Abstract
Background
Multiple clinical practice guidelines recommend rapid evaluation of patients with suspected lung cancer. It is uncertain whether delays in diagnosis and management have a negative effect on outcomes.
Methods
This retrospective study included 551 patients diagnosed with lung cancer through the diagnostic assessment program at the Institut universitaire de cardiologie et de pneumologie de Québec between September 2013 and March 2015. Median wait times between initial referral, diagnosis, and first treatment were calculated and compared with recommended targets. Analyses were performed to evaluate for specific factors associated with longer wait times and for the effect of delays on the outcomes of progression-free survival (pfs), relapse-free survival (rfs) after primary surgical resection, and overall survival (os).
Results
Most patients were investigated and treated within recommended targets. Of the entire cohort, 379 patients were treated at our institution. Of those 379 patients, 311 (82%) were treated within recommended targets. In comparing patients within and outside target times, the only statistically significant difference was found in the distribution of treatment modalities: patients meeting targets were more likely to be treated with surgery or chemotherapy rather than with radiation. The pfs on first treatment modality was influenced by clinical stage, but not by time to therapy [hazard ratio (hr): 1.10; p = 0.65]. The os for the entire cohort was also influenced by stage, but not by delays (hr: 1.04; p = 0.87). For the 209 patients treated by surgery with curative intent, a significant reduction in rfs was associated with male sex and TNM stage, but not with delays (hr: 1.11; p = 0.83). The os after primary surgical resection was also associated with TNM stage, but not with delays (hr: 1.82; p = 0.43).
Conclusions
Recommended targets for wait times in the investigation and treatment of lung cancer can be achieved within a diagnostic assessment program. Compared with radiation treatment, treatment with surgery or chemotherapy is more likely to be completed within targets. Delays in investigation and treatment do not appear to negatively affect the clinical outcomes of os, rfs, and pfs. Prospective studies are needed to evaluate whether efficient work-up and treatment influence other important variables, such as quality of life, cost of care, and access to therapies while performance status is adequate.
Keywords: Lung cancer, wait times, diagnosis, treatment
INTRODUCTION
Lung cancer is the most frequently diagnosed cancer worldwide and the leading cause of cancer-related mortality1. Multiple clinical practice guidelines recommend rapid evaluation, diagnosis, and treatment of patients with suspected lung cancer2–5. The goal is to maximize cure rates for patients with early-stage disease and to rapidly palliate symptoms, improve quality of life, and extend survival in patients with advanced disease.
Cancer Care Ontario guidelines recommend that patients with an abnormal chest radiograph or a high suspicion of lung cancer based on clinical judgment undergo chest computed tomography (ct) within 2 weeks2. Patients referred to a specialist or a diagnostic assessment program (dap) should expect a consultation within 2 weeks. According to the British Thoracic Society, when confirming the diagnosis, pathology results should be available within 2 weeks of the relevant procedure3. Epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) rearrangement testing should be ordered at the time of diagnosis for patients with metastatic disease and should be available within 2 weeks of receiving the specimen in the laboratory4. In patients deemed to be candidates for resection, a maximum of 8 weeks should elapse between the first consultation with a respiratory physician and the surgery, and the surgery should take place within 4 weeks of acceptance on a surgeon’s waiting list3. If needed, adjuvant chemotherapy should be started within 120 days of surgery. When radiotherapy is required, patients should be evaluated by a radiation oncologist within 1 week of referral and should start treatment within 4 weeks if the intent is curative and within 2 weeks if the intent is palliative3. Stage iii patients treated with chemoradiation should begin radiotherapy and platinum-based chemotherapy within 180 days of diagnosis5. Chemotherapy should start within 7 working days of the decision to treat3.
Adherence to the foregoing recommendations has been shown to be relatively poor in multiple countries6–9. However, the effect of timely investigation and treatment for lung cancer on progression-free survival (pfs), relapse-free survival (rfs) after primary surgical resection, and overall survival (os) is unclear10,11. Delays in patient flow through diagnostic imaging and biopsy, and prolonged times from diagnosis to radical radiotherapy have been reported by some authors to result in an increase in tumour size and stage12,13. Others have reported no association between time to diagnosis or treatment and clinical outcomes14,15. Some studies have demonstrated that shorter delays are associated with shorter survival, perhaps reflecting expedited investigation and treatment of advanced disease and greater numbers of symptomatic cases16–18. In contrast, other reports showed that longer time to treatment was a significant negative prognostic factor in patients with stage iii disease and an os of 5 or more years19 and in those with stage ii disease undergoing surgical resection20. Almost all those studies were retrospective and had small sample sizes. A recent review of 693,554 patients diagnosed with non-small-cell lung cancer in United States from 2003 to 2011 showed that shorter wait times were associated with a decreased risk of death in early-stage patients, but poorer survival in patients with advanced disease21.
