Abstract
Objectives
Diverticulitis is a common disease with high clinical burden. We evaluated the joint contribution of multiple lifestyle factors to risks of incident diverticulitis. We also estimated the proportion of diverticulitis preventable by lifestyle modifications.
Methods
We prospectively examined the association between lifestyle factors [red meat, dietary fiber intake, vigorous physical activity (activity with metabolic equivalent≥6), body mass index (BMI), and smoking] and risk of diverticulitis among participants in the Health Professionals Follow-Up Study.
Results
We documented 907 incident cases of diverticulitis during 757,791 person-years. High intake of red meat, low intake of dietary fiber, low vigorous physical activity, high BMI, and smoking were independently associated with increased risks of diverticulitis (all p<0.05). Low-risk lifestyle was defined as average red meat intake <51 grams/day, dietary fiber intake in the top 40% of the cohort (about 23 grams/day), vigorous physical activity in the highest 50% among participants with non-zero vigorous physical activity (roughly 2 hours of exercise weekly), normal BMI between 18.5–24.9 kg/m2, and never-smoker. There was an inverse linear relationship between number of low-risk lifestyle factors and diverticulitis incidence (p for trend<0.001). Compared to men with no low-risk lifestyle factors, the multivariable relative risks of diverticulitis were 0.71 [95% confidence interval (CI):0.59–0.87] for men with 1 low-risk lifestyle factor; 0.66 (95% CI:0.55–0.81) for 2 low-risk factors; 0.50 (95% CI:0.40–0.62) for 3 low-risk factors; 0.47 (95% CI:0.35–0.62) for 4 low-risk factors, and 0.27 (95% CI:0.15–0.48) for 5 low-risk factors. Adherence to a low-risk lifestyle could prevent 50% (95% CI:20–71%) of incident diverticulitis.
Conclusions
Adherence to a low-risk lifestyle is associated with reduced incidence of diverticulitis.
INTRODUCTION
Diverticular disease has been called a “disease of Western Civilization” and is a significant burden in industrialized societies (1, 2). The incidence of diverticulitis has risen drastically to become one of the most common indications for gastrointestinal admission in the US at a cost of more than 2 billion dollars annually (3, 4). Wide geographical and temporal variations in the prevalence of diverticular disease has led to the hypothesis that the increasing incidence may be attributed to modifiable diet and lifestyle factors (5). Given the high disease burden, prevention strategies at the population level are needed to reduce diverticulitis incidence.
Diet and other modifiable lifestyle factors play major roles in the development of diverticulitis (5, 6). Among lifestyle factors, dietary fiber intake is the most studied. Previous research from our group and the United Kingdom have shown that dietary fiber is inversely associated with diverticular diseases (7–9). Beyond dietary fiber, red meat consumption has also been associated with diverticulitis risk (9, 10). Non-dietary lifestyle factors also played important roles. Physical activity, particularly vigorous physical activity, was inversely correlated with diverticulitis incidence (11–13). Two large-scale prospective cohort studies revealed a positive association between obesity and diverticulitis risk (14, 15). Finally, tobacco smoking had been related to diverticulitis (16, 17). Although each of these factors appears to contribute to diverticulitis incidence, their combined influence on overall risk of diverticulitis is unclear (18).
A low-risk lifestyle, defined by adherence to multiple modifiable risk factors associated with low diverticulitis incidence such as low red meat intake, high dietary fiber intake, regular exercise, maintenance of a healthy weight, and abstinence from tobacco smoking, may be an approach to prevent the disease. Currently, no study has examined the combination of multiple lifestyle factors and diverticulitis incidence. In this report, we evaluated the joint contribution of lifestyle risk factors to diverticulitis in the HPFS, a nationwide prospective cohort with detailed assessment on lifestyle factors. We also estimated the overall population burden of diverticulitis that may be attributed to adverse lifestyle.
