INTRODUCTION
Increased physical activity (PA) is associated with the prevention and delayed onset of many noncommunicable chronic conditions, such as heart disease, diabetes, and cancer, and health benefits, for example reduced risk of disease progression, secondary chronic conditions, and mortality, for individuals with chronic conditions.1 PA is also associated with reduced cognitive dysfunction and functional limitations and enhanced mental health and quality of life.2 Thus, identifying strategies to increase activity in high-risk individuals, as in those with at least one major risk factor, and those with chronic conditions remains a priority.
An abundance of technological devices exist to collect and analyze PA data and support the health of high-risk individuals and those with chronic conditions. Devices that are worn on the person, consumer-targeted, and worn continuously to quantify motion during the 24 hour day offer many promising opportunities to advance chronic disease research and clinical practice. These “wearables” include fitness bands, smart watches, and jewelry that log users’ activity metrics (i.e., step counts, energy expenditure) plus a range of other physiological and behavioral factors and can be used with a tablet/smartphone app or website that provides users with a summary of collected data. Wearables are designed and marketed as tools to motivate users to increase PA to improve health by allowing them to easily self-monitor behavior, a key element to successful behavior change.3
In 2013, 84 million wearables were sold; one in ten Americans own a device4 and annual sales are projected to be >$50 billion by 2018.5 This burgeoning industry has been met with both excitement and skepticism. Wearables represent a scalable opportunity to collect activity data in free-living conditions, unobtrusively, with greater frequency, lower cost, and higher volume than ever before.6 Wearables have significant potential to facilitate improvements in health behaviors. However, their capacity to translate external motivations into internal ones for sustainable behavioral changes and clinical value is still unknown.3
This article aims to: (1) summarize current and potential uses for wearables; (2) discuss challenges to using wearables; and (3) identify future directions for researchers and clinicians for chronic disease prevention and management.
CURRENT AND POTENTIAL USES FOR WEARABLES IN CHRONIC DISEASE PREVENTION AND MANAGEMENT
Studies suggest wearables have adequate validity and reliability for activity measurement, use in chronic disease populations is feasible, and most individuals are open to sharing device data with researchers and healthcare systems.7,8 Thus, this is an opportune time to integrate wearables into diverse contexts. Table 1 details potential uses for wearable-derived data for chronic disease research and management within two domains: epidemiological and health promotion.
Table 1.
Current and Potential Uses for Wearables Within Chronic Disease Management in Clinical and Research Settings
| Domain | Current and potential uses |
|---|---|
| Epidemiological | Monitoring and assessing physical activity
|
| Health promotion |
|
PA, physical activity
Epidemiological
Epidemiological uses of wearables for chronic disease development and management include: monitoring activity; describing activity context and correlates; and tracking individual disease trajectories (e.g., return to activity post-surgery).
Epidemiological studies typically rely on self-reported PA estimates, which, generally, have low to moderate validity.9 Over the past decade, the introduction of accelerometers, valid and reliable tools for measuring activity,10 into epidemiological studies has provided fine-grained data on individual movement patterns. However, limited battery capacity results in short, “snapshot” monitoring periods, typically 7 days. Because fluctuations in activity across weeks and months are not captured, information is missed that may be important when planning to intervene. Wearables can be recharged by the user or have coin cell batteries (last ≥ 12 months) translating to continuous, objective, real-time activity data can be collected over longer time periods resulting in greater insight into activity patterns across time. Finally, although data extracted from most wearables (via the application programming interfaces developed by device manufacturers) is crude compared with data extracted from research grade accelerometers, wearables exhibit adequate validity in comparison with research-grade monitors11 and are more cost efficient making them an attractive alternative for larger studies. Total duration and activity accrual patterns likely vary over the course of disease progression or treatment. Wearables can be used across different chronic disease trajectory phases (e.g., pre-versus post-surgery) and linked to medical record data to obtain granular data on how activity frequency, intensity, and duration changes over the disease course and with different treatments. These data could be used to identify crucial intervention time points.
Understanding who is likely to be physically active in different contexts is key to any successful intervention. Wearables provide an opportunity to better understand PA determinants and preferences in real-world settings over chronic conditions’ trajectories to identify specific intervention targets. Combining wearable data with clinical data, patient-reported outcomes (i.e., motivation, symptoms), remote monitoring sensors (i.e., GPS, blood pressure, glucose, body weight), and social media data collected using Ecological Momentary Assessment methodology could provide a more comprehensive, multidimensional understanding of PA across the disease continuum. Ecological Momentary Assessment involves repeated sampling of subjects’ current behaviors and experiences in real time in subjects’ natural environments. Analysis of these data highlights individual differences in behavior, their distribution over time, factors affecting behavior and the mutual associations between them.11 Combining wearable data with Ecological Momentary Assessment data from these other sources could provide more accurate information about activity fluctuations’ antecedents and outcomes and new insight into potential intervention timing, targets, and high-risk subgroups. For example, these methods could reveal individuals engage in less activity on days they report greater fatigue. Thus, researchers may want to design interventions to increase activity and reduce fatigue.
