Abstract
Purpose
To propose a Large coverage Black-Bright blood Interleaved imaging sequence (LaBBI) for 3D dynamic contrast-enhanced MRI (DCE-MRI) of vessel wall.
Methods
LaBBI consists of a 3D black-blood stack-of-star golden angle radial acquisition with high spatial resolution for vessel wall imaging and a 2D bright-blood Cartesian acquisition with high temporal resolution for Arterial Input Function (AIF) estimation. The two acquisitions were performed in an interleaved fashion within a single scan. Simulations, phantom experiments and in vivo tests were performed to investigate the feasibility and performance of the proposed LaBBI.
Results
In simulation test, the estimated Ktrans and νp by LaBBI were more accurate than conventional bright-blood DCE-MRI with lower root mean square error (RMSE) in all the tested conditions. In phantom test, no signal interference was found on the 2D scan in LaBBI. Pharmacokinetic analysis of the three patients data acquired by LaBBI showed that the Ktrans was higher in fibrous tissue (0.0717±0.0279 min−1), while lower in necrotic core (0.0206±0.0040 min−1) and intraplaque hemorrhage (0.0078±0.0007 min−1), compared with normal vessel wall (0.0273±0.0052 min−1).
Conclusion
The proposed LaBBI sequence, with high spatial and temporal resolution, and large coverage blood suppression, was promising to probe the perfusion properties of vessel wall lesions.
Keywords: vessel wall, inflammation, dynamic contrast-enhanced MRI, black-bright blood interleaved imaging
INTRODUCTION
Vessel wall inflammation plays an important role in the development of vascular diseases such as atherosclerosis and aneurysms (1,2). In several studies, vessel wall inflammation has been shown to be associated with clinical vascular events (3,4). Thus, it is important to image vessel wall inflammation in vivo. Dynamic contrast-enhanced MRI (DCE-MRI) is a useful tool to analyze inflammation in carotid atherosclerosis noninvasively (5–10) by using pharmacokinetic modeling.
However, using DCE-MRI to evaluate inflammation in vessel wall is technically challenging, especially in early lesions. There are two competing requirements in DCE data acquisition (11): high temporal resolution bright-blood imaging for arterial input function (AIF) acquisition, and high spatial resolution black-blood imaging for vessel wall depiction. Currently, most studies have made compromises by acquiring only bright-blood images (6,7,10,12,13) for advanced plaques. The bright-blood technique can estimate the AIF directly, but the temporal resolution (~15s) is compromised by the high spatial resolution required for vessel wall imaging and is usually insufficient to extract AIF accurately. More importantly, thin vessel walls with early lesions cannot be quantified reliably due to signal contamination from the luminal signal. Black-blood DCE techniques have also been proposed for vessel wall imaging (9,14) that allow accurate thin vessel wall imaging by suppressing blood signal. However, AIF cannot be extracted directly from the black-blood images and may introduce bias in the pharmacokinetic analysis using the reference region method or population AIF (9,14).
Recently, several techniques enabling interleaved black-bright blood imaging have been introduced (15–17), which can acquire bright-blood images for AIF and black-blood images for vessel wall in a single scan. However, these techniques are based on a double inversion-recovery (DIR) (18) or quadruple inversion-recovery (QIR) (19) prepulse for black-blood imaging. Thus, they are limited to 2D vessel wall imaging and with limited longitudinal coverage. Mendes J. et al, proposed an interleaved 2D–3D bright-blood imaging sequence to perform 3D vessel wall DCE-MRI (20). However, the vessel wall imaging in this sequence may still suffer from luminal signal contamination, since no black-blood prepulse was used.
In this study, we propose a Large coverage Black-Bright blood Interleaved imaging sequence (LaBBI) for vessel wall DCE-MRI. In this sequence, the 3D large coverage high spatial resolution black-blood vessel wall images and 2D high temporal resolution bright-blood images can be acquired in an interleaved fashion within a single scan. In LaBBI, the spatial and temporal resolution of the two acquisitions can be adjusted flexibly to satisfy the requirements of pharmacokinetic analysis on different targets. Simulation, phantom studies and in vivo experiments were performed to test the feasibility of the proposed LaBBI method.
METHODS
Sequence Design
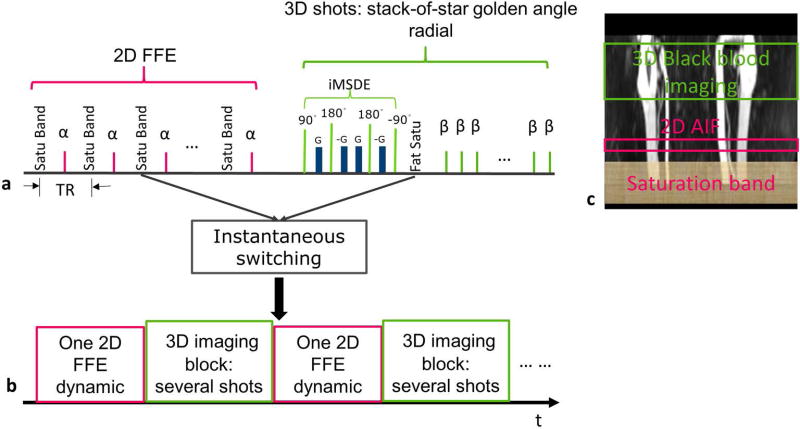
LaBBI is composed of a 2D high temporal resolution bright-blood imaging module and a 3D high spatial resolution black-blood imaging module (Fig. 1a). For the 3D black-blood imaging module, the stack-of-star (SoS) golden radial acquisition (21) was used, which performed Cartesian sampling in the slice (kz) direction with low-high acquisition order and radial sampling in the kx-ky plane. As shown in Fig. 1a, each 3D shot consisted of the iMSDE (improved Motion Sensitized Driven Equilibrium) black-blood prepulse (22) for large coverage 3D blood signal suppression, SPIR (Spectral Presaturation with Inversion Recovery) fat suppression and data acquisition, where the radial spokes with the same angle through all slices were acquired. The 3D imaging slab was centered at the target vessel wall lesion (Fig. 1c). For bright-blood imaging, 2D fast field echo (FFE) Cartesian data acquisition was used, and the 2D imaging slice was positioned perpendicular to a large vessel adjacent to the vessel wall lesion (Fig. 1c). In the 2D acquisition, a spatial saturation band was placed upstream of the 2D imaging slice (Fig. 1c) and performed in each TR (Fig. 1a). The saturation band was used to induce the saturation recovery of blood signal and then eliminate the interference from the non-selective black-blood prepulse. The proposed sequence supports instantaneous switching (23) between bright- and black-blood imaging modules. In our design, one bright-blood imaging dynamic is interleaved with one 3D imaging block containing the user-defined number of 3D shots of black-blood imaging (Fig. 1a, b).
