Abstract
Background
As cure rates for breast cancer improve, there is increasing evidence that late effects of treatment—and impaired fertility in particular—are emerging as important concerns among young breast cancer survivors. Older reports have evaluated the occurrence of amenorrhea after treatment, but few data have been reported about the incidence of biochemical evidence for impaired ovarian function in patients who do not become overtly menopausal.
Methods
We conducted a cross-sectional study evaluating anti-Müllerian hormone (amh) in premenopausal chemotherapy-treated breast cancer survivors and control patients. Random serum levels of amh and other relevant clinical data were collected for 100 premenopausal chemotherapy-treated breast cancer survivors and 76 control subjects. Subgroup analyses were performed for women with regular menstrual cycles at the time of amh testing.
Results
After adjustment for age, amh was significantly lower in the overall group of patients receiving chemotherapy (p = 0.002) and in the subgroup reporting normal cycles (p = 0.03). Cyclophosphamide produced a significant dose-dependent reduction in amh (p < 0.001); trastuzumab was associated with increased amh in survivors with normal cycles. Overall, serum amh in survivors was roughly equivalent to that measured in control patients 12 years older.
Conclusions
Young breast cancer survivors often experience significant impairment of ovarian function despite having normal menstrual cycles after treatment. Those results have important implications for patient counselling and the timing of possible referral to a fertility specialist.
Keywords: Anti-Müllerian hormone, breast cancer survivors, ovarian reserve, chemotherapy
INTRODUCTION
Although breast cancer is typically a disease of elderly women, approximately 6% of cases occur in women less than 40 years of age, and the 5-year survival rate for those women is approximately 85%1–3. Consequently, the long-term toxicities of breast cancer treatment are of increasing relevance, and several studies have found that fertility-related concerns greatly affect quality of life for young breast cancer survivors3–6. Such issues are likely to become more important given that the number of women delaying childbirth is increasing7.
Most young women with breast cancer are treated with adjuvant chemotherapy8. Prior studies have reported that chemotherapy induces premature ovarian failure in 20%–80% of premenopausal women9–13, with the wide range reflecting variations in age at treatment, follow-up duration, the definition of ovarian failure, and the chemotherapy regimen studied. Preferred chemotherapy regimens have changed since the reports of premature ovarian failure first emerged, with greater use of anthracyclines and taxanes (which have a more variable effect on ovarian function) and with the introduction of trastuzumab (for which no published data about ovarian toxicity are available)14.
Moreover, ovarian failure is a crude marker of potential fertility, because fertility could be significantly impaired despite normal menstruation15. Clearly, for breast cancer survivors seeking counselling about post-treatment reproductive options, an earlier actionable indicator of ovarian reserve is needed. Anti-Müllerian hormone (amh), a glycoprotein of the transforming growth factor β family produced by the granulosa cells of the ovary from birth until menopause, is measurable in serum16,17. Serum amh indirectly reflects the oocyte or primordial follicle pool, which declines over time, making it a good marker of ovarian reserve18–20. Unlike inhibin B and follicle-stimulating hormone, serum amh is relatively stable across the menstrual cycle, and amh measurement is less invasive than transvaginal ultrasonic assessment of antral follicle count21.
Studies have already demonstrated a decline in serum amh after chemotherapy for breast cancer15,22–26, although they generally have not had sample sizes or follow-up durations adequate for multivariable analyses of the long-term effects on ovarian function of chemotherapy dose, patient age, and other clinical factors. Many studies have analyzed amenorrheic and cycling patients together, which obscures the distinctive usefulness of amh in addition to menstrual history15,23–26.
Given the limited available data about how serum amh and other clinical factors could be used to counsel patients and to direct posttreatment fertility management in the oncology outpatient setting, the purpose of the present study was to evaluate how combinations of age, menstrual status, chemotherapy agent and dose, and posttreatment serum amh relate to ovarian function in terms that could inform the appropriate timing of specialized fertility evaluation and the feasibility of assisted reproduction.
METHODS
Patient Recruitment
This cross-sectional comparative study identified and enrolled female breast cancer survivors from outpatient oncology clinics at the Princess Margaret Cancer Centre and the Odette Cancer Centre, Sunnybrook Hospital, Toronto, Ontario. Eligible patients had histologically confirmed breast cancer diagnosed when they were 40 years of age or younger, had received curative-intent chemotherapy with or without radiotherapy, and were now 50 years of age or younger. Exclusion criteria included amenorrhea more than 18 months after treatment and any breast cancer recurrence. Control subjects were recruited from two sources: women who had recently undergone surgery for breast cancer with curative intent but who had not yet received any adjuvant systemic therapy, and healthy volunteers. Patients attending surgical clinics for benign breast disease were also approached by direct contact in clinic and by flyer distribution in the clinic areas. Controls had to be premenopausal and between 25 and 50 years of age. Exclusion criteria for healthy volunteers included history or investigation for infertility, or any prior diagnosis of cancer. Exclusion criteria for all patients were oophorectomy, use of oral contraceptives within the preceding 12 weeks, current pregnancy, and another cancer diagnosis (excluding non-melanoma skin cancer).
Data Collection and AMH Testing
For breast cancer survivors, clinical information including age at diagnosis, disease stage, surgical procedures, obstetric history, radiotherapy treatments, chemotherapy regimens, and menstrual patterns before and after chemotherapy were abstracted from the medical record.
