Abstract
Aim
To describe a sensory map of pelvic dermatomes in women with Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS). We hypothesized that if IC/BPS involves changes in central processing, then women with IC/BPS will exhibit sensory abnormalities in neurologic pelvic dermatomes.
Methods
Women with IC/BPS and healthy controls underwent neurologic examination that included evaluation of sharp pain sensitivity and vibration in dermatomes T12, L1, L2, S1–5. Peripheral nervous system sensitivity to pressure, vibration and pinprick were scored using numeric rating scales (NRS). Bilateral comparisons were made with Wilcoxon signed-rank test and comparisons between groups were made by the Mann-Whitney U test.
Results
Total of 74 women with IC/BPS and 36 healthy counterparts were included. IC/BPS and control groups had similar age (43.0±14.1 years and 38.6±15.3 years, p=0.14) and BMI (28.9±8.0 kg/m2 and 26.9±8.4 kg/m2, p=0.24), respectively. Women with IC/BPS reported hyperalgesia (elevated bilateral NRS pain intensity) in all pelvic dermatomes compared to healthy controls. S4-S5 region had the highest pain intensity in all participants. All IC/BPS participants exhibited vibration sensation hypoesthesia, at least unilaterally, in all of the pelvic dermatomes except L1 compared to healthy controls.
Conclusion
This detailed map of neurologic pelvic dermatomes in women with IC/BPS found hyperalgesia in all pelvic dermatomes, and some evidence of vibration sensation hypoesthesia, compared to healthy controls. These findings support the hypothesis that IC/BPS may involve changes in central signal processing biased towards nociception.
Keywords: chronic pelvic pain, interstitial cystitis/bladder pain syndrome, myofascial pelvic pain syndrome, sensory mapping, pelvic dermatomes
Introduction
Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS), a common urologic condition causing CPP, is characterized by pressure or discomfort related to the urinary bladder with at least one other urinary symptom, such as persistent urge to void or urinary frequency.1 Strict clinical and cystoscopic diagnostic criteria by National Institute of Health for IC/BPS have been mostly replaced by clinical diagnosis based on bladder symptoms and pain. However, the cause(s) of IC/BPS remain unknown, precluding clinicians from understanding the mechanisms underlying the development of IC/BPS that might establish effective treatment strategies.
IC/BPS, as a member of the “chronic overlapping pain conditions” family, reflects a systemic disorder or nervous system abnormality.2, 3 Recent data suggest that IC/BPS symptoms may represent one manifestation of an altered central mechanism in the processing of sensory events from the bladder,4 aberrant global neurologic response to earlier stressful/traumatic provocations5, 6 or visceral hyperalgesia.7 However, systemic assessment of global sensory function in patients with IC/BPS compared to controls has not been reported. Previous studies have investigated vibration threshold abnormalities using large fiber modality testing (A-beta fibers) and cutaneous current perception testing8, 9 in C5, T6,10,12, and S3 neurologic dermatomes; small C fiber thermoreceptor stimulation4, 7, 9 in similar dermatomes and extremities, blunt pressure pain7, 10 in T1, T11, L4, S2–3 dermatomes. However, no study to our knowledge provides a thorough, detailed sensory map of the pelvic dermatomes in women with IC/BPS.
We hypothesized that the aberrant neurologic response, suggested by some literature reports, should manifest as sensory abnormalities in neurologic pelvic dermatomes. Demonstration of such neurologic abnormalities on physical examination could help guide clinicians to individualize and optimize the treatment for women with IC/BPS, and provide more understanding of potential pathophysiologic mechanisms. The aim of this study was to perform systemic sensory mapping of pelvic dermatomes in women with IC/BPS.
Methods
Participants
The ICEPAC study was approved by the University Hospitals Cleveland Medical Center (UHCMC) Institutional Review Board (Cleveland, Ohio). ICEPAC consisted of a comprehensive, interdisciplinary evaluation of women with IC/BPS to elucidate the role of central and peripheral nervous system processing in CPP. Specific details and methods of the study have been published.11 General exclusion criteria are listed in Supplemental Table 1. Pelvic dermatomes were mapped in women (aged 18–80 years) with IC/BPS and healthy control women. Classification of IC/BPS was made according to European Society for the Study of Painful Bladder Syndrome recommendations,12 with study participants having at least 6 months of urgency, frequency, and bladder pain clearly linked to bladder filling and emptying. Since as many as 87% of women with IC/BPS also have myofascial pelvic pain (MPP),8 we also categorized women whether or not they had MPP. Women were classified as IC/BPS+MPP, if participants had evidence of MPP on physical examination with a minimum pain score of 4 out of 10 (2 of 5 sites) based on a numeric rating scale (NRS) following digital palpation of five pelvic floor muscles (levator ani (right and left), obturator internus (right and left), perineal body). Detailed methodology of musculoskeletal evaluation in participants has been published.13 Healthy control participants had no known neuropathic, autonomic or chronic pain disorder of any type and were age matched to within ±3 years of study participants.