FIG. 1.
The design scheme of LaBBI. a: Sequence schematic of LaBBI: 2D FFE Cartesian acquisition for AIF with a spatial saturation band performed in each TR; 3D stack-of-star golden angle radial for vessel wall imaging with iMSDE prepulse for blood suppression and fat saturation after iMSDE pulse. b: The interleaved scheme of LaBBI with one 2D bright-blood dynamic interleaved with a designated number of 3D shots. c: The locations of the spatial saturation band, 2D imaging slice and 3D imaging slab.
Image Reconstruction
For the SoS golden angle radial data, the gradient delays in radial sampling was firstly compensated using the method in (24). The radial data was then reconstructed into time series by grouping several blocks of 3D imaging into temporal frames using an established reconstruction method (21), which utilizes parallel imaging and compressed sensing together to reduce undersampling artifacts. The reconstruction equation is as follows:
| [1] |
where m is the image series to be reconstructed, d is the acquired multi-coil data, S are the coil sensitivity maps, F is the non-uniform fast Fourier transform (NUFFT) operator, TVt is the temporal total-variation operator, and λ is the regularization factor. The coil sensitivities were obtained by NUFFT of all radial lines, and λ were selected empirically (21).
Imaging Parameters
All imaging experiments in this study were performed on a 3.0T Philips MR scanner (Achieva TX, Best, the Netherlands). The imaging parameters of LaBBI are shown in Table 1. For the 3D vessel wall imaging module, 15 transverse slices were acquired with a large coverage of 30mm. So the turbo field echo (TFE) factor of the 3D shot was 15, which means that only 15 spokes (duration of about 180ms) were acquired following each iMSDE and SPIR prepulses, so the black-blood contrast and fat suppression can be maintained. Each 3D imaging block was set to contain 3 dummy shots and 10 acquisition shots with the dummy shots to restore the steady-state of the 3D acquisition. The signal evolutions of the 3D imaging during contrast passage with and without dummy shots are demonstrated in Supporting Figure S1. In LaBBI, one 2D dynamic was interleaved with one 3D block, resulting in a temporal resolution of AIF, Taif = 4.7s. Two 3D blocks with 20 radial spokes per slice were used to reconstruct one frame of the 3D black-blood dynamic image series, resulting in a temporal resolution of vessel wall, TVW = 9.4s. The distance between the two imaging modules was about 20mm.
Table 1.
Imaging parameters of LaBBI
| Parameters | 3D black-blood | 2D bright-blood |
|---|---|---|
| TR/TE | 12/5.6 | 19/4.2 |
| Flip angle | 15° | 25° |
| FOV (transverse) | 160×160 mm2 | 150×150 mm2 |
| Spatial resolution | 0.6×0.6 mm2 | 1×1.5 mm2 |
| Slice thickness | 2mm | 2mm |
| Slices | 15 | 1 |
| TFE factor | 15 | NAa |
| Readout | 536 | NA |
| Spokes | 429 | NA |
| Dynamics | NA | 43 |
| Interleaved mode | 1 blockb | 1 dynamic |
| Temporal resolution | 9.4sc | 4.7s |
| Total duration | 3.4min | |
| Interval distanced | 20mm | |
NA: not applicable.
One 3D imaging block contained 3 dummy shots and 10 acquisition shots.
Temporal resolution of the reconstructed 3D images using 20 spokes in each slice per frame.
Interval distance: the distance between the 2D and 3D imaging module.
Simulation Test
Simulations were performed to investigate the accuracy of pharmacokinetic parameters estimated by LaBBI. The traditional bright-blood DCE imaging sequence (6) was also simulated as a comparison.
Both LaBBI and the traditional bright-blood DCE-MRI used the same 2D Cartesian acquisition for AIF, so the AIF Cp was simulated directly using a biexponential equation (25). The contrast agent concentration in the blood was calculated by:
| [2] |
with an assumed hematocrit (Hct) value of 0.4 (25). The changing T1 values of blood after contrast injection can be calculated by:
| [3] |
where r1=4.5L/mmol/s (26), C[Gd] is the concentration of contrast agent, T10 is the pre-contrast T1 value. In this study, T10 was assumed to be 1650ms for blood (27). Then, signal curve of blood (Sigb) can be calculated using the saturation recovery (SR) equation (Eq. [4])
| [4] |
where M0b is the proton density of blood, td is saturation delay time and α is the flip angle. Subsequently, the blood signal curves of the traditional bright-blood DCE (6) and the proposed LaBBI were generated by undersampling the Sigb according to the corresponding temporal resolutions of the two methods. The zero-mean Gaussian noise with standard deviation (SD) = the mean signal of all frames / signal-to-noise ratio (SNR) was then added to the signal curve. The SNR of simulated blood signal was set to be twice that of the vessel wall SNR, which was in agreement with the following in vivo SNR measurements.
For vessel wall imaging, three types of simulations were carried out to simulate the vessel wall dynamic images under different conditions: 9 spheres with 9 evenly distributed Ktrans values ranging from 0.02 to 0.2min−1 and fixed νp =0.05, SNR=30; 9 spheres with 9 evenly distributed νp values ranging from 0.01 to 0.1 and fixed Ktrans =0.1min−1, SNR = 30; 1 sphere with fixed Ktrans =0.1min−1, νp =0.05, and simulated with 10 equally spaced SNR levels ranging from 20 to 40.
With known Cp and given Ktrans, νp values, the uptake curves of the vessel wall (Ct(t)) can be generated using the Patlak model (28):
| [5] |
And the changing T1 values of vessel wall during dynamic imaging can be calculated by Eq. [3] with assumed T10 of 1150ms (17).