METHODS
Study Population
The Health Professionals Follow-Up Study (HPFS) began in 1986 when 51,529 male health professionals aged 40–75 years provided detailed information on lifestyle and medical history. Participants are followed every 2 years since 1986 by self-administered questionnaires. The overall follow-up rate of the cohort is approximately 93% (19). The study was approved by the institutional review board at the Harvard T.H. Chan School of Public Health.
Participants who reported a diagnosis of diverticulosis or its complications, inflammatory bowel diseases, or malignancy of the gastrointestinal tract at baseline were excluded. Men who reported implausible energy intakes were also excluded. Participants who did not answer food frequency questionnaires (FFQ), questions regarding levels of physical activity, or anthropometric measurements at baseline were excluded, leaving 45,203 men in the current analysis (Supplementary Figure 1).
Assessment of Lifestyle Factors and Other Potential Risk Factors
Dietary information was assessed by a validated, semi-quantitative FFQ every four years from 1986 to 2010 (20). The FFQ assessed how often, on average, a participant consumed a particular food during the previous year. Frequencies of food consumption of a standard portion of 131 (in 1986) to 148 (in 2010) specified food items were recorded in 9 categories ranging from “never or less than once per month” to “6 or more times per day.” Unprocessed red meat items included food such as “beef or lamb as main dish” or “hamburger.” For processed red meat, the FFQ included questions such as “bacon” or “beef or pork hot dogs”. We calculated total red meat consumption by summing unprocessed and processed red meat intake.
Dietary fiber intake was computed by multiplying the fiber content of the specified food by its consumption frequency. Dietary fiber contents were derived from the Harvard Food Composition Database, which is based on US Department of Agriculture sources and from manufacturers derived by the Association of Official Analytical Chemists enzyme gravimetric method (7, 21). Dietary variables were energy-adjusted by regression analysis. This approach allowed us to focus on the composition of diets independent of total energy intake (22).
Physical activity was self-reported using a validated, biennial questionnaire (23). Participants were asked to note the average time per week spent in recreational activity (walking, jogging, running, bicycling, lap swimming, tennis, squash or racquet ball, calisthenics, rowing, stair climber or ski machine) during the previous year. Each physical activity was assigned a metabolic equivalent of tasks (MET) score based on energy expenditure (24). Physical activity with a MET score of at least 6 were classified as vigorous, and those with MET score less than 6 was categorized as non-vigorous (11, 13). Height was recorded at baseline in 1986, and body weight was updated biennially. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2).
Assessment of smoking habits had been reported previously (25). Participants provided information about past smoking habits in baseline questionnaire in 1986. Current smoking status and the number of cigarettes smoked daily were updated in the biennial questionnaire. Cumulative pack-years smoked was derived from this information. For participants who did not respond to questionnaire items on red meat intake, dietary fiber intake, physical activity, and weight, their responses from previous cycle were used. This carry-forward was limited to 4 years, which is the cycle length of the FFQ. Intervals with missing data after carry-forward were excluded. We also assessed other factors that had been shown to be risk factors for diverticulitis (16, 26). These factors included the use of non-steroid anti-inflammatory drugs (NSAID), aspirin and acetaminophen which were assessed regularly in the biennial questionnaire.
