber spatula, and the acidic lysate was partially neutralized with 350 μL of 100 mM Na2HPO4 containing 50 μM EDTA, pH 8.0. The cell lysate was centrifuged at 4°C for 1 min at 13,000 x g and the supernatant was collected for assay of ascorbate by high-performance liquid chromatography in duplicate as previously described [17,18]. Intracellular ascorbate concentrations were calculated as a fraction of the intracellular distribution space of 3-O-methylglucose relative to the cell protein content [19] which was taken as 3.6 ± 1.2 μL/mg protein [16].
Viability
Confluent HUVECs were incubated with CFH (0–1 mg/ml) for 18 h. Cell viability was determined by trypan blue exclusion, after washing with EDTA (1mM) to remove extracellular hemoglobin.
Data analyses
Data are expressed as means ± SEM. Determination of significant differences between all groups was done with one-way ANOVA and Tukey post hoc test or Mann-Whitney U, as specified. Significance was defined as p < 0.05.
RESULTS
Cell-free hemoglobin decreases endothelial monolayer electrical resistance
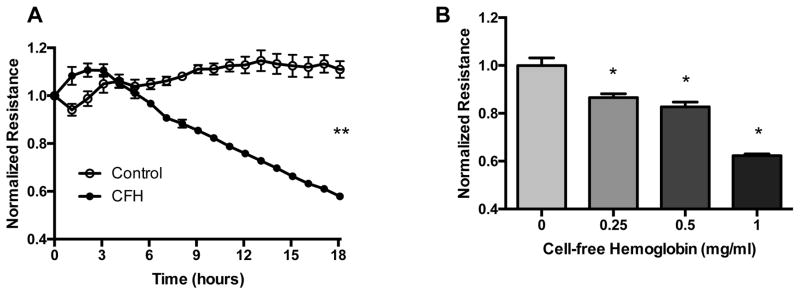
To test whether CFH disrupts endothelial barrier integrity, we measured electrical resistance of a HUVEC monolayer using Electric Cell-substrate Impedance Sensing (ECIS). An intact, tight endothelial barrier presents a high resistance to current flow. When the barrier is disrupted, current flows more easily and is registered as a drop in resistance. We found that CFH caused a time-dependent (Figure 1A) and dose-dependent (Figure 1B) decrease in electrical resistance, consistent with disruption of the endothelial barrier by CFH.
Figure 1. Cell-free hemoglobin decreases electrical resistance across an endothelial cell monolayer.
HUVECs were cultured to confluence on ECIS plates and electrical resistance was measured at 4,000 hz over time in response to 1 mg/mL CFH (A). In addition, CFH decreased electrical resistance at 18 hours in a dose-dependent manner (B). **p<0.05 by Mann Whitney U at 18 hours, *p < 0.05 by ANOVA.
Cell-free hemoglobin increases endothelial macromolecular permeability
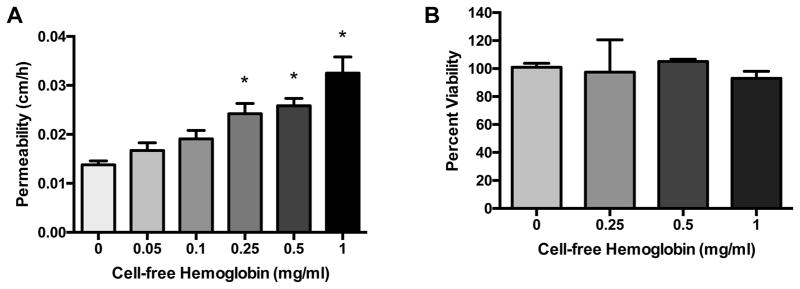
Decreased trans-endothelial resistance following CFH exposure reflects a loss of barrier function, but resistance measurements do not provide any information on the size of particles that might be able to cross the disrupted endothelium. We next tested the hypothesis that CFH would mediate an increase in permeability to larger macromolecules across the endothelial barrier, suggesting more severe damage. The direct effects of cell-free hemoglobin (CFH, 0–1 mg/ml) on HUVEC macromolecular permeability were determined after 18 h (Figure 2). CFH triggered a dose-dependent increase in 14C-inulin transferred from luminal to abluminal chambers in transwell plates compared to cells treated with vehicle (Panel 2A). The highest dose of CFH (1 mg/ml), a clinically relevant concentration directly observed in the plasma of septic patients in vivo, [4,8] caused a more than two-fold increase in 14C-inulin transfer. We next determined whether decreases in barrier integrity were temporally associated with cell death after CFH challenge. CFH did not cause a dose-dependent change in cell viability after 18 h compared to control cells as measured by trypan blue exclusion (Panel 2B). These data suggest that CFH increases endothelial monolayer permeability within 18 h by a mechanism independent of cell death.