The simulated spheres were analytic in the Fourier domain (29), which can be calculated by the corresponding acquisition trajectory and image signal. For traditional bright-blood DCE, Cartesian trajectory was simulated with a temporal resolution of 15s (6). The signal curve of the vessel wall (SigVW_traditional) can be generated based on the spoiled gradient echo (SPGR) equation:
| [6] |
where M0VW is the proton density of vessel wall, E1,traditional = exp(−TRtraditional/T1), TRtraditional is the repetition time, θ is the flip angle. For the 3D vessel wall imaging module in LaBBI, the SoS golden angle radial trajectory was simulated using parameters in Table 1, and the signal of each spoke was generated using Bloch simulation with the iMSDE prepulse represented by a T2 relaxation term , where is duration of iMSDE. The changing T2 values during dynamic imaging were estimated by:
| [7] |
where r2=5.09L/mmol/s (26), and T20 was assumed to be 54ms for vessel wall (30). For each simulation, the zero-mean Gaussian noise was added to the simulated k-space. Then dynamic image frames were reconstructed from the simulated k-space data by Fourier Transform for the Cartesian data and the reconstruction method described above for the SoS golden angle radial data.
For pharmacokinetic analysis of the simulations, AIF was obtained by converting the generated signal curve to concentration curve using Eq. [2–4]. For the traditional bright-blood DCE-MRI, pharmacokinetic analysis was performed based on Eq. [3, 5, 6]. For LaBBI, the steady-state signal equation is (the derivation of the steady-state equation is detailed in the Supporting file):
| [8] |
where K is the total number of acquired slices. Combing Eq. [3, 5, 7, 8], Ktrans and νp can be estimated using the Levenberg-Marquardt curve fit. Then, the Ktrans and νp maps were generated for both methods.
Each simulation was repeated 100 times with independent zero-mean Gaussian noise added. The mean value of each simulated sphere was calculated at every simulation. The estimation accuracy of the pharmacokinetic parameters was evaluated by the normalized root mean square error (RMSE): , where Pe and Pt are the estimated mean values and simulated true values, N is the number of repeated simulations. The average and SD of the 100 repeats were also reported for the three simulation types.
Phantom Study
In this study, phantom scans were performed to test whether there was interference on the 2D bright-blood imaging from the iMSDE prepulse in the black-blood imaging module. A series of tubes with varied concentrations of Gadolinium contrast agent were used to simulate the T1 (300ms–1600ms) prior and after contrast injection. The phantom was set upright, and the two scan slabs in LaBBI were both performed coronally with a distance of about 20mm. For the 2D imaging module, to induce the saturation recovery of signal, a slice selective SR prepulse at the imaging location was used to simulate the saturation band. All other imaging parameters were the same as the in vivo experiments shown in Table 1. The 3D imaging and 2D imaging modules were first performed in an interleaved fashion (LaBBI scan), and then performed separately with the same imaging settings. The agreement of the 2D images acquired by the interleaved and separate scan was investigated by comparing the acquired signal intensities of different tubes using Pearson correlation.
In vivo Experiments
This study was approved by the institutional review board, and written informed consent was obtained from all participating subjects. Three patients (3 males, age of 70, 72 and 47 years for case 1, case 2 and case 3) with carotid artery atherosclerosis were scanned with a custom designed 36-channel neurovascular coil (31). A 3D iMSDE prepared turbo field echo (TFE) black-blood sequence, 3D MERGE (32) was firstly performed to identify the lesion location with FOV=250×160×40mm3, voxel size=0.8×0.8×0.8mm3 and 50 coronal slices. The simultaneous noncontrast angiography and intraplaque hemorrhage (IPH) imaging sequence, SNAP (33), with the same FOV and voxel size to 3D MERGE was also performed. Then, the LaBBI sequence was used to acquire DCE-MRI images with 3D imaging centered at the location with the largest lesion size. Coincident with the third dynamic of the bright-blood imaging, a bolus of 0.1mmol/kg Gd-DTPA (Magnevist; Bayer Schering Pharma, Berlin, Germany) was intravenously injected at a rate of 1.5mL/s, followed by a 20mL saline flush at the same rate. The imaging parameters of LaBBI are shown in Table 1. For two patients with obvious plaque, the post-contrast images were also acquired by a T1-weighted (T1W) inversion recovery (IR) prepared TFE sequence, with an inversion delay time=500ms, and voxel size=0.8×0.8×0.8mm3.
In vivo Image Analysis
On the 2D bright-blood images of LaBBI, a small region of interest (ROI) was placed around the center of carotid artery lumen, and then a clustering algorithm was used to select voxels with strong enhancement to calculate the signal curve of blood to reduce the influence of inflow artifacts (7). By assuming the T10 = 1650ms (27) and Hct=0.4 (25) for blood, the AIF can be acquired by Eq. [2–4].
The 3D SoS golden angle radial data was reconstructed into a dynamic series using Eq. [1] with 20 spokes for each slice per frame. The 3D MERGE and SNAP images were reformatted to 2mm axial slices according to the imaging geometry of LaBBI for analysis. Then, for case 1 and case 2, 7 slices centered at bifurcation were selected from each carotid artery. For case 3, the imaging was targeted at intracranial internal carotid artery with location much higher than the bifurcation, so 5 slices covering artery were selected for each carotid artery.
The ability of the black-blood image of LaBBI to depict vessel wall was evaluated by comparing with 3D MERGE. The frame of LaBBI vessel wall images that visually best depicted vessel wall was selected by one reviewer. Then another experienced reviewer blinded to the image types drew the contours of lumen, outer wall and muscle on the selected slices of LaBBI vessel wall and 3D MERGE using a custom-designed image analysis software (34). The vessel wall area (WA) and maximum wall thickness (MWT) were compared between LaBBI and 3D MERGE using Wilcoxon signed rank test and intra-class correlation coefficient (ICC).