Definition of Low-risk Lifestyle
Low-risk lifestyle was defined for calculation of population-attributable risk (PAR). Five lifestyle factors were considered based on their association with diverticulitis: total red meat intake, dietary fiber intake, vigorous physical activity, BMI, and smoking. For red meat intake, we defined low-risk as average daily red meat intake less than 51 grams per 2000 kcal energy intake based on United States Department of Agriculture Food Pattern recommendation (27). This is roughly fewer than 4 servings of red meat weekly. The standard serving size was 140 grams (5 ounces) for beef or pork as main dish, 85 grams (3 ounces) for other unprocessed red meat, and < 50 grams for processed red meat such as bacon (13 grams) or hot dogs (45 grams). Healthy dietary fiber intake was defined as daily fiber intake in the highest 40% of the cohort (approximately 23 grams of dietary fiber or more daily). For vigorous physical activity, we defined healthy lifestyle as having vigorous physical activity in the highest 50% of the cohort among participants with non-zero vigorous physical activity. This is roughly equivalent to at least two hours of jogging, running, biking, swimming, or playing tennis weekly. We defined optimal weight as a BMI between 18.5 and 24.9 kg/m2, which is the normal BMI range defined by the World Health Organization (28). We also defined participants who never smoked as having low-risk lifestyle. We evaluated the association between number of low-risk lifestyle factors and diverticulitis risk. As a sensitivity analysis, a more lenient low-risk lifestyle was defined as red meat intake fewer than 5 servings per week, dietary fiber intake in the highest 50% of the cohort, any vigorous physical activity, a BMI less than 27.5 kg/m2, and participants who were not smoking currently.
The dichotomized variables may not fully account for the risk gradients in diverticulitis at more extreme values. Therefore, we calculated an expanded lifestyle score by assigning scores of 1 (lowest risk) to 5 (highest risk, 3 for BMI) to the categories of lifestyle factors and summed the scores for every participant (score range, 5–23 points). For this analysis, lowest-risk was defined as red meat intake in the lowest quintile, dietary fiber intake in the highest quintile, vigorous physical activity in the highest quartile among participants with non-zero vigorous physical activity, BMI less than 24.9 kg/m2, and never smoker.
Ascertainment of Diverticulitis
Our primary endpoint was incident diverticulitis. Since 1990, participants who reported newly diagnosed diverticular disease were sent supplementary questionnaires to ascertain date of diagnosis, presenting symptoms, diagnosis procedures, and treatment. Beginning in 2006, we administered a revised supplementary diverticular disease questionnaire to assess diverticulosis, diverticular bleeding, uncomplicated diverticulitis, and complications of diverticulitis including abscess, fistula, perforation, and obstruction. In this study, we focused on diverticulitis, which included uncomplicated diverticulitis and complications of diverticulitis.
Diverticulitis was defined as abdominal pain attributed to diverticular disease which fulfilled one of the following hierarchical criteria: 1) complicated by perforation, abscess, fistula, or obstruction; 2) required antibiotics, hospitalization, or surgical intervention; or 3) abdominal pain categorized as severe or acute; or pain presenting with fever, requiring medication, or evaluated using abdominal computed tomography. We have utilized these criteria in prior analyses, and documented the validity of self-reported diverticulitis in this population (6, 10, 11, 15, 29).
Statistical Analysis
We evaluated the association between five lifestyle factors (total red meat consumption, dietary fiber consumption, vigorous physical activity, BMI, and smoking) and risk of incident diverticulitis. Participant follow-up time accrued from the return of the baseline questionnaire to the first of the following: date of diagnosis of diverticulitis, diverticulosis, diverticular bleeding, death, or the end of follow up (December 31st, 2012). We calculated age-adjusted and multivariable-adjusted hazards ratios to estimate relative risks (RR) and 95% confidence intervals (CI) using stratified Cox proportional hazards models. Total energy intake (quintiles), current regular aspirin use (yes/no), current regular NSAID use (yes/no), and current regular acetaminophen use (yes/no) were adjusted in the multivariable model stratified by age (months) and study period (2-year intervals). Tests for linear trend were performed using the variables of interest as continuous variables. Tests of non-linearity were performed using the likelihood ratio test between models with and without quadratic terms of the variables of interest. We compared the final multivariable model with and without interaction terms created with continuous variables by likelihood ratio tests to test for potential interactions between the five main lifestyle factors.
