Abstract
Introduction
A high proportion of children presenting to pediatric urgent cares are exposed to tobacco smoke. An electronic health record-based clinical decision support system for nurses to facilitate guideline-based tobacco smoke exposure screening and counseling for caregivers who smoke was designed and evaluated.
Design
A mixed-methods, 3-month, prospective study that began in November 2015, data were analyzed in June 2016.
Setting/participants
Five urgent cares that were part of a large children’s hospital in Cincinnati, OH. Participants were urgent care nurses.
Intervention
The clinical decision support system prompted nurses to Ask, Advise, Assess, and Assist caregivers to quit smoking. Monthly feedback reports were also provided.
Main outcome measure
Clinical decision support system use rates, nurses’ attitudes towards tobacco smoke exposure intervention, and percentage of children screened and caregivers counseled.
Results
All nurses used the clinical decision support system. Compared with Month 1, nurses were twice as likely to advise and assess during Months 2 and 3. There was significant improvement in nurses feeling prepared to assist caregivers in quitting. Nurses reported that feedback reports motivated them to use the clinical decision support system, and that it was easy to use. Almost 65% of children were screened for tobacco smoke exposure; 19.5% screened positive. Of caregivers identified as smokers, 26% were advised to quit, and 29% were assessed for readiness to quit. Of those assessed, 67% were interested in quitting, and of those, 100% were assisted.
Conclusions
A clinical decision support system increased rates of tobacco smoke exposure screening and intervention in pediatric urgent cares. Rates might further improve by incorporating all components of the clinical decision support system into the electronic health record.
Clinical trial registration
INTRODUCTION
Each year, >400,000 deaths in the U.S. are attributable to smoking.1–5 In addition, exposure of nonsmokers to tobacco smoke is a serious health hazard. More than 40% of U.S. children aged 3–11 years are exposed to tobacco smoke.6 Tobacco smoke exposure (TSE) places children at increased risk for illnesses including asthma and bronchiolitis.1–5 TSE-related illnesses result in increased pediatric visits to emergency settings including the pediatric emergency department and urgent cares (UC).5 Low-cost cessation interventions delivered in emergency settings are urgently needed to impact public health.7–11
A large percentage of caregivers who are smokers accompany their children in the emergency setting and their children have high rates of TSE-related morbidity.12–17 When caregivers are educated about TSE effect’s on their child’s illness, they may be more motivated to quit smoking.15,18,19 An adult emergency visit is a teachable moment in which cessation interventions are feasible and may be effective in helping smokers quit20,21; thus, cessation interventions may also be effective in the pediatric emergency setting.
The American Academy of Pediatrics exhorts practitioners to help eliminate TSE by advising caregivers to quit smoking at every patient encounter.22 The approach is based on the “5As” of the Clinical Practice Guidelines—Ask about tobacco use, Advise to quit, Assess willingness to quit, and Assist/Arrange with follow-up and resources.7 However, emergency practitioners do not routinely screen or advise caregivers about ways to reduce a child’s TSE7; citing barriers including lack of training, time, standardized protocols, materials, and self-efficacy.23,24
Clinical decision support systems (CDSS) may overcome these barriers and facilitate standardized TSE screening and counseling, and can be triggered from patient-specific data.25–28 Successful CDSS are integrated with the electronic health record (EHR), and dynamically interact with practitioners and existing systems.28–30 Clinical workflow integration remains a major challenge.31–33
An EHR-based TSE reduction CDSS was designed and implemented for use by registered nurses in pediatric UCs.34,35 The CDSS implemented guideline-based assessments of child’s TSE and smoking cessation counseling for caregivers. Nurses were enlisted in the design and implementation of the CDSS. The primary objective is to assess changes in use of the CDSS over time. Secondary objectives are to assess changes in self-reported attitudes and barriers towards TSE intervention in the UC, and the demographics of patients and caregivers who were screened for or received TSE intervention.
METHODS
Study Population
This study was conducted at five separate outpatient UCs, which are part of an academic, pediatric medical center, Cincinnati Children’s Hospital Medical Center (CCHMC). The UC sites had >73,000 visits in 2016. CCHMC has used an enterprise EHR (Epic™)36 since 2009. The study was approved by the CCHMC IRB. All nurse participants completed written informed consent.
Nurses who worked at least one shift per month were eligible to participate; the authors examined all visits (N=14,218) in which study nurses cared for patients during the study period.
The study was conducted in three phases. In Phase I, the CDSS was developed using a mixed-methods approach.35 During Phase II, the CDSS was implemented and its impact on nurses’ assessment of TSE and caregivers’ tobacco use, and delivery of the intervention components was assessed. Nurses may have participated in both of these Phases. In Phase III, the effect of the CDSS on caregivers’ tobacco use was assessed. Herein, the results of Phase II are reported.