For pharmacokinetic analysis, the regions of necrotic core (NC), calcification and IPH were also identified for slices with plaque, with NC and calcification defined on post-contrast phase of LaBBI vessel wall images with post-contrast T1W and SNAP images as reference (35,36) and IPH defined on SNAP images (33). After registration of the LaBBI vessel wall series (37), the contours of lumen, outer wall, NC and calcification defined on one frame of LaBBI vessel wall images with best vessel wall contrast were propagated to other frames. The reformatted SNAP images were registered to the LaBBI vessel wall images, and then the IPH contours were mapped to LaBBI. The remaining region in the plaque were assumed to be fibrous tissue (FT) (37). Then the signals of LaBBI vessel wall images in the defined contour were averaged to generate the uptake curves. Then as done in the simulation, based on Eq. [3, 5, 7, 8] and assumed T10, T20, the mean Ktrans and νp can be estimated for the ROI in each slice. No IPH was observed in the analyzed NC, and pre-contrast T1 of NC, FT and normal vessel wall, muscle was assumed to be 1150ms (17). For IPH, T10 was set to be 500ms (33,38). The pre-contrast T2 values of normal vessel wall, muscle, FT, NC, and IPH reported in (30) were used in the analysis, as shown in Table 2. The calcification was excluded from analysis due to the lack of pre-contrast T1 and T2.
Table 2.
Pharmacokinetic analysis results of the 3 patients imaged by LaBBI
| Normal VW |
Muscle | FT | NC | IPH | |
|---|---|---|---|---|---|
| Pre-contrast T1, T2 (ms)a | 1150, 54 | 1150, 39 | 1150, 56 | 1150, 37 | 500, 107 |
| Number of slicesb | 17 | 38 | 21 | 4 | 2 |
| Ktrans (min−1)c | 0.0273±0.0052 | 0.0206±0.0064 | 0.0717±0.0279 | 0.0206±0.0040 | 0.0078±0.0007 |
| νp | 0.0152±0.0042 | 0.0107±0.0033 | 0.1253±0.0248 | 0.0021±0.0002 | 0.0043±0.0008 |
The pre-contrast T1, T2 values used in the pharmacokinetic analysis of LaBBI data
The number of slices to calculate the slice averaged Ktrans and νpvalues
mean±SD
The SNRs of blood and normal vessel wall were also estimated for the in vivo data. Signal was determined by the mean signal of all the dynamics in the corresponding ROI, while noise was defined as the standard deviation of the mean signal in a peripheral air region in the same frames.
RESULTS
Simulation and phantom studies
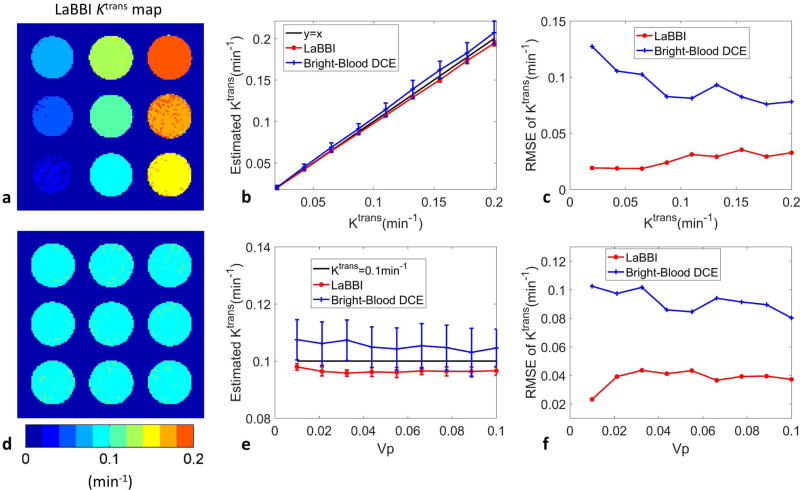
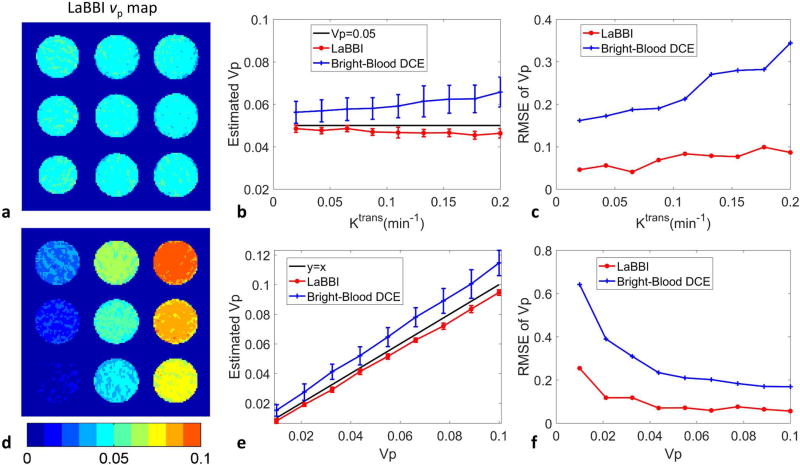
In simulation of different Ktrans from 0.02 to 0.2 min−1, fixed νp=0.05 and fixed Ktrans=0.1 min−1, different νp from 0.01 to 0.1, the average Ktrans and νp maps of the 100 repeated simulations with vessel wall SNR=30, derived from LaBBI are shown in Fig. 2a, d and Fig. 3a, d. LaBBI tended to underestimate the Ktrans and νp values a little, while the bright-blood DCE overestimated the parameters. But the mean Ktrans and νp values estimated by LaBBI were consistently closer to the true values with much smaller RMSE than the bright-blood DCE (Fig. 2b, c, e, f and Fig. 3b, c, e, f). For the fixed Ktrans, the νp values had little influence on its estimation accuracy in LaBBI, while the estimation error of Ktrans tended to be lower at larger νp values in bright-blood DCE (Fig. 2e, f). For the fixed νp, its estimation accuracy was higher at smaller Ktrans values in both LaBBI and bright-blood DCE (Fig. 3b, c).
FIG. 2.
Results of the simulations in Ktrans estimation with LaBBI compared with conventional bright-blood DCE method. For Ktrans ranging from 0.02 to 0.2 min−1, fixed νp = 0.05 and vessel wall SNR=30: a shows the average Ktrans map of 100 repeated simulations of LaBBI; b, c shows the mean and SD, RMSE of Ktrans. For νp ranging from 0.01–0.1, fixed Ktrans = 0.1min−1 and vessel wall SNR=30: d shows the average Ktrans map of 100 repeated simulations of LaBBI; e, f shows the mean and SD, RMSE of Ktrans.
FIG. 3.