Serum amh testing was performed at a random time in relation to the menstrual cycle. The amh assays were run in batches on stored samples using the AMH Gen II ELISA kit (Beckman Coulter, Brea, CA, U.S.A.) with a limit of detection of 0.57 pmol/L. Lower nominal levels of serum amh could be detected as 0.57 pmol/L, but could not be accurately quantified.
Statistics and Data Analysis
Demographic variables in the control and chemotherapy groups were compared using either a Wilcoxon rank-sum test (for all variables except menstrual cycle patterns) or a Fisher exact test (for menstrual cycle patterns).
To account for the limit of detection of the AMH Gen II ELISA kit, amh was modelled by censored linear regression with a lower limit of 0.57 pmol/L in the CensReg package (version 0.5–4) for the R software application (The R Foundation, Vienna, Austria)27–29. For regressions of amh on attained age and either chemotherapy status or use of cyclophosphamide, the best model was determined by a likelihood ratio test. Multivariate models were developed using a stepwise approach (both backward elimination and forward selection) and the Akaike information criterion to determine the optimal model in the MASS package (version 7.3–29) for the R software application30.
In models including all patients (regular and irregular menses), the initial model contained age at diagnosis, attained age, cyclophosphamide dose, menstrual pattern during chemotherapy and at the time of amh testing, and the interaction terms age at diagnosis with cyclophosphamide dose and attained age with menstrual pattern at the time of amh testing. For modelling of the chemotherapy patients with regular menses at the time of amh testing, the initial model contained age at diagnosis, attained age, cyclophosphamide dose, menstrual pattern during chemotherapy, and the interaction term age at diagnosis with cyclophosphamide. The live birth probability after in vitro fertilization (ivf) was determined for each individual using the logistic model proposed by La Marca et al.31, which considers attained age and serum amh.
Ethics approval for the study was granted by the research ethics boards of the University Health Network and Sunnybrook Health Sciences Centre. Informed consent was obtained from all participants.
RESULTS
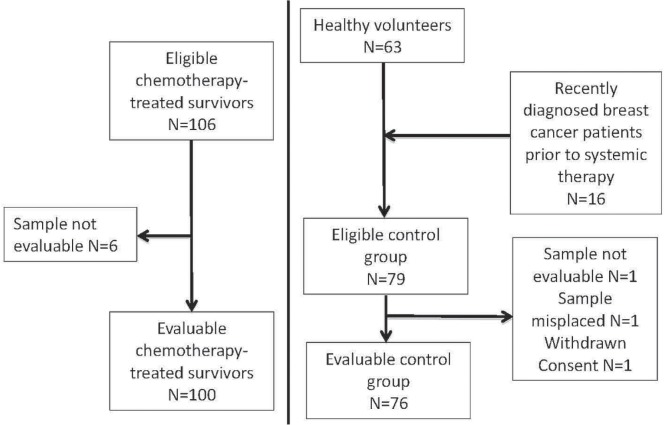
Of the 106 chemotherapy-treated breast cancer survivors with stages i–iii disease who were enrolled and were tested for serum amh, 100 were evaluable (Figure 1). Their median age at initiation of chemotherapy was 36.2 years (range: 28–41 years). Serum amh was tested a median of 2 years after chemotherapy (range: 0.04–13.5 years), at a median age of 39.2 years (range: 28–50 years; Table i).