The aims of the present study were to
-
■
evaluate wait times for diagnosis and treatment of lung cancer in a public hospital,
-
■
determine whether recommended targets were met,
-
■
evaluate whether specific factors were associated with longer wait times, and
-
■
assess the influence on outcomes (pfs, rfs, os) of patient characteristics and of delays.
METHODS
Clinical Setting and Patients
At the Institut universitaire de cardiologie et de pneumologie de Québec, a public academic hospital, a dap was implemented in 2008. Referrals are received from family physicians or other centres in the eastern Quebec area, for a population of approximately 2.3 million. All referrals are triaged by a respirologist within 1 working day, and necessary tests are prioritized and organized quickly by a nurse navigator with access to dedicated investigation booking slots. Almost all patients undergo chest ct imaging before referral or before the first consultation. If needed, most patients will also undergo positron-emission tomography before the consultation appointment. Most patients undergo same-day bloodwork and pulmonary function tests, plus bronchoscopy or endobronchial ultrasonography (or both) if required. Once the histologic diagnosis and disease staging are obtained, the patient is seen again by a respirologist with specific expertise in oncology to discuss treatment options. Referrals to thoracic surgery or radiation oncology are then expedited, and chemotherapy is started or the patient is sent back to the referring centre for treatment.
For the present study, we retrospectively reviewed medical records of all patients who were referred to the dap at the Institut universitaire de cardiologie et de pneumologie de Québec between 22 September 2013 and 7 March 2015. The study was approved by our institutional review board.
Data Extraction
We searched patient medical records for the following general information: age, sex, smoking status, reason for referral, travelling distance between the patient’s home and the hospital, histology, disease stage, EGFR and ALK results, date of initial referral, dates of all consultations, dates of all investigations, discussion (or not) at a tumour board review, and treatments. Staging followed the 7th edition of the TNM classification of malignant tumours22 and was based on the most definitive data available, surgical staging being preferentially used over clinical staging.
Because tests and procedures for most patients were performed before the initial consultation with the respirologist, the date of the first appointment was defined as either the date of the first test or the date of the first consultation (whichever occurred first). The date of diagnosis was defined as the date of release of the final pathology report.
Outcomes included wait times for investigation and treatment, pfs on first treatment modality, rfs after primary surgical resection, and os. The pfs and rfs were measured, respectively, from the time of diagnosis or primary surgical resection to the first date of documented objective progressive disease or death as a result of any cause. The os was measured from the time of diagnosis to the date of death or date the patient was last seen.
Statistical Analysis
Results are presented as means ± standard deviation for continuous variables and proportions for categorical variables. We used chi-square or Fisher exact tests to compare proportions and the one-way analysis of variance to compare continuous variables. Normality of distribution was first assessed with the Shapiro–Wilk test. When the assumption of normality was not met, we first attempted to transform the data. We used the Wilcoxon rank-sum test when no transformation could satisfy normality. Cox proportional hazards regression analyses were performed to model event-free periods (that is, time to death or waiting time). In univariate analyses, baseline characteristics were investigated to identify prognostic factors that might explain time to death or time from referral to first treatment. Only variables with a p value less than 0.20 were retained for inclusion in multivariate regression analyses, where the selection of variables was conducted using a forward approach. The results were considered significant when p values were less than 0.05.