Results of simulations in νp estimation with LaBBI compared with a conventional bright-blood DCE method. For Ktrans ranging from 0.02–0.2 min−1, fixed νp = 0.05 and vessel wall SNR=30: a shows the average νp map of the 100 repeated simulations of LaBBI; b, c shows the mean and SD, RMSE of νp. For νp ranging from 0.01–0.1, fixed Ktrans = 0.1min−1 and vessel wall SNR=30: d shows the average νp map of 100 repeated simulations of LaBBI; e, f shows the mean and SD, RMSE of νp.
Simulation results of different SNR levels, with Ktrans=0.1 min−1, νp=0.05, are shown in Figure 4. An example of the average Ktrans and νp maps of the 100 repeated simulations of LaBBI at SNR=30 of vessel wall are shown in Fig. 4a, d. Higher SNR increased estimation accuracy in both LaBBI and bright-blood DCE methods. However, LaBBI was consistently better than traditional bright-blood DCE in Ktrans and νp estimations for all the tested SNR levels (Fig. 4b, c, e, f), with smaller bias and much lower RMSEs (5%–8% vs. 6%–13% for Ktrans; 5%–8% vs. 19%–23% for νp).
FIG. 4.
Simulation results of Ktrans = 0.1min−1 and νp = 0.05 with vessel wall SNR ranging from 20–40 by LaBBI and conventional bright-blood DCE. a, d shows an example of the average Ktrans and νp map of 100 repeated simulations of LaBBI at SNR=30; b, c, e, f shows the mean and SD, RMSE of Ktrans and νp estimated at different SNR levels. The SNR of AIF was set to be twice that of the vessel wall.
For the phantom study, signal intensity acquired by the 2D SR imaging module in the interleaved acquisition, imitating the LaBBI 2D AIF imaging module, agreed well with that of a separate acquisition (Fig. 5, R=0.99), suggesting that the 3D black-blood prepulse had little influence on the saturation recovery signal.
FIG. 5.
Comparison of the interleaved scan in LaBBI and the separate scan using 2D SR. a: The signal intensity of tubes with different contrast concentrations acquired by separate 2D SR and interleaved 2D SR scans. b: The two signal intensity curves in a were in good agreement (R=0.99).
In vivo experiments
All 3 subjects were successfully imaged. For comparison of LaBBI vessel wall images with 3D MERGE images, totally 38 slices from the 6 carotid arteries were measured. The WA and MWT measured by LaBBI were (mean±SD) 38.66±22.23mm2 and 2.240±1.513mm, and by 3D MERGE were 39.52±19.24 mm2 and 2.245±1.269mm. No significant difference was found between LaBBI and 3D MERGE (WA: P=0.27; MWT: P=0.25). And the ICCs indicate good agreements (WA: 0.952, 95% CI, 0.911–0.975; MWT: 0.944, 95% CI, 0.894–0.970), suggesting the LaBBI sequence itself can be used to depict vessel wall.
Among the 38 slices, plaque (focal wall thickness > 2mm) was detected in 21 slices in 4 carotid arteries, among which, one plaque had NC (case 1), and one plaque had IPH (case 2). For case 1, four high spatial resolution dynamic images of a representative slice with carotid plaque and an example slice of the normal vessel wall acquired using the 3D black-blood imaging module in LaBBI are demonstrated (Fig. 6a, b). Blood suppression was sufficient prior and post contrast in both the diseased and healthy locations. For comparison, the reformatted iMSDE and post-contrast T1W images are also shown in Fig. 6a, b. The locations with and without plaque can be clearly confirmed and a necrotic core (NC) was identified in the diseased slice. High temporal resolution bright-blood dynamic images acquired by the 2D imaging module of LaBBI during contrast agent injection, are also demonstrated (Fig. 6c). For case 2, dynamic black-blood images of the 2 slices with IPH and bright-blood images acquired by LaBBI throughout the contrast injection process are shown in Figure 7. The IPH regions were identified on the SNAP images (Fig. 7a). And for the LaBBI vessel wall images, good blood suppression can be seen for all frames. The LaBBI images of case 3 are shown in Supporting Figure S2.
FIG. 6.
Dynamic images before, during, and after bolus arrival of case 1 with atherosclerotic plaque acquired by LaBBI, as well as the corresponding iMSDE and post-contrast T1W images. a: A typical slice for normal vessel wall in the common carotid artery. b: One representative slice of the atherosclerotic plaque. The dynamic LaBBI vessel wall images in a, b are shown in the same intensity scale. The blue contours outline the outer wall; the red contours outline the lumen; the yellow contours outline the necrotic core. c: The dynamic bright-blood images acquired by LaBBI shown in the same intensity scale. One black-blood image frame was interleaved with two bright-blood image frames. The white arrowheads indicate the artery to extract AIF.
FIG. 7.
Dynamic images before, during, and after bolus arrival of case 2 with intraplaque hemorrhage acquired by LaBBI. a: The slices of IPH with red contours outlining the lumen, blue contours outlining the outer wall and orange contours outlining IPH on SNAP images. The dynamic vessel wall images of LaBBI in a are shown in the same level/window. b: The dynamic bright-blood images of LaBBI, shown in the same intensity scale. The white arrowheads indicate the artery to extract AIF. One black-blood image frame was interleaved with two bright-blood image frames in LaBBI.
For pharmacokinetic analysis, one example of the extracted AIF from the bright-blood images in LaBBI is shown in Figure 8. And the average Ktrans and νp of the measured slices for normal vessel wall, FT, NC, IPH and muscle were summarized in Table 2. Similar Ktrans and νp values were observed for the normal vessel wall and the muscle tissue. Compared to the normal vessel wall, NC and IPH had lower Ktrans and νp, while FT showed higher values. The measured SNR of blood in the bright-blood images of the 3 cases was 63.4±3.8, and the SNR of normal vessel wall was 33.5±3.1.
FIG. 8.
The example AIF extracted from the 2D bright-blood images of LaBBI with temporal resolution of 4.7s.
DISCUSSION
In this study, a large coverage black-bright blood interleaved imaging sequence, LaBBI, was proposed for vessel wall DCE-MRI. The proposed sequence could acquire both high temporal resolution bright-blood images and 3D large coverage high spatial resolution black-blood vessel wall images simultaneously. Simulations, phantom studies, and in vivo experiments were performed to demonstrate the feasibility and advantages of LaBBI.