FIGURE 1.
CONSORT diagram of patient recruitment to the study.
TABLE I.
Demographics and chemotherapy (CTx) exposures for the young breast cancer (BCa) survivors
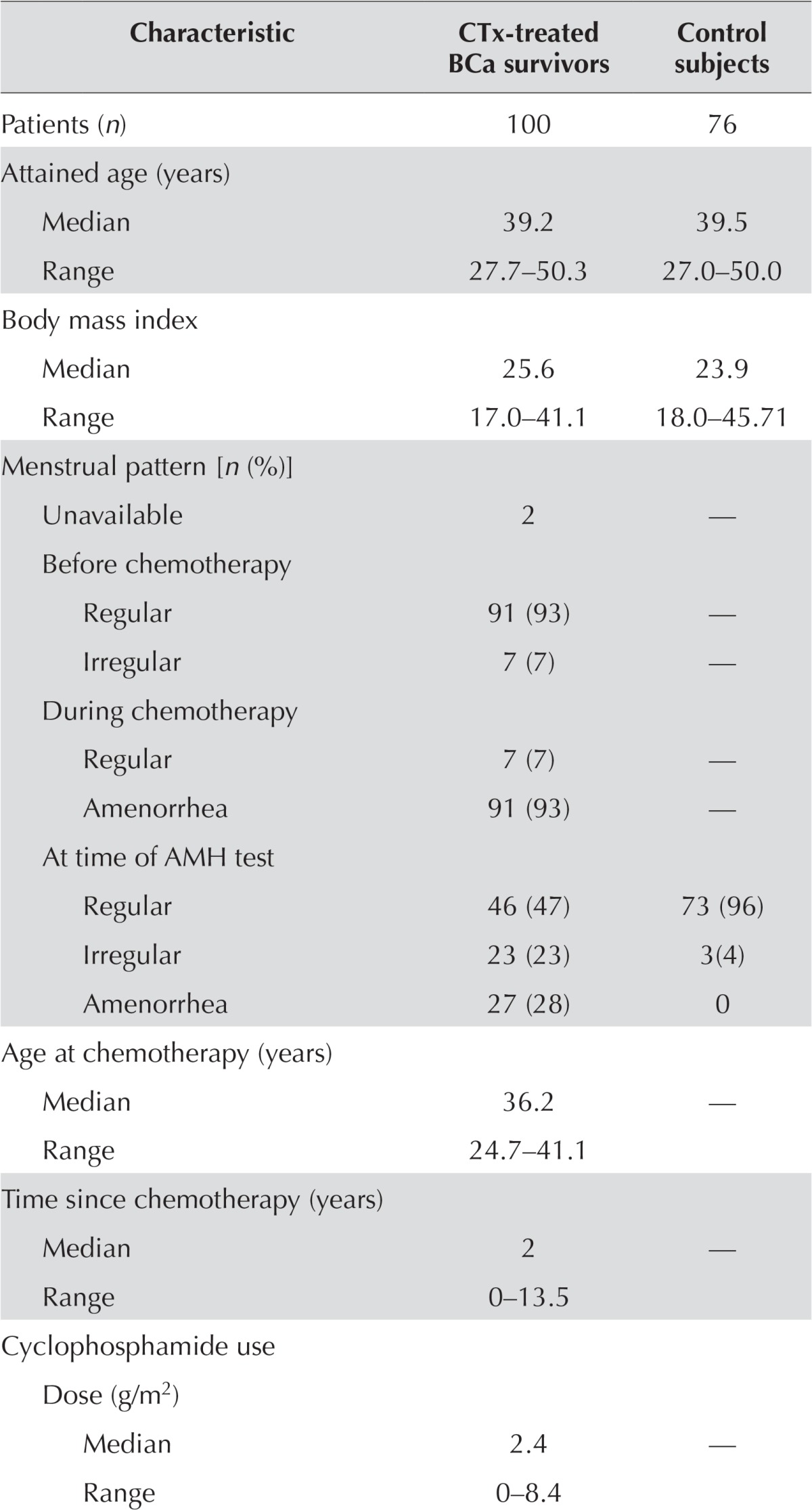
| Characteristic | CTx-treated BCa survivors | Control subjects |
|---|---|---|
| Patients (n) | 100 | 76 |
| Attained age (years) | ||
| Median | 39.2 | 39.5 |
| Range | 27.7–50.3 | 27.0–50.0 |
| Body mass index | ||
| Median | 25.6 | 23.9 |
| Range | 17.0–41.1 | 18.0–45.71 |
| Menstrual pattern [n (%)] | ||
| Unavailable | 2 | — |
| Before chemotherapy | ||
| Regular | 91 (93) | — |
| Irregular | 7 (7) | — |
| During chemotherapy | ||
| Regular | 7 (7) | — |
| Amenorrhea | 91 (93) | — |
| At time of AMH test | ||
| Regular | 46 (47) | 73 (96) |
| Irregular | 23 (23) | 3(4) |
| Amenorrhea | 27 (28) | 0 |
| Age at chemotherapy (years) | ||
| Median | 36.2 | — |
| Range | 24.7–41.1 | |
| Time since chemotherapy (years) | ||
| Median | 2 | — |
| Range | 0–13.5 | |
| Cyclophosphamide use | ||
| Dose (g/m2) | ||
| Median | 2.4 | — |
| Range | 0–8.4 | |
| Dose group [n (%)] | ||
| 0 g/m2 | 4 (4) | |
| <2 g/m2 | 33 (33) | |
| ≥2 g/m2 | 60 (60) | |
| Unavailable | 3 (3) | |
| Tamoxifen use [n (%)] | ||
| Ongoing | 42 (42) | 1 (1) |
| No | 49 (49) | 75 (99) |
| Previous | 9 (9%) | — |
| Trastuzumab use [n (%)] | ||
| Yes | 25 (25) | — |
| No | 74 (74) | — |
| Unavailable | 1 (1) | — |
| Other hormonal agents [n (%)] | ||
| Goserelin | 3 (3)a | — |
| Leuprolide | 7 (7)b | 1 (1) |
| Aromatase inhibitor | 1 (1)c | — |
| Oral contraceptive pill | - | 3 (4) |
| Levonorgestrel-releasing IUD | - | 1 (1) |
| Nil | 90 (90) | 71 (93) |
| AMH (pmol/L) | ||
| Median | 0.57 | 7.47 |
| Range | 0.57–31.34 | 0.57–106.5 |
Concurrent with tamoxifen in 2 patients; concurrent with aromatase inhibitor in 1 patient.
Concurrent with tamoxifen in 6 patients.
Concurrent with goserelin.
AMH = anti-Müllerian hormone; IUD = intrauterine device.
Table ii describes the chemotherapy regimens used. The most common regimens were fec-d (5-fluorouracil–epirubicin–cyclophosphamide–docetaxel) and act (doxorubicin–cyclophosphamide–paclitaxel). Overall, 96% of patients received a cyclophosphamide-containing regimen, and the median cumulative cyclophosphamide dose received was 2.4 g/m2 (range: 0–8.4 g/m2). Adjuvant trastuzumab was given to 25 patients. Of the 98 patients for whom menstrual pattern data were available at the time of amh testing, 46 (47%) reported regular cycles (defined as a cycle length of 22–35 days32), and 27 (28%) reported irregular cycles or fewer than 18 months of amenorrhea in the period immediately after chemotherapy.
TABLE II.
