Abstract
Background
Resource limitations affect the intensity of speech–language pathology (slp) dysphagia interventions for patients with head-and-neck cancer (hnc). The objective of the present study was to assess the feasibility of a prospective clinical trial that would evaluate the effects on health and patient costs of early slp dysphagia intervention for hnc patients planned for curative concurrent chemoradiotherapy (ccrt).
Methods
Patients with hnc planned for curative ccrt were consecutively recruited and received dysphagia-specific intervention before, during, and for 3 months after treatment. Swallowing function, body mass index, health-related quality of life (qol), and out-of-pocket costs were measured before ccrt, at weeks 2 and 5 during ccrt, and at 1 and 3 months after ccrt. Actuarial percutaneous endoscopic gastrostomy (peg) removal rates and body mass index in the study patients and in a time-, age-, and disease-matched cohort were compared.
Results
The study enrolled 21 patients (mean age: 54 years; 19 men). The study was feasible, having a 95% accrual rate, 10% attrition, and near completion of all outcomes. Compared with the control cohort, patients receiving dysphagia intervention trended toward a higher rate of peg removal at 3 months after ccrt [61% (32%–78%) vs. 53% (23%–71%), p = 0.23]. During ccrt, monthly pharmaceutical costs ranged between $239 and $348, with work loss in the range of 18–30 days for patients and 8–12 days for caregivers.
Conclusions
We demonstrated the feasibility of comparing health and economic outcomes in patients receiving and not receiving early slp dysphagia intervention. These preliminary findings suggest that early slp dysphagia intervention for hnc patients might reduce peg dependency despite worsening health. Findings also highlight effects on financial security for these patients and their caregivers.
Keywords: Head-and-neck cancer, dysphagia interventions, patient costs, lost income, quality of life
BACKGROUND
Patients with squamous cell carcinoma of the head and neck frequently undergo radiotherapy (with or without chemotherapy) as a primary curative modality for organ preservation. However, because of anatomic and functional changes, preservation of swallowing function is not always successful1. As a result, about 44% of patients require enteral tube feeding at 3 months after treatment2, and approximately 7% remain tube-dependent at 1 year3.
Emerging evidence suggests that assessment and intervention by a speech–language pathologist (slp) before, during, and shortly after treatment might improve swallowing function and reduce the need for enteral feeding4–6. Earlier dysphagia intervention is also shown to have an overall positive effect on quality of life (qol) for these patients7. Yet because of resource limitations, preventive slp dysphagia intervention for patients with head-and-neck cancer (hnc) is not common and varies from one institution to another8.
Standard practice at our facility is to prophylactically place enteral feeding tubes for all patients before they receive intensified, curative, organ-sparing treatment such as concurrent chemoradiotherapy (ccrt), and to refer to slp for dysphagia assessment only if and when the oncology physician team identifies swallowing problems.
In a climate of limited absolute health dollars, using an economic lens to examine the potential benefit of an intervention from a societal perspective is informative. To date, only two studies have addressed economic aspects of hnc. Their findings suggest that health care costs for these patients are high9 and that, compared with late dysphagia intervention, early intervention might be more cost-effective10. Unfortunately, small samples and a single-country perspective (United States) limited the applicability of those data.
The primary aim of our study was to examine the feasibility of conducting a prospective clinical trial that would assess the effects on health and patient costs of early slp dysphagia intervention for hnc patients planned for curative ccrt. Little has been published addressing the combined perspective of health and economics with respect to this topic. In the present study, we also assessed longitudinal changes in health, qol, overall utility, and out-of-pocket costs before, during, and up to 3 months after treatment. Costs incurred by study patients and their families were compared with costs previously obtained for patients with cancers other than hnc treated in Ontario11.
METHODS
Patients
After research ethics approval was obtained, consecutive newly diagnosed English-speaking patients with squamous cell carcinoma of the pharynx, larynx, or oral cavity, or an unknown hnc primary were recruited prospectively from radiation oncology clinics at a large tertiary care facility, the Princess Margaret Cancer Centre. To be eligible, patients had to be proficient in spoken and written English, had to be planned for organ-preserving curative ccrt without preceding surgery, and had to have no pre-existing dysphagia from causes other than their current hnc.
Outcomes
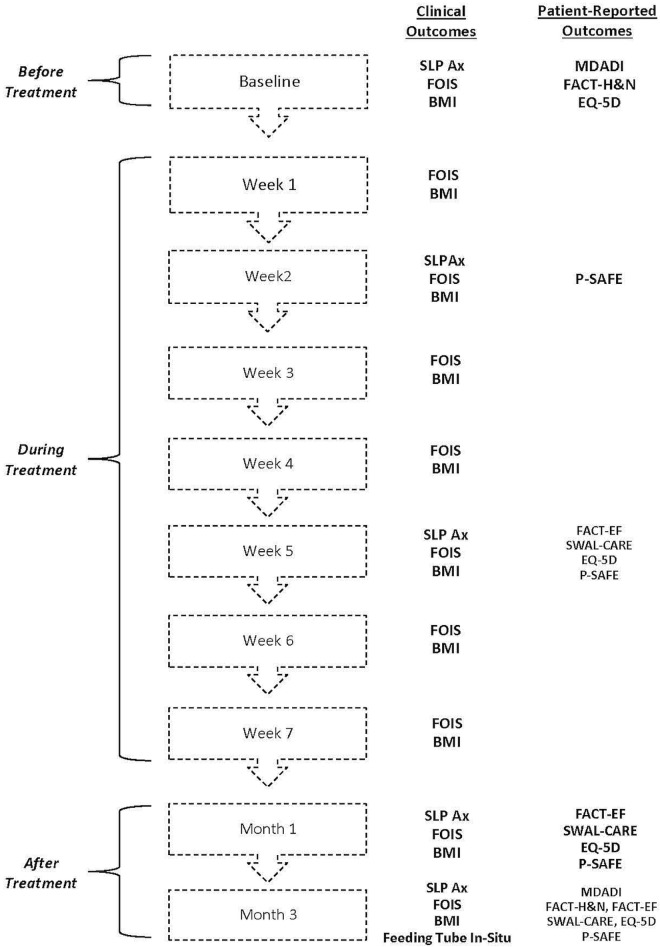
Study feasibility was measured by patient consent rate, acceptability of each outcome, and adherence to the intended schedule for outcome capture. Our primary outcome of clinical interest was delay to removal of an enteral feeding tube after completion of treatment. At various time points, we included 7 health outcomes, among which 5 with established reliability and validity were patient-reported (Figure 1).
FIGURE 1.