RESULTS
Patients
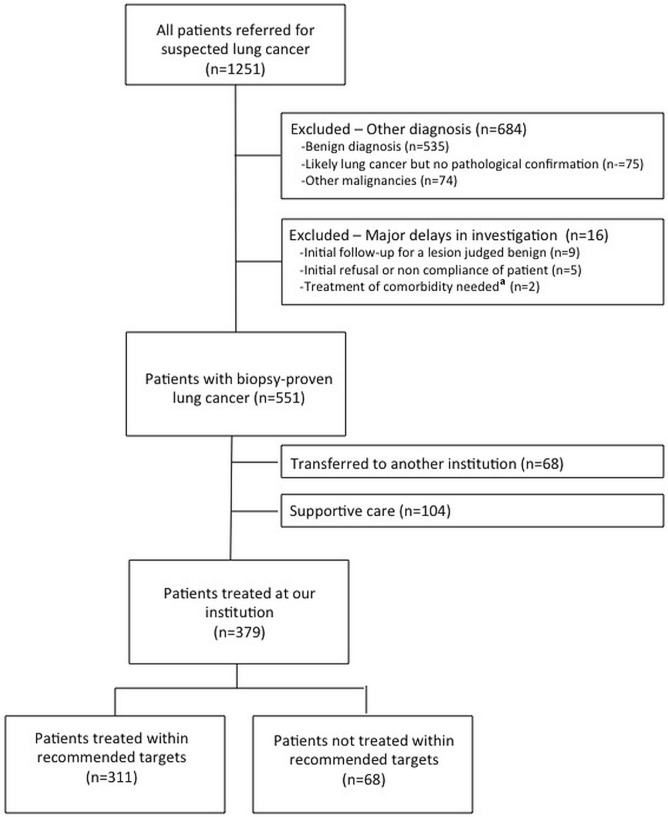
We reviewed the medical records of 1251 consecutive patients who were referred to our dap between September 2013 and March 2015 (Figure 1). After excluding patients with a final diagnosis other than lung cancer and those with major delays in investigation attributable to poor cooperation or concurrent unstable medical conditions, 551 patients with a biopsy-proven diagnosis of lung cancer were included in the analysis. Of those 551 patients, 68 were transferred back to their referring institution for treatment, and 104 were treated with supportive care [43 refused treatment, 15 had a poor performance status (ps), 20 had a rapidly deteriorating ps or died, 20 had comorbidities, and 6 had asymptomatic and indolent multifocal adenocarcinoma]. We therefore analyzed the effect of treatment delays for the remaining 379 patients who were treated at our institution.
FIGURE 1.
Patient flow diagram. aSurgery for severe aortic stenosis needed before thoracic surgery (n = 1), and surgery for a colorectal cancer needed before stereotactic body radiation therapy (n = 1).
Table i shows patient characteristics, diagnostic procedures, and first treatment received. Molecular testing was obtained for 172 patients with metastatic adenocarcinoma; of those 172 patients, 13 (8%) had EGFR-mutated tumours, and 5 (3%) carried ALK rearrangements.
TABLE I.
Patient characteristics
| Characteristic | Patients with biopsy-proven lung cancer | Treated within targets? (of 379 curatively treated) | p Value | |
|---|---|---|---|---|
|
| ||||
| Yes | No | |||
| Patients [n (%)] | 551 | 311 (82) | 68 (18) | |
| Mean age (years) | 66.3±8.6 | 65.3±8.0 | 66.3±8.8 | 0.38 |
| Sex [n (%) men] | 289 (53) | 168 (54) | 34 (50) | 0.59 |
| Previous or current smokers [n (%)] | 519 (94) | 292 (94) | 64 (94) | 1.00 |
| Symptomatic at presentation [n (%)] | 271 (49) | 133 (43) | 25 (37) | 0.42 |
| Hospital distance (km) | ||||
| Median | 48 | 23 | 30 | 0.66 |
| IQR | 14, 119 | 13, 102 | 14, 111 | |
| Histology [n (%)] | ||||
| Adenocarcinoma | 353 (64) | 214 (69) | 42 (62) | 0.47 |
| Neuroendocrine tumour | 53 (10) | 24 (8) | 7 (10) | |
| Othersa | 145 (26) | 73 (23) | 19 (28) | |
| TNM stage [n (%)] | ||||
| I | 180 (32) | 134 (43) | 31 (46) | 0.18 |
| II | 58 (11) | 36 (12) | 13 (19) | |
| III | 121 (22) | 68 (22) | 14 (20) | |
| IV | 192 (35) | 73 (23) | 10 (15) | |
| Investigations per patientb (n) | ||||
| Median | 4 | 4 | 4 | 0.15 |
| IQR | 3, 5 | 3, 5 | 4, 5 | |
| Tumour board review [n (%)] | 56 (10) | 33 (11) | 13 (19) | 0.06 |
| Final diagnostic procedure [n (%)] | ||||
| Flexible bronchoscopy | 124 (22) | 63 (20) | 9 (13) | 0.49 |
| EBUS or EUS | 153 (28) | 75 (24) | 20 (29) | |
| Transthoracic needle biopsy | 125 (22) | 74 (24) | 23 (34) | |
| Thoracoscopy | 81 (15) | 69 (22) | 12 (18) | |
| Biopsy of metastatic site | 31 (6) | 11 (4) | 1 (1) | |
| Lymph node biopsy | 21 (4) | 8 (3) | 2 (3) | |
| Thoracentesis | 14 (3) | 9 (3) | 1(1) | |
| Mediastinoscopy | 2 (<1) | 0 | 2 (1) | |
| First treatment | ||||
| Surgery | 210 (38) | 180 (58) | 30 (44) | <0.0001 |
| Neoadjuvant chemoradiation | 2 (<1) | 1 (<1) | 1 (1) | |
| Definitive chemoradiation | 48 (9) | 39 (13) | 9 (13) | |
| Curative radiation (conventional or SBRT) | 29 (5) | 13 (4) | 16 (24) | |
| Palliative chemotherapy | 67 (12) | 59 (19) | 8 (12) | |
| Palliative radiation | 68 (12) | 19 (6) | 4 (6) | |
| Supportive care or transfer to another institution | 127 (23) | 0 | 0 | |
| Median wait time (days) | ||||
| From referral to diagnosis | 21 | 25 | 28.5 | 0.24 |
| IQR | 13, 37 | 14, 44 | 16, 49 | |
| From referral to first treatment | 56 | 55 | 77 | <0.0001 |
| IQR | 34, 81 | 36, 78 | 56, 97 | |
| From referral to surgery | 70 | 66 | 91 | <0.0001 |
| IQR | 51, 91 | 49, 84 | 74, 116 | |
Squamous cell carcinoma, adenosquamous carcinoma, unclassifiable non-small-cell lung cancer, other rare histologies.
