Abstract
Introduction
Only approximately 25% of stage iv non-small-cell lung cancer (nsclc) patients receive systemic therapy. For such patients, we examined factors affecting referral to a cancer centre (cc) and to medical oncology (mo), and use of systemic therapy.
Methods
Using the Glans–Look Lung Cancer database, we completed a chart review of stage iv nsclc patients diagnosed in Southern Alberta during 2003–2006 and 2010–2011, comparing median overall survival (mos), referral, and treatment in the two cohorts.
Results
Of the 922 patients diagnosed in 2003–2006 and the 560 diagnosed in 2010–2011, 94% and 82% respectively were referred to a cc, with 22% and 23% receiving traditional chemotherapy (tctx). Referral to a cc or mo and use of tctx correlated with survival (p < 0.0001): The mos duration was 11.2 months in those receiving tctx and 1.0 months in those not referred to a cc. The overall mos duration was similar in the two cohorts (4.1 months vs. 3.9 months, p = 0.47). Major reasons for lack of referral to mo included poor functional status, rapid decline, and patient wish, which were similar to the reasons for forgoing tctx. In the two cohorts, 87 (9.4%) and 42 (7.5%) patients received epidermal growth factor inhibitors, with a mos duration of 16.2 months. Multivariable analysis showed that male sex [hazard ratio (hr): 1.16; p = 0.008] and pulmonary embolus (hr: 1.2; p = 0.002) correlated with worse survival. In contrast, receipt of chemotherapy (hr: 0.5; p < 0.001) and enrolment in a clinical trial (hr: 0.76; p = 0.049) correlated with better survival.
Conclusions
Our experience confirms that, over time, uptake of systemic therapy, including tctx and targeted therapy, changed little despite their established efficacy. Most of the factors limiting systemic therapy uptake appear to be non-modifiable at the time of referral. Rapid diagnosis and the availability of well-tolerated drugs for all nsclc patients will likely be the most important factors in increasing systemic therapy uptake in this population.
Keywords: Non-small-cell lung cancer, stage iv, chemotherapy, targeted therapy, immunotherapy, survival, referral patterns, reasons for no treatment
INTRODUCTION
Lung cancer is the most common cause of cancer mortality worldwide, accounting for 25% of all cancer deaths1,2. Non-small-cell lung cancer (nsclc) accounts for 85% of all lung cancer cases, with 40% of the affected patients presenting with stage iv disease3. Large prospective randomized trials and meta-analyses have demonstrated improved survival and quality of life with the use of chemotherapy in advanced nsclc4–6. Recently, significant advances in the understanding of molecular and genomic pathophysiology have opened access to new systemic treatments, including epidermal growth factor receptor (egfr) inhibitors, EML4–ALK inhibitors, and immunotherapy7–12.
With the introduction of targeted therapy and immunotherapy as part of standard treatment in stage iv nsclc, patients who would have not benefited from traditional cytotoxic chemotherapy (tctx) can now benefit from timely referral to medical oncology (mo) and other forms of systemic therapy. Yet despite the demonstrated efficacy of systemic therapy, referral to mo after a diagnosis of advanced nsclc and administration of chemotherapy or targeted therapy remains suboptimal. The referral rates to mo are estimated to be 50%–70% for all nsclc patients and 30%–60% for all advanced nsclc patients13–17.
Factors previously identified to affect referral to oncology for lung cancer patients include age, sex, race, functional status, rural residence, treatment at an academic centre, and perception by the referring physician of lack of benefit—although with conflicting results17–23. Some studies suggest that introduction of targeted agents might have changed the patterns of prescription for first-line therapy16. However, conventional cytotoxic chemotherapy is still an important part of treatment for two reasons: patients on novel therapies, after progression, still receive next-line cytotoxic chemotherapy; and most patients have mutation-negative tumours, rendering them ineligible for first-line targeted therapy options.
Our southern Alberta population-based study used retrospective chart review and correlative methods to evaluate rates of patient referral to mo after a diagnosis of stage iv nsclc, rates of systemic therapy administration (both chemotherapy and targeted agents), and the reasons that a stage iv nsclc patient might not be referred to mo or receive systemic therapy.
METHODS
Using the provincial cancer registry and the donor-funded Glans–Look Lung Cancer database (http://www.glanslook-database.ucalgary.ca), we identified all patients diagnosed with de novo stage iv nsclc in southern Alberta (including the Tom Baker Cancer Centre and its urban and rural catchment areas) between 1 January 2003 and 31 December 2006 and between 1 January 2010 and 31 December 2011. Using the electronic medical system, all patients were subsequently screened for data collection.
Non-small-cell lung cancer was staged according to the 7th edition of the American Joint Committee on Cancer staging manual. The selected time periods were designed to represent cohorts before and after provincial approval of egfr tyrosine kinase inhibitors (tkis) in 2006, the approval of second-line pemetrexed in 2008, and the adoption of routine EGFR mutation status analysis in 2010.
We collected baseline patient characteristics, referral patterns after diagnosis, treatments received, and survival data from medical chart reviews and available records compiled in the Glans–Look Lung Cancer Database. Reasons for not referring to a cancer centre (cc) or to mo and reasons for not offering (tctx) were recorded and encoded. The study was approved by the University of Calgary Conjoint Health Research Ethics Board.