Study flow diagram. SLP Ax = speech–language pathology assessment; FOIS = Functional Oral Intake Scale; BMI = body mass index; MDADI = M.D. Anderson Dysphagia Inventory; FACT-H&N = Functional Assessment of Cancer Therapy–Head and Neck (FACIT.org, Elmhurst, IL, U.S.A.); EQ-5D = EQ-5D-5L (EuroQol Research Foundation, Rotterdam, Netherlands); P-SAFE = Patient Self-Administered Financial Expenditure; FACT-EF = Functional Assessment of Cancer Therapy–Enteral Feeding (FACIT.org); SWAL-CARE = Swallow Quality of Care.
The M.D. Anderson Dysphagia Inventory is a 20-item questionnaire that assesses dysphagia-related qol in patients with hnc12. The Functional Assessment of Cancer Therapy–Head and Neck Cancer (version 4: FACIT.org, Elmhurst, IL, U.S.A.) is a qol questionnaire standardized for all hnc patients. It consists of 28 general and 11 head-and-neck–specific items, each rated on a 5-point ordinal scale13. The Functional Assessment of Cancer Therapy–Enteral Feeding (FACIT.org) is a 20-item enteral feeding–specific qol questionnaire applicable to patients currently using a feeding tube14. The 15-item Swallow Quality of Care questionnaire assesses patient-perceived quality of care and patient satisfaction related to swallowing15. The health state classifier EQ-5D-5L (EuroQol Research Foundation, Rotterdam, Netherlands) is a health utility instrument whose 5 items have been used in hnc populations to target mobility, self-care, usual activities, pain or discomfort (or both), and depression or anxiety (or both)16. The Functional Oral Intake Scale is a 7-point ordinal clinician-derived score (from chart review or patient report) of food intake, ranging from nothing by mouth to a totally unrestricted oral diet17. An additional health outcome was body mass index, derived from height and weight.
Economic outcomes were collected over time using the Patient Self-Administered Financial Expenditure (p-safe) questionnaire. The p-safe items were first used in a Canadian study targeting adult patients with cancers other than hnc11; the questionnaire has since been refined based on both user and analysis feedback during the past 15 years. A formal validation of the tool is currently underway, and the items used in our pilot are those that were incorporated in the validation phase. The p-safe captures information about insurance coverage, patient out-of-pocket costs, lost time from work, travel costs, and perceived financial burden associated with treatment and follow-up care.
Procedures
A part-time research assistant approached eligible patients before the start of their curative cancer treatment (baseline). Consenting patients underwent clinical swallow assessment by a slp. The assessments were repeated at four time points: in weeks 2 and 5 during curative ccrt, and at months 1 and 3 after ccrt (Figure 1). Depending on the swallowing difficulties identified during each swallow assessment, the slp subsequently provided the study patient with customized strategies to address the specific dysphagia. The goal was to maintain a safe and efficient swallow. The recommendations provided were similar to those provided during usual slp care, which included any combination of oral exercises, laryngeal exercises, compensatory postural techniques, and suggested changes to food textures. Study patients also received any additional follow-up that the slp considered necessary. The slp followup occurred either in person during the patient’s regularly scheduled hospital visits or during a brief telephone conversation. The follow-ups were intended to informally monitor any change in the patient’s swallow status and to clarify any issues related to swallowing for the patient.
Patients completed the health and economic questionnaires at multiple time points between baseline and 3 months after ccrt. At the same time points, information about feeding-tube status and weight was collected (Figure 1).
To explore the health benefits to study patients, we compared the number of days without a feeding tube from the end of ccrt therapy to 3 months after treatment had ended for the study patients and for a time-matched patient cohort similar in age, disease site, disease stage, and human papillomavirus status, who received the usual slp swallowing care. The control cohort was derived by random sampling while matching on the foregoing variables among all eligible patients in our existing Anthology of Outcomes database. The Anthology is a prospective quality assurance tool consisting of data from all hnc patients treated with radiotherapy at the Princess Margaret Cancer Centre from 2003 onward18.
To explore out-of-pocket costs incurred by hnc patients, we noted and compared the responses to p-safe items by study patients with published data from other adult patients treated in Ontario for cancers other than hnc11,19.
Analysis
Demographic, feasibility, clinical, and economic data are summarized using descriptive statistics. The health outcome of “gain in feeding tube–free days” at 3 months after curative treatment in study patients was compared using Kaplan–Meier survival analysis in our patients and the matched control cohort. Using the nonparametric Wilcoxon signed-rank test, change over time in health outcomes was assessed from baseline to each time point up to 3 months after curative treatment; change in economic outcomes was assessed from week 2 to 3 months after curative treatment.
A pragmatic sample size of 20 patients was chosen a priori and considered sufficient to inform the feasibility of the study design for a future large clinical trial. Furthermore, the sample was sufficient to explore differences in feeding tube dependency in the study patients and in a time-, age-, and disease-matched cohort, and differences in out-of-pocket costs incurred by the study patients and by patients with non-hnc tumours who incurred similar costs (determined from published reports). Our sample was also considered sufficient to explore the direction and magnitude of change over time in patient health, utility, and out-of-pocket costs.
RESULTS
Over a 6-month period, 22 patients were approached, and 21 (95%) agreed to be enrolled (mean age: 54 years; 19 men; Tables i and ii). Tumour sites were the nasopharynx (n = 4), oropharynx (n = 13), and larynx (n = 2), with 2 unknown primaries (Table iii). Two patients left the study early, one at week 3 during ccrt, and the other at 1 month after ccrt, because of cancer progression. All available data for those patients were retained and analyzed accordingly.
TABLE I.
Patient characteristics
| Characteristic | Patient group | ||
|---|---|---|---|
|
| |||
| Overall | Intervention | Control | |
| Patients (n) | 42 | 21 | 21 |
| Mean age (years) | 54±9.5 | 54.5±8.3 | 53.4±10.7 |
| Sex [n (%) men] | 36 (85.7) | 19 (90.5) | 17 (81) |
| Smoking history [n (%)] | |||
| Nonsmoker | 15 (35.7) | 10 (47.6) | 5 (23.8) |
| Current smoker | 11 (26.2) | 4 (19) | 7 (33.3) |
| Mean pack–years | 32.6±11.3 | 34±16.1 | 31.7±9 |
| Ex-smoker | 15 (35.7) | 7 (33.3) | 8 (38.1) |
| Mean pack–years | 19.5±15.7 | 27.6±17.5 | 12.5±10.5 |
| Alcohol history [n (%)] | |||
| Non-drinker | 9 (21.4) | 5 (23.8) | 4 (19) |
| Light drinking | 14 (33.3) | 5 (23.8) | 9 (42.9) |
| Moderate drinking | 8 (19) | 7 (33.3) | 1 (4.8) |
| Heavy drinking | 8 (19) | 3 (14.3) | 5 (23.8) |
| Ex-drinker | 1 (2.4) | 0 (0) | 1 (4.8) |
| Unknown | 2 (4.8) | 1 (4.8) | 1 (4.8) |
| HPV status [n (%)] | |||
| Negative | 4 (9.5) | 1 (4.8) | 3 (14.3) |
| Positive | 26 (61.9) | 14 (66.7) | 12 (57.1) |
| Not available | 12 (28.6) | 6 (28.6) | 6 (28.6) |
HPV = human papillomavirus.