Computed tomography imaging, position-emission tomography, bone scan, cerebral imaging, abdominal ultrasonography, flexible bronchoscopy, endobronchial ultrasonography, endoscopic ultrasonography, transthoracic biopsy, thoracentesis, lymph node biopsy, biopsy of metastatic site, mediastinoscopy.
IQR = interquartile range; EBUS = endobronchial ultrasonography; EUS = endoscopic ultrasonography; SBRT = stereotactic body radiation therapy.
Wait Times
Table i also shows the median times from referral to diagnosis (21 days), to first treatment (56 days), and to surgery (70 days). Overall, when considering treatment for which the intention was to increase pfs or os (surgery, definitive radiation, definitive chemoradiation, and palliative chemotherapy), 311 of the 379 patients (82%) started their first treatment within recommended targets. In comparing the characteristics of patients who did and did not receive their first treatment within targets, the only difference was treatment modality. Patients meeting targets were more likely to be treated with surgery (58% vs. 44%) or palliative chemotherapy (19% vs. 12%) rather than with curative radiation (4% vs. 24%, p < 0.0001). Notably, before January 2014, patients with early-stage lung cancer who were unfit for surgery had to be referred to another institution in Montreal for stereotactic body radiation therapy, which might explain some of the delay. In the group not meeting targets, there was also a trend for more patients to be discussed at tumour board reviews (19% vs. 11%, p = 0.06). Table ii presents other median wait times for investigation and treatment.
TABLE II.
Median wait times for investigation and treatment
| Investigation or treatment interval | Pts (n) | Recommended target (days) | Measured wait [median (IQR) days] | Patients within target [n (%)] |
|---|---|---|---|---|
| Investigation | 551 | |||
| Referral to first appointment | 551 | 14 | 6 (4, 10) | 461 (84) |
| Referral to chest computed tomography imaging | 189a | 14 | 5 (1, 7) | 174 (92) |
| Biopsy to pathology result | 551 | 14 | 3 (2, 4) | 550 (99.8) |
| Request for EGFR and ALK testing to result | 172 | 14 | 5 (3, 6) | 169 (98) |
| Treatment | 379 | |||
| Respirology consultation to time of surgery | 210 | 56 | 59 (41, 80) | 100 (48) |
| Operative decision to time of surgery | 210 | 28 | 8 (2, 21) | 180 (86) |
| Surgery to commencing adjuvant chemotherapy | 42 | 120 | 44 (36, 55) | 41 (98) |
| RO referral to RO consultation | 211 | 7 | 4 (2, 7) | 166 (79) |
| RO referral to commencing definitive radiation | 65b | 28 | 26 (22, 34) | 41 (63) |
| Diagnosis to commencing definitive chemoradiation | 48 | 180 | 27 (13, 34) | 48 (100) |
| RO referral to commencing palliative radiation | 96 | 14 | 8 (6, 16) | 69 (72) |
| Decision for chemotherapy to commencing chemotherapy | 117c | 7 wd | 4 wd (2, 6) | 103 (88) |
Most patients had undergone chest computed tomography imaging before referral.
Includes stereotactic body radiation therapy, radical external-beam radiation, and concurrent chemoradiation.
Includes palliative chemotherapy and sequential chemoradiation (radiation planning was usually the limiting factor for concurrent chemoradiation).