Descriptive statistics were used to summarize demographic information and referral and treatment patterns. We defined overall survival as the period from diagnosis to date of death. We used Kaplan–Meier survival analyses to assess overall survival for each of the subcohorts and in comparisons of the subcohorts. Log-rank statistics were used to test the separation of survival by referral patterns and treatments. Cox proportional hazards models were used to compare the influence of specific variables on survival in terms of risk ratios. Multivariate logistic analyses were used to identify variables that significantly influenced referral patterns. The multivariate analysis included these variables: diagnosis era (2003–2006 vs. 2010–2011), age, sex, distance from a cc by postal code of the patient’s home address, enrolment in a clinical trial, presence of pulmonary embolus, and receipt of chemotherapy. A p value less than 0.05 was considered statistically significant for all analyses. All statistical analyses were performed using the SAS software application (version 9.3: SAS Institute, Cary, NC, U.S.A.).
RESULTS
The 922 patients from 2003–2006 and 560 from 2010–2011 who met the inclusion criteria had a median age of 69 years (range: 20–97 years). Table i summarizes patient and disease characteristics, which were similar in the two cohorts.
TABLE I.
Baseline patient characteristics
| Characteristic | Cohort | |
|---|---|---|
|
| ||
| 2003–2006 | 2010–2011 | |
| Patients (n) | 922 | 560 |
| Sex [n (%)] | ||
| Men | 475 (52) | 292 (52) |
| Women | 447 (48) | 268 (48) |
| Median age (years) | ||
| Median | 69 | 70 |
| Range | 32–96 | 20–97 |
| Smoking status [n (%)] | ||
| Current smoker | 297 (32) | 162 (29) |
| Former smoker | 492 (53) | 263 (47) |
| Nonsmoker | 92 (10) | 55 (10) |
| Unknown | 41 (5) | 80 (14) |
| Pulmonary embolism [n (%)] | 385 (42) | 209 (37) |
| ECOG functional status [n (%)] | ||
| 0 | 48 (5) | 33 (6) |
| 1 | 147 (16) | 88 (16) |
| 2 | 120 (13) | 52 (9) |
| 3 | 99 (11) | 38 (7) |
| 4 | 18 (2) | 6 (1) |
| Unknown | 490 (53) | 343 (61) |
| Other malignancies present [n (%)] | 154 (17) | 93 (17) |
| Tumour histology [n (%)] | ||
| Adenocarcinoma | 398 (43) | 294 (53) |
| Adenosquamous: 6 (<1) | 4 (<1) | |
| Adenocarcinoma in situ or bronchioalveolar carcinoma | 8 (1) | 3 (<1) |
| Squamous cell carcinoma | 169 (18) | 94 (17) |
| Large cell | 25 (3) | 11 (2) |
| Not otherwise specified | 316 (34) | 140 (25) |
| Unknown | 0 (0) | 14 (3) |
| EGFR receptor status [n (%)] | ||
| Not done | 920 (100) | 230 (41) |
| Positive | 114 (20) | |
| Negative | 193 (34) | |
| Unknown | 23 (4) | |
Tables ii and iii summarize, respectively, patterns of referral and treatment (Figure 1). Compared with the 2010–2011 cohort, the 2003–2006 cohort included more patients who were referred to a cc (94% vs. 82%, p < 0.0001), but in both groups, a similar proportion of patients received cytotoxic chemotherapy (22% vs. 23%). Also, a similar proportion of patients in the two cohorts received egfr tkis (9.2% vs. 7.5%, p = 0.5). No patient in either cohort received immunotherapy.
TABLE II.
Referral patterns in the 2003–2006 and 2010–2011 cohorts
| Referral variable | Cohort | |
|---|---|---|
|
| ||
| 2003–2006 (n=922) | 2010–2011 (n=520) | |
| Patients referred to cancer centre [n (%) of the cohort] | 869 (94) | 460 (88) |
| Referral made by ... [n (%) of referred pts] | ||
| Family physician | 82 (9) | 78 (17) |
| Surgeon | 101 (12) | 44 (10) |
| Respirologist | 346 (40) | 146 (32) |
| Internist | 244 (28) | 140 (30) |
| Other or unknown | 126 (14) | 52 (11) |
| Physician initially seen at cancer centre [n (%) of referred pts] | ||
| Medical oncologist (MO) | 193 (22) | 121 (26) |
| Radiation oncologist (RO) | 623 (72) | 281 (61) |
| Both MO and RO | 42 (5) | 13 (<1) |
| Respirologist | 8 (1) | 28 (6) |
| Unknown | 3 (<1) | 4 (<1) |
| Patients referred to MO [n (%) of the cohort] | 511 (59) | 285 (62) |
| Referral to MO made by ... [n (%) of referred pts] | ||
| Family physician | 26 (5) | 34 (12) |
| Surgeon | 47 (9) | 16 (3) |
| Respirologist | 85 (17) | 45 (16) |
| Internist | 83 (16) | 21 (7) |
| Radiation oncologist | 236 (46) | 114 (40) |
| Emergency physician | 2 (<1) | 0 (0) |
| Others | 32 (6) | 55 (19) |
| Patients attending the medical oncology clinic | 471 | 263 |
| As a percentage of ... | ||
| Those referred to the cancer centre | 54 | 57 |
| The cohort | 51 | 51 |
Pts = patients.
TABLE III.