Figure 2. Cell-free hemoglobin increases endothelial macromolecular permeability without altering viability.
HUVECs were cultured to confluence on transwell plates and treated with cell-free hemoglobin (CFH, 0–1 mg/ml) for 18 h. CFH increased transfer of 14C-inulin (A) in a dose-dependent manner. Cell viability as measured by Trypan blue exclusion was not affected by CFH treatment for 18 hours (B). *p < 0.05 by ANOVA.
Cell-free hemoglobin depletes intracellular ascorbate
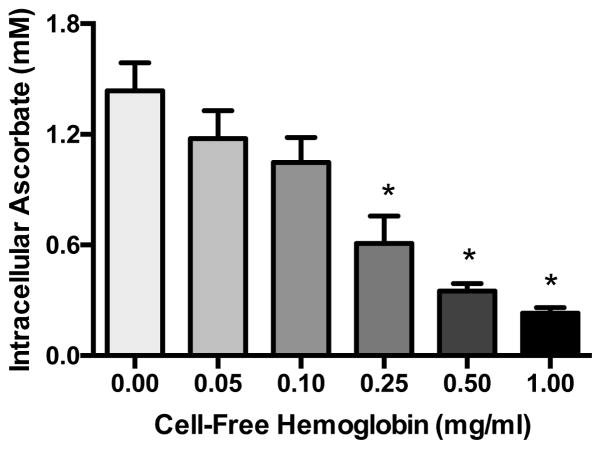
Because we have previously shown that intracellular ascorbate is critical for maintenance of endothelial barrier integrity [20,21,22]. we tested whether CFH altered levels of intracellular ascorbate. Confluent HUVECs were challenged with increasing doses of CFH (0–1 mg/ml) for 18 h and intracellular ascorbate was measured by HPLC. CFH triggered significant dose-dependent decreases in intracellular ascorbate levels (Figure 3).
Figure 3. Cell-free hemoglobin depletes intracellular ascorbate.
Confluent HUVECs were challenged with CFH (0–1 mg/ml) in antioxidant-free medium for 18 h, then intracellular ascorbate was measured by HPLC. *p < 0.05 by ANOVA.
Ascorbate inhibits cell-free hemoglobin-induced permeability increases
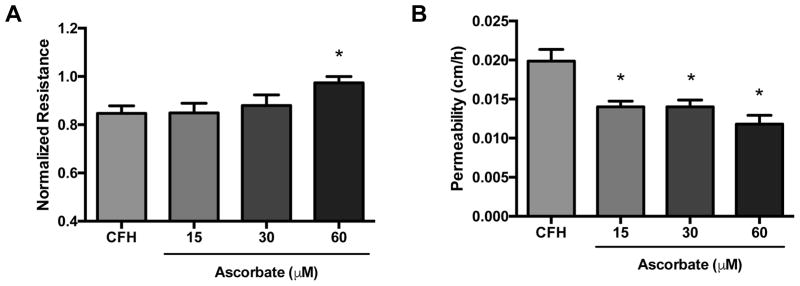
To determine whether ascorbate blocks increases in endothelial permeability triggered by CFH, confluent HUVECs grown in ascorbate-free medium were loaded with ascorbate (0–60 μM) for 15 min prior to challenge with CFH (0.3 mg/ml). After 18 h, ascorbate prevented decreases in monolayer resistance caused by CFH (Figure 4A). Similarly, transfer of 14C-inulin was significantly lower in cells pre-treated with ascorbate compared to those untreated (Figure 4B).
