Abstract
Physical and chemical factors influencing the anti-leukemic l-asparaginase enzyme production by Bacillus subtilis VUVD001 were optimized using multi-stage optimization on the basis of preliminary experimental outcomes obtained by conventional one-factor-at-a-time approach using shake flasks. Process variables namely carbon, nitrogen sources, pH and temperature were taken into consideration during response surface methodology (RSM) optimization. The finest enzyme activity of 0.51 IUml−1 obtained by OFAT method was enhanced by 3.2 folds using RSM optimization. Artificial neural network (ANN) modelling and genetic algorithm (GA) based optimizations were further carried out to improve the enzyme drug yield. Results were also validated by conducting experiments at optimum conditions determined by RSM and GA optimization methods. The novel bacterium yielded in 2.88 IUml−1 of enzyme activity at optimum process variables determined by GA optimization, i.e., 0.5% glucose, 8.0% beef extract, 8.3 pH and 49.9 °C temperature. The study explored the optimized culture conditions for better yielding of anti-leukemic enzyme drug from a new bacterial source namely Bacillus subtilis VUVD001.
Electronic supplementary material
The online version of this article (10.1007/s13205-017-1020-2) contains supplementary material, which is available to authorized users.
Keywords: Artificial neural networks, Bioprocess optimization, Genetic algorithm, l-Asparaginase, Statistical optimization methods
Introduction
For the therapy of deadly diseases like acute myelocytic leukaemia, acute lymphoblastic leukaemia, acute myelomonocytic leukaemia, lymphosarcoma treatment, reticulosarcoma, chronic lymphocytic leukaemia, melanoarcoma, and Hodgkin disease one of the potent chemotherapeutic drugs is l-asparaginase (Verma et al. 2007; Stecher et al. 1999). Due to its antioxidant property (Maysa et al. 2010) and its ability to reduce up to 90% of food acrylamide levels it also has its application in food industry. A hypersensitivity reaction caused by this enzyme restricts its continuous use (Reynolds and Taylor 1993) and it also results in neutralization of the drug effect or an anaphylactic shock because of development of anti-asparaginase antibody. Recently, to conquer the drawbacks above said renewed l-asparaginases from other wide-ranging sources and regulated preparations have been accepted. Escherichia coli and Erwinia chrysanthemi l-asparaginase enzymes have been highly efficient in lymphoblastic leukaemia therapy, acute leukaemia and lymphosarcoma (Graham 2003) for long days with exceptional remedial response (Duval et al. 2002). This resulted to test new microbial sources to ascertain strains proficient of manufacturing novel enzyme for ALL therapy with more yield. The l-asparaginase associated toxicity is partially traceable to the same enzyme’s glutaminase activity (Howard and Carpenter 1972). l-glutamine is essential not only in the function of l-asparagine by but also in numerous other metabolic pathways (Prager and Bachynsky 1968). In recent time major research is concerted on microbial l-asparaginase production which is glutaminase free. Some attempts were also made to reveal the key residues of enzyme that are involved in the binding of l-asparagine as well as l-glutamine substrates using different in silico approaches like homology modelling of enzyme drug structure, molecular docking studies with enzyme substrates and molecular dynamic simulation studies, etc., (Erva et al. 2013, 2015; Rajulapati and Erva 2015; Reddy et al. 2015, 2016). Though quite a good number of microbial l-asparaginases are intracellular a few are extracellular which are advantageous. The fermentative synthesis of l-asparaginase is highly influenced by both culture conditions and composition of the medium. With the minimal cost, raise in productivity of anticancer enzyme is possible either by optimization of fermentation conditions or by the strain improvement. From the past few decades, statistical experimental designs are in use for optimization purpose in many bioprocesses which can be imposed on a number of stages. The competent and strategic RSM experimental tool can be applied to establish optimal process conditions in multi variable biotechnology practices. Sometimes applicability of RSM to all modelling and optimization studies is difficult for which alternatives were ANN modelling and GA optimization. ANN mimics the brain which takes whole ‘black box’ methodology for data modelling. GAs are optimization algorithms which are unorthodox search based and help in the direct search for an elucidation to a problem by imitating part of the process of natural evolution. Through a given set of alternatives, GA performs direct random searches to find the finest choice with regard to the specified criterion for goodness of fit, which is expressed as a fitness function. The use of ANNs and GAs is well established in environmental biotechnology and biochemical engineering, for modelling of analytical biochemistry signals, chromatographic spectral pattern recognition, cancer research, genomic and proteomic sequence functional analyses, analysis of alterations in soil microbial community composition, etc., (Almeida 2002). Unavailability of scientific literature on modelling and statistical optimization studies using Bacillus subtilis VUVD001 for l-asparaginase production gave the scope to do the present work where process variables are optimized for l-asparaginase synthesis using Bacillus subtilis VUVD001 with an importance to the noteworthy parameters (temperature, pH, glucose, and beef extract).