TABLE II.
Additional characteristics of the intervention patient group
| Characteristic | Value [n (%)] |
|---|---|
| Marital status | |
| Married | 13 (61.9) |
| Common law | 2 (9.5) |
| Single, never married | 2 (9.5) |
| Widowed | 1 (4.8) |
| Separated | 2 (9.5) |
| Not available | 1 (4.8) |
| Living situation | |
| Lives alone | 3 (14.3) |
| Lives with ... | |
| 1 Other person | 10 (47.6) |
| 2 Other people | 3 (14.3) |
| 3 Other people | 2 (9.5) |
| More than 3 other people | 2 (9.5) |
| Not available | 1 (4.8) |
| Highest level of education | |
| Some high school | 4 (19) |
| Completed high school | 5 (23.8) |
| Some college or university | 5 (23.8) |
| Completed university or college | 5 (23.8) |
| Not available | 2 (9.5) |
| Pre-tax family income last year | |
| <$5,000 | 1 (4.8) |
| $10,000–$14,999 | 1 (4.8) |
| $15,000–$19,999 | 1 (4.8) |
| $30,000–$39,999 | 1 (4.8) |
| $40,000–$49,999 | 3 (14.3) |
| $60,000–$79,999 | 5 (23.8) |
| >$80,000 | 6 (28.6) |
| Not available | 3 (14.3) |
TABLE III.
Disease and treatment characteristics
| Characteristic | Patient group | ||
|---|---|---|---|
|
| |||
| Overall (n=42) | Intervention (n=21) | Control (n=21) | |
| Site [n (%)] | |||
| Nasopharynx | 10 (23.8) | 4 (19) | 6 (28.6) |
| Oropharynx | 26 (61.9) | 13 (61.9) | 13 (61.9) |
| Larynx | 3 (7.1) | 2 (9.5) | 1 (4.8) |
| Unknown primary | 3 (7.1) | 2 (9.5) | 1 (4.8) |
| Tumour stage [n (%)] | |||
| T0 | 3 (7.1) | 2 (9.5) | 1 (4.8) |
| T1 | 10 (23.8) | 5 (23.8) | 5 (23.8) |
| T2 | 14 (33.3) | 7 (33.3) | 7 (33.3) |
| T3 | 11 (26.2) | 5 (23.8) | 6 (28.6) |
| T4 | 2 (4.8) | 0 (0) | 2 (9.5) |
| T4a | 2 (4.8) | 2 (9.5) | 0 (0) |
| Lymph node status [n (%)] | |||
| N0 | 3 (7.1) | 1 (4.8) | 2 (9.5) |
| N1 | 3 (7.1) | 2 (9.5) | 1 (4.8) |
| N2 | 4 (9.5) | 0 (0) | 4 (19) |
| N2a | 3 (7.1) | 1 (4.8) | 2 (9.5) |
| N2b | 15 (35.7) | 8 (38.1) | 7 (33.3) |
| N2c | 10 (23.8) | 6 (28.6) | 4 (19) |
| N3 | 4 (9.5) | 3 (14.3) | 1 (4.8) |
| Stage [n (%)] | |||
| II | 2 (4.8) | 1 (4.8) | 1 (4.8) |
| III | 8 (19) | 4 (19) | 4 (19) |
| IVA | 28 (66.7) | 12 (57.1) | 16 (76.2) |
| IVB | 4 (9.5) | 4 (19) | 0 (0) |
| Squamous cell histology [n (%)] | 42 (100) | 21 (100) | 21 (100) |
| Radiation treatment | |||
| Mean treatments (n fractions) | 34.9±0.3 | 35±0.2 | 34.9±0.4 |
| Total dose (Gy) | 69.7 (1.7) | 69.9 (0.4) | 69.5 (2.3) |
| Chemotherapy | |||
| Mean total cisplatin dose (mg) | 409.3 ±114.5 | 380.3 ±118.1 | 438.3 ±105.6 |
Success in capturing the clinician-administered and patient-reported health outcomes is shown in Tables iv and v respectively. With respect to the surveillance schedules of 1 and 3 months after ccrt for all study patients, successful administration of clinical tests deviated from the targets by medians of 5 days (range: 0–19 days) and 12 days (range: 0–22 days) respectively. Clinician tests were occasionally missed when a patient’s oncology appointment was rescheduled and the part-time research assistant was unaware or unavailable (Table iv). Whenever possible, missing clinical data were then extracted from the patient record. That approach proved helpful for data points related to food intake and weight. Relative to the clinician-administered data, fewer patient-reported data were missed at the various time points (Table v). Although administration of the questionnaires was also aligned with the regularly scheduled clinic appointments for the patients, the outcomes did not depend on in-person patient visits because we invited missed patients to submit completed questionnaires by mail. Of the 7 missed patients, 3 agreed to mail their questionnaires, thereby reducing by almost half the overall missed data points at month 3.
TABLE IV.
Successful capture of clinician-administered health outcomes, by study time point
| Assessment | Patients whose assessment data were captured at ... | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||
| Baseline (n=21) | Week 1 (n=21) | Week 2 (n=21) | Week 3 (n=20) | Week 4 (n=20) | Week 5 (n=20) | Week 6 (n=20) | Week 7 (n=20) | Month 1 (n=19) | Month 3 (n=19) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | |
| Functional Oral Intake Scale | 21 | 100 | 20 | 95.2 | 20 | 95.2 | 20 | 100 | 20 | 100 | 19 | 95 | 20 | 100 | 20 | 100 | 17 | 89.5 | 16 | 84.2 |
| Speech–language pathology assessment | 21 | 100 | NA | 20 | 95.2 | NA | NA | 14 | 70 | NA | NA | 14 | 73.7 | 12 | 63.2 | |||||
| Speech–language pathology impression | 21 | 100 | NA | 20 | 95.2 | NA | NA | 14 | 70 | NA | NA | 14 | 73.7 | 12 | 63.2 | |||||
| Body mass index | 20 | 95.2 | 21 | 100 | 21 | 100 | 20 | 95.2 | 20 | 100 | 20 | 100 | 20 | 100 | 20 | 100 | 14 | 73.7 | 12 | 63.2 |
NA = not available.