Figure 4. Ascorbate prevents increases in endothelial permeability caused by CFH.
HUVECs were cultured to confluence in antioxidant-free medium on transwell plates and loaded with ascorbate (0–60 uM) for 15 min prior to challenge with cell-free hemoglobin (CFH, 0.3 mg/ml) for 18 h. Ascorbate significantly blocked CFH mediated decrease in electrical resistance (A) and the increased transfer of 14C-inulin (B). *p < 0.05 by ANOVA.
DISCUSSION
In this study we show that CFH decreases monolayer integrity, increases macromolecular permeability, and decreases intracellular ascorbate in human endothelial cells (HUVEC). These effects can be reversed by pre-treatment with ascorbate at physiologic concentrations. These results provide a mechanistic link between the observations that CFH causes endothelial dysfunction [23] and that ascorbate (vitamin C) is critical for maintenance of endothelial barrier integrity [12,20,21,22]. The observation that CFH can deplete intracellular ascorbate may have particular relevance to conditions such as sepsis, which are characterized by both increased circulating CFH [4] and ascorbate depletion [14].
Hemoglobin, a potent oxidant once released from the confines of the red blood cell, is a driver of vascular dysfunction in a variety of disease states. For example, in the setting of chronic hemolysis, the vascular effects of CFH on larger muscular arteries are mediated by reductions in nitric oxide bioavailability [5]. In sickle cell disease, the severity of hemolysis with release of CFH is associated with pulmonary hypertension [24,25,26], which is mediated, in part, by nitric oxide depletion by CFH [5]. Similarly, hemodialysis is associated with episodic release of CFH and decreased NO bioavailability leading to vascular dysfunction [27]. While much is known about the effects of CFH on vascular dysfunction in chronic hemolysis, relatively little is know about its acute effects on endothelial permeability. CFH decreases monolayer electrical resistance in dermal microvascular endothelial cells through a MyD88-dependent mechanism [23] and in bovine lung microvascular endothelial cells specifically in response to hemoglobin alpha [28] but no cellular mechanism of these effects was explored. Our data extend these studies to demonstrate that CFH can mediate endothelial permeability through depletion of intracellular ascorbate. Together these studies support that CFH can acutely increase microvascular permeability and identifies a novel mechanism, vitamin C depletion, to explain this effect.
Ascorbate is a critical cofactor for maintenance of a healthy endothelium. Our group has previously shown that depletion of intracellular ascorbate in cultured endothelial cells leads to increased paracellular permeability [21,22]. Ascorbate helps to tighten the endothelial barrier through stabilization of the tubulin cytoskeleton [22]. Despite this understanding of the critical role of ascorbate in endothelial barrier integrity, little is known about the specific factors that cause intracellular ascorbate depletion. Intracellular ascorbate stores are depleted as ascorbate functions as a reducing agent (electron donor) in an oxidative milieu to reduce oxidant stress. In this study, we demonstrate that CFH is one stimulus that can deplete intracellular ascorbate in cultured endothelial cells. CFH also increases endothelial permeability which can be attenuated by treatment with ascorbate, confirming that the mechanism of CFH-induced permeability is through ascorbate depletion. This conclusion is supported by prior data showing that CFH can be reduced by ascorbate [15]. Overall, our findings may have particular relevance to conditions characterized by both ascorbate depletion and liberation of CFH, such as sepsis [4,8,13,14]. In fact, ascorbate treatment has been [29] and is currently being studied (NCT02734147, NCT02106975) in patients with sepsis.
In summary, we show that CFH depletes intracellular ascorbate leading to increased paracellular permeability of endothelial cells. These effects can be attenuated by ascorbate supplementation. These findings may have clinical relevance to conditions associated with both ascorbate depletion and CFH release such as sepsis.
Highlights.