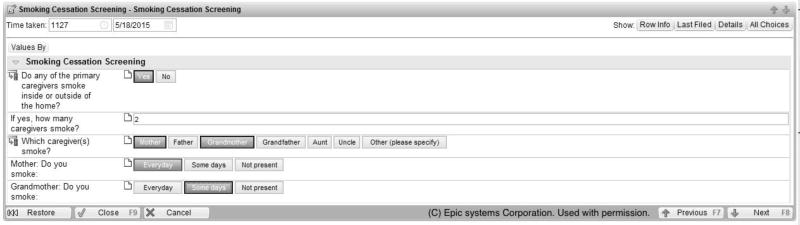
The CDSS was added to nursing documentation after the chief complaint to ensure smooth workflow integration. The CDSS contained non-mandatory prompts for the nurse to screen (Ask) if the child was exposed to tobacco smoke (Figure 1). Screening only occurred per visit; if multiple caregivers or children were present, they were screened as a unit; and the same caregiver and child could be screened again in a subsequent visit. If the caregivers said yes to the question: Do any of the primary caregivers smoke inside or outside of the home? and that caregiver was present, then an alert was triggered for the nurse to provide smoking cessation education (Advise). Nurses were given options for when to counsel or to give opt-out reasons (e.g., too busy, parent refused). Counseling prompts were provided outside of the EHR in a research database, REDCap.37 This was to ensure that no caregiver data were stored in the child’s record. Following the Advise step, nurses completed the Assess step, and then Assist/Arrange steps with options of fax referral to the Quitline; links to smokefree.gov or smokefreeTXT; and provision of a packet of written TSE and cessation materials. If a primary caregiver smoked but was not present, then an information packet was offered to give to the caregiver. Full details are available elsewhere.34,35
Figure 1.
Smoking cessation screening.
Monthly feedback reports were designed and provided.38 Feedback reports displayed individual and aggregate compliance with each of the intervention components.
A 3-month, prospective cohort study was conducted, starting in November 2015. Follow-up assessments occurred at 1 and 3 months after implementation, and exit interviews with nurses were completed by March 2016. Nurses were required to complete a 30-minute webinar before using the CDSS that included information about the effects of TSE in children, the importance of screening and counseling caregivers, and instructions on how to use the CDSS. The webinar was available to nurses for up to 8 weeks prior to the implementation, and remained accessible throughout the study period.
Measures
Nurses completed a baseline questionnaire covering demographics and current and former smoking status. Nurses’ practice behaviors related to providing caregivers with the 5As were measured by 16 items.24 At baseline, 1, and 3 months post-training, nurses completed a 17-item assessment of attitudes and perceived barriers related to TSE screening and counseling. All items were measured using 5-point Likert scales (i.e., behaviors, 1=never to 5=always; attitudes, 1=strongly agree to 5=strongly disagree; and barriers, 1=a large barrier to 5=not a barrier; Appendix).
Usability ratings of the CDSS (e.g., ease of use, amount of time), were collected at 3 months, and were based on a 3- or 5-point Likert scale (Appendix). Prompts to complete the follow-up assessments were sent via email at 1 and 3 months.
Exit interviews were conducted within 1 month of study completion with six study nurses to assess their views on the CDSS; verbatim responses were audio-recorded, transcribed, and evaluated. Two investigators (MMG and JG) individually reviewed and performed content analysis of the transcripts before manually structuring the data into major themes. Coding was conducted within each theme. Coding was compared and discrepancies were resolved through discussions and review of raw data. The themes, suggestions, salient points, and supporting quotations were reviewed and used to make decisions about how to enhance the CDSS and make its use sustainable in the future.
Nurses received gift cards of $50 for each survey plus $40 for the exit interview.
The primary outcome was change in rates of each of the 5As. EHR and REDCap data was extracted from all children seen in all UC sites during the 3-month study period. Multiple visits were included.
Secondary outcomes were changes in nurses’ self-reported attitudes and barriers towards TSE intervention. Frequencies and sociodemographics of children whose caregivers were screened and were eligible to receive intervention components are reported.
Statistical Analysis
Analyses were conducted in June 2016. Descriptive statistics were calculated for all variables, including means with associated standard deviations or medians with interquartile ranges for continuous variables and frequencies with percentages for categorical variables. Histograms and box plots were used to evaluate distributions for normality. Generalized estimating equations examined changes in study nurses’ behavior regarding the 5As over time, and differences by child characteristics; appropriate link functions were used based upon the dependent variable of interest. Generalized estimating equations models were used to include all encounters by nurses, while accounting for the clustering effect, and to examine changes over time. To examine the changes in use of the CDSS over time, a first order autoregressive correlation structure was used. Nurse identifier was included as a repeated effect in the model. ORs with 95% CIs and p-values for the differences in least square means were adjusted for multiple comparisons using the Tukey–Kramer method. For ratings of nurse self-efficacy and perceived barriers, the first two levels of each Likert scale were combined and compared with the lowest three levels. Statistical significance was set at α<0.05. SAS, version 9.4, was used to conduct all analyses.