Treatment patterns in the 2003–2006 and 2010–2011 cohorts
| Treatment variable | Cohort | |
|---|---|---|
|
| ||
| 2003–2006 (n=922) | 2010–2011 (n=560) | |
| Patients deemed to be candidates for CTx [n (%) of the cohort] | 324 (35) | 197 (35) |
| Patients who received ... | ||
| Systemic therapy (n) | 225 | 150 |
| As a percentage (%) of ... | ||
| Those referred to medical oncology | 44 | 52 |
| The cohort | 24 | 27 |
| First-line cytotoxic CTx (n) | 204 | 130 |
| As a percentage (%) of ... | ||
| Those referred to medical oncology | 40 | 46 |
| The cohort | 22 | 23 |
| Patients who received EGFR TKI [n (%) of the cohort] | 87 (9) | 42 (8) |
| Received both CTx and EGFR TKI (n) | 66 | 22 |
| Received EGFR TKI only (n) | 21 | 20 |
| Type of first-line cytotoxic CTx received [n (%) of those receiving first-line CTx] | ||
| Platinum doublet | 155 (76) | 102 (78) |
| Platinum doublet plus targeted agent | 20 (10) | 1 (1) |
| Non-platinum doublet | 6 (3) | 0 (0) |
| Platinum triplet | 1 (<1) | 1 (1) |
| Single agent | 11 (5) | 9 (7) |
| Single agent plus targeted agent | 3 (1) | 0 (0) |
| Other or unknown | 8 (4) | 17 (13) |
| Patients who required dose reduction or discontinuation because of toxicity [n (%)] | 46 of 204 (23) | 22 of 61 (36) |
| Patients who ... [n (%) of the cohort] | ||
| Received second-line systemic therapy | 37 (4) | 41 (7) |
| Received third-line systemic therapy | 7 (1) | 11 (2) |
| Received radiation therapy | 648 (71) | 291 (52) |
| Patients who enrolled in clinical trials [n (%) of the cohort] | 54 (6) | 9 (2) |
CTx = chemotherapy; EGFR = epidermal growth factor receptor; TKI = tyrosine kinase inhibitor.
FIGURE 1.
Patients who were diagnosed with stage IV non-small-cell lung cancer, and the proportions of those patients who were referred to a cancer centre, who were referred to a medical oncologist, and who received chemotherapy, 2003–2006 and 2010–2011 cohorts.
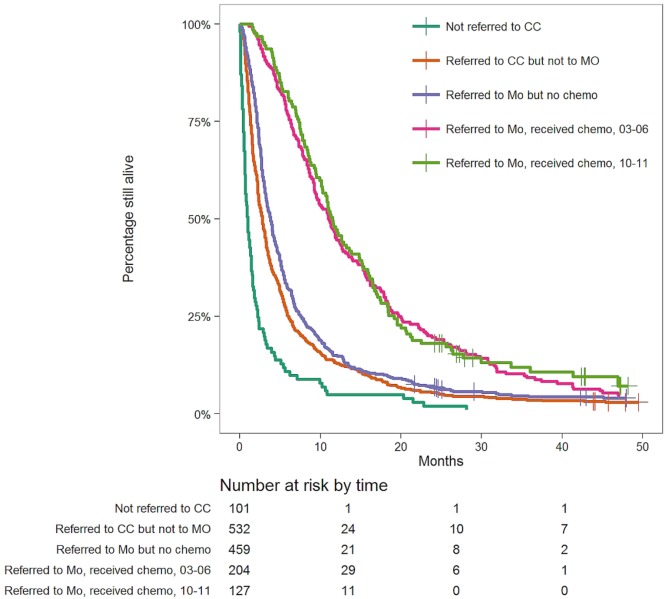
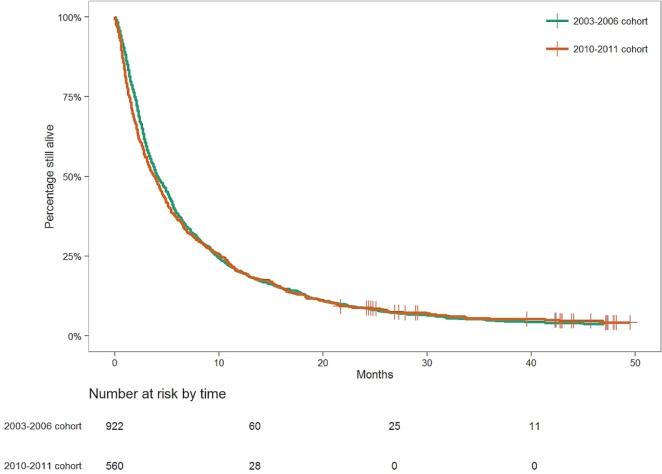
Considering all patients, median overall survival (mos) was 1.0 month in those not referred to a cc [95% confidence interval (ci): 0.7 months to 1.4 months], 2.8 months in those referred to a cc but not to mo (95% ci: 2.4 months to 3.1 months), and 3.8 months in those who were referred to mo but who did not receive (tctx) (95% ci: 3.4 months to 4.2 months). In contrast, the mos in patients receiving tctx was 11.1 months for the 2003–2006 cohort (95% ci: 9.4 months to 12.7 months) and 11.5 months for the 2010–2011 cohort (95% ci: 10.2 months to 14.8 months). Differences in mos between the foregoing five groups were all statistically significant (log-rank p < 0.0001, Figure 2), except between the two groups receiving tctx. The mos in the 2003–2006 and 2010–2011 cohorts was similar (4.1 months vs. 3.9 months, p = 0.47; Figure 3).