Experimental
Production and optimization of l-asparaginase by RSM
Bacillus subtilis VUVD001 has been isolated from Vignans University, Vadlamudi, Guntur district, Andhra Pradesh, India (NCBI accession number KT894158). Crude l-asparaginase enzyme activity measurement was done by ammonia developed quantification spectroscopically. Standard Nesslerization technique was employed for l-asparaginase activity assessment by quantifying the total ammonia liberated during l-asparagine hydrolysis spectrometrically at 480 nm. One unit (IU) of l-asparaginase activity is defined as the magnitude of enzyme which releases 1 μmol of ammonia per minute under the typical assay conditions (Wriston and Yellin 1973). The submerged fermentation approach was used for the production l-asparaginase using the media components in the range of KH2PO4 0.5–2.0%, MgSO4·7H2O 0.5–2.0%, CaCl2·2H2O 0.5–2.0%, l-asparagine 1.0–4.0%, beef extract 1.0–5.0% and glucose 0.5–3.0% and incubated in a orbital shaker at 35 °C with an agitation rate of 200 rpm for 6 days and the enzyme activity was measured. Later the effect of pH (4–7), temperature (20–35 °C), size of inoculum (0.5–4.0% V/V) and agitation speed (50–250 rpm) were also evaluated using OFAT optimization methodology (Ashok and Kumar 2017; Doriya and Kumar 2016; Erva et al. 2017; Xu et al. 2003).
The investigational RSM design was drawn in the choice of each autonomous bioprocess parameter at three levels. Amongst the total parameters evaluated for their significance on l-asparaginase activity in OFAT method (results are not shown here), four variables (pH, temperature, glucose and beef extract) were found to be potent. Using the method of least squares, the response function was approximated by a second degree polynomial of quadratic and interaction effects (Rajulapati et al. 2011). The ranges of glucose (A), beef extract (B), pH (C) and temperature (D) was considered for experimental study using the full factorial face centred central composite design (FCCCD). Table 1 describes the real ranges of coded factors which were obtained on the basis of OFAT method outcomes (data is not shown here). For designing of experiments as well as analysis of experimental design, design-expert7® statistical software package was used (Stat- Ease Inc, USA). Overall 30 experiments were designed (Table 2) and the l-asparaginase synthesis data were analyzed using analysis of variance (ANOVA) to distinguish significance of individual variables. The statistical correlation was deliberated by the second-order polynomial equation between the finale objective (l-asparaginase activity) and the independent variables,
| 1 |
where Y = predicted response, B 0 = intercept term, B i = linear effect, B ii = squared effect and B ij = interaction effect. The linear, quadratic and interactive effect of autonomous bioprocess parameters on ultimate objective can be approximated using Eq (1). The substantial response surface plots specify the response function on z-axis where the other two axes (X and Y) indicating the two autonomous process parameters while retaining the other invariable at the center point. All experimentations were performed in triplicate and computations were carried out to determine the mean values.
Table 1.
Variables used in experimental design
| Name | Code | Lower limit (− 1) | Upper limit (+ 1) |
|---|---|---|---|
| Glucose (%) | A | 0.5 | 2.5 |
| Beef extract (%) | B | 2 | 8 |
| pH | C | 4 | 10 |
| Temp (°C) | D | 30 | 50 |
Table 2.