Chemotherapy regimens used in young breast cancer survivors
| Regimen | Patients [n (%)] |
|---|---|
| 5-Fluorouracil, epirubicin, cyclophosphamide, docetaxel (FEC-D) | 34 (34) |
| Doxorubicin, cyclophosphamide, paclitaxel (ACT) | 30 (30) |
| Cyclophosphamide, epirubicin, 5-fluorouracil (CEF) | 9 (9) |
| Docetaxel, cyclophosphamide (TC) | 7 (7) |
| Doxorubicin, cyclophosphamide (AC) | 4 (4) |
| Cyclophosphamide, methotrexate, 5-fluorouracil (CMF) | 3 (3) |
| 5-Fluorouracil, epirubicin, cyclophosphamide (FEC) | 3 (3) |
| Doxorubicin, cyclophosphamide, docetaxel (ACD) | 2 (2) |
| Other | 8 (8) |
| TOTAL | 100 |
Of the 79 eligible control subjects (63 healthy volunteers and 16 pretreatment breast cancer patients), 76 were evaluable (Figure 1). Median age was 39.5 years (range: 27–50 years). Of the control subjects, 73 (96%) reported regular menstrual cycles. There was no significant difference in attained age between the control subjects and the chemotherapy-treated breast cancer survivors (p = 0.20).
Effect of Chemotherapy on Serum AMH
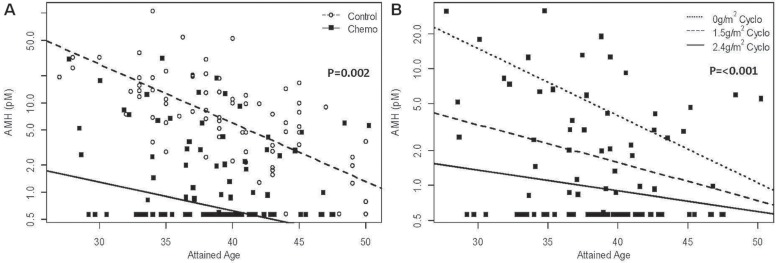
Figure 2(A) shows the decline in serum amh with advancing age in chemotherapy-treated survivors and control subjects. In the survivors overall, chemotherapy significantly lowered serum amh (average reduction: 2.33 log units; 95% confidence interval: −3.809 log units to −0.861 log units; p = 0.002 compared with the control subjects). However, the amh results were substantially dispersed, such that 16% of chemotherapy-treated survivors had serum amh levels within the 95% confidence interval of the logAMH regression line for the control subjects.
FIGURE 2.
Censored regression of anti-Müllerian hormone (AMH) versus attained age (A) in all chemotherapy (chemo)–treated patients and controls, and (B) in patients stratified by cumulative dose of cyclophosphamide.
Figure 2(B) illustrates the significant effect of increasing cyclophosphamide exposure on ovarian reserve. After adjusting for attained age, serum amh was significantly lower in patients receiving a total cyclophosphamide dose of 1.5 g/m2 or more (p < 0.001). In addition, an increasing cyclophosphamide dose significantly reduced the association between increasing attained age and serum amh level (p = 0.012) by lowering serum amh in younger survivors.
Clinical Factors Associated with Serum AMH in Chemotherapy-Treated Patients
In the survivor cohort overall, univariable analysis found significantly lower levels of serum amh with increasing age at diagnosis (p = 0.002), with the presence of amenorrhea during chemotherapy (p < 0.001 compared with regular cycles), with irregular cycles immediately after chemotherapy (p = 0.008 compared with regular cycles), and with irregular cycles (p = 0.003) or amenorrhea (p = 0.001) at the time of the amh test (Table iii). We observed a trend toward decreasing serum amh with attained age (p = 0.092).
TABLE III.
Univariate and multivariate regression analyses of the effect of indicated predictors on log(anti-Müllerian hormone) among young chemotherapy (CTx)–treated breast cancer survivors
| Predictor | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| β | 95% CI | p Value | β | 95% CI | p Value | |
| Age at diagnosis | −0.075 | −0.121 to −0.029 | 0.002 | −0.061 | −0.099 to −0.023 | 0.002 |
| Cyclophosphamide use (g/m2) | −0.050 | −0.18 to 0.081 | 0.455 | −0.111 | −0.214 to −0.008 | 0.035 |
| Menstrual patterna | ||||||
| Irregular or amenorrhea during CTx | −1.215 | −1.870 to −0.561 | <0.001 | −0.953 | −1.465 to −0.441 | <0.001 |
| Irregular at AMH test | −0.695 | −1.154 to −0.237 | 0.0030 | −0.724 | −1.057 to −0.392 | <0.001 |
| Attained age | −0.037 | −0.080 to 0.006 | 0.092 | |||
| Body mass index | −0.010 | −0.045 to 0.025 | 0.569 | |||
| Menstrual patterna | ||||||
| Irregular before CTx | 0.358 | −0.401 to 1.112 | 0.356 | |||
| Amenorrhea after CTx | −4.939 | −1570 to 1561 | 0.9951 | |||
| Irregular after CTx | −0.516 | −0.900 to −0.133 | 0.0083 | |||
| Amenorrhea <18 months at AMH test | −1.247 | −1.800 to −0.695 | <0.001 | |||
| Tamoxifen exposure | −0.112 | −0.530 to 0.305 | 0.598 | |||
| Trastuzumab exposure | 0.247 | −0.227 to 0.721 | 0.307 | |||
| Years since CTx | 0.054 | −0.012 to 0.120 | 0.112 | |||
Referenced against regular menstrual cycles.
CI = confidence interval; CTx = chemotherapy; AMH = anti-Müllerian hormone.