FIGURE 2.
Kaplan–Meyer survival curves for selected patient subgroups: those diagnosed with stage IV non-small-cell lung cancer, but not referred to a cancer centre (both study periods); those referred to a cancer centre, but not referred to a medical oncologist (both study periods); those referred to a medical oncologist, but not having received chemotherapy (both study periods); those referred to a medical oncologist and having received chemotherapy in 2003–2006; those referred to a medical oncologist and having received chemotherapy in 2010–2011. CC = cancer centre; MO = medical oncologist.
FIGURE 3.
Median survival of patients with stage IV non-small-cell lung cancer diagnosed in Southern Alberta in 2003–2006 and 2010–2011.
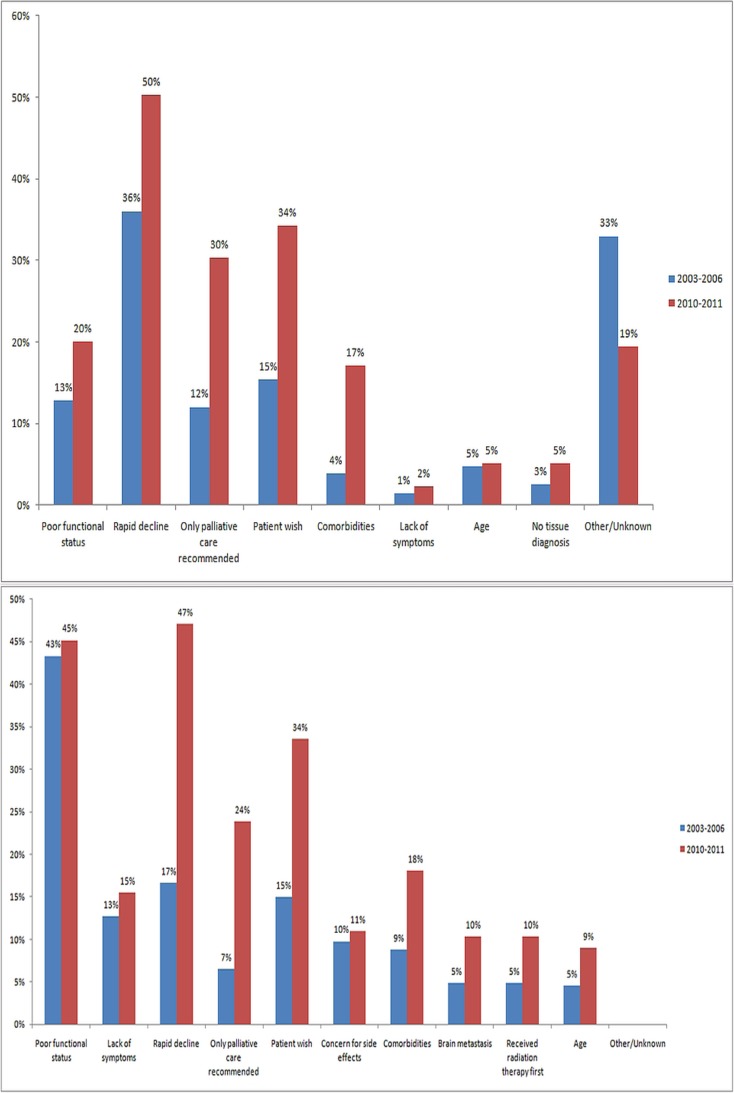
The two panels of Figure 4 illustrate the reasons that patients were not referred to mo, and the reasons that patients were not given tctx, as documented by a physician in a consultation report. Poor performance status or rapid decline in functional status, followed by patient wish, were the main reasons in both instances. Reasons for not referring to mo included poor functional status (13% in 2003–2006 vs. 20% in 2010–2011), rapid decline (36% vs. 50%), palliative care recommended as only therapy (12% vs. 30%), and patient wish (15% vs. 34%). Reasons for not giving chemotherapy were similar: poor functional status (43% vs. 45%), lack of symptoms (13% vs. 15%), rapid decline (17% vs. 47%), palliative care recommended as only therapy (7% vs. 24%), patient wish (15% vs. 34%), concern for side-effects (10% vs. 11%), and comorbidities (9% vs. 18%).
FIGURE 4.
(Top panel) Reasons for not referring a patient to a medical oncologist (denominator = patients who were referred to cancer centre, but not to a medical oncologist). (Bottom panel) Reasons for deciding against chemotherapy (denominator = patients who were referred to a medical oncologist, but who did not receive chemotherapy). Blue bars = 2003–2006 cohort; red bars = 2010–2011 cohort.
Multivariable analysis showed that male sex [hazard ratio (hr): 1.16; p = 0.008] and presence of pulmonary embolus (hr: 1.2; p = 0.002) correlated with worse survival. Meanwhile, receipt of chemotherapy (hr: 0.5; p < 0.001) and enrolment in a clinical trial (hr: 0.76; p = 0.049) correlated with better survival.
DISCUSSION
This study, conducted in a large population-based provincial sample of stage iv lung cancer patients attending a tertiary cancer centre, stratified patients into two cohorts: those diagnosed before and after major changes in treatment paradigms. The two cohorts, from 2003–2006 and 2010–2011, showed no significant differences in demographic and disease characteristics; no improvements in rates of referral to mo, in uptake of cytotoxic chemotherapy, or in use of egfr tkis; and no improvements in survival despite the introduction of major novel treatment options between the two time periods.