RESULTS
A total of 43 eligible nurses were approached; 98% were white, non-Hispanic; 100% were female; 37 (86%) consented, and 35 (81%) participated at baseline. The mean age was 36.9 (SD=9.2) years. Twenty-six (74.3%) had been in practice >5 years. Twenty-seven (77.1%) were never smokers, and all were current nonsmokers.
At baseline, ten (28.6%) respondents indicated that they always/often asked about child TSE, six (17.1%) reported that they always/often asked caregivers about their smoking status, and seven (20.0%) always/often documented caregivers’ tobacco use on pediatric charts. Almost 23% (n=8) reported that they always/often advised caregivers to not smoke around their child; four (11.4%) always/often advised caregivers to quit smoking. Five (14%) indicated that they provided specific assistance to caregivers; three (8.6%) had given educational materials; and two (5.7%) had ever recommended pharmacotherapy. Most (94%) were interested in learning cessation intervention techniques.
All cessation attitudes and barriers are summarized in Table 1. At baseline, 30 (85.7%) strongly agreed/agreed that nurses should advise caregivers to quit, and 97% reported that nurses can be effective in helping caregivers to quit smoking. Nurses reported that caregiver resistance (n=18; 51.4%), lack of caregiver materials (n=17; 48.6%), caregiver disinterest (n=15; 42.9%), and lack of training (n=15; 42.9%) were the highest barriers.
Table 1.
Nurse Attitudes and Barriers
| Attitudes/Barriers | Baseline | Month 1 | Month 3 | 3 month vs baseline |
|---|---|---|---|---|
|
| ||||
| Strongly agree/Agree N (%) |
Strongly agree/Agree N (%) |
Strongly agree/Agree N (%) |
OR (95% CI) |
|
| Attitudes | ||||
| I am interested in learning new ways | 33 (94.3) | 28 (80.0) | 23 (69.7) | 0.13 (0.02, 0.72) |
| Appropriate for nurse to assess and document | 32 (91.4) | 30 (85.7) | 27 (81.8) | 0.42 (0.08, 2.24) |
| Nurses should advise parents | 30 (85.7) | 25 (71.4) | 28 (84.8) | 0.96 (0.20, 4.66) |
| It is the doctors’ job to advise | 5 (14.3) | 5 (14.3) | 9 (27.3) | 2.31 (0.74, 7.23) |
| Doctors, nurses and respiratory therapists work together | 35 (100) | 31 (88.6) | 31 (93.9) | NE |
| Nurses can be effective | 34 (97.1) | 28 (80.0) | 31 (93.9) | 0.46 (0.02, 9.1) |
|
|
||||
| Barriers | Large (1–2) N (%) | Large (1–2) N (%) | Large (1–2) N (%) | 3 month vs baseline |
|
|
||||
| Parent resistance | 30 (85.7) | 28 (80.0) | 25 (75.8) | 0.53 (0.12, 2.41) |
| Amount of time | 25 (71.4) | 18 (51.4) | 17 (51.5) | 0.43 (0.16, 1.20) |
| Resistance by doctors | 3 (8.6) | 1 (2.9) | 2 (6.1) | 0.66 (0.10, 4.12) |
| Concerns about effectiveness | 11 (31.4) | 11 (31.4) | 8 (24.2) | 0.31 (0.26, 2.15) |
| Parent anger | 26 (74.3) | 19 (54.3) | 13 (39.4) | 0.24 (0.07, 0.77) |
| Parent disinterest | 28 (80.0) | 30 (85.7) | 27 (81.8) | 0.37 (0.29, 4.37) |
| Feat will change relationship | 14 (40.0) | 10 (28.6) | 10 (30.3) | 0.74 (0.28, 1.92) |
| Lack of training | 28 (80.0) | 12 (34.3) | 6 (18.2) | 0.06 (0.01, 0.26) |
| Lack of parent materials | 30 (85.7) | 3 (8.6) | 3 (9.1) | 0.02 (0.003, 0.09) |
| Lack of referral resources | 23 (65.7) | 3 (8.6) | 3 (9.1) | 0.05 (0.01, 0.22) |
Notes: Boldface indicates statistical significance (p<0.05).
NE, non-estimable
All (100%) and 94% of the nurses completed the 1- and 3-month assessment, respectively. Compared with baseline: there was significant improvement in nurses feeling very prepared to assist caregivers in quitting smoking at Month 3 (14% vs 85%, p<0.001); and decreased odds of rating caregiver anger (OR=0.24 [95% CI=0.07, 0.77]), lack of training (OR=0.06 [95% CI=0.01, 0.26]), lack of materials (OR=0.02 [95% CI=0.003, 0.09]), and lack of referral resources (OR=0.05 [95% CI=0.01, 0.22] as large barriers to incorporating cessation activities into their routine care at Month 3.