Sensory Mapping Procedures
Peripheral neuropathy screen
Participants underwent screening for peripheral neuropathy by examination of low-threshold mechanoreceptors (LTMs, large myelinated A-beta nerve fibers) using vibration and 2-point discrimination sensation, thinly myelinated small A-delta fibers using pinprick sharpness sensation, and unmyelinated small C fibers using measurement of suprathreshold heat sensation (115°F–120°F) in the hands and feet. Vibration sensation and pinprick sharpness were assessed over the dorsum of the index finger (dermatome C6) and great toe (dermatome L5) using a tuning fork and the sharp end of a broken wooden cotton swab, respectively (testing described in14). A NRS of 0–4 was used to rate vibration, pinprick, and thermal sensation with 0 defined as “clearly normal”, 1, “minimally abnormal” (questionable, not clearly normal), 2 “clearly mildly abnormal”, 3 “moderately abnormal”, and 4 as “severely abnormal”. Two-point discrimination was assessed using the dull wooden end of 2 cotton swabs and a measurement taken of the smallest distance still experienced as 2 separate sensations. Examiners were blinded as to the status of the participant (healthy control or pelvic pain subject).
Mechanical pain “sharpness” and intensity determination
A digital force gauge (Model FDIX, Wagner Instruments, Greenwich, CT) coupled to a broken wooden cotton swab was used to test pinprick sensation of pelvic dermatomes from the sharp edge of the wooden cotton swab touching the skin with 0.56 Newtons (57 grams) pressure on the algometer (Figure 1). Participants were familiarized with the difference between “sharpness” and “pain intensity” by first exerting the same pinprick pressure on the forehead to ensure that they could differentiate the sensation of sharpness from that of the pain that results from a pinprick. Pelvic dermatomes were tested using the progression T12, L1, L2, S2, S1, S3, S4, and S5. “Sharpness” followed by “pain intensity” during pinprick testing were assessed using a NRS from 0 “no sensation” to 10 “very sharp” for sharpness anchored to the forehead defined as “10”, and from 0 “no pain” to 10 “worst imaginable pain” for pain intensity without any anchor.
Figure 1. Pelvic dermatomes and locations of sensory testing.
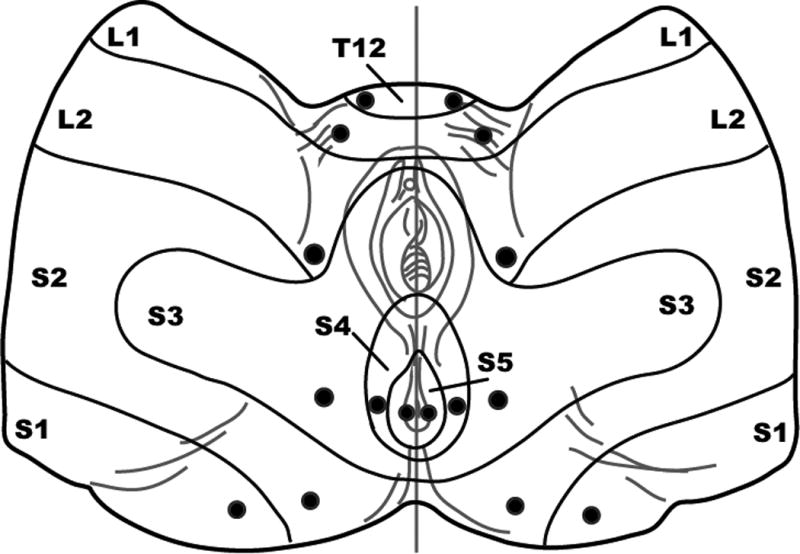
Filled circles indicate testing sites.
Vibration threshold detection
Vibratory sensation disappearance threshold was assessed with a quantitative 64 Hz tuning fork15 and the method of limits for each pelvic dermatome, first on the right side then on the left side, using the same progression, except that S5 was not included. Vibration thresholds were characterized using a NRS from 0 to 4 with zero representing “normal or no sensation impairment” and 4 representing “severe loss of sensation or severe sensation impairment”.