TABLE V.
Successful capture of patient-reported outcomes, by study time point
| Assessment | Patients whose assessment data were captured at ... | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Baseline (n=21) | Week 2 (n=21) | Week 5 (n=20) | Month 1 (n=19) | Month 3 (n=19) | ||||||
|
|
|
|
|
|
||||||
| (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | |
| EQ-5D-5La | 21 | 100 | NA | 15 | 75 | 14 | 73.7 | 15 | 78.9 | |
| Functional Assessment of Cancer Therapyb module | ||||||||||
| Enteral Feedingc | NA | NA | 13 | 92.9 | 14 | 87.5 | 3 | 75 | ||
| Head and Neck Cancer | 21 | 100 | NA | NA | NA | 15 | 78.9 | |||
| M.D. Anderson Dysphagia Inventory | 21 | 100 | NA | NA | NA | 15 | 78.9 | |||
| Swallow Quality of Care | NA | NA | 17 | 85 | 14 | 73.7 | 15 | 78.9 | ||
| Patient Self-Administered Financial Expenditure | NA | 20 | 95.2 | 17 | 85 | 15 | 78.9 | 15 | 78.9 | |
EuroQol Research Foundation, Rotterdam, Netherlands.
FACIT.org, Elmhurst, IL. U.S.A.
Applicable only to patients with a feeding tube in situ (14 in week 5, 16 in month 1, 4 in month 3).
NA = not available.
Health outcomes for study patients, including swallow and weight, worsened at subsequent time points (Table vi). Likewise, head-and-neck and swallow-related qol outcomes both significantly worsened from baseline to 3 months after ccrt (Tables vii and viii). Health utility also worsened over time, but significantly so only at week 5 during ccrt. Patient satisfaction with dysphagia care increased, with the highest satisfaction at month 3.
TABLE VI.
Health outcomes, by time point
| Assessment | Outcome at ... | ||||
|---|---|---|---|---|---|
|
| |||||
| Baseline (n=21) | Week 2 (n=21) | Week 5 (n=20) | Month 1 (n=19) | Month 3 (n=19) | |
| Functional Oral Intake Scale (score) | |||||
| Median | 7 | 7a | 3b | 3c | 6a |
| Range | 5–7 | 3–7 | 1–6 | 1–7 | 3–7 |
| Speech–language pathology impression [n (%)] | a | c | c | c | |
| Unimpaired | 20 (95.2) | 12 (57.1) | 0 (0) | 3 (15.8) | 2 (10.5) |
| Mild dysphagia | 1 (4.8) | 4 (19) | 3 (15) | 5 (26.3) | 5 (26.3) |
| Moderate dysphagia | 0 (0) | 3 (14.3) | 6 (30) | 4 (21.1) | 5 (26.3) |
| Severe dysphagia | 0 (0) | 1 (4.8) | 5 (25) | 2 (10.5) | 0 (0) |
| Not available | 0 (0) | 1 (4.8) | 6 (30) | 5 (26.3) | 7 (36.8) |
| Weight (kg) | |||||
| Median | 83.5 | 80.0c | 78.9b | 77.4c | 65.4c |
| Range | 57.9–122 | 55.6–119 | 55.3–111.8 | 53–95.1 | 54–92.5 |
| Body mass index (kg/m2), | |||||
| Median | 27.1 | 26.9c | 25.76c | 24.7c | 23.1c |
| Range | 18.5–36.8 | 18.1–35.9 | 18.1–33.8 | 18.6–32.6 | 18.4–32 |
Significant at p < 0.05 compared with baseline.
Significant at p < 0.001 compared with baseline.
Significant at p < 0.01 compared with baseline.
TABLE VII.
Patient reported outcomes, by time point
| Assessment | Outcome at ... | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Baseline (n= 21) | Week 2 (n=21) | Week 5 (n=20) | Month 1 (n=19) | Month 3 (n=19) | ||||||
|
|
|
|
|
|
||||||
| Median | Range | Median | Range | Median | Range | Median | Range | Median | Range | |
| Functional Assessment of Cancer Therapya | ||||||||||
| Head and Neck Cancer module | ||||||||||
| Personal well-being | 25 | 14–28 | NA | NA | NA | 23 | 6–27b | |||
| Social and family well-being | 24 | 1.2–29 | 24 | 17–29 | ||||||
| Emotional well-being | 18 | 7.2–22 | 20 | 14–24b | ||||||
| Functional well-being | 20 | 7–28 | 19 | 4–21 | ||||||
| General module | 83.3 | 61–104 | 84 | 48–94 | ||||||
| Additional concerns | 34 | 14–39 | 20 | 8–33c | ||||||
| TOTAL | 117 | 76.6–140 | 105 | 59–125b | ||||||
| M.D. Anderson Dysphagia Inventory | ||||||||||
| Global | 5 | 1–5 | NA | NA | NA | 4 | 2–5c | |||
| Total score | 86.3 | 52.6–100 | 71.1 | 35.8–88.4c | ||||||
| Swallow Quality of Care | ||||||||||
| Clinical advice | NA | NA | 47 | 10–77 | 51.5 | 24–76 | 63 | 20–87 | ||
| General advice | 44 | 0–72 | 40 | 4–68 | 52 | 20–88 | ||||
| Patient satisfaction | 55 | 25–60 | 45 | 10–60 | 57.5 | 0–60 | ||||
| EQ-5D-5Ld index score | 0.9 | 0.7–1 | NA | 0.8 | 0.3–0.9b | 0.8 | 0.6–0.9 | 0.8 | 0.6–1 | |
FACIT.org, Elmhurst, IL. U.S.A.
Significant at p < 0.05 compared with baseline.
Significant at p < 0.01 compared with baseline.
EuroQol Research Foundation, Rotterdam, Netherlands. NA = not applicable.
TABLE VIII.
Functional Assessment of Cancer Therapy–Enteral Feedinga, assessmentb by time point
| Variable | Week 5 (n=14) | Month 1 (n=16) | Month 3 (n=4) | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Median | Range | Median | Range | Median | Range | |
| Total score | 50 | 39–69 | 50.5 | 31–67 | 44 | 39–64 |
EuroQol Research Foundation, Rotterdam, Netherlands.