In multivariable analyses, serum amh was significantly lower with increasing age at diagnosis (p = 0.002), with increasing cyclophosphamide dose (p = 0.035), and with the presence of irregular cycles or amenorrhea during chemotherapy (p < 0.001) and at the time of amh testing (p < 0.001, Table iii).
Analysis of Patients with Regular Cycles
Compared with the control subjects, chemotherapy-treated patients reporting regular menstrual cycles at the time of amh testing had significantly lower serum amh [reduced by 1.53 log units; 95% confidence interval: −2.93 log units to −0.13 log units; p = 0.03; Figure 3(A)]. Figure 3(B) shows the effect of cyclophosphamide dose on serum amh in survivors reporting regular cycles, adjusted for attained age. That analysis again revealed a significant effect of the cumulative cyclophosphamide dose on serum amh (p = 0.006), and the secondary effect of an attenuation of the association between serum amh and attained age with increasing cyclophosphamide exposure (p = 0.038) by lowering serum amh in younger survivors.
FIGURE 3.
Censored regression of anti-Müllerian hormone (AMH) versus attained age (A) in patients with regular menstrual cycles at the time of AMH testing, and (B) in patients with regular menstrual cycles stratified by cumulative dose of cyclophosphamide.
Univariate analyses of survivors with regular menstrual cycles at the time of amh testing found that serum amh was significantly lowered with increasing age at diagnosis (p = 0.041), with increasing attained age (p = 0.042), and with the presence of amenorrhea during chemotherapy (p = 0.012 compared with regular cycles during chemotherapy, Table iv). In multivariable analyses, serum amh declined significantly with increasing attained age (p = 0.002) and with the presence of irregular menstrual cycles or amenorrhea during chemotherapy (p < 0.001 compared with regular menstrual cycles during chemotherapy, Table iv).
TABLE IV.
Univariate and multivariate analyses of young chemotherapy (CTx)–treated breast cancer survivors reporting regular menses at the time of posttreatment anti-Müllerian hormone testing
| Predictor | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| β | 95% CI | p Value | β | 95% CI | p Value | |
| Attained age | −0.042 | −0.083 to 0.001 | 0.042 | −0.057 | −0.094 to −0.020 | 0.002 |
| Irregular cycles or amenorrhea during CTxa | −0.781 | −1.392 to −0.169 | 0.012 | −0.983 | −1.548 to −0.417 | <0.001 |
| Age at diagnosis | −0.050 | −0.097 to −0.002 | 0.041 | — | — | |
| Body mass index | 0.006 | −0.033 to 0.046 | 0.749 | — | — | |
| Cyclophosphamide use (g/m2) | −0.045 | −0.16 to 0.079 | 0.477 | — | — | |
| Irregular cycles before CTxa | 0.607 | −0.361 to 1.58 | 0.219 | — | — | |
| Irregular cycles after CTxa | −0.404 | −0.904 to −0.096 | 0.114 | — | — | |
| Tamoxifen exposure | 0.256 | −0.268 to 0.780 | 0.338 | — | — | |
| Trastuzumab exposure | 0.592 | 0.067 to 1.116 | 0.027 | — | — | |
| Years after CTx | −0.015 | −0.086 to 0.056 | 0.67 | — | — | |
Referenced against regular menstrual cycles.
CI = confidence interval.
In survivors with regular menstrual cycles, we observed an association between trastuzumab exposure and higher serum amh (p = 0.027).
Ovarian “Aging” and Probability of Live Births with In Vitro Fertilization
Table v shows the age-equivalent serum amh levels derived from the multivariate regression for chemotherapy-treated patients and for control subjects. It can be seen that for most age strata, the average serum amh in breast cancer survivors was approximately equivalent to the average level seen in control subjects 12 years older.
TABLE V.
Age-equivalent anti-Müllerian hormone (AMH) in breast cancer survivors reporting regular menstrual cycles and in control subjectsa
| CTx-treated breast cancer survivors | Control subjects | |||
|---|---|---|---|---|
|
|
|
|||
| Attained age (years) | Average serum AMH (pmol/L) | Equivalent attained age (years) | Expected serum AMH for ageb (pmol/L) | |
|
| ||||
| 50th percentile | 3rd–97th percentile | |||
| 25 | 9.48 | 36 | 11.78 | 0.93–54.76 |
| 30 | 5.06 | 42 | 7.14 | 0.36–37.49 |
| 35 | 2.70 | >45 | 3.07 | 0.14–17.21 |
| 40 | 1.44 | >45 | 3.07 | 0.14–17.21 |
Model of ovarian aging based on a regression of serum AMH versus attained age in CTx-treated patients with regular menses at time of AMH testing.
Obtained from population-based age-related nomograms published by Almog et al., 201133.
CTx = chemotherapy.
A study evaluating factors predicting successful ivf-assisted live birth found that attained age and serum amh are the two factors most predictive of live birth with ivf, and the authors described a model to estimate the probability of live birth based on those factors31. Applied to all survivors in the present study, that model estimated that patients treated before age 31 with an attained age of less than 40 years would have a probability of successful ivf-assisted live birth in the range of 13%–27%. However, in patients who received chemotherapy when 31 years of age or older, the probability of live birth at an attained age of less than 40 years was only in the 5%–9% range. When the model was limited to the subset of patients with regular menstrual cycles at the time of amh testing, the estimated live birth probability was 26%–27% for patients receiving chemotherapy before the age of 31 years and having an attained age of less than 40 years; patients receiving chemotherapy at 31 years of age and older had a predicted ivf-associated live birth probability in the 5%–18% range.