Statistical Analysis
GraphPad Prism for Windows v6.04 (GraphPad Software, Inc., La Jolla, CA) was used for statistical analysis. Age and body mass index (BMI) for healthy vs. all IC/BPS were compared using unpaired t-tests. A two-tailed Mann-Whitney test was used for between-group comparisons of upper and lower extremity values (global screen for peripheral neuropathy), pelvic floor musculature and dermatomes from healthy and IC/BPS groups. Differences between the left and right sides, where applicable, were evaluated using Wilcoxon matched-pairs signed-rank test. Values are expressed as mean ± standard deviation, unless otherwise noted. Differences within groups and between groups were considered significant at p<0.05.
Results
Total of 74 women with IC/BPS and 36 healthy counterparts were included in this analysis after the ICEPAC enrollment was completed. Women with IC/BPS had a mean age of 43.0±14.1 years and BMI of 28.9±8.0 kg/m2. Healthy control participants had a mean age of 38.6±15.3 years and BMI of 26.9±8.4 kg/m2. There were no differences in age (p=0.14) or BMI (p=0.24) between healthy controls and IC/BPS groups. Women with IC/BPS described themselves as Caucasian (82%), African American (15%) or Asian/other (3%). High prevalence of MPP in women with IC/BPS in our study (40 out of 74 participants) was consistent with prevalence in published literature. Healthy controls described themselves as Caucasian (72%), African American (14%) or Asian/other (14%). Pain in the levator ani (bilaterally), obturator internus (bilaterally), and perineal body for all participants with IC/BPS or IC/BPS+MPP was significantly higher (all comparisons, p<0.01) than for each of these sites in healthy women (Supplemental Figure 1).
Global screen for peripheral neuropathy
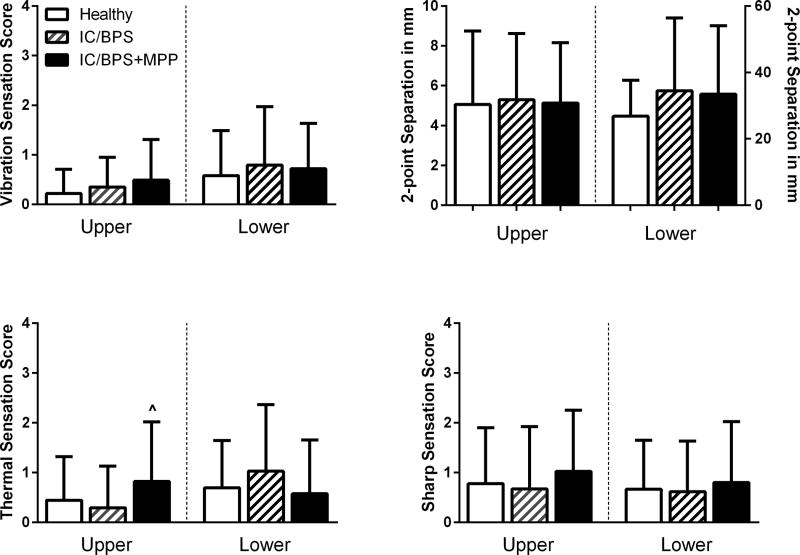
No study participants and healthy controls had any evidence of peripheral neuropathies in either upper or lower extremities. Moreover, no difference was found in vibration, 2-point discrimination, thermal, and sharpness sensation in study participants with and without IC/BPS (Figure 2). Having MPP did not change these findings significantly, except we found a reduced thermal sensation (p=0.02) in the hand of women with IC/BPS+MPP compared to that of women with IC/BPS.
Figure 2. Global screen for peripheral neuropathy.
Mean score and distance values [±SD] for vibration disappearance threshold, 2-point discrimination, and thermal and sharpness sensation in upper (hand) and lower (foot) peripheral sites. ^= p<0.05 IC/BPS vs IC/BPS+MPP.
Sensory mapping of pelvic dermatomes
Sharp pain intensity
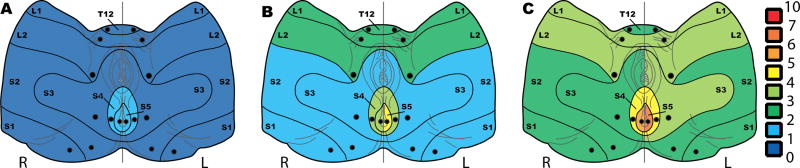
Pain intensity associated with pinprick was examined in all pelvic dermatomes (Figure 3, Table 1). Women with IC/BPS, with or without MPP, reported hyperalgesia (elevated bilateral NRS pain intensity) in all pelvic dermatomes. Comparisons between the IC/BPS and IC/BPS+MPP groups indicated that hyperalgesia was even more prominent in women with both IC/BPS and MPP. The pain intensity was higher in some pelvic dermatomes L2 (left and right), S2 (right), S3 (left), S5 (right and left) in women with IC/BPS+MPP than in women with IC/BPS alone (all p≤0.05). S4–S5 region had the highest pain intensity in both healthy controls and women with IC/BPS with or without MPP. Participants with IC/BPS reported higher pain intensity in response to pinprick on the left than right in pelvic dermatomes S4 and S5 (IC/BPS, p=0.01 and p=0.003 respectively; IC/BPS+MPP, p=0.03 and p=0.002 respectively).