Little is known about how activity patterns before, during, and after medical treatments predict health outcomes. As daily activity may be a proxy of overall health and functioning,12 granular data from wearables could be combined with clinical (i.e., hospitalizations, disease progression markers) or functional (i.e., fitness, strength, balance) data to identify onset of disease or functional limitations prior to clinical symptoms. For example, activity may decline prior to hospitalization or with disease complication (i.e., edema, neuropathy) onset. Activity patterns may also be an indicator of symptoms, treatment adherence and short- and long-term disease prognosis.13 Activity may decline more rapidly prior to disease onset or in individuals with a more aggressive disease. Changes in activity may also be indicative of treatment-related side effects resulting in poorer treatment adherence. Consequently, PA data from wearables could be used to monitor disease onset, treatment adherence, and predict prognostic outcomes amongst high-risk and chronic disease populations in the hospital and outpatient setting.
To date, machine learning algorithms have been developed to predict functional status including post-surgery body area changes in movement, walking stability, gait speed, and fall detection14 using research-grade accelerometer and gyroscope data. As wearables’ storage capacity increases, more data could be collected and used to refine algorithms to track high-risk individuals and identify activity patterns changes indicative of increased complications, more rapid disease progression, poor treatment adherence or disease self-management. Finally, combining wearables, clinical, and functional data with biological datasets (e.g., genomewide association study data; NIH precision medicine initiative) could provide greater insight into interactions between genetics and behavior in disease onset and progression.
Health Promotion
A review of behavior change techniques in 13 wearables and their associated mobile apps15 found they use many of the same techniques employed in typical PA behavior change interventions (i.e., self-monitoring, goal-setting, social support). Additionally, individuals with chronic conditions perceive wearables as useful and acceptable.16 Thus, wearables may be a low-cost, feasible, and accessible way for promoting PA. However, most existing efficacious wearables studies provide other behavior change supports including social support, financial incentives, and health coaching.17 Hence, wearables’ ability, alone, to produce clinically meaningful, sustained changes in activity without external motivation and accountability (e.g., reporting progress to study team) in high-risk individuals and those with chronic conditions, is unknown.3 This is similar to the limited efficacy of providing a scale without additional support for weight loss. Wearables integration within a theory-driven intervention strategy should be carefully considered as wearables, by themselves, will not work for all individuals for a variety of reasons (i.e., demographics, psychosocial factors, motivation) and may need to be combined with additional intervention components. Adopting a stepped-care approach to provide additional intervention tools and supports to those who need them may be necessary to observe meaningful, sustained behavior change.
Cancer Prevention and Control Case Study
Wearables could be implemented and integrated into clinical practice as a monitoring and intervention tool. Table 2 provides example research questions and clinical implications of answering these questions across the themes addressed within this section using cancer prevention and control as a case study. Briefly, wearables may allow for more personalized care and targeted interventions delivered with optimal timing to improve health and quality of life for high-risk individuals and those with chronic conditions.
Table 2.
Potential Clinical Implications of Using Wearables in Chronic Disease Management: Cancer Prevention and Control Case Study
| Domain | Example research questions | Potential clinical implications |
|---|---|---|
| Epidemiological | Monitoring and assessing PA
|
|
| PA promotion |
|
|
PA, physical activity; EHR, electronic health record
Challenges and Future Directions
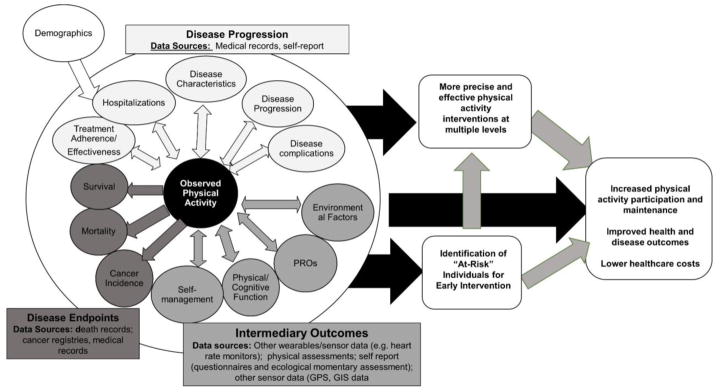
Significant work is needed to understand the best and most efficient ways to fully integrate wearables into care (Table 3). However, in the future it is possible that wearables data are able to be used in conjunction with other data sources to increase PA, improve health and disease outcomes, and reduce healthcare costs. Figure 1 depicts what this ideal could look like. The relationships between activity data obtained from wearables and data related to disease progression, intermediary endpoints and disease outcomes obtained from other data sources could be integrated and examined unidirectionally and bidirectionally by individual characteristics to identify high-risk individuals for early intervention. These big datasets could be used to create more precise and effective multi-level (i.e., individual, systems wide) PA interventions with oversight from clinical and research staff to increase activity, improve health and disease outcomes and lower healthcare costs for high-risk individuals and those with chronic conditions.