Experimental design and response
| S. no | Glucose (%) | Beef extract (%) | pH | Temperature (°C) | l-Asparaginase activity (IUml−1) | ||
|---|---|---|---|---|---|---|---|
| RSM experimental | RSM predicted | ANN predicted | |||||
| 1 | 0.5 | 2 | 4 | 30 | 0.24 ± 0.04 | 0.34 | 0.35 |
| 2 | 1.5 | 5 | 7 | 40 | 1.11 ± 0.12 | 1.09 | 1.19 |
| 3 | 2.5 | 8 | 10 | 50 | 0.19 ± 0.02 | 0.2 | 0.20 |
| 4 | 0.5 | 8 | 4 | 30 | 0.35 ± 0.03 | 0.44 | 0.35 |
| 5 | 2.5 | 5 | 7 | 40 | 0.21 ± 0.11 | 0.59 | 0.21 |
| 6 | 2.5 | 8 | 4 | 50 | 0.41 ± 0.02 | 0.41 | 0.41 |
| 7 | 0.5 | 8 | 4 | 50 | 1.2 ± 0.01 | 1.12 | 1.20 |
| 8 | 1.5 | 5 | 7 | 30 | 0.89 ± 0.02 | 0.91 | 0.89 |
| 9 | 0.5 | 2 | 4 | 50 | 0.99 ± 0.01 | 0.94 | 1.01 |
| 10 | 2.5 | 2 | 4 | 50 | 0.22 ± 0.03 | 0.22 | 0.19 |
| 11 | 1.5 | 5 | 7 | 40 | 0.86 ± 0.02 | 1.09 | 1.19 |
| 12 | 1.5 | 5 | 4 | 40 | 0.35 ± 0.01 | 0.58 | 0.41 |
| 13 | 1.5 | 5 | 7 | 40 | 0.78 ± 0.02 | 1.09 | 1.19 |
| 14 | 0.5 | 5 | 7 | 40 | 1.56 ± 0.01 | 1.27 | 1.56 |
| 15 | 2.5 | 2 | 4 | 30 | 0.25 ± 0.02 | 0.08 | 0.24 |
| 16 | 2.5 | 8 | 4 | 30 | 0.29 ± 0.03 | 0.18 | 0.28 |
| 17 | 1.5 | 8 | 7 | 40 | 1.21 ± 0.05 | 1.25 | 1.22 |
| 18 | 1.5 | 5 | 7 | 40 | 1.38 ± 0.01 | 1.09 | 1.19 |
| 19 | 1.5 | 5 | 7 | 40 | 1.41 ± 0.02 | 1.09 | 1.19 |
| 20 | 0.5 | 2 | 10 | 50 | 1.09 ± 0.09 | 1.3 | 1.05 |
| 21 | 1.5 | 5 | 7 | 50 | 1.03 ± 0.06 | 1.1 | 1.03 |
| 22 | 2.5 | 8 | 10 | 30 | 0.5 ± 0.11 | 0.42 | 0.50 |
| 23 | 2.5 | 2 | 10 | 50 | 0.41 ± 0.05 | 0.2 | 0.43 |
| 24 | 0.5 | 8 | 10 | 30 | 0.96 ± 0.03 | 1.06 | 0.96 |
| 25 | 1.5 | 5 | 7 | 40 | 1.27 ± 0.04 | 1.09 | 1.19 |
| 26 | 1.5 | 2 | 7 | 40 | 1.14 ± 0.02 | 1.2 | 1.13 |
| 27 | 1.5 | 5 | 10 | 40 | 1.01 ± 0.01 | 0.88 | 1.00 |
| 28 | 2.5 | 2 | 10 | 30 | 0.31 ± 0.02 | 0.5 | 0.34 |
| 29 | 0.5 | 2 | 10 | 30 | 1.27 ± 0.09 | 1.15 | 1.29 |
| 30 | 0.5 | 8 | 10 | 50 | 1.25 ± 0.02 | 1.3 | 1.25 |
Modelling using artificial neural networks (ANNs)
ANN models imitate the role of a biological network, made up of neurons and are applied to decipher composite functions in diverse applications. Simple synchronous processing elements are included in NN which are motivated by the biological nerve systems. Neurons are the basic unit of ANN and they are linked to one another by synapses, and a weight factor is allied with every synapse (Zhang and Friedrich 2003). Back-propagation (BP) is one of the trendiest algorithms in ANN which is used in this study, with one hidden layer enhanced with numerical optimization technique named Levenberg-Marquardt (LM) (Arcaklioglu et al. 2004).
Process optimization by GA
A theoretical universal search and optimization technique called GA copies the metaphor of natural biological evolution. GA works on a population of likely solutions implying the principle of survival of the fittest to produce sequentially superior estimations to a solution. A fresh set of estimation is produced at each generation by the process of individual selection as per their fitness level in the domain of problem and their replication using rented operators from natural genetics. This practice directs to the progression of individual populations that better suited for their environment compared to the individuals from which they were created, just as in normal adaptation process.
The GA optimization begins with initialization of the population of solutions P(t). The population size was 16 (4 × no. of variables) and the initial population type chosen was double. In the present optimization for the process of the selection among the available methods, Rank method opted. The scattered option was used as crossover operator and other constraints used for reproduction and mutations are 0.8 crossover rate and constraint dependent mutations function. Other approximated parameters were forward migration direction, 0.2 migration fraction and 20 as migration interval. The stopping criterion usually advises the upper limit of iterations or verifies if the finest solution attained is acceptable. Values considered for stopping criteria includes ceiling iteration number of 400 (100 × no. of variables), infinite time limit, infinite fitness limit, 50 stall generations, infinite stall time limit, function tolerance and nonlinear constraint tolerance of 10−6 (Rajulapati and Narasu 2011).