Figure 3. Sensory mapping of pelvic dermatomes: hyperalgesia in women with IC/BPS based on pain sensation in response to pin prick.
Heat maps of mean pain scores in A. healthy, B. IC/BPS, and C. IC/BPS+MPP participants.
Table 1.
Hyperalgesia in Women with IC/BPS Based on Pain Sensation in Response to Pinprick (Mean ± [SD] scores)
| Dermatome | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T12 | L1 | L2 | S1 | S2 | S3 | S4 | S5 | |||||||||
| R | L | R | L | R | L | R | L | R | L | R | L | R | L | R | L | |
|
|
||||||||||||||||
| Healthy | 0.3 [0.5] | 0.4 [0.6] | 0.4 [0.7] | 0.4 [0.4] | 0.6 [1.1] | 0.6 [1.4] | 0.4 [0.9] | 0.4 [1.0] | 0.5 [1.2] | 0.6 [1.6] | 0.5 [0.9] | 0.6 [1.2] | 1.5 [2.3] | 1.5 [2.219] | 1.8 [2.2] | 1.6 [2.3] |
|
|
||||||||||||||||
| IC/BPS | 2.5 [3.1] | 2.2 [3.0] | 2.8 [2.9] | 2.89 [3.09] | 2.7 [2.8] | 2.3 [2.8] | 1.5 [1.6] | 1.4 [1.6] | 1.8 [2.0] | 1.7 [1.9] | 1.7 [2.2] | 2.0 [2.6] | 3.3# [2.9] | 3.8# [2.7] | 3.9# [3.3] | 4.3# [3.4] |
|
|
||||||||||||||||
| IC/BPS +MPP | 2.3 [2.7] | 2.9 [2.9] | 3.8 [2.6] | 3.6 [2.9] | 4.0^ [3.0] | 4.0^ [3.0] | 2.1 [2.6] | 2.0 [2.5] | 2.5^ [2.8] | 2.8 [3.0] | 3.0 [2.9] | 3.3^ [2.9] | 4.1# [3.6] | 5.0# [3.4] | 5.9 ^# [3.1] | 6.7 ^# [3.0] |
Values were determined using a numeric rating scale (0–10) with 0 defined as “normal” and 10 as “worst pain imaginable.”
L = left, R = right. Cells shaded indicated dermatomes that were statistically different from the same dermatome of healthy controls, p<0.05.
= p<0.05 IC/BPS+MPP vs. IC/BPS;
= p<0.05 left vs. right.
Pinprick sharpness
The sensation of sharpness in pelvic dermatomes (Supplemental Table 2) did not differ between IC/BPS participants and healthy controls, except reduced NRS sharpness for both left (p=0.03) and right (p=0.04) S2 dermatomes in IC/BPS participants compared to healthy controls.
Vibration
All IC/BPS participants exhibited vibration sensation hypoesthesia, at least unilaterally, in all of the pelvic dermatomes except L1 in comparison to healthy controls (Table 2). Vibration sensation deficiencies in IC/BPS participants with or without MPP tended to be mild, but most pronounced in dermatomes S1–3. No differences in vibration sensation were identified at any pelvic dermatomes when compared between IC/BPS and IC/BPS+MPP groups.
Table 2.