There were 19,749 visits over the 3-month study period; 14,218 of these visits were seen by study nurses (median, 366 [interquartile range,150–647] per nurse). All nurses used the CDSS more than once; 9,209 (65%) children were screened for TSE and 1,801 (19.5%) screened positive.
Of children whose caregivers smoked, the median age was 4 [interquartile range, 1–9] years; 52% were female; 56% were non-Hispanic white, 31% were non-Hispanic black, and 4% were Hispanic; 75% had Medicaid; 1,267 (70%) of the caregivers were daily smokers; 1,002 (56%) reported that one caregiver smoked; 395 (22%) reported that two or more caregivers smoked; 174 (10%) reported that the child was exposed to other smokers besides the primary caregivers. Of children seen by non-study nurses, 278 (5%) were screened for TSE, and 52 (19%) screened positive.
An alert was displayed for 1,782 of the UC visits. Of these, nurses acknowledged 1,490 (83.6%), selected open order set in 222 (12.5%), and took no action in 70 (3.9%). The highest response rate was for “Yes, I’ll counsel now” (n=888; 50%).
Nurses were more likely to screen children who: were aged >2 years (OR=1.1 [95%CI=1.0, 1.2]); were Hispanic (OR=1.6 [95% CI=1.4, 1.9]); and had commercial insurance (OR=1.2 [95%CI=1.1, 1.4]). Nurses had greater odds of Advise and Assist/Arrange step for blacks compared with whites (both OR=1.3 [95% CI=1.0, 1.7]). There were no differences on the 5As steps by sex of the child (Table 2).
Table 2.
Differences in Nurses’ Ask, Advise, Assess, Assist/Arrange Behaviors by Child Characteristics
| N (%) | Ask | Advise | Assess | Assist/Arrange | |
|---|---|---|---|---|---|
|
|
|||||
| Characteristics | OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
|
| Child age, years | |||||
| >2 | 8,429 (59.3) | 1.08 (1.00, 1.16) | 0.88 (0.72, 1.06) | 0.98 (0.80, 1.21) | 0.99 (0.83, 1.17) |
| 0 – 2 | 5,789 (40.7) | ref | ref | ref | ref |
| Child sex | |||||
| Female | 6,976 (49.1) | 1.00 (0.95, 1.06) | 0.99 (0.82, 1.19) | 1.03 (0.88, 1.20) | 1.0 (0.83, 1.20) |
| Male | 7,242 (50.9) | ref | ref | ref | ref |
| Child race | Of 11,842a | ||||
| African American | 4,164 (35.2) | 0.93 (0.82, 1.06) | 1.32 (1.03, 1.70) | 1.11 (0.92, 1.34) | 1.31 (1.00, 1.72) |
| Caucasian | 7,678 (64.8) | ref | ref | ref | ref |
| Child ethnicity | Of 13,963b | ||||
| Hispanic | 956 (93.2) | 1.64 (1.40, 1.92) | 1.29 (0.50, 3.32) | 1.65 (0.75, 3.63) | 1.77 (0.81, 3.89) |
| Non-Hispanic | 13,007 (6.8) | ref | ref | ref | ref |
| Child insurance | Of 14,173c | ||||
| Commercial/Self-pay/Other | 6,173 (43.6) | 1.23 (1.11, 1.37) | 0.64 (0.45, 0.90) | 0.30 (0.23, 0.40) | 0.31 (0.18, 0.50) |
| Medicaid/Government | 8,000 (56.4) | ref | ref | ref | ref |
Notes: Boldface indicates statistical significance (p<0.05).
Child race: Other N=1,728, Missing N=648.
Child ethnicity: Missing N=255.
Child insurance: Missing N=45.
Of the 1,801 children identified as being exposed to tobacco smoke, 473 of their caregivers (26.3%) were advised to quit, and 516 (28.6%) were assessed for readiness to quit. Of those, 346 (67%) said they were interested in quitting in the next 30 days, and all 346 (100%) were assisted as follows (may have received more than one): written information: 83.5%; smokefree.gov: 52%; smokefreeTXT:16.2%; quitline: 7.5%; and not specified: 40%.
Across all follow-up periods, the total practice-level use of the CDSS for Ask, Advise, Assess, and Assist/Arrange was 65% (range, 59%–69%), 26% (range, 20%–32%), 29% (range, 21%–35%), and 67% (range, 61%–81%), respectively, of all eligible visits (Table 3). Over time, there were increases in Advise and Assess, but decreases in Ask and Assist/Arrange. Specifically, compared with Month 1, nurses were almost twice as likely to advise during Month 2: OR=1.7 (95% CI=1.0, 2.8) and Month 3: OR=2.0 (95% CI=1.0, 3.8), and twice as likely to assess during Month 2: OR=1.9 (95% CI=1.1, 3.1) and Month 3: OR=2.2 (95% CI=1.2, 4.1). However, they were 1.4 times less likely to Ask in Month 3 compared with Month 2: OR=0.7 (95% CI=0.56, 0.91) and about 2.5 times less likely to Assist/Arrange in Month 2 compared with Month 1: OR=0.4 (95% CI=0.24, 0.61) and Month 3: OR=0.4 (95% CI=0.28, 0.62). UC volumes were progressively busier over time, with a 14% increase in patient volume during Month 3 compared with Month 1.