Results and discussion
RSM optimization
RSM is an efficient method in which the principal aim is to run swiftly and impressively along the path of augment towards the universal habitat of the best, identifying the finest probable segment for running fermentation. Glucose (A), beef extract (B), pH (C) and temperature (D) were the four self-regulating variables used for this rationale. For final response of l-asparaginase activity [Eq. (2)] use of RSM furnished the consequent quadratic regression equation. Table 1 stands for the range of process parameters; investigational design and the outcomes gained for ultimate objective are presented in Table 2. Interpretation of results of this experimental study depicts that, the finale enzyme drug activity was assemblage on the blend of glucose, beef extract, pH and temperature. Assessment of the predicted response values with investigational outcomes signifies that the data were in reasonable harmony. Optimized bioprocess parameter values for boosting up of finale objective were recognized as 0.51, 7.99, 7.56 and 49.73 for glucose, beef extract, pH and temperature, respectively.
| 2 |
where Y is the activity of l-asparaginase.
ANOVA was executed to confirm the model suitability and Table 3 describes the results.
Table 3.
ANOVA analysis
| Source | Sum of squares | Df | Mean square | F value | P value prob > F | |
|---|---|---|---|---|---|---|
| Model | 4.79 | 1 | 0.34 | 5.68 | 0.0009 | Significant |
| A—glucose | 2.08 | 1 | 2.08 | 34.52 | < 0.0001 | |
| B—beef extract | 0.011 | 1 | 0.011 | 0.18 | 0.6787 | |
| C—pH | 0.40 | 1 | 0.40 | 6.67 | 0.0208 | |
| D—temp | 0.17 | 1 | 0.17 | 2.76 | 0.1175 | |
| AB | 5.625E−005 | 1 | 5.625E−005 | 9.332E−004 | 0.9760 | |
| AC | 0.15 | 1 | 0.15 | 2.49 | 0.1354 | |
| AD | 0.21 | 1 | 0.21 | 3.47 | 0.0821 | |
| BC | 0.033 | 1 | 0.033 | 0.55 | 0.4688 | |
| BD | 6.0006E−003 | 1 | 6.0006E−003 | 0.100 | 0.7566 | |
| CD | 0.20 | 1 | 0.20 | 3.32 | 0.0883 | |
| A2 | 0.065 | 1 | 0.065 | 1.08 | 0.3149 | |
| B2 | 0.045 | 1 | 0.045 | 0.74 | 0.4025 | |
| C2 | 0.34 | 1 | 0.34 | 5.68 | 0.0308 | |
| D2 | 0.018 | 1 | 0.018 | 0.30 | 0.5917 | |
| Residual | 0.90 | 15 | 0.060 | |||
| Lack of fit | 0.55 | 10 | 0.055 | 0.77 | 0.6623 | Non-significant |
| Pure error | 0.36 | 5 | 0.071 | |||
| Cor total | 5.70 | 29 |
ANOVA analysis recommends that the RSM model with computed 5.68F value for the quadratic regression model is significant and Prob > F value is < 0.0001, which is smaller than 0.05. A poor coefficient of variation (CV) value of suggests superior uniformity to the experimentation and the accomplished CV value of 30.51% authenticates an elevated steadiness of the experimental trials. R 2 denotes the CV of response under trial whose values always reside between 0 and 1; with nearer to 1 for the more vigorous statistical model and healthier is the prediction of response (Montgomery and Myers 1995). The R 2 value of 0.8413 for finale objective signifies that the RSM model can explicate 84.13% of discrepancy in the response and only 15.87% of the disparities for ultimate objective are not explained by it. Based on the P values, significance of distinctive parameters is assessed, with superior significant terms holding a lesser P value and in this case A, C and C2 was found to be significant (Table 3).
Mutual effects of process variables
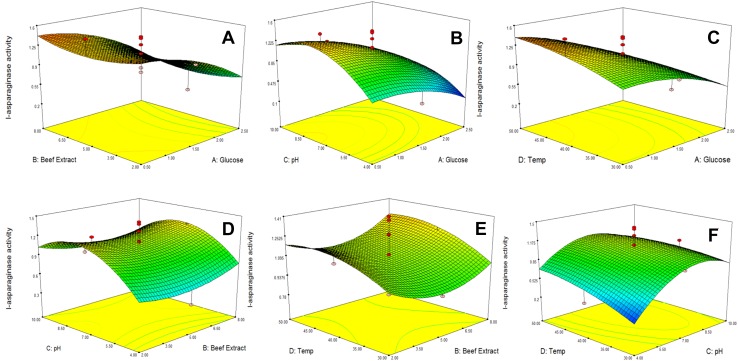
Figure 1a–f describes the four process variables with their mutual effects on final response as response surface plots. The greatest enzyme drug activity can also be attained from the subsequent multi interaction blends by maintaining other process parameters stagnant at optimal levels.