Hypoesthesia in Women with IC/BPS Based on Vibration Sensation (Mean ± [SD] scores)
| Dermatome | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T12 | L1 | L2 | S1 | S2 | S3 | S4 | ||||||||
| R | L | R | L | R | L | R | L | R | L | R | L | R | L | |
|
|
||||||||||||||
| Healthy | 0.1 [0.4] | 0.1 [0.4] | 0.2 [0.4] | 0.2 [0.5] | 0.2 [0.5] | 0.1 [0.4] | 0.5 [0.9] | 0.6 [1.1] | 0.4 [0.7] | 0.5 [0.9] | 0.4 [0.7] | 0.3 [0.6] | 0.2 [0.5] | 0.2 [0.5] |
|
|
||||||||||||||
| IC/BPS | 0.4 [0.6] | 0.4 [0.7] | 0.4 [0.6] | 0.1 [0.4] | 0.6 # [1.0] | 0.8 # [1.1] | 1.1 [1.2] | 1.0 [1.2] | 0.7 [1.1] | 1.0 [1.1] | 1.0 [1.3] | 0.7 [1.0] | 0.7 [1.1] | 0.9 [1.3] |
|
|
||||||||||||||
| IC/BPS +MPP | 0.4 [0.9] | 0.4 [0.8] | 0.4 [0.7] | 0.4 [0.7] | 0.8 [1.1] | 0.6 [1.06] | 1.1 [1.3] | 1.2 [1.2] | 1.0 [1.3] | 1.1 [1.3] | 1.1 [1.3] | 0.9 [1.3] | 0.7 [1.2] | 0.6 [1.1] |
Values are determined using a numeric rating scale (0–4) with 0 defined as “normal” and 4 as “severe loss of sensation”.
L = left, R = right. Cells shaded gray indicated dermatomes that are statistically different than the same dermatome of healthy controls, p<0.05.
= p<0.05 left vs. right.
Discussion
This study presents a detailed map of neurologic pelvic dermatomes and cutaneous sensation in women with IC/BPS. The main findings include: (1) Women with IC/BPS displayed hyperalgesia based on pin prick sensation in all pelvic dermatomes compared to healthy controls; 2) Hyperalgesia in pelvic dermatomes was even more prominent in IC/BPS women with MPP; and 3) Patients with IC/BPS exhibited some evidence of vibration sensation hypoesthesia, although not as uniform as with hyperalgesia; 4) no evidence supported a more distant neuropathy involving hands or feet in any modality. These findings support somatic neural sensory processing abnormalities beyond bladder and pelvic muscle afferents as pathologic manifestations of IC/BPS.
A questionnaire-based whole body pain mapping study suggested that a form of central sensitization may develop in individuals with IC/BPS.16 The current study moves our evidence base from the entirely subjective self-report captured by definition in any questionnaire study, to the more semi-quantitative assessment afforded in an examination study. Thus the current study strengthens the Tripp et al. conclusions drawn by directly demonstrating hyperalgesia in pelvic dermatomes on physical examination in women with IC/BPS in the absence of evidence for a generalized neurologic abnormality, such as a peripheral neuropathy.
Lowenstein et al. compared thermal and vibratory sensory thresholds in IC/BPS patients and asymptomatic controls. However, it is difficult to make comparisons in the vibration sensation reported in our study to those of Lowenstein et al.9 due to different methodologies. Our findings of no difference in vibration sensation on the right side of suprapubic dermatome T12 in participants with IC/BPS, with or without MPP, are similar to those of Lowenstein et al.,9 although they used a singular, midline test site. In contrast to Lowenstein et al., we found decreased vibration sensation in most other dermatomes, maximal at S3, whereas they found no difference. We used a 64 Hz tuning fork designed for assessment of the large A-beta cutaneous mechanoreceptors. This could explain the different findings, based on the known characteristics of different tuning forks stimulating cutaneous receptors differentially.17 Our findings suggest a consistent decrease in vibration sensation in women with one or more of these pain disorders, indicating either impairment of A-beta fibers themselves, or more likely, altered processing of these signals in the central nervous system anywhere from the spinal cord to the cortex.
Cutaneous sensory threshold mapping studies in potential bladder pain referral areas such as the suprapubic area (T10–T12) have reported reduced sensitivity to thermal stimuli,9 no difference in response to cutaneous current perception,8 and hyperalgesia to blunt pressure stimuli.10 These stimuli all are mediated by small C fiber nociceptors,18 whereas that of “sharp pain”, used in the present study, is mediated by small A-delta fibers.18 Our findings lend further weight to the body of evidence suggesting increased afferent excitability and central sensitization, and now extending to A-delta fibers, in the pathologic state of IC/BPS. As some degree of pelvic floor pain was reported in the majority of IC/BPS participants in our study, and (by definition) all IC/BPS participants report bladder pain, a convergence of nociceptive signals from the bladder and pelvic floor may lead to altered processing of both noxious and non-noxious stimuli. Additional acute stimuli, such as “sharp pain” provide input to an already primed spinal cord and pain is perceived as being stronger than it would in a healthy individual. This sensitized state is restricted to the nociceptive system, as response to non-noxious stimuli such as vibration, appears hyposensitive, presumably reflecting the different neurophysiology of noxious and non-noxious nociceptive neurons,19 or perhaps reflecting impaired function of non-nociceptive pathways paralleling the upregulation of nociceptive pathways.