Our study showed that the rate of referral to mo remains suboptimal at 60%, although that rate is comparable to rates reported from other Canadian provinces24,25. Although one study conducted in the United States showed a referral rate of 88%, that study excluded patients surviving less than 2 months, those with previous malignancies, and those without full coverage for the entire period of the study23. Documented reasons for not making a referral appear largely non-modifiable at the time of referral (Figure 4); however, other complex systematic factors also likely contribute. Despite guidelines that assist primary care physicians in referring advanced lung cancer patients, therapeutic nihilism among primary care physicians might be persistent21,26–28. A delay in diagnosis might also have contributed, because data suggest that, within the current Canadian infrastructure, wait time from abnormal imaging to diagnosis and treatment of lung cancer remains in excess of 60–90 days across the country29–32. We note that, in our study, most patients were first seen by a radiation oncologist, before a medical oncologist. A delay or lack of referral could also potentially occur when physicians other than medical oncologists, who see patients as part of cancer care, might not be sufficiently informed of possible systemic therapy options, and patients engage in local therapies such as radiation therapy or a “watch and wait” approach instead25. It is particularly important that patients and non-oncologist physicians are informed of the available systemic therapy options other than tctx and radiation. Further studies are required to clarify whether patients with rapidly declining or suboptimal functional status could benefit from novel treatments.
In our cohort, the rate of chemotherapy administration remained poor at 22%–23%, without a significant change over time. The median survival duration of 3–5 months in our patients is similar to the median survival duration reported in patients with stage iv nsclc treated with best supportive care alone, and reflects a low rate of chemotherapy use in our cohort33. Some studies from British Columbia and Ontario suggest that, during a similar period, the proportion of stage iv nsclc patients who received chemotherapy increased to 35%–45% from less than 25% over time33,34. One Canadian study reports up to 50% administration of systemic therapy between 2009 and 201235. However, other centres in Canada reported no significant changes in systemic therapy administration, which is similar to the findings in our study24,36. The discrepancy between studies might in part reflect a different population composition—that is, the Alberta population has a significantly smaller proportion of people of Asian ethnicity, a higher proportion of smokers, and a lower frequency of EGFR mutation compared with the other two provinces37–39. Internationally, the published rates of chemotherapy administration have all shown a favourable trend over time, although they vary widely, from less than 10% to more than 50% in developed countries14,16,17,40. Our outcomes would therefore be considered an outlier in comparison with those from other large retrospective studies.
Our results suggest that adoption of new, less-toxic therapeutic agents for advanced nsclc might be delayed in southern Alberta. For instance, provincial funding approval for pemetrexed in the second-line setting was given in 2008; in the first-line setting, it was given in 2014. In our study, only 6 patients in the 2003–2006 cohort and 28 patients in the 2010–2011 cohort received pemetrexed. Furthermore, EGFR mutation testing and administration of tkis were underutilized in both cohorts, despite landmark trials in 2003 and 20108,41–43. In particular, between 2010 and 2011, approximately 20% of patients tested positive for an EGFR mutation, and yet only 8% received an appropriate tki in the first-line setting. Uptake remains far inferior to what has been reported in other centres44,45, indicating a need for quality assurance processes to promote adherence to the standard of care. Part of the process should entail education for physicians involved in the diagnostic process with respect to appropriate work-up within an acceptable timeframe, and introduction of reflex tumour marker testing. A similar pattern of delayed uptake of PD-1 inhibitors in the first- and second-line settings has been observed, despite recent data proving efficacy and tolerability, because of the national and provincial funding approval process; as of November 2017, first-line use of PD-1 inhibitors had just recently been approved in Canada; PD-1 inhibitors for second-line use had become publicly funded only in early 2017. Taken together, those patterns highlight the importance of timely incorporation of more tolerable systemic options into practice both at the provincial level and at the level of individual oncologists. Although education and more-inclusive referral to mo are essential to appropriate patient selection for systemic therapy, timely regulatory approval and funding for effective and tolerable systemic therapies will likely also play a significant role in improving outcomes, given that few other clinical factors that affect administration of systemic therapy are modifiable at the time of referral.
Our study results reveal that, although the proportion of patients receiving second-line systemic therapy increased over time, most patients did not receive further therapy after their first-line treatment. Since 2015, immunotherapy with PD-1 inhibitors has become a standard second-line treatment option that is superior to docetaxel10–12. In the keynote-024 trial, approximately 40% of the patients who initially received chemotherapy crossed over to immunotherapy, and in the CheckMate-026 trial, almost 60% of patients who progressed on first-line therapy received second-line therapy12,46. Those percentages far surpass the rate of second-line systemic therapy administration in our cohort and suggest that novel therapies have the potential to allow for continued sequential therapies in many patients with stage iv nsclc. Furthermore, given the results of recently reported trials, PD-1 inhibitors have become a standard first-line treatment option in stage iv nsclc showing PD-L1 staining of 50% or more46–48. Although the clinical trials selected patients with an Eastern Cooperative Oncology Group performance status of 0 or 1, immunotherapy with PD-1 inhibitors has the potential to benefit any patient who otherwise would have been unsuitable for, or would have chosen not to take, chemotherapy because of concern for toxicity. We previously presented data suggesting that physicians are more willing to try PD-1 inhibitors in patients whose performance status is significantly worse than in the original clinical trials (Nixon NA, Li H, D’Silva A, Bebb G, Verma S. Single institution experience of nivolumab for second-line therapy of non–small cell lung cancer. Presented at the 2nd Annual Young Investigators Forum in Non–Small Cell Lung Cancer; Atlanta, GA, U.S.A.; 9–11 March 2017). Ensuring rapid screening, diagnosis, and treatment will be key to ensuring that patients are offered immunotherapy and non-chemotherapy systemic therapy options in a timely fashion.