The LaBBI sequence was designed to solve the technical problems in vessel wall DCE-MRI. For vessel wall, the imaging target is small, especially in early lesions, and a submillimeter in-plane spatial resolution, such as 0.6×0.6 mm2 is usually needed (9,10,14,17). The vessel wall lesion may have a large distribution along the diseased artery. Sufficient imaging coverage is important to cover the whole plaque. Vessel wall signal may also be contaminated by luminal blood signal, especially following bolus arrival, making imaging of early lesions or the region near lumen in advanced plaques difficult. Therefore, blood signal should be suppressed in vessel wall DCE imaging (9,14,17). While for AIF acquisition, the blood should not be suppressed, and the high temporal resolution is required for accurate pharmacokinetic analysis of DCE data (11). However, high spatial resolution or large coverage is not needed since AIF can be acquired from one slice of large vessels. In LaBBI scan, a 2D high temporal resolution bright-blood acquisition was interleaved with a 3D large coverage high spatial resolution black-blood acquisition, meeting the different technical requirements of vessel wall imaging and AIF acquisition in vessel wall DCE-MRI.
In the 3D imaging module of LaBBI, a black-blood prepulse, iMSDE (22) was used. Compared with DIR (18) or QIR (19) prepulse, iMSDE has the advantage of large coverage blood suppression, which enables large coverage 3D black-blood imaging in LaBBI. The iMSDE prepulse will introduce T2 weighting into the vessel wall images. Unlike the traditional DCE analysis method, the T2 effect should be considered in the conversion from image signal to contrast concentration, as was done in the simulation and in vivo experiments.
In addition, the 3D imaging module for LaBBI used the SoS golden-angle radial trajectory for acquisition. Utilizing a parallel imaging and compressed sensing combination reconstruction method (21), both high in-plane spatial resolution (0.6×0.6 mm2) and large coverage (30m) of the vessel wall imaging were achieved with a temporal resolution of 4.7s for AIF and 9.4s for the vessel wall. However, compared with traditional Cartesian acquisitions (6) the reconstructed images from the SoS golden-angle radial data may have undersampling artifacts, which may introduce bias into the pharmacokinetic modeling. To address these concerns, simulation experiments considering the interleaved acquisition, T2 effects of iMSDE, SoS golden-angle radial sampling, and reconstruction were carried out. The results showed that the proposed LaBBI sequence had a consistently lower RMSE of estimated kinetic parameters than the conventional bright-blood DCE protocol (6) under all tested conditions. Notably, the simulation experiments did not consider vessel wall signal contamination from lumen in bright-blood DCE, which could further introduce bias into its pharmacokinetic modeling. Therefore, LaBBI, with the ability to acquire high temporal resolution AIF and large coverage high spatial resolution black-blood vessel wall images, has unique advantages for vessel wall DCE-MRI.
A common issue in interleaved black-bright imaging techniques is the difficulty in eliminating the interference of black-blood imaging on the AIF acquisition (15–17,20). In the proposed LaBBI sequence, the non-selective black-blood imaging pulses, iMSDE, will influence the blood signal, and disturb the AIF acquisition. Therefore, a spatial saturation band was used and placed upstream of the 2D imaging slice, eliminating possible interference by inducing saturation recovery of the blood signal. In the phantom test, good agreement of the signal intensities of the 2D SR images acquired by the separate and interleaved scans proved that the interference from iMSDE was successfully eliminated.
In this study, the feasibility of LaBBI was demonstrated on three subjects with carotid atherosclerosis. LaBBI has been shown to successfully evaluate normal thin vessel walls, slightly thickened vessel walls and vessel walls with advanced plaque containing necrotic cores and IPH, with sufficient blood suppression in both pre- and post-contrast images. And the LaBBI vessel wall images can be used to depict vessel wall with good agreement of plaque burden measurements with 3D MERGE. For pharmacokinetic analysis, the normal vessel wall had similar Ktrans and νp values to muscle, which agreed with the published work (39). The fibrous tissue which usually had active inflammation or enriched neovasculature (6) showed higher Ktrans and νp values, while the necrotic core and IPH, where little contrast agent uptake occurred, had low Ktrans and νp values, which echoed the findings of previous studies (37).
Notably, the proposed interleaved acquisition scheme of LaBBI is also flexible for different applications and adjustable for practical imaging demands. For example, to further improve the temporal resolution of AIF, the interleaved scheme can be adjusted by reducing the number of 3D shots between the 2D imaging dynamics, or the spatial resolution of 2D imaging can be decreased when imaging large vessels. To further enlarge the acquisition coverage of LaBBI for intracranial or femoral artery imaging, the 3D imaging orientation can be changed to a coronal or sagittal orientation with proper adjustment to avoid placing the 2D imaging slice and the spatial saturation band on the targeted vessel wall lesion. Furthermore, the 3D imaging module for LaBBI can also be adjusted to acquire 3D isotropic images for curved arteries, such as the intracranial arteries.
There are several limitations in this study. First, the in vivo experiments lacked histological validation due to the unavailability of histological specimens. However, LaBBI is theoretically similar to the previously histologically validated DCE methods (6,7). And the simulation studies demonstrated that LaBBI should have more accurate quantifications than the conventional method benefiting from the higher temporal resolution. Therefore, LaBBI should be able to quantify neovasculature and inflammation of the vessel wall in a similar way to the previously validated work. Second, the assumed pre-contrast T1, T2 values were used in the pharmacokinetic analysis of LaBBI. In spite of the possible bias introduced by the assumed pre-contrast T1 and T2 values, the pharmacokinetic analysis results of the in vivo data had similar findings to previously published work (17,37,39) in regards to normal vessel wall, fibrous tissue, necrotic core and IPH, suggesting the feasibility of LaBBI to characterize perfusion properties of vessel wall lesion. However, in the future study, it’s necessary to add pre-contrast T1, T2 quantification (30,40,41) in vessel wall DCE-MRI. Furthermore, the proposed sequence should be further investigated in future studies with larger study population.
CONCLUSIONS
The proposed LaBBI sequence for DCE-MRI of vessel wall has the ability to acquire 2D high temporal resolution bright-blood images and 3D large coverage high spatial resolution black-blood vessel wall images simultaneously. The results of simulation, phantom, and in vivo experiments suggest that LaBBI is accurate in pharmacokinetic modeling and feasible for in vivo vessel wall imaging. Therefore, the LaBBI sequence may be a better tool for the characterization of vessel wall perfusion.