Table 3.
Challenges and Future Directions in Using Wearables in Research and Clinical Settings
| Participant/Patient challenges | Challenges | Potential solutions/Future directions |
|---|---|---|
| Participant/Patient |
|
|
| Device |
|
|
| Clinical/Research setting |
|
|
PA, physical activity; EHR, electronic health record
Figure 1.
Conceptual model of how wearables could ideally be integrated into research and clinical practice for chronic disease prevention and management.
To reach this ideal, many challenges must be overcome related to the: (1) participant/patient, (2) devices, and (3) clinical/research settings (Table 3). Engaging multi-level and -sector stakeholders from the outset can help circumvent many challenges. Understanding individual patient perspectives on the burden of intensive data collection methods is important. Further, understanding clinicians’ interest in wearable data and preferences for data presentation is necessary. Wearables developers should also be engaged, in the event activity output or algorithms are needed. Finally, researchers should work with health information specialists and statisticians to develop methods to integrate and harmonize data from wearables and other sources and sensors.
An important challenge is how to derive meaningful outcomes from wearables data, alone, and combined with other data sources to inform clinical decision making. Currently, machine learning approaches are being employed to make sense of the large data volume from wearables using research-grade devices primarily under controlled laboratory conditions. The next step is applying algorithms to free-living data from commercially available devices. This will support intervention research and the ability to understand the magnitude of changes in PA observed via wearables needed for health improvements.
Understanding how to use data from wearables to monitor and predict PA and disease or condition onset will create innumerable opportunities to provide patients with proactive interventions to prevent disease, reduce severity or control progression to a clinical syndrome. Building methods for just-in-time adaptive interventions to intervene on activity in real time based on patient profiles, real-world triggers (i.e., proximity to gyms), patient-reported outcomes (i.e., high fatigue, low motivation) or activity data itself (i.e., not meeting activity threshold by noon) should be a priorty.18 Additionally, innovative study designs including single case designs, multiphase optimization strategy,19 and sequential multiple assignment designs19 should be used to identify and adapt the most effective PA intervention components, or component sequence, to understand what works for whom, in what contexts, for what outcomes. Finally, dynamic systems modeling methods can help maximize just-in-time adaptive interventions delivery and make better use of intensive longitudinal data from wearables combined with potential intervention mediators.20
CONCLUSIONS
Wearables offer an unobtrusive way to collect rich data on PA. These data can be linked to other data sources and used to help monitor and promote activity behavior change and maintenance. Despite wearables’ promise, many questions and challenges need to be overcome (Tables 2 and 3) before they can be leveraged to enhance clinical care and improve health outcomes in high-risk individuals and those with chronic conditions. Studies using innovative methodology to understand whether these devices can be used for the purposes discussed are needed. Further, engaging diverse researchers (i.e., exercise science, public health, bioinformatics, statistics, methodology) and stakeholders (i.e., patients, clinicians, electronic health record and wearable companies’ representatives) to understand challenges with using wearables and discover solutions is important. Ultimately, addressing these issues is central to leveraging wearables to improve public health, clinical care, and health outcomes and reduce disease risk, burden, and healthcare costs.
Acknowledgments
Phillips is supported by the National Cancer Institute (K07CA196840); Cadmus-Bertram is supported by K07CA178870; Buman is supported by R01CA198971, R21NR016046, and R18DK109516; Lynch is supported by a Fellowship from the National Breast Cancer Foundation (ECF-15-013).
Footnotes
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee I-M, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. doi: 10.1016/S0140-6736(12)61031-9. https://doi.org/10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. CMAJ. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. https://doi.org/10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel MS, Asch DA, Volpp KG. Wearable devices as facilitators, not drivers, of health behavior change. JAMA. 2015;313(5):459–460. doi: 10.1001/jama.2014.14781. https://doi.org/10.1001/jama.2014.14781. [DOI] [PubMed] [Google Scholar]
- 4.Internicola I. Activity Trackers Get Smarter at Measuring Your Fitness. [Accessed December 6, 2015];Scientific American. 2014 Dec 22; www.scientificamerican.com/article/activity-trackers-get-smarter-at-measuring-your-fitness/
- 5.Gahndi M, Wang T. The Future of Biosensing Wearables. [Accessed December 6, 2015];RockHealth. 2014 Jun 9; http://rockhealth.com/2014/06/future-biosensing-wearables.