Cell-free hemoglobin (CFH) increases endothelial barrier permeability
Endothelial ascorbate is depleted following exposure to CFH
Treatment of endothelial cells with ascorbate attenuates CFH mediated permeability
Acknowledgments
Funding: NIH HL126671, HL117676 to JAB, NIH HL103836 and HL135849 to LBW
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee WL, Liles WC. Endothelial activation, dysfunction and permeability during severe infections. Curr Opin Hematol. 2011;18:191–196. doi: 10.1097/MOH.0b013e328345a3d1. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg NM, Steinberg BE, Slutsky AS, Lee WL. Broken barriers: a new take on sepsis pathogenesis. Sci Transl Med. 2011;3:88ps25. doi: 10.1126/scitranslmed.3002011. [DOI] [PubMed] [Google Scholar]
- 3.Radeva MY, Waschke J. Mind the gap: mechanisms regulating the endothelial barrier. Acta Physiol (Oxf) 2017 doi: 10.1111/apha.12860. [DOI] [PubMed] [Google Scholar]
- 4.Janz DR, Bastarache JA, Peterson JF, Sills G, Wickersham N, May AK, Roberts LJ, 2nd, Ware LB. Association between cell-free hemoglobin, acetaminophen, and mortality in patients with sepsis: an observational study. Critical Care Medicine. 2013;41:784–790. doi: 10.1097/CCM.0b013e3182741a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest. 2017;127:750–760. doi: 10.1172/JCI89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121:1276–1284. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rifkind JM, Mohanty JG, Nagababu E. The pathophysiology of extracellular hemoglobin associated with enhanced oxidative reactions. Front Physiol. 2014;5:500. doi: 10.3389/fphys.2014.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janz DR, Bastarache JA, Rice TW, Bernard GR, Warren MA, Wickersham N, Sills G, Oates JA, Roberts LJ, 2nd, Ware LB. Randomized, Placebo-Controlled Trial of Acetaminophen for the Reduction of Oxidative Injury in Severe Sepsis: The Acetaminophen for the Reduction of Oxidative injury in Severe Sepsis Trial. Critical Care Medicine. 2014 doi: 10.1097/CCM.0000000000000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bastarache JA, Janz DR, Rice TW, Bernard GR, Warren MA, Wickersham N, Sills G, Oates JA, Roberts lJ, Dikalov SI, Ware LB. Plasma Levels of Oxidized Ferryl (4+) Hemoglobin Are Elevated in Severe Sepsis and Are Reduced by Acetaminophen. a Hemoprotein Reductant. Am J Respir Crit Care Med. 2015;191:A2555. [Google Scholar]
- 10.Ware LB, Fessel JP, May AK, Roberts LJ., 2nd Plasma biomarkers of oxidant stress and development of organ failure in severe sepsis. Shock. 2011;36:12–17. doi: 10.1097/SHK.0b013e318217025a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.May JM, Harrison FE. Role of vitamin C in the function of the vascular endothelium. Antioxid Redox Signal. 2013;19:2068–2083. doi: 10.1089/ars.2013.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.May JM, Qu ZC. Nitric oxide mediates tightening of the endothelial barrier by ascorbic acid. Biochem Biophys Res Commun. 2011;404:701–705. doi: 10.1016/j.bbrc.2010.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson JX, Wu F. Vitamin C in sepsis. Subcell Biochem. 2012;56:67–83. doi: 10.1007/978-94-007-2199-9_5. [DOI] [PubMed] [Google Scholar]
- 14.Voigt K, Kontush A, Stuerenburg HJ, Muench-Harrach D, Hansen HC, Kunze K. Decreased plasma and cerebrospinal fluid ascorbate levels in patients with septic encephalopathy. Free Radic Res. 2002;36:735–739. doi: 10.1080/10715760290032557. [DOI] [PubMed] [Google Scholar]