Overall, a patient’s functional status and rate of deterioration were some of the most common reasons for not referring the patient to mo or offering chemotherapy. Although age alone accounted for less than 10% of the reasons given for patients not pursuing chemotherapy, advanced age could have contributed to other reasons, such as patient wish, palliative care only indicated, or comorbidities. However, it is appropriate that the biologic, rather than physical, age of the patient be considered in determining appropriate therapy. Among the reasons for deciding against chemotherapy, lack of symptoms constituted 13%–15%. Older evidence suggests that deferred initiation of chemotherapy until after symptoms appear could be associated with a survival outcome similar to that with immediate initiation in metastatic solid malignancies49. That hypothesis might have to be re-examined as contemporary and more tolerable therapies are developed and increasingly incorporated. For example, a Canadian retrospective study showed that patients with stage iiib or iv nsclc who followed a watch-and-wait strategy often missed the window of opportunity for systemic therapy25. Notably, in our study, “patient wish” was documented as the reason for no referral or chemotherapy in about 15%–34% of patients with stage iv nsclc. With the advent of novel well-tolerated treatment options, a fully informed consent process requires that oncologists and other physicians counsel patients carefully about the benefits and limitations of available treatment options.
The strengths of our study include the fact that all patients diagnosed with stage iv nsclc in southern Alberta and assigned to the Tom Baker Cancer Centre were included, creating a large sample representative of the provincial population. Limitations of the study include an imbalance in the size of the cohorts from 2003–2006 and 2010–2011 (attributable to data availability in the cancer registry). However, despite the differences in cohort size, we observed no significant imbalance in patient and disease characteristics between the groups. We also included only patients diagnosed with de novo stage iv nsclc; patients who had localized nsclc and whose disease recurred were not included because their treatment choices and prognosis remained variable, depending on the treatments initially received. Our results were also limited by what the oncologists chose to record in charts and by other potential confounders not identifiable through the available data. Lastly, the study was conducted in a single cancer centre, limiting its generalizability, but still providing valuable information about outcomes in a tertiary Canadian centre and in comparison with other Canadian and international experiences. Finally, our study was not designed to review the effect that the 2008 reorganization of health care delivery into a single centralized provincial model—Alberta Health Services—might have had on the metrics presented here.
CONCLUSIONS
Systemic therapies remain the only option that can significantly prolong survival in patients with stage iv nsclc. However, the use of chemotherapy and newer systemic lung cancer therapies in southern Alberta remains suboptimal, with underutilization of newer targeted agents. We identified many potential barriers to systemic therapy and suboptimal referral rates to a cc and to mo. Our study shows that several areas in the referral and treatment process show potential for improvement, including rapid diagnosis, patient and physician education, and optimization of access to novel systemic therapies. Ensuring that the frontline physicians who diagnose nsclc are informed about the potential benefits of systemic therapy at an advanced stage is crucial for increasing oncology referrals and guaranteeing that patients make well-informed decisions. Nevertheless, the non-modifiable nature of the most common reasons for systemic treatment not being given suggests that the biggest effect in increasing uptake will be the adoption of more-tolerable, less-toxic agents, possibly exemplified by the recent wave of new immunomodulating monoclonal antibodies.
ACKNOWLEDGMENTS
This study was supported by patient–donors who contributed to the creation and maintenance of the Glans–Look Lung Cancer Database.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: JJK has received honoraria from AstraZeneca, Merck, and Janssen. DGB reports grants from AstraZeneca, grants from Boehringer Ingelheim, and honoraria from Roche, Novartis, Lilly, Pfizer, Bristol–Myers Squibb, and Merck. The remaining authors have no conflicts to disclose.