Table 3.
Changes in Nurses’ Ask, Advise, Assess, Assist/Arrange Behaviors
| AOR (95% CI) p-valuea |
|||||||
|---|---|---|---|---|---|---|---|
| Behavior | Month 1 N=4,788 |
Month 2 N=4,712 |
Month 3 N=4,718 |
Total | Month 2 vs Month 1 |
Month 3 vs Month 1 |
Month 3 vs Month 2 |
| Ask | 3,290/4,788 | 3,121/4,712 | 2,798/4,718 | 9,209/14,218 | 0.95 | 0.68 | 0.71 |
| (68.7%) | (66.2%) | (59.3%) | (64.8%) | (0.65, 1.38) | (0.44, 1.04) | (0.56, 0.91) | |
| 0.94 | 0.08 | 0.003 | |||||
| Screened positive | 692/4,788 | 577/4,712 | 532/4,718 | 1,801/14,218 | – | – | – |
| (14.4%) | (12.3%) | (11.3%) | (12.7%) | ||||
| Advise | 137/692 | 167/577 | 169/532 | 473/1,801 | 1.70 | 1.99 | 1.17 |
| (19.8%) | (32.0%) | (31.8%) | (26.3%) | (1.01, 2.84) | (1.04, 3.8) | (0.79, 1.73) | |
| 0.04 | 0.04 | 0.61 | |||||
| Assess | 143/692 | 187/577 | 186/532 | 516/1,801 | 1.87 | 2.19 | 1.17 |
| (20.7%) | (32.0%) | (35.0%) | (28.6%) | (1.12, 3.1) | (1.17, 4.08) | (0.79, 1.75) | |
| 0.01 | 0.009 | 0.61 | |||||
| Assist/Arrange | 116/143 | 114/187 | 116/186 | 346/516 | 0.38 | 0.41 | 1.08 |
| (81.1%) | (61.0%) | (62.4%) | (67.1%) | (0.24, 0.61) | (0.28, 0.62) | (0.64, 1.82) | |
| <0.0001 | <0.001 | 0.94 | |||||
Notes: Boldface indicates statistical significance (p<0.05).
Tukey-Kramer Adjustment for Multiple Comparisons
All study nurses completed usability assessments of the CDSS (Appendix) at 3 months. Nurses rated the CDSS four out of five for: “easy to use" (56%); “how easily it will fit into the UC workflow” (50%); “useful in helping to address smoking” (63%); “usefulness of feedback reports” (50%); and “likely to continue using the CDSS in daily workflow” (48%). The majority (54%) rated the CDSS a score of three for “length of time to use,” indicating it was neither too long nor too short.
Nurses said that the CDSS was easy to use, user friendly, and took under 3 minutes to complete. All nurses stated that the CDSS increased the number of caregivers they counseled and that it prompted them to assess for TSE. Nurses felt most caregivers were receptive, and that the CDSS facilitated conversations. Nurses reported that caregivers were not interested in the Quitline, but many seemed open to smokefree.gov and smokefreeTXT, and all were receptive to information packets.
All nurses agreed that the CDSS should continue to be used. However, they felt that the research database was inconvenient, and the intervention components should be integrated into the EHR. Most nurses (83%) said the feedback reports were helpful.
DISCUSSION
This study adds to the growing literature supporting the use of CDSS to increase standardized TSE screening and interventions in the pediatric healthcare setting.39–41 This model has the potential to improve the delivery and outcomes of evidence-based TSE screening and treatment in the UC setting. The use of the CDSS resulted in the identification of large numbers of children who were exposed to tobacco smoke and there were increases in the proportion of caregivers who were advised to quit and assessed for readiness to quit. However, the absolute rates of the 5As behaviors was lower than expected which may be because the prompts were not mandatory. Prior studies demonstrate that rates of tobacco treatment by pediatric practitioners are higher when there are mandatory prompts and treatment algorithms in the EHR, standardized TSE education for practitioners, and availability of educational materials.23,42,43 Contrary to prior studies that found an increase in the Assist step by nurses and physicians,38,44,45 there were decreases in rates of compliance with the Assist/Arrange step by nurses over the study period. This may have been because of increased patient volumes and tighter time constraints on the nurses. Assist/Arrange rates may be improved by placing all 5As steps within the EHR as suggested by the nurses during the exit interviews.
The reported findings that children were more likely to be screened if they were older, Hispanic, and had commercial insurance is consistent with other research,40 but because children who were exposed were more likely to be non-Hispanic white and Medicaid recipients, these results indicate that all demographic groups need to be screened.