DISCUSSION
The effect of breast cancer treatment on fertility is of growing importance for young breast cancer survivors. Partridge et al.4 reported that fertility after treatment was a significant concern for approximately 60% of young women with early breast cancer and that fertility concerns would influence the treatment decisions of 26% of women6. As cure rates continue to improve, clinical advances to develop less gonadotoxic therapy and to intervene more effectively so that survivors can achieve their reproductive goals will be required. Guidelines from the American Society of Clinical Oncology recommend that, before initiation of adjuvant chemotherapy, fertility issues be discussed with all cancer patients of reproductive age34,35. In practice, however, such discussions are hampered by a lack of pretreatment fertility preservation options and difficulty in quantifying the effects of cancer therapy on fertility.
Earlier studies have often evaluated survivors with regular menses and with amenorrhea together, leading to uncertainty about the extent to which amh testing adds additional information beyond a clinical history of menstrual regularity. Our study is one of a few that has analyzed women with regular menstrual cycles separately from those with irregular cycles or amenorrhea, finding that serum amh is still significantly lower in the latter group than in age-adjusted controls and illustrating the utility of amh measurement for evaluating ovarian reserve in patients without symptoms of premature ovarian failure. Further, our study is the first to identify the transient loss of regular menses during chemotherapy as an unfavourable factor associated with lower amh after chemotherapy, even after adjustment for other clinical variables that affect ovarian function. That observation suggests that, in survivors for whom a pretreatment serum amh value is not available, a history of loss of regular menses during chemotherapy should be a signal that ovarian reserve is diminished, even if normal menses recover afterward.
To our knowledge, ours is also the first study to report the association between trastuzumab and ovarian function. In 25 patients who received adjuvant trastuzumab, no significant overall effect on serum amh was observed, providing some early tentative evidence that this agent does not appear to adversely affect oocyte reserve. Interestingly, in patients reporting regular cycles, the use of trastuzumab was associated with statistically significantly higher serum amh levels than were measured in chemotherapy-treated patients not receiving that agent. Our view is that, although this finding is encouraging, our small sample of trastuzumab-treated patients prevents a definitive conclusion in this regard. The finding will have to be reproduced in other cohorts to provide reassurance that it is robust. A significant effect of tamoxifen therapy on serum amh was not found, which is consistent with the findings of Su et al. 22, although Partridge et al.25 demonstrated lower serum amh in women taking tamoxifen.
The average overall effect of chemotherapy in our study was to lower serum amh to levels similar to those found in untreated women approximately 12 years older. Earlier smaller studies have also reported lower amh levels in cancer survivors than in control subjects15,22–25,36–39. In the subgroup of patients with regular menstrual cycles at the time of amh testing, factors with a significant positive effect on serum amh were younger attained age and regular menstrual cycles during chemotherapy; specifically, compared with no loss of menses during treatment, a history of losing and then regaining normal cycles predicted worse outcome. The magnitude of the decline in serum amh suggests that, for most survivors in their thirties who want to conceive, specialized evaluation of reproductive options should not be delayed.
Currently, ivf would be one of the major treatment options available to survivors with impaired fertility, and we found that a combination of random amh testing in an outpatient setting and basic clinical parameters could potentially help in counselling patients about the effects of treatment on their ovarian function and clarify the urgency with which specialized reproductive consultation and intervention should be considered. La Marca et al.31 evaluated clinical factors associated with successful live birth after ivf in the general population, and found that serum amh and age were the only baseline patient characteristics that predicted successful live birth after ivf. Application of their model to our study population suggests that survivors with regular menses who received chemotherapy before age 31 and who are currently less than 40 years of age would have a probability of ivf-assisted live birth of 26%–27%, a rate very similar to that reported in the La Marca cohort of women 31–37 years of age attending their fertility clinic (28%)31. However, survivors treated after age 31 had substantially lower rates of predicted ivf success. Taken together with the age-equivalence findings for serum amh, the latter observation suggests that, rather than wait until after treatment to address fertility preservation, patients 31–40 years of age at diagnosis could be targeted for clinical studies of pretreatment interventions such as oocyte or ovarian tissue cryopreservation. Which patients can be advised that they could try to get pregnant without assisted reproduction, and their likelihood of success, is less clear. But we did find that 16% of survivors had serum amh levels within the 95% confidence interval for the control subjects, suggesting that a trial of natural conception is appropriate for some.
Our study has limitations that warrant consideration. We did not have pretreatment measurements of serum amh, which could have been useful for comparison. Dillon et al.38 measured pre- and posttreatment serum amh in young female cancer survivors and demonstrated that pretreatment serum amh was significantly associated with posttreatment serum amh. Similar findings have been reported by other investigators38–42, demonstrating that, for patients with reduced ovarian reserve before treatment, chemotherapy moves them further toward a perimenopausal state. In addition, although the ivf live-birth model provides some broad insight into the predicted outcome of ivf for our survivors, the model was not developed specifically for chemotherapy-treated patients, and other age-related clinical factors besides ovarian reserve could contribute to the likelihood of ivf success. Those results should therefore be interpreted with caution. The association between serum amh, age, and oocyte retrieval rate after controlled ovarian stimulation is better understood than the probability of live birth43,44. Further, the substantial variation observed in serum amh could be explained by unmeasured confounders, including pretreatment serum amh, that we were unable to evaluate. In particular, emerging data suggest that biologic differences in cyclophosphamide metabolism might contribute to the posttreatment serum amh level45. Finally, another potential limitation of our study is that BRCA mutation status was not collected, and some reports have suggested that carriers of the BRCA1 mutation might have impaired ovarian reserve even before receiving chemotherapy46,47.