Fig. 1.
Interaction effects of process parameters on response
Artificial neural network model development
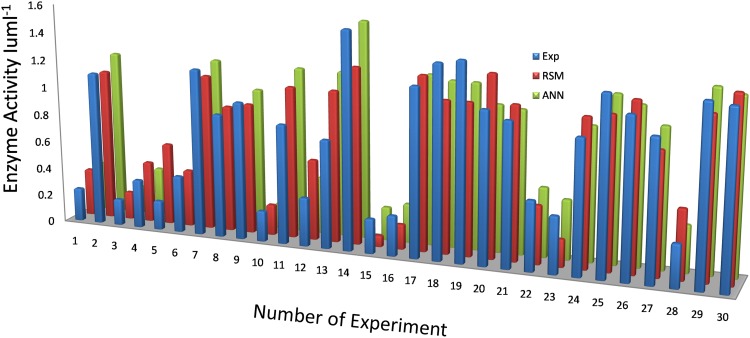
With 4 inputs and one output using feed forward back-propagation network and TRAINLM training function training, testing and validation of NN were carried out. Table 2 describes the results. The outcomes found from the analysis were very pleasing, and an elevated regression value of 0.9506 was attained. The subsequent performance curve was gained on training, testing, and validation of the data shown in the Fig. 2 using MATLAB 2009a. Regression plot showing the output versus target was attained with ten hidden nodes and 0.9506 regression value of was accomplished which shows the model validation. Table 2 shows the experimental and predicted data from statistical regression and ANN.
Fig. 2.
Comparison of RSM and ANN outcomes
ANN strategy for modelling was also applied for production of l-glutaminase from Bacillus cereus MTCC 1305 (Singh et al. 2013), Bacillus subtilis RSP-GLU (Sathish and Prakasham 2010) and also for the production of l-asparaginase using Aspergillus terreus MTCC 1782 (Gurunathan and Sahadevan 2012) and achieved a reasonably good models than RSM.
Genetic algorithm based process optimization and model validation
RSM projected best promising solutions for anti-leukemic enzyme synthesis. For validating the RSM model, experimentation was carried out at the best ten suggested solutions and a finale response of 1.62 IUml−1 was obtained which is pretty nearer to predicted response value of 1.57 IUml−1 (Table 4). As the experimental outcomes attained were as good as the RSM predicted values the RSM model is validated. After statistical optimization Bacillus subtilis VUVD001 resulted in an ultimate enzyme activity of 1.62 IUml−1.
Table 4.
Best ten solutions from RSM optimization
| S. no | Glucose (%) | Beef extract (%) | pH | Temp (°C) | RSM predicted values | Experimental values |
|---|---|---|---|---|---|---|
| 1 | 0.5 | 8 | 7.63 | 47.85 | 1.561 | 1.55 ± 0.01 |
| 2 | 0.5 | 7.98 | 7.33 | 48.13 | 1.562 | 1.57 ± 0.01 |
| 3 | 0.51 | 7.88 | 7.18 | 49.71 | 1.560 | 1.58 ± 0.005 |
| 4 | 0.51 | 7.99 | 7.78 | 48.9 | 1.562 | 1.54 ± 0.02 |
| 5 | 0.55 | 7.98 | 7.63 | 49.1 | 1.560 | 1.55 ± 0.01 |
| 6 | 0.5 | 7.99 | 7.61 | 49.85 | 1.572 | 1.62 ± 0.03 |
| 7 | 0.5 | 7.99 | 7.02 | 48.73 | 1.561 | 1.59 ± 0.01 |
| 8 | 0.54 | 7.99 | 7.41 | 49.01 | 1.564 | 1.55 ± 0.02 |
| 9 | 0.5 | 8 | 7.96 | 49.77 | 1.561 | 1.57 ± 0.005 |
| 10 | 0.56 | 7.95 | 7.4 | 49.97 | 1.562 | 1.54 ± 0.02 |
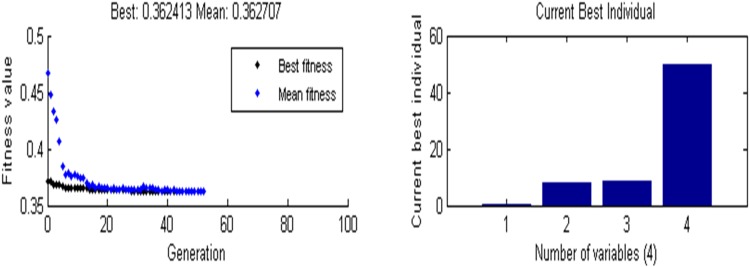
The nonlinear statistical regression equation obtained from RSM was optimized using GA and the plausible results are described in Table 5 and Fig. 3. Utmost response (enzyme activity) of 2.88 IUml−1 was achieved at following optimum process conditions, i.e., Glucose 0.5%, Beef extract 8.0%, pH of 8.3 and temperature of 49.9 °C. At these conditions, the predicted maximum enzyme activity is 2.76 IUml−1 which is comparable to previous sources of l-asparaginase production both in submerged and solid substrate fermentation. Mukherjee and group described a ceiling value of 0.57 IUml−1 using Enterobacter aerogenes without l-asparagine in medium (Mukherjee et al. 2000). Bhaskar and co-workers, on the other hand, attained an upper level of l-asparaginase activity with the use of ANN coupled GA optimization with same organism (Baskar et al. 2011). They also attained 19.129 IUml−1 of enzyme activity using tri sodium citrate (1.88%) as