Nerve branches from the S2, S3, and S4 nerve roots innervate the bladder, pelvic floor muscles and pelvic dermatomes with S3 and S4 supporting bladder function20. Uncontrolled studies of neuromodulation at S3 have found improvements in pain, urgency and frequency in some women with IC/BPS.21 Hyperalgesia in response to mechanical stimuli combined with loss of vibration sensation, similar to that reported here, has been illustrated in large studies of mixed chronic pain conditions.22 Evaluation for abnormalities in neurologic pelvic dermatomes may guide the clinicians to individualize and optimize the treatment, based on the assumption, that loss of vibration sensation indicates changes in the “pain gate”,23 and greater central sensitization. Early treatment with pain pathway modulators such as tricyclics, anxiolytics, or neuromodulating agents may be most effective in this setting. Early recognition of changes in sensory perceptions in women with prodromal bladder pain and urinary symptoms24 with early neuromodulating therapy could forestall the development of full clinical presentation of IC/BPS. Evidence of hyperalgesia in pelvic dermatomes could also help clinicians to more carefully assess for MPP, since our findings revealed more prominent hyperalgesia in pelvic dermatomes in women with both IC/BPS and MPP. Eventually, we will aim develop the diagnosis algorithm or assessment scale that will take into account abnormal neurologic findings in women with CPP during the evaluation and management.
Beyond the gate control theory, the reduction in non-nociceptive signal sensitivity that accompanies increased sensitivity to nociceptive signals may provide further support for an evolving concept of brain function. Central pathways tend to bias brain function towards prior solutions to problems encountered, sometimes referenced as the “Einstellung Effect”.25 Thus, even at the most distal peripheral level, signal processing may be altered to match what the central nervous system expects, perpetuating prior experience bias, and creating a kind of “einstellung signaling bias” which would reduce the probability of considering other signal sets. If true, such a nervous system construction would explain the “pain gate” in a broader context, and explain why chronic pain and other chronic states are so difficult to change.
This study has several limitations. Our study protocol allowed participants to continue their pain medication treatment regimen. Therefore, we cannot comment whether or not medications for IC/BPS contributed to participants’ responses to various stimuli in pelvic dermatomes. Although this approach may have impacted participants’ perception, we concluded that allowing for “wash-out” time from medications prior to neurologic evaluation would have impeded our study recruitment efforts. In addition, analgesic medication withdrawal could have its own undefined impact on pain thresholds. There is no accepted standard neurologic protocol to evaluate women with CPP. Therefore, our study methodology was different from that used in some of the other studies, hampering direct comparisons. However, our detailed neurologic examination was developed by a neurologist with significant expertise in pain evaluation and standardized by our study advisory board and investigators with expertise in pain evaluation, including IC/BPS. We chose this methodology examining hyperalgesia because it could be adapted to clinical practice for better phenotyping of IC/BPS patients. This requires little specialized equipment and could also be utilized in future studies. The cross-sectional study design does not allow any inference regarding the critical questions of causation and chronology - does bladder pain lead to abnormal sensation or vice-versa, etc. Our protocol is not able to assess for global hyperalgesia beyond the pelvic dermatomes, as we applied different approach in screening for peripheral neuropathies and evaluating hyperalgesia in pelvic dermatomes. Additionally, high proportion of our participants had other overlapping pain syndromes such as fibromyalgia, irritable bowel syndrome, migraines, and others. We did not evaluate the impact of these conditions on hyperalgesia in the pelvis or globally in our participants. Despite these limitations, the study does provide findings of a structured examination of women with IC/BPS and healthy control subjects.
Conclusion
This study thoroughly mapped sensory function of the pelvic dermatomes in women with IC/BPS and healthy participants. Women with IC/BPS experience hypersensitivity to sharp pain of the pelvic dermatomes without a change to the pattern seen in healthy controls, which may be the result of central sensitization and convergence of noxious stimuli at the spinal dorsal horn. Conversely, vibration, a non-noxious stimulus applied to pelvic dermatomes of women with IC/BPS revealed hypoesthesia compared to normal healthy women, again without change in the pattern seen in healthy control participants, also supportive of changes in afferent processing. These two findings together may suggest a single neural bias enhancing nociceptive stimuli and diminishing non-nociceptive stimuli, without changing the overall preset dermatomal pattern seen in healthy subjects. Our study was not designed to assess cause and effect relationships of these findings to chronic pelvic pain. Nonetheless, these findings have important implications for both the physiology of IC/BPS and in guiding treatment decisions.