Supplementary Material
Supporting Figure S1: The static tissue magnetization acquired by the 3D imaging of LaBBI during contrast passage. a: The signal evolution without dummy shot. b: The signal evolution with 3 dummy shots before the acquisition shots.
Supporting Figure S2: Dynamic images before, during, and after bolus arrival of case 3 with vessel wall thickening acquired by LaBBI. a: The slice of thickened wall with red contours outlining the lumen, and blue contours outlining the outer wall. The dynamic vessel wall images of LaBBI in a are shown in the same level/window. b: The dynamic bright-blood images of LaBBI, shown in the same intensity scale. The white arrowheads indicate the artery to extract AIF. One black-blood image frame was interleaved with two bright-blood frames.
Acknowledgments
The authors acknowledge all the patients and healthcare workers who participated in this study. We thank Zachary Miller from University of Washington for his help with English language editing.
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Chalouhi N, Ali MS, Jabbour PM, et al. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab. 2012;32(9):1659–1676. doi: 10.1038/jcbfm.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336(14):973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 4.Tulamo R, Frosen J, Hernesniemi J, Niemela M. Inflammatory changes in the aneurysm wall: a review. J Neurointerv Surg. 2010;2(2):120–130. doi: 10.1136/jnis.2009.002055. [DOI] [PubMed] [Google Scholar]
- 5.Kerwin W, Hooker A, Spilker M, et al. Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation. 2003;107(6):851–856. doi: 10.1161/01.cir.0000048145.52309.31. [DOI] [PubMed] [Google Scholar]
- 6.Kerwin WS, O'Brien KD, Ferguson MS, Polissar N, Hatsukami TS, Yuan C. Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology. 2006;241(2):459–468. doi: 10.1148/radiol.2412051336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerwin WS, Oikawa M, Yuan C, Jarvik GP, Hatsukami TS. MR imaging of adventitial vasa vasorum in carotid atherosclerosis. Magn Reson Med. 2008;59(3):507–514. doi: 10.1002/mrm.21532. [DOI] [PubMed] [Google Scholar]
- 8.Calcagno C, Robson PM, Ramachandran S, et al. SHILO, a novel dual imaging approach for simultaneous HI-/LOw temporal (Low-/Hi-spatial) resolution imaging for vascular dynamic contrast enhanced cardiovascular magnetic resonance: numerical simulations and feasibility in the carotid arteries. J Cardiovasc Magn Reson. 2013;15:42. doi: 10.1186/1532-429X-15-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Ricks J, Rosenfeld M, Kerwin WS. Progression of experimental lesions of atherosclerosis: assessment by kinetic modeling of black-blood dynamic contrast-enhanced MRI. Magn Reson Med. 2013;69(6):1712–1720. doi: 10.1002/mrm.24415. [DOI] [PubMed] [Google Scholar]
- 10.Gaens ME, Backes WH, Rozel S, et al. Dynamic contrast-enhanced MR imaging of carotid atherosclerotic plaque: model selection, reproducibility, and validation. Radiology. 2013;266(1):271–279. doi: 10.1148/radiol.12120499. [DOI] [PubMed] [Google Scholar]
- 11.Evelhoch JL. Key factors in the acquisition of contrast kinetic data for oncology. J Magn Reson Imaging. 1999;10(3):254–259. doi: 10.1002/(sici)1522-2586(199909)10:3<254::aid-jmri5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Dong L, Kerwin WS, Chen H, et al. Carotid Artery Atherosclerosis: Effect of Intensive Lipid Therapy on the Vasa Vasorum-Evaluation by Using Dynamic Contrast-enhanced MR Imaging. Radiology. 2011;260(1):224–231. doi: 10.1148/radiol.11101264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun J, Song Y, Chen H, et al. Adventitial perfusion and intraplaque hemorrhage: a dynamic contrast-enhanced MRI study in the carotid artery. Stroke. 2013;44(4):1031–1036. doi: 10.1161/STROKEAHA.111.000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calcagno C, Vucic E, Mani V, Goldschlager G, Fayad ZA. Reproducibility of black blood dynamic contrast-enhanced magnetic resonance imaging in aortic plaques of atherosclerotic rabbits. J Magn Reson Imaging. 2010;32(1):191–198. doi: 10.1002/jmri.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan Z, Xie J, He Y, et al. Black-blood dynamic contrast-enhanced carotid artery wall MRI with SRDIR preparation. J Cardiovasc Magn Reson. 2013:246. [Google Scholar]
- 16.Zhou Z, Fan Z, Xie Y, Li D, Sharif B. Improved Spatial and Temporal Resolution Black-Blood Dynamic Contrast-Enhanced Carotid Artery Wall MRI Using Compressed Sensing; Proceedings of the 22nd Annual Meeting of ISMRM; 2014. p. 3919. [Google Scholar]
- 17.Wu T, Wang J, Song Y, et al. HOmologous Black-Bright-Blood and Flexible Interleaved Imaging Sequence (HOBBI) for Dynamic Contrast Enhanced MRI of the Vessel Wall. Magn Reson Med. 2015;73(5):1754–1763. doi: 10.1002/mrm.25287. [DOI] [PubMed] [Google Scholar]
- 18.Edelman RR, Chien D, Kim D. Fast Selective Black Blood MR Imaging. Radiology. 1991;181(3):655–660. doi: 10.1148/radiology.181.3.1947077. [DOI] [PubMed] [Google Scholar]
- 19.Yarnykh VL, Yuan C. T1-insensitive flow suppression using quadruple inversion-recovery. Magn Reson Med. 2002;48(5):899–905. doi: 10.1002/mrm.10292. [DOI] [PubMed] [Google Scholar]