carbon source (Baskar et al. 2009). Another group has accomplished 15.1 IUml−1 of activity with 1.4% of l-asparagine in medium using Bacillus sp.RKS 20 (Mahajan et al. 2014). In this particular study Bacillus subtilis VUVD001 gave comparable levels of l-asparaginase activity against E. coli ATCC 11303 (Kenari et al. 2011), Pseudomonas plecoglossicida RS1, Cladosporium sp., Pectobacterium carotovorum MTCC 1428 (Kumar et al. 2009) and Erwinia aroideae (Peterson and Ciegler 1969) using RSM only. Some l-asparaginase sources took as longer as 120 h of production time to gain the same levels of enzyme activity that were obtained by Bacillus subtilis VUVD001. The findings of the present study are unlike our previous findings from Enterobacter aerogenes MTCC 111 which involved the use of l-asparagine inducer (Erva et al. 2017; Reddy et al. 2017). Moreover, this strain is producing the anti-leukemic enzyme at higher temperatures, i.e., 49.9 °C. Though this organism proved its efficacy to produce lactase (Venkateswarulu et al. 2017), this is the first report describing the optimum bioprocess variables for l-asparaginase synthesis by Bacillus subtilis VUVD001 with lower fermentation time (30 h) with no use of l-asparagine inducer in shake flask submerged fermentation, which are incredibly vital in view of industry. Experimental values were compared with predicted responses by RSM and Neural Network (Table 2 and Fig. 2). In the present study, genetic algorithm gave more accurate predicted values and the optimized response value compared to RSM optimization (Table 6).
Table 5.
Optimized process variables by GA for maximum l-asparaginase activity
| Glucose (%) | Beef extract (%) | pH | Temp (°C) | Experimental enzyme activity (IUml−1) | Predicted enzyme activity (IUml−1) |
|---|---|---|---|---|---|
| A | B | C | D | Y | Y |
| 0.51 | 7.99 | 7.56 | 49.75 | 2.88 ± 0.02 | 2.76 |
Fig. 3.
GA optimization based fitness value and best individual (process parameter)
Table 6.
Comparison of RSM and GA optimization for maximum l-asparaginase activity
| S. no | Method of optimization | Experimental enzyme activity (IUml−1) | Predicted enzyme activity (IUml−1) |
|---|---|---|---|
| 1 | RSM | 1.62 ± 0.03 | 1.57 |
| 2 | GA | 2.88 ± 0.02 | 2.76 |
Conclusion
The outcome of this anti-leukemic enzyme drug optimization study provides the central idea on levels of fermentation parameters using novel bacterial source namely, Bacillus subtilis VUVD001. An investigational yield of 1.62 IUml−1 from RSM optimized bioprocess parameters is quite nearer to predicted RSM activity. RSM optimization resulted in 3.2 fold augmentation of l-asparaginase activity in contrast to OFAT approach. Further, the anticancer enzyme drug yield was enhanced by 5.6 times (2.88 IUml−1) with no l-asparagine inducer, lower carbon source concentrations in reasonably less amount of fermentation time using multi-stage optimization. These findings propose Bacillus subtilis VUVD001 as a novel and prospective microbial source for anti-leukemic enzyme drug production in comparison to other producers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (DOCX 15452 kb)
Acknowledgements
The authors would like to thank Vignan’s Foundation for Science Technology and Research (VFSTR) University, Vadlamudi, Guntur, Andhra Pradesh for the facilities provided to carry out the experiments and computational work.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13205-017-1020-2) contains supplementary material, which is available to authorized users.