Supplementary Material
Mean pain [±SD] of the obturator internus muscles, levator ani and perineal body are illustrated. Left (L) and Right (R); * = p<0.05 compared to healthy control; ^ = p<0.05 compared to IC/BPS; # = p<0.05 L vs R side of dermatome.
Values are determined using a numeric rating scale (0–10) with 0 defined as “no sensation” and 10 defined as “very sharp.” Sharpness was anchored to that of a pinprick to the forehead. L = left, R = right. Cells shaded gray indicated dermatomes that are statistically different than the same dermatome of healthy controls, p<0.05.
Acknowledgments
Funding: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; R01DK083538), Advancing a Healthier Wisconsin Endowment Grant # 5520298. Dr. Sanses is supported K12 HD43489 from the National Institute of Child Health and Human Development.
Footnotes
The study was performed at University Hospitals Cleveland Medical Center and Case Western University, Cleveland, Ohio.
References
- 1.Hanno PM, Erickson D, Moldwin R, Faraday MM, American Urological A. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193(5):1545–53. doi: 10.1016/j.juro.2015.01.086. Epub 2015/01/28. [DOI] [PubMed] [Google Scholar]
- 2.Yunus MB. Editorial review: an update on central sensitivity syndromes and the issues of nosology and psychobiology. Current rheumatology reviews. 2015;11(2):70–85. doi: 10.2174/157339711102150702112236. Epub 2015/07/04. [DOI] [PubMed] [Google Scholar]
- 3.NINDS. National Institute of Neurological Disorders and Stroke. Bethesda, Maryland: Aug 13–14, 2012. A Workshop on Chronic Overlapping Pain Conditions. [Google Scholar]
- 4.Ness TJ, Lloyd LK, Fillingim RB. An endogenous pain control system is altered in subjects with interstitial cystitis. J Urol. 2014;191(2):364–70. doi: 10.1016/j.juro.2013.08.024. Epub 2013/08/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenton BW. Limbic associated pelvic pain: a hypothesis to explain the diagnostic relationships and features of patients with chronic pelvic pain. Med Hypotheses. 2007;69(2):282–6. doi: 10.1016/j.mehy.2006.12.025. Epub 2007/02/13. [DOI] [PubMed] [Google Scholar]
- 6.Twiss C, Kilpatrick L, Craske M, Buffington CA, Ornitz E, Rodriguez LV, et al. Increased startle responses in interstitial cystitis: evidence for central hyperresponsiveness to visceral related threat. J Urol. 2009;181(5):2127–33. doi: 10.1016/j.juro.2009.01.025. Epub 2009/03/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ness TJ, Powell-Boone T, Cannon R, Lloyd LK, Fillingim RB. Psychophysical evidence of hypersensitivity in subjects with interstitial cystitis. J Urol. 2005;173(6):1983–7. doi: 10.1097/01.ju.0000158452.15915.e2. Epub 2005/05/10. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald MP, Koch D, Senka J. Visceral and cutaneous sensory testing in patients with painful bladder syndrome. Neurourol Urodyn. 2005;24(7):627–32. doi: 10.1002/nau.20178. Epub 2005/09/21. [DOI] [PubMed] [Google Scholar]
- 9.Lowenstein L, Kenton K, Mueller ER, Brubaker L, Heneghan M, Senka J, et al. Patients with painful bladder syndrome have altered response to thermal stimuli and catastrophic reaction to painful experiences. Neurourol Urodyn. 2009;28(5):400–4. doi: 10.1002/nau.20676. Epub 2009/02/05. [DOI] [PubMed] [Google Scholar]
- 10.Lai HH, Gardner V, Ness TJ, Gereau RWt. Segmental hyperalgesia to mechanical stimulus in interstitial cystitis/bladder pain syndrome: evidence of central sensitization. J Urol. 2014;191(5):1294–9. doi: 10.1016/j.juro.2013.11.099. Epub 2013/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chelimsky T, Chelimsky G, McCabe NP, Louttit M, Hijaz A, Mahajan S, et al. Interstitial Cystitis - Elucidation of Psychophysiologic and Autonomic Characteristics (the ICEPAC Study): design and methods. J Pain Res. 2014;7:243–53. doi: 10.2147/JPR.S58853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van de Merwe JP, Nordling J, Bouchelouche P, Bouchelouche K, Cervigni M, Daha LK, et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur Urol. 2008;53(1):60–7. doi: 10.1016/j.eururo.2007.09.019. Epub 2007/09/29. [DOI] [PubMed] [Google Scholar]