We calculated the proportion of diverticulitis that was attributable to non-adherence to each individual low-risk lifestyle factor as well as to all five low-risk lifestyle factors after keeping all other variables constant. The PAR was calculated by estimating the hazard ratio from the multivariable Cox proportional hazard model while controlling for other covariates (30, 31). The PAR can be interpreted as the proportion of diverticulitis that would have been prevented had all participants adhered to a healthy, low-risk lifestyle. Statistical analyses were conducted with SAS version 9.4 (SAS Institute Inc., NC). Significance was set as p value less than 0.05 in a two-sided test.
RESULTS
We identified 907 incident cases of diverticulitis over 757,791 person-years of follow-up. The age-standardized baseline characteristics according to categories of red meat intake, dietary fiber intake, vigorous physical activity, BMI, and smoking status are summarized in Table 1. Healthy behaviors, including vigorous physical activity, lower BMI, lower red meat, higher dietary fiber intake and less smoking tended to correlate with each other.
Table 1.
Characteristics of Men in Health Professionals Follow-Up Study (HPFS), 1986–2012
| Characteristics1 | Full HPFS Cohort | Lifestyle Factors | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Total red meat, quintiles | Dietary Fiber, quintiles | Vigorous physical activity, quartiles2 | Body mass index | Smoking | ||||||||||||
|
|
|
|
|
|
||||||||||||
| 1 | 3 | 5 | 1 | 3 | 5 | 0 | Q2 | Q4 | < 25 | 25–29.9 | ≥30 | Never | 5–19 PY | ≥40 PY | ||
| Age, years | 62 | 63 | 62 | 61 | 60 | 62 | 65 | 64 | 62 | 60 | 63 | 62 | 62 | 62 | 61 | 66 |
| Total red meat intake, servings/week | 6.2 | 1.2 | 5.2 | 14 | 8.4 | 6.4 | 3.5 | 7.0 | 5.9 | 5.0 | 5.5 | 6.5 | 7.7 | 5.9 | 6.0 | 7.9 |
| Total red meat intake, g/day | 72 | 15 | 63 | 150 | 95 | 74 | 42 | 80 | 69 | 59 | 64 | 75 | 88 | 69 | 70 | 88 |
| Dietary fiber, g/day | 23 | 28 | 22 | 20 | 15 | 22 | 34 | 22 | 23 | 25 | 24 | 22 | 22 | 24 | 23 | 20 |
| All physical activity, MET-hours/week | 29 | 32 | 29 | 28 | 24 | 29 | 35 | 18 | 26 | 66 | 32 | 28 | 22 | 31 | 30 | 21 |
| Vigorous physical activity, MET-hours/week | 13 | 18 | 12 | 8.9 | 8.3 | 13 | 18 | 0 | 9.3 | 55 | 16 | 11 | 7.1 | 14 | 14 | 7 |
| Body mass index, kg/m2 | 26 | 25 | 26 | 27 | 26 | 26 | 25 | 26 | 26 | 25 | 23 | 27 | 33 | 26 | 26 | 27 |
| Ever smokers, % | 47 | 43 | 47 | 51 | 55 | 47 | 41 | 51 | 48 | 43 | 44 | 49 | 52 | 0 | 100 | 100 |
| Pack-years among ever smokers | 24 | 20 | 23 | 27 | 28 | 23 | 20 | 27 | 22 | 19 | 22 | 24 | 26 | 0 | 11 | 54 |
| Alcohol intake, g/day | 12 | 8.8 | 12 | 13 | 18 | 11 | 7.1 | 12 | 11 | 12 | 11 | 12 | 11 | 9.0 | 13 | 17 |
| Regular aspirin use, % | 47 | 48 | 47 | 46 | 46 | 48 | 46 | 46 | 49 | 46 | 44 | 48 | 52 | 45 | 49 | 50 |
| Regular NSAID use, % | 15 | 12 | 16 | 17 | 17 | 16 | 12 | 15 | 16 | 17 | 13 | 16 | 21 | 14 | 16 | 15 |
| Regular acetaminophen use, % | 8.2 | 6.6 | 8.4 | 9.6 | 9.0 | 8.5 | 6.9 | 8.8 | 8.2 | 6.7 | 7.4 | 8.5 | 11 | 7.5 | 9.1 | 9.2 |
MET, metabolic equivalent of tasks; NSAID, non-steroid anti-inflammatory drugs
All values other than age is directly standardized to age distribution (in 5-year age group) of all the participants. Mean is presented for continuous variables.