- 20.Mendes J, Parker DL, McNally S, DiBella E, Bolster BD, Jr, Treiman GS. Three-dimensional dynamic contrast enhanced imaging of the carotid artery with direct arterial input function measurement. Magn Reson Med. 2014;72(3):816–822. doi: 10.1002/mrm.24993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng L, Grimm R, Block KT, et al. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med. 2014;72(3):707–717. doi: 10.1002/mrm.24980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Yarnykh VL, Yuan C. Enhanced Image Quality in Black-Blood MRI Using the Improved Motion-Sensitized Driven-Equilibrium (iMSDE) Sequence. J Magn Reson Imaging. 2010;31(5):1256–1263. doi: 10.1002/jmri.22149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henningsson M, Mens G, Koken P, Smink J, Botnar RM. A new framework for interleaved scanning in cardiovascular MR: Application to image-based respiratory motion correction in coronary MR angiography. Magn Reson Med. 2015;73(2):692–696. doi: 10.1002/mrm.25149. [DOI] [PubMed] [Google Scholar]
- 24.Yang M, Qi H, Zhang S, et al. A noval method of correcting off-center errors for radial acquisition with arbitrary angle; Proceedings of the 23rd Annual Meeting of ISMRM; 2015. p. 3717. [Google Scholar]
- 25.Jaspers K, Aerts HJ, Leiner T, et al. Reliability of pharmacokinetic parameters: small vs. medium-sized contrast agents. Magn Reson Med. 2009;62(3):779–787. doi: 10.1002/mrm.22035. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki M, Shibata E, Kanbara Y, Ehara S. Enhancement effects and relaxivities of gadolinium-DTPA at 1.5 versus 3 Tesla: a phantom study. Magn Reson Med Sci. 2005;4(3):145–149. doi: 10.2463/mrms.4.145. [DOI] [PubMed] [Google Scholar]
- 27.Lu H, Clingman C, Golay X, van Zijl PC. Determining the longitudinal relaxation time (T-1) of blood at 3.0 tesla. Magnet Reson Med. 2004;52(3):679–682. doi: 10.1002/mrm.20178. [DOI] [PubMed] [Google Scholar]
- 28.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical Evaluation of Blood-to-Brain Transfer Constants from Multiple-Time Uptake Data. J Cereb Blood Flow Metab. 1983;3(1):1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- 29.Koay CG, Sarlls JE, Ozarslan E. Three-dimensional analytical magnetic resonance imaging phantom in the Fourier domain. Magn Reson Med. 2007;58(2):430–436. doi: 10.1002/mrm.21292. [DOI] [PubMed] [Google Scholar]
- 30.Biasiolli L, Lindsay AC, Chai JT, Choudhury RP, Robson MD. In-vivo quantitative T2 mapping of carotid arteries in atherosclerotic patients: segmentation and T2 measurement of plaque components. J Cardiovasc Magn Reson. 2013;15:69. doi: 10.1186/1532-429X-15-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Li R, Hayes C, Balu N, Zhao X, Yuan C. A New Designed 36-Channel Neurovascular Coil at 3T; Proceedings of the 20th Annual Meeting of ISMRM; 2012. p. 2787. [Google Scholar]
- 32.Balu N, Yarnykh VL, Chu B, Wang J, Hatsukami T, Yuan C. Carotid Plaque Assessment Using Fast 3D Isotropic Resolution Black-Blood MRI. Magn Reson Med. 2011;65(3):627–637. doi: 10.1002/mrm.22642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JN, Bornert P, Zhao HL, et al. Simultaneous noncontrast angiography and intraPlaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magn Reson Med. 2013;69(2):337–345. doi: 10.1002/mrm.24254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerwin W, Xu D, Liu F, et al. Magnetic resonance imaging of carotid atherosclerosis: plaque analysis. Top Magn Reson Imaging. 2007;18(5):371–378. doi: 10.1097/rmr.0b013e3181598d9d. [DOI] [PubMed] [Google Scholar]
- 35.Takaya N, Cai JM, Ferguson MS, et al. Intra- and interreader reproducibility of magnetic resonance imaging for quantifying the lipid-rich necrotic core is improved with gadolinium contrast enhancement. J Magn Reson Imaging. 2006;24(1):203–210. doi: 10.1002/jmri.20599. [DOI] [PubMed] [Google Scholar]
- 36.Balu N, Sun J, Liu J, Chen S, Chen H, Yuan C. Assessment of Calcification Size and Juxtaluminal Status Using Gray-Blood 3D Vessel Wall MRI; Proceedings of the 23rd Annual Meeting of ISMRM; 2015. p. 2652. [Google Scholar]
- 37.Chen H, Cai J, Zhao X, et al. Localized measurement of atherosclerotic plaque inflammatory burden with dynamic contrast-enhanced MRI. Magn Reson Med. 2010;64(2):567–573. doi: 10.1002/mrm.22369. [DOI] [PubMed] [Google Scholar]
- 38.Zhu DC, Ferguson MS, DeMarco JK. An optimized 3D inversion recovery prepared fast spoiled gradient recalled sequence for carotid plaque hemorrhage imaging at 3.0 T. Magn Reson Imaging. 2008;26(10):1360–1366. doi: 10.1016/j.mri.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Jaspers K, Leiner T, Dijkstra P, et al. Optimized pharmacokinetic modeling for the detection of perfusion differences in skeletal muscle with DCE-MRI: effect of contrast agent size. Med Phys. 2010;37(11):5746–5755. doi: 10.1118/1.3484057. [DOI] [PubMed] [Google Scholar]
- 40.Chai JT, Biasiolli L, Li L, et al. Quantification of Lipid-Rich Core in Carotid Atherosclerosis Using Magnetic Resonance T2 Mapping: Relation to Clinical Presentation. JACC Cardiovasc Imaging. 2016 doi: 10.1016/j.jcmg.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coolen BF, Poot DH, Liem MI, et al. Three-dimensional quantitative T1 and T2 mapping of the carotid artery: Sequence design and in vivo feasibility. Magn Reson Med. 2016;75(3):1008–1017. doi: 10.1002/mrm.25634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure S1: The static tissue magnetization acquired by the 3D imaging of LaBBI during contrast passage. a: The signal evolution without dummy shot. b: The signal evolution with 3 dummy shots before the acquisition shots.
Supporting Figure S2: Dynamic images before, during, and after bolus arrival of case 3 with vessel wall thickening acquired by LaBBI. a: The slice of thickened wall with red contours outlining the lumen, and blue contours outlining the outer wall. The dynamic vessel wall images of LaBBI in a are shown in the same level/window. b: The dynamic bright-blood images of LaBBI, shown in the same intensity scale. The white arrowheads indicate the artery to extract AIF. One black-blood image frame was interleaved with two bright-blood frames.