CONCLUSIONS
We demonstrated that, in young breast cancer survivors, serum amh is, on average, approximately equivalent to that in a normal population 12 years older, and that patients treated after age 31 have a low expected rate of fertility after chemotherapy. Large studies are needed in future to evaluate the success of fertility preservation strategies and ivf in survivors and to identify the biologic factors associated with retained fertility in such individuals.
ACKNOWLEDGMENTS
This study was funded by the Canadian Breast Cancer Foundation (grant no. 1006175).
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Web resource] Lyon, France: International Agency for Research on Cancer; 2013. [Accessible at: http://globocan.iarc.fr/Default.aspx; cited 15 April 2017] [Google Scholar]
- 2.Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009;36:237–49. doi: 10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thewes B, Meiser B, Taylor A, et al. Fertility- and menopause-related information needs of younger women with a diagnosis of early breast cancer. J Clin Oncol. 2005;23:5155–65. doi: 10.1200/JCO.2005.07.773. [DOI] [PubMed] [Google Scholar]
- 4.Partridge AH, Gelber S, Peppercorn J, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22:4174–83. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 5.Kondapalli LA, Dillon KE, Sammel MD, et al. Quality of life in female cancer survivors: is it related to ovarian reserve? Qual Life Res. 2014;23:585–92. doi: 10.1007/s11136-013-0473-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruddy KJ, Gelber SI, Tamimi RM, et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol. 2014;32:1151–6. doi: 10.1200/JCO.2013.52.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Practice Committee of the American Society for Reproductive Medicine Aging and infertility in women. Fertil Steril. 2006;86(suppl 4):S248–52. doi: 10.1016/j.fertnstert.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Pritchard KI. Adjuvant therapy of the very young woman. Breast. 2007;16(suppl 2):S136–46. doi: 10.1016/j.breast.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Bianco AR, Del Mastro L, Gallo C, et al. Prognostic role of amenorrhea induced by adjuvant chemotherapy in premenopausal patients with early breast cancer. Br J Cancer. 1991;63:799–803. doi: 10.1038/bjc.1991.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–29. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 11.Hortobagyi GN, Buzdar AU, Marcus CE, Smith TL. Immediate and long-term toxicity of adjuvant chemotherapy regimens containing doxorubicin in trials at M.D. Anderson Hospital and Tumor Institute. NCI Monogr. 1986:105–9. [PubMed] [Google Scholar]
- 12.Levine MN, Bramwell VH, Pritchard KI, et al. Randomized trial of intensive cyclophosphamide, epirubicin, and fluorouracil chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1998;16:2651–8. doi: 10.1200/JCO.1998.16.8.2651. [DOI] [PubMed] [Google Scholar]
- 13.Goldhirsch A, Gelber RD, Castiglione M. The magnitude of endocrine effects of adjuvant chemotherapy for premenopausal breast cancer patients. The International Breast Cancer Study Group. Ann Oncol. 1990;1:183–8. doi: 10.1093/oxfordjournals.annonc.a057718. [DOI] [PubMed] [Google Scholar]
- 14.Amir E, Freedman O, Allen L, Colgan T, Clemons M. Defining ovarian failure in amenorrheic young breast cancer patients. Breast. 2010;19:545–8. doi: 10.1016/j.breast.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Lutchman Singh K, Muttukrishna S, Stein RC, et al. Predictors of ovarian reserve in young women with breast cancer. Br J Cancer. 2007;96:1808–16. doi: 10.1038/sj.bjc.6603814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajpert-De Meyts E, Jorgensen N, Graem N, Muller J, Cate RL, Skakkebaek NE. Expression of anti-Mullerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab. 1999;84:3836–44. doi: 10.1210/jcem.84.10.6047. [DOI] [PubMed] [Google Scholar]
- 17.Massague J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- 18.La Marca A, Volpe A. Anti-Mullerian hormone (amh) in female reproduction: is measurement of circulating amh a useful tool? Clin Endocrinol (Oxf) 2006;64:603–10. doi: 10.1111/j.1365-2265.2006.02533.x. [DOI] [PubMed] [Google Scholar]
- 19.Grynnerup AG, Lindhard A, Sorensen S. The role of anti-Mullerian hormone in female fertility and infertility—an overview. Acta Obstet Gynecol Scand. 2012;91:1252–60. doi: 10.1111/j.1600-0412.2012.01471.x. [DOI] [PubMed] [Google Scholar]
- 20.Bozza C, Puglisi F, Lambertini M, Osa EO, Manno M, Del Mastro L. Anti-Mullerian hormone: determination of ovarian reserve in early breast cancer patients. Endocr Relat Cancer. 2014;21:R51–65. doi: 10.1530/ERC-13-0335. [DOI] [PubMed] [Google Scholar]
- 21.Practice Committee of the American Society for Reproductive Medicine Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2012;98:1407–15. doi: 10.1016/j.fertnstert.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 22.Su HI, Sammel MD, Green J, et al. Antimullerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors. Cancer. 2010;116:592–9. doi: 10.1002/cncr.24746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson RA, Themmen AP, Al-Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod. 2006;21:2583–92. doi: 10.1093/humrep/del201. [DOI] [PubMed] [Google Scholar]