Encouragingly, the nurses provided information and resources to 100% of caregivers who were interested in quitting. The CDSS was acceptable to nurses, although the use of an external database was seen as a deterrent. Data from the exit interviews indicated that nurses wanted continued use of the CDSS. Future research should evaluate whether the incorporation of the full CDSS within the child’s EHR improves Assist/Arrange rates.
Limitations
The current study has several limitations. First, this pilot study was conducted with a small convenience sample of UC nurses in one urban tertiary care children’s hospital. The results may not be representative of larger populations of UC nurses. Second, the UCs have a large, comprehensive EHR system, and therefore nurse practice, recommendations and suggestions may be skewed towards the capabilities of this clinical setting. Third, the timeframe allowed for the intervention was limited because of administrative reasons; thus, the sustainability of changes in practice beyond the pilot period was assessed. Finally, lower than expected rates of positive TSE screens (prior work in the pediatric emergency department setting shows rates of as high as 48%) was observed14–17; although this may have been because of differences in the screening questions used between studies.
CONCLUSIONS
This study demonstrates the feasibility of a CDSS in the pediatric UC setting for reducing TSE. The EHR modification for screening, and the integration of an interruptive alert led to increases in TSE screening and counseling during UC visits. Rates of CDSS use were lower than expected, but there is need for screening of all demographic groups. Rates of use may be improved by incorporating all CDSS components into the EHR. Future research is warranted to modify the CDSS, and assess how the use of CDSS affects clinical outcomes for children exposed to tobacco smoke, and cessation rates in caregivers.
Supplementary Material
Acknowledgments
Funded by the NIH National Cancer Institute (Grant Number R21CA184337).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial disclosures were reported by the authors of this paper.
References
- 1.Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. Exposure of the U.S. population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. JAMA. 1996;275(16):1233–1240. https://doi.org/10.1001/jama.1996.03530400021033. [PubMed] [Google Scholar]
- 2.Bek K, Tomac N, Delibas A, Tuna F, Tezic HT, Sungur M. The effect of passive smoking on pulmonary function during childhood. Postgrad Med J. 1999;75(884):339–341. doi: 10.1136/pgmj.75.884.339. https://doi.org/10.1136/pgmj.75.884.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham J, O’Connor GT, Dockery DW, Speizer FE. Environmental tobacco smoke, wheezing, and asthma in children in 24 communities. Am J Respir Crit Care Med. 1996;153(1):218–224. doi: 10.1164/ajrccm.153.1.8542119. https://doi.org/10.1164/ajrccm.153.1.8542119. [DOI] [PubMed] [Google Scholar]
- 4.Gold DR, Wang X, Wypij D, Speizer FE, Ware JH, Dockery DW. Effects of cigarette smoking on lung function in adolescent boys and girls. N Engl J Med. 1996;335(13):931–937. doi: 10.1056/NEJM199609263351304. https://doi.org/10.1056/NEJM199609263351304. [DOI] [PubMed] [Google Scholar]
- 5.Tutka P, Wielosz M, Zatonski W. Exposure to environmental tobacco smoke and children health. Int J Occup Med Environ Health. 2002;15(4):325–335. [PubMed] [Google Scholar]
- 6.Homa DM, Neff LJ, King BA, et al. Vital signs: disparities in nonsmokers’ exposure to secondhand smoke--United States, 1999–2012. MMWR Morb Mortal Wkly Rep. 2015;64(4):103–108. [PMC free article] [PubMed] [Google Scholar]
- 7.Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Human Services. Public Health Service; 2008. [Google Scholar]
- 8.Bartecchi CE, MacKenzie TD, Schrier RW. The human costs of tobacco use. N Engl J Med. 1994;330(13):907–912. doi: 10.1056/NEJM199403313301307. https://doi.org/10.1056/NEJM199403313301307. [DOI] [PubMed] [Google Scholar]
- 9.Schroeder SA. What to do with a patient who smokes. JAMA. 2005;294(4):482–487. doi: 10.1001/jama.294.4.482. https://doi.org/10.1001/jama.294.4.482. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein SL, Boudreaux ED, Cydulka RK, et al. Tobacco control interventions in the emergency department: a joint statement of emergency medicine organizations. Ann Emerg Med. 2006;48(4):e417–426. doi: 10.1016/j.annemergmed.2006.02.018. https://doi.org/10.1016/j.annemergmed.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Cepeda MS, Boston R, Farrar JT, Strom BL. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol. 2003;158(3):280–287. doi: 10.1093/aje/kwg115. https://doi.org/10.1093/aje/kwg115. [DOI] [PubMed] [Google Scholar]