REFERENCES
- 1.GLOBOCAN 2012 . Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012 [Web page] Lyon, France: IARC; 2012. [Available at: http://globocan.iarc.fr/Default.aspx; cited 10 November 2017] [Google Scholar]
- 2.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2013. Toronto, ON: Canadian Cancer Society; 2013. [Google Scholar]
- 3.Socinski MA, Crowell R, Hensing TE, et al. on behalf of the American College of Chest Physicians Treatment of non–small cell lung cancer, stage iv: accp evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(suppl):277S–89S. doi: 10.1378/chest.07-1381. [DOI] [PubMed] [Google Scholar]
- 4.nsclc Meta-Analyses Collaborative Group Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26:4617–25. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non–Small Cell Lung Cancer Collaborative Group. BMJ. 1995;311:899–909. doi: 10.1136/bmj.311.7010.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delbaldo C, Michiels S, Syz N, Soria JC, Le Chevalier T, Pignon JP. Benefits of adding a drug to a single-agent or a 2-agent chemotherapy regimen in advanced non-small-cell lung cancer: a meta-analysis. JAMA. 2004;292:470–84. doi: 10.1001/jama.292.4.470. [DOI] [PubMed] [Google Scholar]
- 7.Rosell R, Carcereny E, Gervais R, et al. on behalf of the Spanish Lung Cancer Group in collaboration with the Groupe Français de Pneumo-Cancérologie and the Associazione Italiana Oncologia Toracica Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation–positive non-small-cell lung cancer (eurtac): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 8.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 9.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [Erratum in: N Engl J Med 2015;373:1582] [DOI] [PubMed] [Google Scholar]
- 10.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1–positive, advanced non-small-cell lung cancer (keynote-010): a randomised controlled trial. Lancet. 2016;387:1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 13.Pai RK, Rathinam S, Sharma V, et al. Postoperative oncological referral patterns for adjuvant treatment of patients undergoing curative resections for non-small-cell lung cancer at a regional thoracic centre. Eur J Cardiothorac Surg. 2010;37:782–6. doi: 10.1016/j.ejcts.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 14.Stevens W, Stevens G, Kolbe J, Cox B. Lung cancer in New Zealand: patterns of secondary care and implications for survival. J Thorac Oncol. 2007;2:481–93. doi: 10.1097/JTO.0b013e31805fea3a. [DOI] [PubMed] [Google Scholar]
- 15.Winget M, Stanger J, Gao Z, Butts C. Predictors of surgery and consult with an oncologist for adjuvant chemotherapy in early stage nsclc patients in Alberta, Canada. J Thorac Oncol. 2009;4:629–34. doi: 10.1097/JTO.0b013e31819ccf26. [DOI] [PubMed] [Google Scholar]
- 16.Ritzwoller DP, Carroll NM, Delate T, et al. Patterns and predictors of first-line chemotherapy use among adults with advanced non-small cell lung cancer in the cancer research network. Lung Cancer. 2012;78:245–52. doi: 10.1016/j.lungcan.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Earle CC, Neumann PJ, Gelber RD, Weinstein MC, Weeks JC. Impact of referral patterns on the use of chemotherapy for lung cancer. J Clin Oncol. 2002;20:1786–92. doi: 10.1200/JCO.2002.07.142. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg ER, Chute CG, Stukel T, et al. Social and economic factors in the choice of lung cancer treatment. A population-based study in two rural states. N Engl J Med. 1988;318:612–17. doi: 10.1056/NEJM198803103181006. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg ER, Dain B, Freeman D, Yates J, Korson R. Referral of lung cancer patients to university hospital cancer centers. A population-based study in two rural states. Cancer. 1988;62:1647–52. doi: 10.1002/1097-0142(19881015)62:8<1647::AID-CNCR2820620832>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 20.Smith TJ, Penberthy L, Desch CE, et al. Differences in initial treatment patterns and outcomes of lung cancer in the elderly. Lung Cancer. 1995;13:235–52. doi: 10.1016/0169-5002(95)00496-3. [DOI] [PubMed] [Google Scholar]
- 21.Wassenaar TR, Eickhoff JC, Jarzemsky DR, Smith SS, Larson ML, Schiller JH. Differences in primary care clinicians’ approach to non–small cell lung cancer patients compared with breast cancer. J Thorac Oncol. 2007;2:722–8. doi: 10.1097/JTO.0b013e3180cc2599. [DOI] [PubMed] [Google Scholar]
- 22.Hardy D, Liu CC, Xia R, et al. Racial disparities and treatment trends in a large cohort of elderly black and white patients with nonsmall cell lung cancer. Cancer. 2009;115:2199–211. doi: 10.1002/cncr.24248. [DOI] [PubMed] [Google Scholar]
- 23.Goulart BH, Reyes CM, Fedorenko CR, et al. Referral and treatment patterns among patients with stages iii and iv non-small-cell lung cancer. J Oncol Pract. 2013;9:42–50. doi: 10.1200/JOP.2012.000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawe DE, Pond GR, Ellis PM. Assessment of referral and chemotherapy treatment patterns for elderly patients with non-small-cell lung cancer. Clin Lung Cancer. 2016;17:563–72.e2. doi: 10.1016/j.cllc.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Noonan K, Tong KM, Laskin J, et al. Evaluation of a “watch and wait” approach for chemotherapy in patients with newly diagnosed advanced non-small cell lung cancer from a diverse community population. Clin Oncol (R Coll Radiol) 2015;27:505–13. doi: 10.1016/j.clon.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Abdolmohammadi A, Sears W, Rai S, Pan J, Alexander J, Kloecker G. Survey of primary care physicians on therapeutic approaches to lung and breast cancers. South Med J. 2014;107:437–42. doi: 10.14423/SMJ.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 27.Alberts WM, Bepler G, Hazelton T, Ruckdeschel JC, Williams JH, Jr, on behalf of the American College of Chest Physicians Lung cancer. Practice organization. Chest. 2003;123(suppl):332S–7S. doi: 10.1378/chest.123.1_suppl.332S. [DOI] [PubMed] [Google Scholar]