Pts = patients; IQR = interquartile range; RO = radiation oncology; wd = working days.
Outcomes
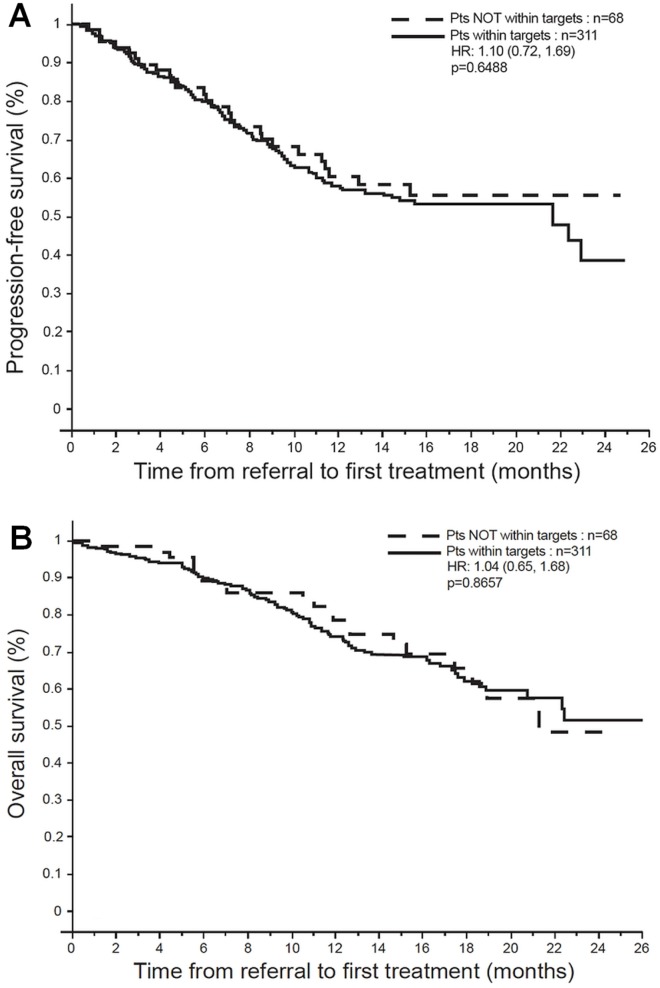
In the multivariable analyses for the overall cohort, pfs on first treatment modality was influenced only by TNM stage [hazard ratios (hrs), with stage i as referent: 1.9 for stage ii, 6.3 for stage iii, and 17.4 for stage iv; p < 0.0001). However, no statistical difference was evident for patients treated within or outside targets [hr: 1.10; p = 0.65; Figure 2(A)]. The os for the overall cohort was also affected by TNM stage (hrs: 1.7 for stage ii, 4.7 for stage iii, and 10.3 for stage iv; p < 0.0001), but not by delays [hr: 1.04; p = 0.87; Figure 2(B)].
FIGURE 2.
Outcomes for the entire cohort. (A) Progression-free survival on first treatment modality. (B) Overall survival. Pts = patients; HR = hazard ratio (with confidence limits).
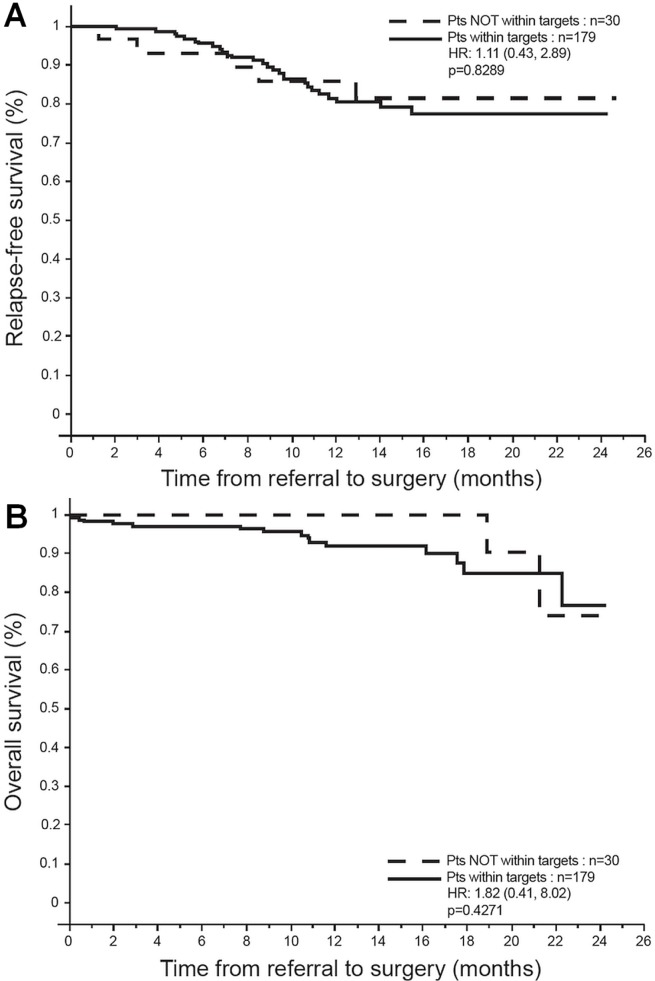
After 1 patient who underwent palliative surgery for stage iv disease was excluded, 209 patients were treated by primary surgical resection with curative intent. Female sex (hr: 0.31; p = 0.007) and TNM stage (hrs: 2.3 for stage ii, 5.5 for stage iii; p = 0.001) influenced rfs, but time to surgery did not [hr: 1.11; p = 0.83; Figure 3(A)]. The os for surgical patients was also influenced by TNM stage (hr: 4.8 for stage iii; p = 0.005), but not by wait time [hr: 1.82; p = 0.43; Figure 3(B)].