Applicable only to patients with a feeding tube in situ.
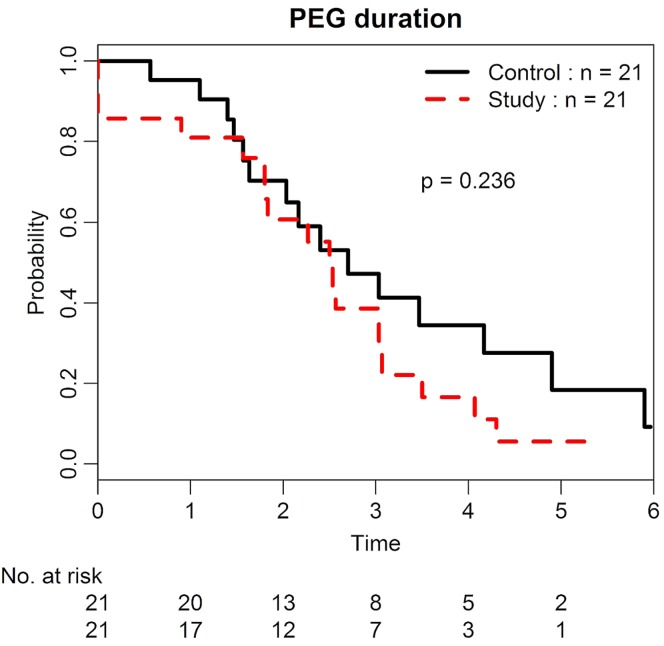
Although not statistically significant, the feeding tube removal rate was observed to be higher in the study group than in the matched control cohort at 3 months after ccrt [61% (32%–78%) vs. 53% (23%–71%), p = 0.23; Figure 2]. In contrast, a trend toward greater weight loss was observed in the study patients compared with patients from the matched cohort (11.7 kg vs. 9.4 kg, p = 0.58).
FIGURE 2.
Duration of percutaneous endoscopic gastrostomy (PEG) in the study and control groups.
The out-of-pocket patient costs varied over time (Table ix). Notably, monthly patient costs for pharmaceuticals ranged between $348 (week 2) and $239 (week 5) during active treatment and declined by approximately 50% in follow-up (months 1 and 3). Supplement costs were low during ccrt, but peaked at $780 during month 1 after ccrt. Device costs also peaked at $250 by month 1. Patient work loss was lowest at week 2 (mean: 18 days), remaining high (mean: 22–28 days) at all subsequent time points. For caregivers, work loss peaked at 5 weeks (mean: 12 days), but by month 3 after ccrt was low (mean: 3 days). Lost time from work was observed for both hnc patients and their caregivers, with lost work time peaking at week 5 (27 days) and remaining fairly constant for patients at 1 and 3 months after ccrt (22 and 28 days respectively). Lost caregiver work time peaked at week 5 (12 days) and dropped afterward to 7 and 3 days at 1 and 3 months respectively after ccrt (Table x). Travel costs such as parking peaked during ccrt (mean: $699–$868), but dropped after ccrt (mean: $92–$248; Table xi). Patient-reported financial burden (costs and lost income) was “significant” or “unmanageable” for 33% and 20% of patients respectively during ccrt, dropping to 21% and 16% after ccrt (Table xii).
TABLE IX.
Patient out-of-pocket costs, assessed by Patient Self-Administered Financial Expenditure, by time point
| Cancer-related expenditure | Week 2 (n=21) | Week 5 (n=20) | Month 1 (n=19) | Month 3 (n=19) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | |
| Prescription drugs | ||||||||
| No | 2 | 9.5 | 1 | 5 | 3 | 15.8 | 7 | 36.8 |
| Yes | ||||||||
| Paid by self | 6 | 28.6 | 7 | 35 | 4 | 21.1 | 3 | 15.8 |
| Paid by private insurance | 5 | 23.8 | 3 | 15 | 3 | 15.8 | 2 | 10.5 |
| Paid by gov’t | 2 | 9.5 | 2 | 10 | 2 | 10.5 | 1 | 5.3 |
| Paid by self and private insurance OR gov’t | 4 | 19.1 | 3 | 15 | 1 | 5.3 | 1 | 5.3 |
| Paid by private insurance and gov’t | 0 | 0 | 1 | 5 | 2 | 10.5 | 1 | 5.3 |
| Paid by self, private insurance, and gov’t | 1 | 4.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not available | 1 | 4.8 | 3 | 15 | 4 | 21.1 | 4 | 21.1 |
| Mean amount if paid by self ($) | 347.54±512.62 | 238.5±192.40 | 167.00±104.99 | 106.93±85.42 | ||||
| Vitamins and supplements, including special diets | ||||||||
| No | 14 | 66.7 | 8 | 40 | 9 | 47.4 | 9 | 47.4 |
| Yes | ||||||||
| Paid by self | 4 | 19 | 4 | 20 | 3 | 15.8 | 2 | 10.5 |
| Paid by private insurance | 0 | 0 | 1 | 5 | 0 | 0 | 1 | 5.3 |
| Paid by gov’t | 1 | 4.8 | 2 | 10 | 2 | 10.5 | 3 | 5.3 |
| Paid d by other | 0 | 0 | 2 | 10 | 0 | 0 | 0 | 0 |
| Not available | 2 | 9.5 | 3 | 15 | 5 | 26.3 | 4 | 21.1 |
| Mean amount if paid by self ($) | 73.75±84.79 | 110.67±84.51 | 780.00±593.97 | 20.00 | ||||
| Accommodation and meals | ||||||||
| No | 14 | 66.7 | 13 | 65 | 14 | 73.7 | 15 | 78.9 |
| Yes | ||||||||
| Paid by self | 5 | 23.8 | 2 | 10 | 0 | 0 | 0 | 0 |
| Paid by gov’t | 0 | 0 | 1 | 5 | 1 | 5.3 | 0 | 0 |
| Paid by other | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 0 |
| Not available | 2 | 9.5 | 3 | 15 | 4 | 21.1 | 4 | 21.1 |
| Mean amount if paid by self ($) | 107.00±129.75 | 250.00±212.13 | — | — | ||||
| Devices or equipment | ||||||||
| No | 15 | 71.4 | 14 | 70 | 9 | 47.4 | 12 | 63.2 |
| Yes | ||||||||
| Paid by self | 4 | 19 | 1 | 5 | 3 | 15.8 | 0 | 0 |
| Paid by gov’t | 0 | 0 | 2 | 10 | 3 | 15.8 | 3 | 15.8 |
| Not available | 2 | 9.5 | 3 | 15 | 4 | 21.1 | 4 | 21.1 |
| Amount if paid by self ($) | 186.30±132.52 | 100.00 | 250.00±304.14 | — | ||||
TABLE X.