- 15.Vollaard NB, Reeder BJ, Shearman JP, Menu P, Wilson MT, Cooper CE. A new sensitive assay reveals that hemoglobin is oxidatively modified in vivo. Free radical biology & medicine. 2005;39:1216–1228. doi: 10.1016/j.freeradbiomed.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Ulker E, Parker WH, Raj A, Qu ZC, May JM. Ascorbic acid prevents VEGF-induced increases in endothelial barrier permeability. Mol Cell Biochem. 2016;412:73–79. doi: 10.1007/s11010-015-2609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hastie LE, Patton WF, Hechtman HB, Shepro D. H2O2-induced filamin redistribution in endothelial cells is modulated by the cyclic AMP-dependent protein kinase pathway. J Cell Physiol. 1997;172:373–381. doi: 10.1002/(SICI)1097-4652(199709)172:3<373::AID-JCP11>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.May JM, Qu ZC, Mendiratta S. Protection and recycling of alpha-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch Biochem Biophys. 1998;349:281–289. doi: 10.1006/abbi.1997.0473. [DOI] [PubMed] [Google Scholar]
- 19.Jones W, Li X, Qu ZC, Perriott L, Whitesell RR, May JM. Uptake, recycling, and antioxidant actions of alpha-lipoic acid in endothelial cells. Free Radic Biol Med. 2002;33:83–93. doi: 10.1016/s0891-5849(02)00862-6. [DOI] [PubMed] [Google Scholar]
- 20.Meredith ME, Qu ZC, May JM. Ascorbate reverses high glucose- and RAGE-induced leak of the endothelial permeability barrier. Biochemical and biophysical research communications. 2014;445:30–35. doi: 10.1016/j.bbrc.2014.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker WH, Qu ZC, May JM. Intracellular Ascorbate Prevents Endothelial Barrier Permeabilization by Thrombin. J Biol Chem. 2015;290:21486–21497. doi: 10.1074/jbc.M115.662098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker WH, Rhea EM, Qu ZC, Hecker MR, May JM. Intracellular ascorbate tightens the endothelial permeability barrier through Epac1 and the tubulin cytoskeleton. Am J Physiol Cell Physiol. 2016;311:C652–C662. doi: 10.1152/ajpcell.00076.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lisk C, Kominsky D, Ehrentraut S, Bonaventura J, Nuss R, Hassell K, Nozik-Grayck E, Irwin DC. Hemoglobin-induced endothelial cell permeability is controlled, in part, via a myeloid differentiation primary response gene-88-dependent signaling mechanism. American journal of respiratory cell and molecular biology. 2013;49:619–626. doi: 10.1165/rcmb.2012-0440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 25.De Castro LM, Jonassaint JC, Graham FL, Ashley-Koch A, Telen MJ. Pulmonary hypertension associated with sickle cell disease: clinical and laboratory endpoints and disease outcomes. Am J Hematol. 2008;83:19–25. doi: 10.1002/ajh.21058. [DOI] [PubMed] [Google Scholar]
- 26.Damy T, Bodez D, Habibi A, Guellich A, Rappeneau S, Inamo J, Guendouz S, Gellen-Dautremer J, Pissard S, Loric S, Wagner-Ballon O, Godeau B, Adnot S, Dubois-Rande JL, Hittinger L, Galacteros F, Bartolucci P. Haematological determinants of cardiac involvement in adults with sickle cell disease. Eur Heart J. 2016;37:1158–1167. doi: 10.1093/eurheartj/ehv555. [DOI] [PubMed] [Google Scholar]
- 27.Meyer C, Heiss C, Drexhage C, Kehmeier ES, Balzer J, Muhlfeld A, Merx MW, Lauer T, Kuhl H, Floege J, Kelm M, Rassaf T. Hemodialysis-induced release of hemoglobin limits nitric oxide bioavailability and impairs vascular function. J Am Coll Cardiol. 2010;55:454–459. doi: 10.1016/j.jacc.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 28.Dull RO, DeWitt BJ, Dinavahi R, Schwartz L, Hubert C, Pace N, Fronticelli C. Quantitative assessment of hemoglobin-induced endothelial barrier dysfunction. J Appl Physiol (1985) 2004;97:1930–1937. doi: 10.1152/japplphysiol.00102.2004. [DOI] [PubMed] [Google Scholar]
- 29.Fowler AA, 3rd, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, Farthing CA, Larus TL, Martin E, Brophy DF, Gupta S, Fisher BJ, Natarajan R N Medical Respiratory Intensive Care Unit. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12:32. doi: 10.1186/1479-5876-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]