- 12.Merianos AL, Dixon CA, Mahabee-Gittens EM. Secondhand Smoke Exposure, Illness Severity and Resource Utilization in Pediatric Emergency Department Patients with Respiratory Illnesses. J Asthma. doi: 10.1080/02770903.2016.1265127. In press. Online December 8, 2016. https://doi.org/10.1080/02770903.2016.1265127. [DOI] [PMC free article] [PubMed]
- 13.Merianos AL, Dixon CA, Mahabee-Gittens EM. Tobacco Smoke Exposure-Related Illnesses Among Pediatric Emergency Department Patients. J Pediatr Health Care. 2017;31(2):161–166. doi: 10.1016/j.pedhc.2016.07.001. https://doi.org/10.1016/j.pedhc.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahabee-Gittens EM, Huang B. ED environmental tobacco smoke counseling. Am J Emerg Med. 2005;23(7):916–918. doi: 10.1016/j.ajem.2005.07.001. https://doi.org/10.1016/j.ajem.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Mahabee-Gittens M. Smoking in parents of children with asthma and bronchiolitis in a pediatric emergency department. Pediatr Emerg Care. 2002;18(1):4–7. doi: 10.1097/00006565-200202000-00002. https://doi.org/10.1097/00006565-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Mahabee-Gittens EM, Gordon JS, Krugh ME, Henry B, Leonard AC. A smoking cessation intervention plus proactive quitline referral in the pediatric emergency department: a pilot study. Nicotine Tob Res. 2008;10(12):1745–1751. doi: 10.1080/14622200802443494. https://doi.org/10.1080/14622200802443494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahabee-Gittens EM, Khoury JC, Ho M, Stone L, Gordon JS. A smoking cessation intervention for low-income smokers in the ED. Am J Emerg Med. 2015;33(8):1056–1061. doi: 10.1016/j.ajem.2015.04.058. https://doi.org/10.1016/j.ajem.2015.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarvis MJ. The association between having children, family size and smoking cessation in adults. Addiction. 1996;91(3):427–434. https://doi.org/10.1111/j.1360-0443.1996.tb02292.x. [PubMed] [Google Scholar]
- 19.Winickoff JP, Hibberd PL, Case B, Sinha P, Rigotti NA. Child hospitalization: an opportunity for parental smoking intervention. Am J Prev Med. 2001;21(3):218–220. doi: 10.1016/s0749-3797(01)00355-5. https://doi.org/10.1016/S0749-3797(01)00355-5. [DOI] [PubMed] [Google Scholar]
- 20.Bernstein SL, D'Onofrio G, Rosner J, et al. Successful Tobacco Dependence Treatment in Low-Income Emergency Department Patients: A Randomized Trial. Ann Emerg Med. 2015;66(2):140–147. doi: 10.1016/j.annemergmed.2015.03.030. https://doi.org/10.1016/j.annemergmed.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernstein SL, Rosner J, Toll B. A Multicomponent Intervention Including Texting to Promote Tobacco Abstinence in Emergency Department Smokers: A Pilot Study. Acad Emerg Med. 2016;23(7):803–808. doi: 10.1111/acem.12990. https://doi.org/10.1111/acem.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Academy of Pediatrics. Policy statement--Tobacco use: a pediatric disease. Pediatrics. 2009;124(5):1474–1487. doi: 10.1542/peds.2009-2114. https://doi.org/10.1542/peds.2009-2114. [DOI] [PubMed] [Google Scholar]
- 23.Geller AC, Brooks DR, Woodring B, et al. Smoking cessation counseling for parents during child hospitalization: a national survey of pediatric nurses. Public Health Nurs. 2011;28(6):475–484. doi: 10.1111/j.1525-1446.2011.00954.x. https://doi.org/10.1111/j.1525-1446.2011.00954.x. [DOI] [PubMed] [Google Scholar]
- 24.Deckter L, Mahabee-Gittens EM, Gordon JS. Are pediatric ED nurses delivering tobacco cessation advice to parents? J Emerg Nurs. 2009;35(5):402–405. doi: 10.1016/j.jen.2007.10.018. https://doi.org/10.1016/j.jen.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garg AX, Adhikari NK, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293(10):1223–1238. doi: 10.1001/jama.293.10.1223. https://doi.org/10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- 26.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. doi: 10.1136/bmj.38398.500764.8F. https://doi.org/10.1136/bmj.38398.500764.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blumenthal D, Glaser JP. Information technology comes to medicine. N Engl J Med. 2007;356(24):2527–2534. doi: 10.1056/NEJMhpr066212. https://doi.org/10.1056/NEJMhpr066212. [DOI] [PubMed] [Google Scholar]
- 28.Marcy TW, Kaplan B, Connolly SW, Michel G, Shiffman RN, Flynn BS. Developing a decision support system for tobacco use counselling using primary care physicians. Inform Prim Care. 2008;16(2):101–109. doi: 10.14236/jhi.v16i2.681. https://doi.org/10.14236/jhi.v16i2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wears RL, Berg M. Computer technology and clinical work: still waiting for Godot. JAMA. 2005;293(10):1261–1263. doi: 10.1001/jama.293.10.1261. https://doi.org/10.1001/jama.293.10.1261. [DOI] [PubMed] [Google Scholar]