- 6.Ginexi EM, Riley W, Atienza AA, Mabry PL. The promise of intensive longitudinal data capture for behavioral health research. Nicotine Tob Res. 2014;16(suppl 2):S73–S75. doi: 10.1093/ntr/ntt273. https://doi.org/10.1093/ntr/ntt273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cadmus-Bertram L, Marcus BH, Patterson RE, Parker BA, Morey BL. Use of the Fitbit to measure adherence to a physical activity intervention among overweight or obese, postmenopausal women: self-monitoring trajectory during 16 weeks. JMIR Mhealth Uhealth. 2015;3(4):e96. doi: 10.2196/mhealth.4229. https://doi.org/10.2196/mhealth.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg D, Renz A. Physical activity and sedentary behavior in men with prostate cancer. Group Health Research Institute Annual Birnbaum Research Rounding; Seattle, WA: 2015. [Google Scholar]
- 9.Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exer Sport. 2000;71(suppl 2):1–14. doi: 10.1080/02701367.2000.11082780. https://doi.org/10.1080/02701367.2000.11082780. [DOI] [PubMed] [Google Scholar]
- 10.Trost SG, Mciver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005;37(suppl 11):S531–S543. doi: 10.1249/01.mss.0000185657.86065.98. https://doi.org/10.1249/01.mss.0000185657.86065.98. [DOI] [PubMed] [Google Scholar]
- 11.Lee J-M, Kim Y, Welk GJ. Validity of consumer-based physical activity monitors. Med Sci Sports Exerc. 2014;46(9):1840–1848. doi: 10.1249/MSS.0000000000000287. https://doi.org/10.1249/MSS.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 12.Marszalek J, Morgulec-Adamowicz N, Rutkowska I, Kosmol A. Using ecological momentary assessment to evaluate current physical activity. Biomed Res Int. 2014;2014:915172. doi: 10.1155/2014/915172. https://doi.org/10.1155/2014/915172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maddocks M, Byrne A, Johnson CD, Wilson RH, Fearon KC, Wilcock A. Physical activity level as an outcome measure for use in cancer cachexia trials: a feasibility study. Support Care Cancer. 2010;18(12):1539–1544. doi: 10.1007/s00520-009-0776-2. https://doi.org/10.1007/s00520-009-0776-2. [DOI] [PubMed] [Google Scholar]
- 14.Ferriolli E, Skipworth RJ, Hendry P, et al. Physical activity monitoring: a responsive and meaningful patient-centered outcome for surgery, chemotherapy, or radiotherapy? J Pain Symptom Manage. 2012;43(6):1025–1035. doi: 10.1016/j.jpainsymman.2011.06.013. https://doi.org/10.1016/j.jpainsymman.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Shull PB, Jirattigalachote W, Hunt MA, Cutkosky MR, Delp SL. Quantified self and human movement: a review on the clinical impact of wearable sensing and feedback for gait analysis and intervention. Gait Posture. 2014;40(1):11–19. doi: 10.1016/j.gaitpost.2014.03.189. https://doi.org/10.1016/j.gaitpost.2014.03.189. [DOI] [PubMed] [Google Scholar]
- 16.Lyons EJ, Lewis ZH, Mayrsohn BG, Rowland JL. Behavior change techniques implemented in electronic lifestyle activity monitors: a systematic content analysis. J Med Internet Res. 2014;16(8):e192. doi: 10.2196/jmir.3469. https://doi.org/10.2196/jmir.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mercer K, Giangregorio L, Schneider E, Chilana P, Li M, Grindrod K. Acceptance of commercially available wearable activity trackers among adults aged over 50 and with chronic illness: a mixed-methods evaluation. JMIR mHealth and uHealth. 2016;4(1):e7. doi: 10.2196/mhealth.4225. https://doi.org/10.2196/mhealth.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kostkova P, Coventry LM, D’Ambrosio A, Gesù OB, Sullivan AN. Behavior change with fitness technology in sedentary adults: a review of the evidence for increasing physical activity. Front Public Health. 2017;4:289. doi: 10.3389/fpubh.2016.00289. https://doi.org/10.3389/fpubh.2016.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nahum-Shani I, Smith SN, Tewari A, et al. Just in time adaptive interventions (JITAIS): An organizing framework for ongoing health behavior support. [Accessed 22 May 2017];Methodology Center Technical Report. https://methodology.psu.edu/media/techreports/14-126.pdf. Published 2014.
- 20.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med. 2007;32(suppl 5):S112–118. doi: 10.1016/j.amepre.2007.01.022. https://doi.org/10.1016/j.amepre.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riley WT, Martin CA, Rivera DE. The importance of behavior theory in control system modeling of physical activity sensor data. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:6880–6883. doi: 10.1109/EMBC.2014.6945209. https://doi.org/10.1109/EMBC.2014.6945209. [DOI] [PMC free article] [PubMed] [Google Scholar]