Lost patient and caregiver income, assessed by Patient Self-Administered Financial Expenditure, by time point
| Variable | Week 2 (n=21) | Week 5 (n=20) | Month 1 (n=19) | Month 3 (n=19) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | |
| Patient employment status | ||||||||
| Employed full-time | 13 | 61.9 | 9 | 45 | 8 | 42.1 | 9 | 47.4 |
| Employed part-time | 1 | 4.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Retired | 3 | 14.3 | 2 | 10 | 3 | 15.8 | 2 | 10.5 |
| Homemaker | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5.3 |
| On disability | 2 | 9.5 | 4 | 20 | 3 | 15.8 | 2 | 10.5 |
| Unemployed | 1 | 4.8 | 2 | 10 | 1 | 5.3 | 1 | 5.3 |
| Not available | 1 | 4.8 | 3 | 15 | 4 | 21.1 | 4 | 21.1 |
| Took time off work in last 30 days to receive treatment related to cancer | ||||||||
| Yes | 10 | 47.6 | 6 | 30 | 6 | 31.6 | 6 | 31.6 |
| Not available | 1 | 4.8 | 3 | 15 | 3 | 15.8 | 10 | 52.6 |
| Mean days off worka | 18±9.7 | 27±4.5 | 22±12.2 | 28±4.5 | ||||
| Quit work in last 30 days because of illness | ||||||||
| Yes | 4 | 19 | 3 | 15 | 2 | 10.5 | 1 | 5.3 |
| Not available | 1 | 4.8 | 3 | 15 | 3 | 15.8 | 10 | 52.6 |
| If took time off work ... | ||||||||
| With full pay | 4 | 19 | 2 | 33.3 | 2 | 33.3 | 0 | 0 |
| With partial pay | 2 | 9.5 | 2 | 33.3 | 2 | 33.3 | 1 | 5.3 |
| Without pay | 3 | 14.3 | 2 | 33.3 | 2 | 33.3 | 3 | 15.8 |
| With partial pay and using personal days, including sick days | 1 | 4.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not available | 1 | 4.8 | 0 | 0 | 0 | 0 | 15 | 78.9 |
| Friends or family took time off work in last 30 days related to patient’s treatment | ||||||||
| Yes | 10 | 47.6 | 8 | 40 | 6 | 31.6 | 5 | 26.3 |
| Not available | 1 | 4.8 | 3 | 15 | 5 | 26.3 | 5 | 26.3 |
| Mean days off work | 9.2±8.5 | 12±9.8 | 7.4±8.5 | 3±1.4 | ||||
This estimate could be a slightly high, because a typical work schedule consists of about 22 work days per month, but some patients clearly assumed that a full month off work represented 30 lost work days.
TABLE XI.
Patient travel cost and parking, assessed by Patient Self-Administered Financial Expenditure, by time point
| Variable | Week 2 (n=21) | Week 5 (n=20) | Month 1 (n=19) | Month 3 (n=19) |
|---|---|---|---|---|
| Mean trips to PMH or UHN in last 30 days (n) | 19.3±9 | 24.2±12.7 | 5.5±7.7 | 2±1.5 |
| Mean one-way distance (km) | 60.1±54.1 | 59.6±54.8 | 71.8±57.2 | 62.4±57.3 |
| Mean fare or parking cost ($) | 32.13±43.51 | 38.65±65.32 | 21.46±16.20 | 20.31±9.35 |
| TOTAL (mean $) | ||||
| Parking | 32 | 39 | 21 | 20 |
| Travel | 667 | 829 | 227 | 72 |
| Parking and travel | 699 | 868 | 248 | 92 |
$0.575/km per Canada Revenue Agency, 2014.
PMH = Princess Margaret Hospital; UHN = University Health Network.
TABLE XII.
Patient financial burden, assessed by Patient Self-Administered Financial Expenditure, by time point
| Financial burden caused by out-of-pocket expenditures | Week 2 (n=21) | Week 5 (n=20) | Month 1 (n=19) | Month 3 (n=19) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | |
| Not a burden at all | 3 | 14.3 | 2 | 10 | 4 | 21.1 | 5 | 26.3 |
| Only a slight burden | 5 | 23.8 | 7 | 35 | 3 | 15.8 | 3 | 15.8 |
| Somewhat of a burden | 4 | 19 | 4 | 20 | 4 | 21.1 | 4 | 21.1 |
| Significant burden, but manageable | 7 | 33.3 | 2 | 10 | 3 | 15.8 | 3 | 15.8 |
| Unmanageable burden | 0 | 0 | 2 | 10 | 1 | 5.3 | 0 | 0 |
| Not available | 2 | 9.5 | 3 | 15 | 4 | 21.1 | 4 | 21.1 |
In comparison with costs reported for patients having other common solid cancersa in Ontario, costs related to pharmaceuticals, supplements, devices, and hospitalization were higher for patients with hnc. Furthermore, a relatively higher proportion of hnc patients were employed during ccrt treatment. However, of all patients with any cancer who remained employed, hnc patients took more days off work.
DISCUSSION
Dysphagia is a common sequela for patients with hnc treated with radiotherapy, with or without chemotherapy. Despite provincial20–24 and international25,26 guidelines that advocate for slp dysphagia intervention for those patients, limited resources reduce slp intervention.
In the present study, we assessed the feasibility of conducting a longitudinal clinical study to determine the effect on patient health and enteral feeding duration of early slp dysphagia intervention in adult hnc patients planned for organ-sparing curative ccrt. We did not measure the effect of our intervention on expenses because we had no comparative data for hnc patients.
Our findings identified a motivated patient group, resulting in an almost perfect accrual rate and minimal attrition at 3 months after ccrt. Furthermore, completed tests and questionnaires were successfully captured within an acceptable allowance of the targeted time. Missed clinician-administered tests after ccrt were a challenge. Clearly, a future clinical trial will have to ensure more research staff resources and improved communication with clinic booking clerks. Fewer patient-reported health outcomes were missed because of the option for mailing questionnaires as needed. To summarize, with adjustments to follow-up clinic visits by patients, our findings demonstrated that a longitudinal study design is feasible and should be well-received by patients with hnc.