FIGURE 3.
Outcomes after primary surgical resection. (A) Relapse-free survival. (B) Overall survival. Pts = patients; HR = hazard ratio (with confidence limits).
DISCUSSION
The present retrospective study shows that recommended targets for wait times in the investigation and treatment of lung cancer can be achieved in a rapid dap within a public health care system. Median times from referral to diagnosis, to first treatment, and to surgery were, respectively, 21, 56, and 70 days. The only factor associated with longer wait times was treatment modality (radiation as opposed to surgery and chemotherapy), although there was also a trend toward longer waits if the case was discussed at a tumour board review.
The observed delay before radiation start can be partly explained (as previously mentioned) by the fact that stereotactic body radiation therapy was available only at another institution during part of the study.
We feel that the delay associated with tumour board review, which takes place once per week at our institution, is a reflection of those cases being more complicated and requiring more tests and referrals to multiple specialties.
As expected, TNM stage and sex had a prognostic influence. However, in this particular cohort, delays in investigation and treatment did not appear to negatively affect the clinical outcomes of os, rfs, and pfs.
Many societies recommend rapid evaluation, diagnosis, and treatment of patients with suspected lung cancer2–5, with the rationale that earlier diagnosis and treatment lead to improved prognosis. However, the targets recommended in the clinical practice guidelines that we used in the present study are based on expert opinion. Those targets are arbitrary and might need to be revisited in large cohorts of patients, given that the issue of optimal targets cannot, for obvious reasons, be addressed in randomized trials.
In our study, the difference in median time from referral to first treatment for patients meeting and not meeting targets was small (55 days vs. 77 days). That small difference might explain the inability of the present work to identify an effect of wait time on clinical outcomes. In patients with lung cancer, os might be so uniformly poor that a small effect cannot be detected. In our study, 57% of patients had locally advanced or metastatic disease at presentation. Long management delays are most frequently found in less-symptomatic patients, and hence potentially carry a better prognosis; in contrast, patients with more advanced disease often have more severe symptoms that require expedited investigation and earlier initiation of treatment23. Time to treatment might become a more important factor for patients with more rapidly growing tumours or with longer survival times.
The goal of rapid investigation and treatment would be to maximize cure rates for patients with early-stage disease, to increase the number of patients with resectable disease, and to avoid tumour growth and upstaging. Our data did not allow us to evaluate whether patients presenting with curable disease became incurable because of delays in investigation. Nevertheless, even if our wait times were acceptable, 20 of the 551 patients included in the study (4%), mainly having stage iv disease, experienced ps deterioration or death before being able to start treatment. Also, we calculated delay based on referral by the family physician, and not from the start of symptoms or the first abnormal imaging. Hence, we did not capture an important part of the patient pathway that might have had an influence on outcome.
In our population, 57% of patients had locally advanced or metastatic disease at presentation. After excluding 3 patients who were treated aggressively with surgery or curative chemoradiation (or both) for oligometastatic disease from among the 152 patients with stage iv disease who were not referred back to their institution, we observed that first-line palliative chemotherapy was administered to 81 of the remaining 149 patients (54%). Comparably, earlier studies have reported that up to 32% of patients with metastatic non-small-cell lung cancer could not receive treatment because of rapidly deteriorating ps24 and had hypothesized that efficient work-up could lead to increased uptake of treatment25.