- 24.Yu B, Douglas N, Ferin MJ, et al. Changes in markers of ovarian reserve and endocrine function in young women with breast cancer undergoing adjuvant chemotherapy. Cancer. 2010;116:2099–105. doi: 10.1002/cncr.25037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Partridge AH, Ruddy KJ, Gelber S, et al. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril. 2010;94:638–44. doi: 10.1016/j.fertnstert.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 26.Anders C, Marcom PK, Peterson B, et al. A pilot study of predictive markers of chemotherapy-related amenorrhea among premenopausal women with early stage breast cancer. Cancer Invest. 2008;26:286–95. doi: 10.1080/07357900701829777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greene WH. Econometric Analysis. 6th ed. Upper Saddle River, NJ: Prentice Hall; 2008. [Google Scholar]
- 28.Kleiber C, Zeileis A. Applied Econometrics with R. New York, NY: Springer; 2008. [DOI] [Google Scholar]
- 29.Tobin J. Estimation of relationships for limited dependent variables. Econometrica. 1958;26:24–36. doi: 10.2307/1907382. [DOI] [Google Scholar]
- 30.Venables WN, Ripley BD. Modern Applied Statistics with S. 4th ed. New York, NY: Springer; 2002. [DOI] [Google Scholar]
- 31.La Marca A, Nelson SM, Sighinolfi G, et al. Anti-Mullerian hormone-based prediction model for a live birth in assisted reproduction. Reprod Biomed Online. 2011;22:341–9. doi: 10.1016/j.rbmo.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Fraser IS, Critchley HO, Munro MG, Broder M, on behalf of the Writing Group for the Menstrual Agreement Process A process designed to lead to international agreement on terminologies and definitions used to describe abnormalities of menstrual bleeding. Fertil Steril. 2007;87:466–76. doi: 10.1016/j.fertnstert.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 33.Almog B, Shehata F, Suissa S, et al. Age-related normograms of serum antimüllerian hormone levels in a population of infertile women: a multicenter study. Fertil Steril. 2011;95:2359–63. doi: 10.1016/j.fertnstert.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 34.Lee SJ, Schover LR, Partridge AH, et al. on behalf of the American Society of Clinical Oncology American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 35.Loren AW, Mangu PB, Beck LN, et al. on behalf of the American Society of Clinical Oncology Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–10. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gracia CR, Sammel MD, Freeman E, et al. Impact of cancer therapies on ovarian reserve. Fertil Steril. 2012;97:134–40.e1.. doi: 10.1016/j.fertnstert.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bath LE, Wallace WH, Shaw MP, Fitzpatrick C, Anderson RA. Depletion of ovarian reserve in young women after treatment for cancer in childhood: detection by anti-Mullerian hormone, inhibin B and ovarian ultrasound. Hum Reprod. 2003;18:2368–74. doi: 10.1093/humrep/deg473. [DOI] [PubMed] [Google Scholar]
- 38.Dillon KE, Sammel MD, Prewitt M, et al. Pretreatment antimullerian hormone levels determine rate of post-therapy ovarian reserve recovery: acute changes in ovarian reserve during and after chemotherapy. Fertil Steril. 2013;99:477–83. doi: 10.1016/j.fertnstert.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henry NL, Xia R, Schott AF, McConnell D, Banerjee M, Hayes DF. Prediction of postchemotherapy ovarian function using markers of ovarian reserve. Oncologist. 2014;19:68–74. doi: 10.1634/theoncologist.2013-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruddy KJ, O’Neill A, Miller KD, et al. Biomarker prediction of chemotherapy-related amenorrhea in premenopausal women with breast cancer participating in E5103. Breast Cancer Res Treat. 2014;144:591–7. doi: 10.1007/s10549-014-2891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson RA, Rosendahl M, Kelsey TW, Cameron DA. Pretreatment anti-Mullerian hormone predicts for loss of ovarian function after chemotherapy for early breast cancer. Eur J Cancer. 2013;49:3404–11. doi: 10.1016/j.ejca.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson RA, Cameron DA. Pretreatment serum anti-Mullerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab. 2011;96:1336–43. doi: 10.1210/jc.2010-2582. [DOI] [PubMed] [Google Scholar]
- 43.Broer SL, Mol B, Dolleman M, Fauser BC, Broekmans FJ. The role of anti-Mullerian hormone assessment in assisted reproductive technology outcome. Curr Opin Obstet Gynecol. 2010;22:193–201. doi: 10.1097/GCO.0b013e3283384911. [DOI] [PubMed] [Google Scholar]
- 44.Bhide P, Shah A, Gudi A, Homburg R. The role of anti-Mullerian hormone as a predictor of ovarian function. The Obstetrician and Gynaecologist. 2012;14:161–6. doi: 10.1111/j.1744-4667.2012.00112.x. [DOI] [Google Scholar]
- 45.Su HI, Sammel MD, Velders L, et al. Association of cyclophosphamide drug-metabolizing enzyme polymorphisms and chemotherapy-related ovarian failure in breast cancer survivors. Fertil Steril. 2010;94:645–54. doi: 10.1016/j.fertnstert.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ ovarian cancer risks. J Clin Oncol. 2010;28:240–4. doi: 10.1200/JCO.2009.24.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith KR, Hanson HA, Hollingshaus MS. BRCA1 and BRCA2 mutations and female fertility. Curr Opin Obstet Gynecol. 2013;25:207–13. doi: 10.1097/GCO.0b013e32835f1731. [DOI] [PMC free article] [PubMed] [Google Scholar]