- 28.Del Giudice ME, Young SM, Vella ET, et al. Guideline for referral of patients with suspected lung cancer by family physicians and other primary care providers. Can Fam Physician. 2014;60:711–6. e376–82. [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung WY, Butler JR, Kliewer EV, et al. Analysis of wait times and costs during the peri-diagnostic period for non–small cell lung cancer. Lung Cancer. 2011;72:125–31. doi: 10.1016/j.lungcan.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Evans SM, Earnest A, Bower W, Senthuren M, McLaughlin P, Stirling R. Timeliness of lung cancer care in Victoria: a retrospective cohort study. Med J Aust. 2016;204:75.e1–9. doi: 10.5694/mja15.01026. [DOI] [PubMed] [Google Scholar]
- 31.Lo DS, Zeldin RA, Skrastins R, et al. Time to treat: a system redesign focusing on decreasing the time from suspicion of lung cancer to diagnosis. J Thorac Oncol. 2007;2:1001–6. doi: 10.1097/JTO.0b013e318158d4b6. [DOI] [PubMed] [Google Scholar]
- 32.Van de Vosse D, Chowdhury R, Boyce A, Halperin R. Wait times experienced by lung cancer patients in the BC Southern Interior to obtain oncologic care: exploration of the intervals from first abnormal imaging to oncologic treatment. Cureus. 2015;7:e330. doi: 10.7759/cureus.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho C, Ramsden K, Zhai Y, et al. Less toxic chemotherapy improves uptake of all lines of chemotherapy in advanced non-small-cell lung cancer: a 10-year retrospective population-based review. J Thorac Oncol. 2014;9:1180–6. doi: 10.1097/JTO.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 34.Yu JL, Simmons C, Victor JC, et al. Impact of new chemotherapeutic and targeted agents on survival in stage iv non–small cell lung cancer. Oncologist. 2011;16:1307–15. doi: 10.1634/theoncologist.2011-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brule SY, Al-Baimani K, Jonker H, et al. Palliative systemic therapy for advanced non–small cell lung cancer: investigating disparities between patients who are treated versus those who are not. Lung Cancer. 2016;97:15–21. doi: 10.1016/j.lungcan.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Sacher AG, Le LW, Lau A, Earle CC, Leighl NB. Real-world chemotherapy treatment patterns in metastatic non-small cell lung cancer: are patients undertreated? Cancer. 2015;121:2562–9. doi: 10.1002/cncr.29386. [DOI] [PubMed] [Google Scholar]
- 37.Statistics Canada . NHS Focus on Geography Series. “Provinces and Territories” [Web page] Ottawa, ON: Statistics Canada; 2016. [Available at: http://www12.statcan.gc.ca/nhs-enm/2011/as-sa/fogs-spg/Pages/ProvinceSelector.cfm?lang=E&level=2; cited 10 November 2017] [Google Scholar]
- 38.Murphy A. The Risks of Tobacco Use on Overall Health (slide presentation). Edmonton, AB: Alberta Health Services, Tobacco Reduction Program; 2011. [Available online at: http://www.albertahealthservices.ca/assets/about/scn/ahs-scn-cvs-vrr-risk-of-tobacco-use-on-overall-health-slide-set.pdf; cited 10 November 2017] [Google Scholar]
- 39.British Columbia, Ministry of Health Services . BC’s tobacco control strategy: targeting our efforts [Web page]. Victoria, BC: Government of British Columbia; 2004. [Available at: https://www2.gov.bc.ca/assets/gov/government/ministries-organizations/ministries/health/targetingoureffort.pdf; cited 10 November 2017] [Google Scholar]
- 40.Earle CC, Venditti LN, Neumann PJ, et al. Who gets chemotherapy for metastatic lung cancer? Chest. 2000;117:1239–46. doi: 10.1378/chest.117.5.1239. [DOI] [PubMed] [Google Scholar]
- 41.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation–positive non-small-cell lung cancer (optimal, ctong-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 42.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. on behalf of the saturn investigators Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–9. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 43.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 44.Keam B, Kim DW, Park JH, et al. How molecular understanding affects to prescribing patterns and clinical outcome of gefitinib in non-small cell lung cancer? 10 year experience of single institution. Cancer Res Treat. 2013;45:178–85. doi: 10.4143/crt.2013.45.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takano T, Fukui T, Ohe Y, et al. EGFR mutations predict survival benefit from gefitinib in patients with advanced lung adenocarcinoma: a historical comparison of patients treated before and after gefitinib approval in Japan. J Clin Oncol. 2008;26:5589–95. doi: 10.1200/JCO.2008.16.7254. [DOI] [PubMed] [Google Scholar]
- 46.Socinski M, Creelan B, Horn L, et al. CheckMate 026: a phase 3 trial of nivolumab vs investigator’s choice of platinum-based doublet chemotherapy as first-line therapy for stage iv/recurrent programmed death ligand 1–positive nsclc [abstract LBA7_PR]. Ann Oncol. 2016;27(suppl 6) [Google Scholar]
- 47.Langer C, Gaddgeel SM, Borghaei H, et al. Randomized, phase 2 study of carboplatin and pemetrexed with or without pembrolizumab as first-line therapy for advanced nsclc: keynote-021 cohort G [abstract LBA46_PR]. Ann Oncol. 2016;27(suppl 6) doi: 10.1093/annonc/mdw435.45. [DOI] [Google Scholar]
- 48.Reck M, Rodriguez-Abreu D, Robinson AG, et al. on behalf of the keynote-024 investigators Pembrolizumab versus chemotherapy for PD-L1–positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 49.Carden CP, Rosenthal MA. Immediate versus delayed chemotherapy in patients with asymptomatic incurable metastatic cancer. Asia Pac J Clin Oncol. 2007;3:187–98. [Google Scholar]