Although our study did not show a significant effect of shorter time to treatment on clinical outcomes, we still believe that shorter wait times have a positive effect with respect to patient anxiety, mental well-being, satisfaction, physical functioning, quality of life, and cost of care. The psychological stress while under investigation is considerable. Shorter work-up times are also especially important for patients being investigated for a suspicion of lung cancer who turn out to have a benign disease—which was the case for 535 of the 1251 patients identified in our study (43%).
Screening for lung cancer using low-dose ct imaging has been proved to lessen mortality26 and is recommended in high-risk patients in many countries, including Canada27, where it has yet to be widely implemented. Consequently, patients in our trial were referred mainly by family physicians because of suspicious signs or symptoms (49% of our cohort) or because of abnormal radiography or ct findings discovered incidentally. If ct-based screening were to be instituted, the number of patients requiring further investigation could expand substantially. However, asymptomatic patients presenting with early disease likely represent a completely different population. In such circumstances, wait time might have a different prognostic effect than it does in symptomatic patients.
Our study has limitations. Its design as a single-centre retrospective cohort study explains why some clinical data, such as ps and comorbidities, were not available. Data about patient quality of life and satisfaction are also lacking. Although our sample was larger than is seen in most other similar studies, it is still relatively small. We were not able to identify all the variables that could have led to increased wait times, such as patient choice, access to transportation, or repeated biopsies because of inadequate samples. We also excluded patients with unconfirmed pathology diagnoses and patients who were not treated. The latter population would likely have a poor ps, multiple comorbidities, and poorer outcomes. Furthermore, 68 of the 551 included patients (12%) were transferred back to their referring centre and were lost to follow-up, so that we could not assess the effects of rapid access and standardized care from a population level. Finally, we did not separately analyze patients with non-small-cell lung cancer and small-cell lung cancer (8% of the total cohort), even if, for the latter population, wait times are likely to be shorter and their prognosis, poorer.
CONCLUSIONS
Our study shows that adherence to guidelines for recommended wait times in the investigation and treatment of lung cancer is feasible with the implementation of a dap. Compared with patients undergoing radiation, those undergoing surgery or palliative chemotherapy were more likely to be treated within targets. We were not able to demonstrate an effect of shorter delays on pfs, rfs after primary surgical resection, or os. Nevertheless, reducing wait times should continue to be a goal, because shorter waits might influence other important variables such as quality of life, cost of care, access to therapies while ps is still adequate, and access to targeted care with timely biomarker testing. Prospective studies are needed to answer those questions.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012 v1.0. Lyon, France: International Agency for Research on Cancer; 2013. iarc CancerBase No. 11. [Google Scholar]
- 2.Del Giudice ME, Young SM, Vella ET, et al. Guideline for referral of patients with suspected lung cancer by family physicians and other primary care providers. Can Fam Physician. 2014;60:711–16. e376–82. [PMC free article] [PubMed] [Google Scholar]
- 3.bts recommendations to respiratory physicians for organising the care of patients with lung cancer. The Lung Cancer Working Party of the British Thoracic Society Standards of Care Committee. Thorax. 1998;53(suppl 1):S1–8. doi: 10.1136/thx.53.suppl_1.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guideline. J Clin Oncol. 2014;32:3673–9. doi: 10.1200/JCO.2014.57.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Quality Council of Ontario (cqco). Treating NSC Lung Cancer According to Guidelines [Web page]. Toronto, ON: CQCO; n.d.. [Available at: http://www.csqi.on.ca/cms/one.aspx?portalId=351209&pageId=354778; cited 3 November 2017] [Google Scholar]
- 6.Rolke HB, Bakke PS, Gallefoss F. Delays in the diagnostic pathways for primary pulmonary carcinoma in southern Norway. Respir Med. 2007;101:1251–7. doi: 10.1016/j.rmed.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Buccheri G, Ferrigno D. Lung cancer: clinical presentation and specialist referral time. Eur Respir J. 2004;24:898–904. doi: 10.1183/09031936.04.00113603. [DOI] [PubMed] [Google Scholar]