Our findings also explored changes in health over time. Study patients experienced declining swallowing function that was worse at 1 month after therapy, but recovered to near-normal at 3 months. Compared with a matched control group who did not receive early slp dysphagia intervention, study patients were less likely to be dependent on tube feeding by 3 months. The benefit of early intervention has been shown before, with slp swallowing therapy interventions that were more intense (daily rather than as indicated from clinical testing)4–6. However, our study is the first to show a reciprocal and gradual decline in patient body mass index despite the positive transition to an oral diet. Identification of a decline in weight is critical, given the potential effect of nutrition on overall patient recovery27. That finding suggests that, alongside intervention from a slp to address swallowing status, there is need for a dietitian to continue active monitoring of the patient’s nutrition status after treatment. Study patients also reported a decline in cancer-specific and dysphagia-related qol, although satisfaction with dysphagia care improved over time. Satisfaction for qol and overall care have the potential to influence how adherent patients will be, especially with behavioural dysphagia therapy28. Those outcomes are therefore important confounding variables and have to be included in future therapeutic clinical trials.
Our study sought preliminary information about out-of-pocket patient costs, an outcome that has not previously been reported in hnc. Out-of-pocket expenses reported by our patients were higher than those reported for adult patients with other solid tumours11,19. However, lost time from work among our study patients was similar to time lost by breast cancer patients29,30.
Generally, the greater costs in hnc patients might be explained by either greater cancer severity or greater treatment intensity. However, we cannot rule out the possibility that some of the differences might relate to recent changes in the levels of public coverage for health care in Ontario. Our earlier work was based on public and private coverage in 2001–200311,19,31. Although we did not see a substantial change in insurance coverage rates from then to the time of our study11, it is possible that patients in the present study had partial or reduced coverage or increased co-payments. Private sector behaviour suggests that, in Canada, insurers and corporations are managing increasing pharmaceutical-and devices-related health care costs for their employees by increasing co-payments and lowering service limits. In particular, the relatively higher out-of-pocket costs for supplements in hnc are likely a result of the high cost of the dietary supplements needed for enteral feeding, much of which would be borne by patients once they are discharged home. Given that supplement costs are relatively specific to hnc, it is not surprising that costs in that category are much higher than are seen in other common solid cancers (breast, colorectal, lung, and prostate)11,19.
We note that our study patients reported more lost time from work than is seen in patients with other cancers29,30. Perhaps our capture of multiple time points allowed us to more accurately assess lost-time peaks, which our earlier studies missed with the inclusion of only one time point per person.
Patient travel costs were determined by the required number of clinic visits and the duration of cancer treatment. The costs reported by our study group were similar to those reported for other oncology patients11. Patient-perceived financial burden was similar to that reported in Ontario in the early 2000s in non-hnc patients11,19, with up to 33% of the study subjects reporting “significant” or “unmanageable” financial burden.
Limitations
Although the demographic details for our patients are representative of the general hnc population referred for organ-sparing treatment1,3–6, our study is, in keeping with the primary aim of feasibility, limited by sample size. Differences between the groups or changes over time in health and out-of-pocket patient costs are therefore exploratory and hypothesis-generating. Furthermore, because of the small sample, we are unable to assess the effects of individual patient characteristics or cancer treatments on either patient health or incurred costs.
CONCLUSIONS
The present study demonstrated that conducting a longitudinal study to assess the benefit of early slp dysphagia intervention for patients with hnc is feasible and likely to be well accepted by patients. We identified design strategies to minimize missed tests and to ensure comprehensive capture of both potential benefits and harms from dysphagia therapy. Although underpowered, our findings suggest that early slp dysphagia intervention benefits patient health with reduced use of an enteral feeding tube at 3 months after ccrt. Our findings also suggest that ccrt is perhaps more detrimental to hnc patients and their caregivers than to patients with other solid tumours with respect to out-of-pocket costs and lost income.
Having established the feasibility of our study design, a larger randomized trial to more fully assess the effects of early slp dysphagia intervention and ccrt on patient health and costs is now warranted.
ACKNOWLEDGMENTS
This pilot study received funding support from the Canadian Centre for Applied Research in Cancer Control (arcc), which is funded by the Canadian Cancer Society. RM is also supported by a Canada Research Chair (tier ii) in Swallowing Disorders.
Footnotes
Specifically, breast, colorectal, lung, and prostate.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Hutcheson KA, Lewin JS, Barringer DA, et al. Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer. 2012;118:5793–9. doi: 10.1002/cncr.27631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiley SG, Hargunani CA, Skoner JM, Holland JM, Wax MK. Swallowing function after chemoradiation for advanced stage oropharyngeal cancer. Otolaryngol Head Neck Surg. 2006;134:455–9. doi: 10.1016/j.otohns.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 3.Setton J, Lee NY, Riaz N, et al. A multi-institution pooled analysis of gastrostomy tube dependence in patients with oropharyngeal cancer treated with definitive intensity-modulated radiotherapy. Cancer. 2015;121:294–301. doi: 10.1002/cncr.29022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carnaby-Mann G, Crary MA, Schmalfuss I, Amdur R. “Pharyngocise”: randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head-and-neck chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:210–19. doi: 10.1016/j.ijrobp.2011.06.1954. [DOI] [PubMed] [Google Scholar]