- 30.Hesse BW, Shneiderman B. eHealth research from the user's perspective. Am J Prev Med. 2007;32(5 Suppl):S97–103. doi: 10.1016/j.amepre.2007.01.019. https://doi.org/10.1016/j.amepre.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, Cain C, Young S, Chockley N, Burstin H. The adoption gap: health information technology in small physician practices. Understanding office workflow can help realize the promise of technology. Health Aff (Millwood) 2005;24(5):1364–1366. doi: 10.1377/hlthaff.24.5.1364. https://doi.org/10.1377/hlthaff.24.5.1364. [DOI] [PubMed] [Google Scholar]
- 32.Wachter RM. Expected and unanticipated consequences of the quality and information technology revolutions. JAMA. 2006;295(23):2780–2783. doi: 10.1001/jama.295.23.2780. https://doi.org/10.1001/jama.295.23.2780. [DOI] [PubMed] [Google Scholar]
- 33.Elson RB. Clinical Practice Guidelines. Dis Manage Health Outcomes. 1997;1(2):63–74. https://doi.org/10.2165/00115677-199701020-00001. [Google Scholar]
- 34.Mahabee-Gittens EM, Dexheimer JW, Khoury JC, Miller JA, Gordon JS. Development and Testing of a Computerized Decision Support System to Facilitate Brief Tobacco Cessation Treatment in the Pediatric Emergency Department: Proposal and Protocol. JMIR Res Protoc. 2016;5(2):e64. doi: 10.2196/resprot.4453. https://doi.org/10.2196/resprot.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahabee-Gittens EM, Dexheimer JW, Gordon JS. Development of a Tobacco Cessation Clinical Decision Support System for Pediatric Emergency Nurses. Comput Inform Nurs. 2016;34(12):560–569. doi: 10.1097/CIN.0000000000000267. https://doi.org/10.1097/CIN.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epic EMR [computer program] Verona, WI: Epic Systems Corporation; [Google Scholar]
- 37.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. https://doi.org/10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bentz CJ, Bayley KB, Bonin KE, et al. Provider feedback to improve 5A’s tobacco cessation in primary care: a cluster randomized clinical trial. Nicotine Tob Res. 2007;9(3):341–349. doi: 10.1080/14622200701188828. https://doi.org/10.1080/14622200701188828. [DOI] [PubMed] [Google Scholar]
- 39.Jenssen BP, Shelov ED, Bonafide CP, Bernstein SL, Fiks AG, Bryant-Stephens T. Clinical Decision Support Tool for Parental Tobacco Treatment in Hospitalized Children. Appl Clin Inform. 2016;7(2):399–411. doi: 10.4338/aci-2015-12-ra-0169. https://doi.org/10.4338/ACI-2015-12-RA-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharifi M, Adams WG, Winickoff JP, Guo J, Reid M, Boynton-Jarrett R. Enhancing the electronic health record to increase counseling and quit-line referral for parents who smoke. Acad Pediatr. 2014;14(5):478–484. doi: 10.1016/j.acap.2014.03.017. https://doi.org/10.1016/j.acap.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Jenssen BP, Bryant-Stephens T, Leone FT, Grundmeier RW, Fiks AG. Clinical Decision Support Tool for Parental Tobacco Treatment in Primary Care. Pediatrics. 2016;137(5):e20154185. doi: 10.1542/peds.2015-4185. https://doi.org/10.1542/peds.2015-4185. [DOI] [PubMed] [Google Scholar]
- 42.Blaine K, Rogers J, Winickoff JP, et al. Engaging in secondhand smoke reduction discussions with parents of hospitalized pediatric patients: a national survey of pediatric nurses in the United States. Prev Med. 2014;62:83–88. doi: 10.1016/j.ypmed.2014.01.021. https://doi.org/10.1016/j.ypmed.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 43.McMillen R, O'Connor KG, Groner J, Tanski S, Park ER, Klein JD. Changes and Factors Associated with Tobacco Counseling: Results from the AAP Periodic Survey. Acad Pediatr. 2017;17(5):504–514. doi: 10.1016/j.acap.2017.01.002. https://doi.org/10.1016/j.acap.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Mathias JS, Didwania AK, Baker DW. Impact of an electronic alert and order set on smoking cessation medication prescription. Nicotine Tob Res. 2012;14(6):674–681. doi: 10.1093/ntr/ntr265. https://doi.org/10.1093/ntr/ntr265. [DOI] [PubMed] [Google Scholar]
- 45.Adsit RT, Fox BM, Tsiolis T, et al. Using the electronic health record to connect primary care patients to evidence-based telephonic tobacco quitline services: a closed-loop demonstration project. Transl Behav Med. 2014;4(3):324–332. doi: 10.1007/s13142-014-0259-y. https://doi.org/10.1007/s13142-014-0259-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.