Vigorous physical activity is categorized as 0 for participants without vigorous physical activity plus 4 quartiles (Q1–Q4) among participants with non-zero vigorous physical activity.
The five lifestyle factors were each independently associated with diverticulitis risk. Men in the highest quintile of red meat intake had a multivariable RR of 1.43 (95% CI: 1.10–1.85, p for trend = 0.026), after adjusting for dietary fiber intake, vigorous physical activity, BMI, smoking, and other potential confounders (Table 2). Men in the highest quintile of dietary fiber intake had a multivariable RR of 0.77 (95% CI: 0.60–0.98 p for trend = 0.005) compared with men in the lowest quintile of dietary fiber intake. Similarly, participants in the highest quartile of vigorous physical activity had a multivariable RR of 0.73 (95% CI: 0.58–0.92, p for trend = 0.003) compared to men without vigorous physical activity. Obese men (BMI at least 30 kg/m2) had a multivariable RR of 1.21 (95% CI: 0.97–1.49, p for trend = 0.036) for diverticulitis. Finally, participants with a smoking history ≥ 40 pack-years had a RR of 1.27 (95% CI: 1.01–1.58, p for trend = 0.003) compared to never smoker. Regular NSAID user had a RR of 1.42 (95% CI: 1.20–1.67, p < 0.001) for diverticulitis compared to participants who did not use NSAID regularly. There were no interactions between these factors on diverticulitis incidence.
Table 2.
Lifestyle Factors and Risk of Incident Diverticulitis
| Lifestyle Factors | Pearson-Years | Cases, No. | Incidence per 100,000 Person-Years1 | Age-adjusted RR2 (95% CI) | p for trend3 | Multivariable RR4 (95% CI) | p for trend3 |
|---|---|---|---|---|---|---|---|
| Total red meat intake | |||||||
| 1st quintile | 158,221 | 131 | 82 | 1.00 [Reference] | <0.001 | 1.00 [Reference] | 0.026 |
| 2nd quintile | 147,704 | 180 | 120 | 1.45 (1.16, 1.83) | 1.31 (1.04, 1.65) | ||
| 3rd quintile | 148,006 | 207 | 138 | 1.62 (1.30, 2.03) | 1.43 (1.13, 1.80) | ||
| 4th quintile | 152,080 | 178 | 116 | 1.40 (1.12, 1.76) | 1.21 (0.94, 1.56) | ||
| 5th quintile | 151,780 | 211 | 137 | 1.65 (1.32, 2.05) | 1.43 (1.10, 1.85) | ||
|
| |||||||
| Dietary fiber intake | |||||||
| 1st quintile | 151,096 | 213 | 136 | 1.00 [Reference] | <0.001 | 1.00 [Reference] | 0.005 |
| 2nd quintile | 151,410 | 199 | 132 | 0.95 (0.78, 1.15) | 1.00 (0.82, 1.21) | ||
| 3rd quintile | 151,377 | 197 | 128 | 0.92 (0.76, 1.12) | 1.02 (0.83, 1.24) | ||
| 4th quintile | 151,949 | 167 | 106 | 0.76 (0.62, 0.93) | 0.88 (0.71, 1.09) | ||
| 5th quintile | 151,959 | 131 | 83 | 0.59 (0.48, 0.74) | 0.77 (0.60, 0.98) | ||
|
| |||||||
| Vigorous physical activity5 | |||||||
| No vigorous activity | 314,327 | 452 | 141 | 1.00 [Reference] | <0.001 | 1.00 [Reference] | 0.003 |
| 1st quartile | 106,695 | 125 | 116 | 0.85 (0.69, 1.04) | 0.88 (0.72, 1.08) | ||