References
- Almeida JS. Predictive non-linear modeling of complex data by artificial neural networks. Curr Opin Biotechnol. 2002;13:72–76. doi: 10.1016/S0958-1669(02)00288-4. [DOI] [PubMed] [Google Scholar]
- Arcaklıoğlu E, Çavuşoğlu A, Erişen A. Thermodynamic analyses of refrigerant mixtures using artificial neural networks. Appl Energy. 2004;78:219–230. doi: 10.1016/j.apenergy.2003.08.001. [DOI] [Google Scholar]
- Ashok A, Kumar DS. Different methodologies for sustainability of optimization techniques used in submerged and solid state fermentation. 3 Biotech. 2017;7:301. doi: 10.1007/s13205-017-0934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskar G, Dharmendira Kumar M, Anand Prabu A, Renganathan S, Yoo C. Optimization of carbon and nitrogen sources for l-asparaginase production by Enterobacter aerogenes using response surface methodology. Chem Biochem Eng Q. 2009;23:393–397. [Google Scholar]
- Baskar G, Rajasekar V, Renganathan S. Modeling and optimization of l-asparaginase production by Enterobacter Aerogenes using artificial neural network linked genetic algorithm. Int J Chem Eng Appl. 2011;2:98. [Google Scholar]
- Doriya K, Kumar DS. Isolation and screening of l-asparaginase free of glutaminase and urease from fungal sp. 3 Biotech. 2016;6:239. doi: 10.1007/s13205-016-0544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval M, et al. Comparison of Escherichia coli–asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized European Organisation for Research and Treatment of Cancer—Children’s Leukemia Group phase 3 trial. Blood. 2002;99:2734–2739. doi: 10.1182/blood.V99.8.2734. [DOI] [PubMed] [Google Scholar]
- Erva RR, Rajulapati SB, Reddy CVK, Reddy KD, Sugunakar YJ. Omics of asnA gene from Enterobactor aerogenes KCTC2190. Int J Fundam Appl Sci. 2013;2:30–32. [Google Scholar]
- Erva RR, Rajulapati SB, Potla Durthi C, Bhatia M, Pola M. Molecular dynamic simulations of Escherichia colil-asparaginase to illuminate its role in deamination of asparagine and glutamine residues. 3 Biotech. 2015;6:2. doi: 10.1007/s13205-015-0339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erva RR, Goswami AN, Suman P, Vedanabhatla R, Rajulapati SB. Optimization of l-asparaginase production from novel Enterobacter sp., by submerged fermentation using response surface methodology. Prep Biochem Biotechnol. 2017;47:219–228. doi: 10.1080/10826068.2016.1201683. [DOI] [PubMed] [Google Scholar]
- Graham ML. Pegaspargase: a review of clinical studies. Adv Drug Deliv Rev. 2003;55:1293–1302. doi: 10.1016/S0169-409X(03)00110-8. [DOI] [PubMed] [Google Scholar]
- Gurunathan B, Sahadevan R. Optimization of culture conditions and bench-scale production of l-asparaginase by submerged fermentation of Aspergillus terreus MTCC 1782. J Microbiol Biotechnol. 2012;22:923–929. doi: 10.4014/jmb.1112.12002. [DOI] [PubMed] [Google Scholar]
- Howard JB, Carpenter FH. l-asparaginase from Erwinia carotovora substrate specificity and enzymatic properties. J Biol Chem. 1972;247:1020–1030. [PubMed] [Google Scholar]
- Kenari SLD, Alemzadeh I, Maghsodi V. Production of l-asparaginase from Escherichia coli ATCC 11303: optimization by response surface methodology. Food Bioprod Process. 2011;89:315–321. doi: 10.1016/j.fbp.2010.11.002. [DOI] [Google Scholar]
- Kumar S, Pakshirajan K, Dasu VV. Development of medium for enhanced production of glutaminase-free l-asparaginase from Pectobacterium carotovorum MTCC 1428. Appl Microbiol Biotechnol. 2009;84:477–486. doi: 10.1007/s00253-009-1973-0. [DOI] [PubMed] [Google Scholar]
- Mahajan RV, Mihooliya KN, Saran S, Saxena RK (2014) l-asparaginase from Bacillus sp. RKS-20: process optimization and application in the inhibition of acrylamide formation in fried foods. J Proteins Proteomics 5
- Maysa E, Amira M, Gamal E, Sanaa T, Sayed E. Production, immobilization and anti-tumor activity of l-asparaginase of Bacillus sp R36. J Am Sci. 2010;6:157–165. [Google Scholar]
- Montgomery DC, Myers RH. Response surface methodology: process and product optimization using designed experiments. Hoboken: Wiley; 1995. [Google Scholar]