- 5.Carroll WR, Locher JL, Canon CL, Bohannon IA, McColloch NL, Magnuson JS. Pretreatment swallowing exercises improve swallow function after chemoradiation. Laryngoscope. 2008;118:39–43. doi: 10.1097/MLG.0b013e31815659b0. [DOI] [PubMed] [Google Scholar]
- 6.Kotz T, Federman AD, Kao J, et al. Prophylactic swallowing exercises in patients with head and neck cancer undergoing chemoradiation: a randomized trial. Arch Otolaryngol Head Neck Surg. 2012;138:376–82. doi: 10.1001/archoto.2012.187. [DOI] [PubMed] [Google Scholar]
- 7.Kulbersh BD, Rosenthal EL, McGrew BM, et al. Pretreatment, preoperative swallowing exercises may improve dysphagia quality of life. Laryngoscope. 2006;116:883–6. doi: 10.1097/01.mlg.0000217278.96901.fc. [DOI] [PubMed] [Google Scholar]
- 8.Krisciunas GP, Sokoloff W, Stepas K, Langmore SE. Survey of usual practice: dysphagia therapy in head and neck cancer patients. Dysphagia. 2012;27:538–49. doi: 10.1007/s00455-012-9404-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waters TM, Logemann JA, Pauloski BR, et al. Beyond efficacy and effectiveness, conducting economic analyses during clinical trials. Dysphagia. 2004;19:109–19. doi: 10.1007/s00455-003-0507-7. [DOI] [PubMed] [Google Scholar]
- 10.Focht KL, Simpson K, Martin-Harris B. Business case for pretreatment swallowing exercises. Otolaryngol Head Neck Surg. 2011;145:P161–2. doi: 10.1177/0194599811415823a91. [DOI] [Google Scholar]
- 11.Longo CJ, Fitch M, Deber RB, Williams AP. Financial and family burden associated with cancer treatment in Ontario, Canada. Support Care Cancer. 2006;14:1077–85. doi: 10.1007/s00520-006-0088-8. [DOI] [PubMed] [Google Scholar]
- 12.Chen AY, Frankowski R, Bishop-Leone J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M.D. Anderson Dysphagia Inventory. Arch Otolaryngol Head Neck Surg. 2001;127:870–6. [PubMed] [Google Scholar]
- 13.List MA, D’Antonio LL, Cella DF, et al. The performance status scale for head and neck cancer patients and the functional assessment of cancer therapy-head and neck scale. A study of utility and validity. Cancer. 1996;77:2294–301. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2294::AID-CNCR17>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 14.Stevens CS, Lemon B, Lockwood GA, Waldron JN, Bezjak A, Ringash J. The development and validation of a quality-of-life questionnaire for head and neck cancer patients with enteral feeding tubes: the qol-ef. Support Care Cancer. 2011;19:1175–82. doi: 10.1007/s00520-010-0934-6. [DOI] [PubMed] [Google Scholar]
- 15.McHorney CA, Robbins J, Lomax K, et al. The swal–qol and swal–care outcomes tool for oropharyngeal dysphagia in adults: iii. Documentation of reliability and validity. Dysphagia. 2002;17:97–114. doi: 10.1007/s00455-001-0109-1. [DOI] [PubMed] [Google Scholar]
- 16.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil. 2005;86:1516–20. doi: 10.1016/j.apmr.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 18.Wong K, Huang SH, O’Sullivan B, et al. Point-of-care outcome assessment in the cancer clinic: audit of data quality. Radiother Oncol. 2010;95:339–43. doi: 10.1016/j.radonc.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Longo CJ, Deber R, Fitch M, Williams AP, D’Souza D. An examination of cancer patients’ monthly “out-of-pocket” costs in Ontario, Canada. Eur J Cancer Care (Engl) 2007;16:500–7. doi: 10.1111/j.1365-2354.2007.00783.x. [DOI] [PubMed] [Google Scholar]
- 20.Alberta Health Services (ahs) Alberta Health Services > Info > Cancer guidelines > Head and Neck [Web page] Edmonton, AB: AHS; 2015. [Available at: http://www.albertahealthservices.ca/info/cancerguidelines.aspx; cited 14 November 2016] [Google Scholar]
- 21.BC Cancer Agency (bcca) BC Cancer Agency > Health Professionals Info > Cancer Management Guidelines > Head & Neck Cancer > Head & Neck [Web page] Vancouver, BC: BCCA; n.d.. [Available at: http://www.bccancer.bc.ca/health-professionals/clinical-resources/cancer-management-guidelines/head-neck/head-neck; cited 14 November 2016] [Google Scholar]
- 22.Cancer Care Nova Scotia . Halifax, NS: Cancer Care Nova Scotia; n.d.. Cancer Care Nova Scotia > Health Professionals > Resources & Tools > Cancer Management Guidelines > Approved Guidelines [Web page, “Head and Neck Cancers” section] [Available at: http://www.cancercare.ns.ca/en/home/healthprofessionals/resourcestools/cancermanagementguidelines/approvedgdline.aspx; cited 14 November 2016] [Google Scholar]
- 23.Gilbert R, Devries-Aboud M, Winquist E, Waldron J, McQuestion M. The Management of Head and Neck Cancer in Ontario. Toronto, ON: Cancer Care Ontario; 2009. Evidence-based series 5–3. [Available online at: https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=58592: cited 27 October 2017] [Google Scholar]
- 24.Saskatchewan Cancer Agency . Saskatchewan Cancer Agency > Professionals > Clinical Practice Guidelines > Head and Neck Cancer Guidelines [Web page] Regina, SK: Saskatchewan Cancer Agency; 2015. [Available at: http://www.saskcancer.ca/Default.aspx?DN=eac690eb-f6e4-4873-a2c8-0a3e4f7651dc; cited 14 November 2016] [Google Scholar]
- 25.Cohen EE, LaMonte SJ, Erb NL, et al. American Cancer Society head and neck cancer survivorship care guideline. CA Cancer J Clin. 2016;66:203–39. doi: 10.3322/caac.21343. [Erratum in: CA Cancer J Clin 2016;66:351] [DOI] [PubMed] [Google Scholar]
- 26.National Institute for Health and Clinical Excellence (nice) Home > NICE Guidance > Conditions and Diseases > Cancer > Head and Neck Cancers [Web page] London, UK: NICE; 2016. [Available at: https://www.nice.org.uk/guidance/conditions-and-diseases/cancer/head-and-neck-cancers; cited 14 November 2016] [Google Scholar]
- 27.Lee YJ, Zheng LF, Tham I, Kanagalingam J, Hobbs C. An audit on nutritional status using weight as a marker of nutrition in head and neck cancer patients undergoing radiotherapy. Oral Oncol. 2013;49(suppl 1):S138–9. doi: 10.1016/j.oraloncology.2013.03.374. [DOI] [Google Scholar]
- 28.Shinn EH, Basen-Engquist K, Baum G, et al. Adherence to preventive exercises and self-reported swallowing outcomes in post-radiation head and neck cancer patients. Head Neck. 2013;35:1707–12. doi: 10.1002/hed.23255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azzani M, Roslani AC, Su TT. The perceived cancer-related financial hardship among patients and their families: a systematic review. Support Care Cancer. 2015;23:889–98. doi: 10.1007/s00520-014-2474-y. [DOI] [PubMed] [Google Scholar]
- 30.Lauzier S, Maunsell E, Drolet M, et al. Wage losses in the year after breast cancer: extent and determinants among Canadian women. J Natl Cancer Inst. 2008;100:321–32. doi: 10.1093/jnci/djn028. [DOI] [PubMed] [Google Scholar]
- 31.Longo CJ, Bereza BG. A comparative analysis of monthly out-of-pocket costs for patients with breast cancer as compared with other common cancers in Ontario, Canada. Curr Oncol. 2011;18:e1–8. doi: 10.3747/co.v18i1.681. [DOI] [PMC free article] [PubMed] [Google Scholar]