| 2nd quartile | 117,437 | 136 | 114 | 0.81 (0.67, 0.99) | 0.87 (0.72, 1.06) | ||
| 3rd quartile | 107,958 | 94 | 87 | 0.61 (0.48, 0.76) | 0.67 (0.53, 0.84) | ||
| 4th quartile | 111,374 | 100 | 93 | 0.63 (0.51, 0.79) | 0.73 (0.58, 0.92) | ||
|
| |||||||
| Body mass index | |||||||
| BMI < 25.0 kg/m2 | 325,490 | 321 | 97 | 1.00 [Reference] | <0.001 | 1.00 [Reference] | 0.036 |
| BMI 25.0–29.9 kg/m2 | 350,146 | 454 | 127 | 1.27 (1.10, 1.46) | 1.15 (0.99, 1.33) | ||
| BMI ≥ 30.0 kg/m2 | 82,155 | 132 | 157 | 1.46 (1.19, 1.80) | 1.21 (0.97, 1.49) | ||
|
| |||||||
| Smoking | |||||||
| Never smoker | 399,444 | 433 | 108 | 1.00 [Reference] | <0.001 | 1.00 [Reference] | 0.003 |
| 0.1–4.9 pack-years | 43,539 | 32 | 74 | 0.70 (0.48, 1.01) | 0.69 (0.48, 1.01) | ||
| 5–19.9 pack-years | 130,870 | 160 | 123 | 1.13 (0.94, 1.36) | 1.11 (0.92, 1.33) | ||
| 20–39.9 pack-years | 117,745 | 172 | 143 | 1.32 (1.10, 1.58) | 1.21 (1.00, 1.45) | ||
| ≥ 40 pack-years | 66,193 | 110 | 163 | 1.48 (1.19, 1.85) | 1.27 (1.01, 1.58) | ||
BMI, body mass index; CI, confidence interval; RR, relative risks.
Incidence is directly standardized to age distribution (in 5-year age group) of all the participants.
Adjusted for age and questionnaire cycle.
Calculated using continuous variables.
Additionally adjusted for total energy intake (quintiles), regular use of aspirin, NSAID, acetaminophen, red meat, dietary fiber, vigorous physical activity, body mass index, and smoking status.
Vigorous physical activity is categorized as 0 for participants without vigorous physical activity plus 4 quartiles (Q1–Q4) among participants with non-zero vigorous physical activity.
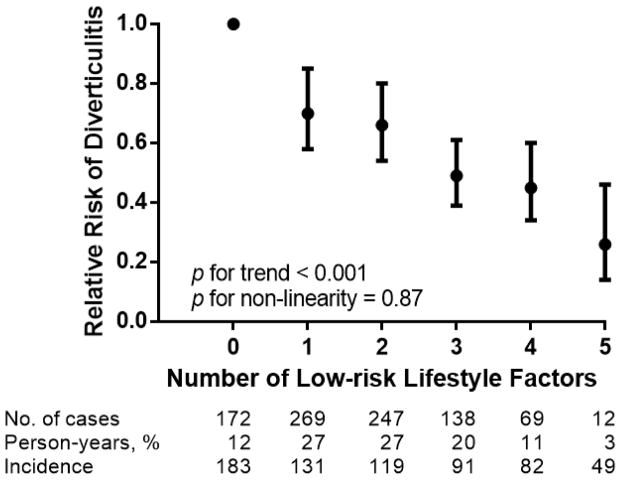
Overall, the number of low-risk lifestyle factors was inversely correlated with diverticulitis risk (Table 3, p for trend < 0.001). The age-standardized incidences of diverticulitis were 183 cases/100,000 person-years among men with no low-risk or healthy factors, 131 cases/100,000 person-years among men with one low-risk factor, 119 cases/100,000 person-years among men with two healthy factors, 91 cases/100,000 person-years among men with three healthy factors, 82 cases/100,000 person-years among men with four low-risk factors, and 49 cases/100,000 person-years among participants with all 5 healthy factors. Compared with men with no healthy lifestyle factor (12% of the cohort), men with five healthy factors (3% of the cohort) had a multivariable-adjusted RR of 0.27 (95% CI: 0.15–0.48) for diverticulitis (Figure 1). We found no evidence of a non-linear association between number of low-risk lifestyle factors and diverticulitis incidence (p for non-linearity = 0.87). The effects of changing from high-risk lifestyle to each low-risk lifestyle factors, sub-grouped by smoking status, are summarized in Supplementary Figure 2.