- Mukherjee J, Majumdar S, Scheper T. Studies on nutritional and oxygen requirements for production of l-asparaginase by Enterobacter aerogenes. Appl Microbiol Biotechnol. 2000;53:180–184. doi: 10.1007/s002530050006. [DOI] [PubMed] [Google Scholar]
- Peterson R, Ciegler A. l-asparaginase production by Erwinia aroideae. Appl Microbiol. 1969;18:64–67. doi: 10.1128/am.18.1.64-67.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager MD, Bachynsky N. Asparagine synthetase in asparaginase resistant and susceptible mouse lymphomas. Biochem Biophys Res Commun. 1968;31:43–47. doi: 10.1016/0006-291X(68)90028-4. [DOI] [PubMed] [Google Scholar]
- Rajulapati SB, Erva RR. Chemical and bioprocess engineering: trends and developments. Boca Raton: CRC Press; 2015. Homology modeling of l-asparaginase enzyme from Enterobactor aerogenes KCTC2190. [Google Scholar]
- Rajulapati SB, Narasu LM. Neural network modeling and optimization of [alpha]-amylase production from spoiled starch rich vegetables. J Chem Biol Phys Sci. 2011;2:201. [Google Scholar]
- Rajulapati SB, Narasu L, Vundavilli P (2011) Optimization of α-amylase production from Aspergillus Niger using spoiled starch rich vegetables by response surface methodology and genetic algorithm. In: India Conference (INDICON), 2011 Annual IEEE, IEEE. pp 1–9
- Reddy ER, Babu RS, Durthi CP (2015) Structural modelling and molecular dynamics study on Erwinaze towards its substrates. In: International Conference on Advances in Biotechnology (BioTech). Proceedings, 2015. Global Science and Technology Forum, p 166
- Reddy ER, Babu RS, Chandrasai PD, Madhuri P. Exploration of the binding modes of l-asparaginase complexed with its amino acid substrates by molecular docking, dynamics and simulation 3. Biotech. 2016;6:105. doi: 10.1007/s13205-016-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy ER, Babu RS, durthi Chandrasai P, Madhuri P. Neural network modeling and genetic algorithm optimization strategy for the production of l-asparaginase from Novel Enterobacter sp. J Pharm Sci Res. 2017;9:124–130. [Google Scholar]
- Reynolds D, Taylor J. The fungal holomorph: a consideration of mitotic meiotic and pleomorphic speciation. Wallingford: CAB International; 1993. [Google Scholar]
- Sathish T, Prakasham RS. Enrichment of glutaminase production by Bacillus subtilis RSP-GLU in submerged cultivation based on neural network—genetic algorithm approach. J Chem Technol Biotechnol. 2010;85:50–58. doi: 10.1002/jctb.2267. [DOI] [Google Scholar]
- Singh P, Shera SS, Banik J, Banik RM. Optimization of cultural conditions using response surface methodology versus artificial neural network and modeling of l-glutaminase production by Bacillus cereus MTCC 1305. Biores Technol. 2013;137:261–269. doi: 10.1016/j.biortech.2013.03.086. [DOI] [PubMed] [Google Scholar]
- Stecher A, De Deus PM, Polikarpov I, Abrahao-Neto J. Stability of l-asparaginase: an enzyme used in leukemia treatment. Pharm Acta Helv. 1999;74:1–9. doi: 10.1016/S0031-6865(99)00009-6. [DOI] [PubMed] [Google Scholar]
- Venkateswarulu T, Prabhakar KV, Kumar RB. Optimization of nutritional components of medium by response surface methodology for enhanced production of lactase. 3 Biotech. 2017;7:202. doi: 10.1007/s13205-017-0805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma N, Kumar K, Kaur G, Anand S. l-asparaginase: a promising chemotherapeutic agent. Crit Rev Biotechnol. 2007;27:45–62. doi: 10.1080/07388550601173926. [DOI] [PubMed] [Google Scholar]
- Wriston JC, Jr, Yellin T. l-asparaginase: a review. Adv Enzymol Relat Areas Mol Biol. 1973;39:185–248. doi: 10.1002/9780470122846.ch3. [DOI] [PubMed] [Google Scholar]
- Xu C-P, Kim S-W, Hwang H-J, Choi J-W, Yun J-W. Optimization of submerged culture conditions for mycelial growth and exo-biopolymer production by Paecilomyces tenuipes C240. Process Biochem. 2003;38:1025–1030. doi: 10.1016/S0032-9592(02)00224-8. [DOI] [Google Scholar]
- Zhang Z, Friedrich K. Artificial neural networks applied to polymer composites: a review. Compos Sci Technol. 2003;63:2029–2044. doi: 10.1016/S0266-3538(03)00106-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (DOCX 15452 kb)