- 8.Koyi H, Hillerdal G, Branden E. Patient’s and doctors’ delays in the diagnosis of chest tumors. Lung Cancer. 2002;35:53–7. doi: 10.1016/S0169-5002(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 9.Vidaver RM, Shershneva MB, Hetzel SJ, Holden TR, Campbell TC. Typical time to treatment of patients with lung cancer in a multisite, US-based study. J Oncol Pract. 2016;12:e643–53. doi: 10.1200/JOP.2015.009605. [DOI] [PubMed] [Google Scholar]
- 10.Olsson JK, Schultz EM, Gould MK. Timeliness of care in patients with lung cancer: a systematic review. Thorax. 2009;64:749–56. doi: 10.1136/thx.2008.109330. [DOI] [PubMed] [Google Scholar]
- 11.Jensen AR, Mainz J, Overgaard J. Impact of delay on diagnosis and treatment of primary lung cancer. Acta Oncol. 2002;41:147–52. doi: 10.1080/028418602753669517. [DOI] [PubMed] [Google Scholar]
- 12.Byrne SC, Barrett B, Bhatia R. The impact of diagnostic imaging wait times on the prognosis of lung cancer. Can Assoc Radiol J. 2015;66:53–7. doi: 10.1016/j.carj.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 13.O’Rourke N, Edwards R. Lung cancer treatment waiting times and tumour growth. Clin Oncol (R Coll Radiol) 2000;12:141–4. doi: 10.1053/clon.2000.9139. [DOI] [PubMed] [Google Scholar]
- 14.Yorio JT, Xie Y, Yan J, Gerber DE. Lung cancer diagnostic and treatment intervals in the United States: a health care disparity? J Thorac Oncol. 2009;4:1322–30. doi: 10.1097/JTO.0b013e3181bbb130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aragoneses FG, Moreno N, Leon P, Fontan EG, Folque E, on behalf of the Bronchogenic Carcinoma Cooperative Group of the Spanish Society of Pneumology and Thoracic Surgery. Influence of delays on survival in the surgical treatment of bronchogenic carcinoma. Lung Cancer. 2002;36:59–63. doi: 10.1016/S0169-5002(01)00458-5. [DOI] [PubMed] [Google Scholar]
- 16.Salomaa ER, Sallinen S, Hiekkanen H, Liippo K. Delays in the diagnosis and treatment of lung cancer. Chest. 2005;128:2282–8. doi: 10.1378/chest.128.4.2282. [DOI] [PubMed] [Google Scholar]
- 17.Myrdal G, Lambe M, Hillerdal G, Lamberg K, Agustsson T, Stahle E. Effect of delays on prognosis in patients with non–small cell lung cancer. Thorax. 2004;59:45–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Bozcuk H, Martin C. Does treatment delay affect survival in non-small cell lung cancer? A retrospective analysis from a single UK centre. Lung Cancer. 2001;34:243–52. doi: 10.1016/S0169-5002(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Correa CR, Hayman JA, et al. Time to treatment in patients with stage iii non–small cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;74:790–5. doi: 10.1016/j.ijrobp.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coughlin S, Plourde M, Guidolin K, et al. Is it safe to wait? The effect of surgical wait time on survival in patients with non-small cell lung cancer. Can J Surg. 2015;58:414–18. doi: 10.1503/cjs.007015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anggondowati T, Ganti AK, Watanabe-Galloway S, Haynatzki G, Islam KM. Effect of time to treatment on survival in non–small cell lung cancer [abstract 8542]. J Clin Oncol. 2016;34 [Available online at: https://meetinglibrary.asco.org/record/126383/abstract; cited 22 October 2017] [Google Scholar]
- 22.Goldstraw P, Crowley J, Chansky K, et al. on behalf of the International Association for the Study of Lung Cancer International Staging Committee. The iaslc lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [Erratum in: J Thorac Oncol 2007;2:985] [DOI] [PubMed] [Google Scholar]
- 23.Vinas F, Ben Hassen I, Jabot L, Monnet I, Chouaid C. Delays for diagnosis and treatment of lung cancers: a systematic review. Clin Respir J. 2016;10:267–71. doi: 10.1111/crj.12217. [DOI] [PubMed] [Google Scholar]
- 24.Tabchi S, Kassouf E, Florescu M, et al. Factors influencing treatment selection and survival in advanced lung cancer. Curr Oncol. 2017;24:e115–22. doi: 10.3747/co.24.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brule SY, Al-Baimani K, Jonker H, et al. Palliative systemic therapy for advanced non–small cell lung cancer: investigating disparities between patients who are treated versus those who are not. Lung Cancer. 2016;97:15–21. doi: 10.1016/j.lungcan.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Aberle DR, Adams AM, Berg CD, et al. on behalf of the National Lung Screening Trial research team Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewin G, Morissette K, Dickinson J, et al. on behalf of the Canadian Task Force on Preventive Health Care Recommendations on screening for lung cancer. CMAJ. 2016;188:425–32. doi: 10.1503/cmaj.151421. [DOI] [PMC free article] [PubMed] [Google Scholar]