- 13.Sanses TV, Chelimsky G, McCabe NP, Zolnoun D, Janata J, Elston R, et al. The Pelvis and Beyond: Musculoskeletal Tender Points in Women With Chronic Pelvic Pain. Clin J Pain. 2016;32(8):659–65. doi: 10.1097/AJP.0000000000000307. Epub 2015/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walk D, Sehgal N, Moeller-Bertram T, Edwards RR, Wasan A, Wallace M, et al. Quantitative sensory testing and mapping: a review of nonautomated quantitative methods for examination of the patient with neuropathic pain. Clin J Pain. 2009;25(7):632–40. doi: 10.1097/AJP.0b013e3181a68c64. Epub 2009/08/21. [DOI] [PubMed] [Google Scholar]
- 15.Pestronk A, Florence J, Levine T, Al-Lozi MT, Lopate G, Miller T, et al. Sensory exam with a quantitative tuning fork: rapid, sensitive and predictive of SNAP amplitude. Neurology. 2004;62(3):461–4. doi: 10.1212/01.wnl.0000106939.41855.36. Epub 2004/02/12. [DOI] [PubMed] [Google Scholar]
- 16.Tripp DA, Nickel JC, Wong J, Pontari M, Moldwin R, Mayer R, et al. Mapping of pain phenotypes in female patients with bladder pain syndrome/interstitial cystitis and controls. Eur Urol. 2012;62(6):1188–94. doi: 10.1016/j.eururo.2012.05.023. Epub 2012/05/29. [DOI] [PubMed] [Google Scholar]
- 17.Harada N, Griffin MJ. Factors influencing vibration sense thresholds used to assess occupational exposures to hand transmitted vibration. British journal of industrial medicine. 1991;48(3):185–92. doi: 10.1136/oem.48.3.185. Epub 1991/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ziegler EA, Magerl W, Meyer RA, Treede RD. Secondary hyperalgesia to punctate mechanical stimuli. Central sensitization to A-fibre nociceptor input. Brain. 1999;122(Pt 12):2245–57. doi: 10.1093/brain/122.12.2245. Epub 1999/12/03. [DOI] [PubMed] [Google Scholar]
- 19.Price DD, Greenspan JD, Dubner R. Neurons involved in the exteroceptive function of pain. PAIN. 2003;106(3):215–9. doi: 10.1016/j.pain.2003.10.016. Epub 2003/12/09. [DOI] [PubMed] [Google Scholar]
- 20.Juenemann KP, Lue TF, Schmidt RA, Tanagho EA. Clinical significance of sacral and pudendal nerve anatomy. J Urol. 1988;139(1):74–80. doi: 10.1016/s0022-5347(17)42297-x. Epub 1988/01/01. [DOI] [PubMed] [Google Scholar]
- 21.Peters KM. Neuromodulation for the treatment of refractory interstitial cystitis. Rev Urol. 2002;4(Suppl 1):S36–43. Epub 2006/09/21. [PMC free article] [PubMed] [Google Scholar]
- 22.Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. PAIN. 2010;150(3):439–50. doi: 10.1016/j.pain.2010.05.002. Epub 2010/07/16. [DOI] [PubMed] [Google Scholar]
- 23.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–9. doi: 10.1126/science.150.3699.971. Epub 1965/11/19. [DOI] [PubMed] [Google Scholar]
- 24.Warren JW, Wesselmann U, Greenberg P, Clauw DJ. Urinary symptoms as a prodrome of bladder pain syndrome/interstitial cystitis. Urology. 2014;83(5):1035–40. doi: 10.1016/j.urology.2014.01.012. Epub 2014/03/29. [DOI] [PubMed] [Google Scholar]
- 25.Thomas C, Didierjean A. Magicians fix your mind: How unlikely solutions block obvious ones. Cognition. 2016;154:169–73. doi: 10.1016/j.cognition.2016.06.002. Epub 2016/06/20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean pain [±SD] of the obturator internus muscles, levator ani and perineal body are illustrated. Left (L) and Right (R); * = p<0.05 compared to healthy control; ^ = p<0.05 compared to IC/BPS; # = p<0.05 L vs R side of dermatome.
Values are determined using a numeric rating scale (0–10) with 0 defined as “no sensation” and 10 defined as “very sharp.” Sharpness was anchored to that of a pinprick to the forehead. L = left, R = right. Cells shaded gray indicated dermatomes that are statistically different than the same dermatome of healthy controls, p<0.05.