Table 3.
Number of Low-Risk Lifestyle Factors and Risk of Incident Diverticulitis
| Number of Low-Risk Lifestyle Factors1 | Pearson-Years (%) | Cases, No. | Incidence per 100,000 Person-Years2 | Age-adjusted RR3 (95% CI) | p for trend4 | Multivariable RR5 (95% CI) | p for trend4 |
|---|---|---|---|---|---|---|---|
| 0 | 91,410 (12%) | 172 | 183 | 1.00 [Reference] | <0.001 | 1.00 [Reference] | <0.001 |
| 1 | 201,485 (27%) | 269 | 131 | 0.70 (0.58, 0.85) | 0.71 (0.59, 0.87) | ||
| 2 | 205,159 (27%) | 247 | 119 | 0.66 (0.54, 0.80) | 0.66 (0.55, 0.81) | ||
| 3 | 150,828 (20%) | 138 | 91 | 0.49 (0.39, 0.61) | 0.50 (0.40, 0.62) | ||
| 4 | 83,264 (11%) | 69 | 82 | 0.45 (0.34, 0.60) | 0.47 (0.35, 0.62) | ||
| 5 | 25,645 (3%) | 12 | 49 | 0.26 (0.14, 0.46) | 0.27 (0.15, 0.48) |
CI, confidence interval; NSAID, non-steroid anti-inflammatory drug; RR, relative risks.
Low risk factors: red meat intake < 51 grams daily, dietary fiber intake in highest 40% of the cohort, vigorous physical activity in the top 2 quartiles after excluding participants with no vigorous physical activity, normal body weight, and never smoker.
Incidence is directly standardized to age distribution (in 5-year age group) of all the participants.
Adjusted for age and questionnaire cycle.
Calculated using continuous variables.
Additionally adjusted for and total energy intake (quintiles), regular use of aspirin, NSAID, and acetaminophen.
Figure 1. Number of low-risk factors and risk of diverticulitis.
Low-risk lifestyle factors were defined as total red meat intake less than 51 grams daily, dietary fiber intake in the highest 40% of the cohort, vigorous physical activity in the highest 50% of the cohort among participants with non-zero vigorous physical activity, normal body mass index between 18.5 and 24.9 kg/m2, and never smoker. Number of low-risk lifestyle factors were linearly and inversely associated with risk of diverticulitis. Error bars indicate 95% confidence intervals.
The individual PAR for non-adherence to low-risk lifestyle factors ranged from 7% for smoking to 21% for vigorous physical activity (Table 4). In the entire cohort, the PAR for non-adherence to healthy lifestyle was 50% (95% CI: 20% to 71%), suggesting that 50% of diverticulitis could have been prevented had all men pursued a healthy lifestyle. When regular NSAID use was included in the model, the PAR for non-adherence to healthy lifestyle was 53% (95% CI: 23% to 74%). In a sensitivity analysis using a more lenient definition of low-risk lifestyle, the PAR for non-adherence to healthy lifestyle was 31% (95% CI: 8% to 51%, Supplementary Table 1). When we used an expanded lifestyle score to estimate PAR, the results were similar to the PAR estimation based on binary lifestyle factors (Supplementary Figure 3).
Table 4.
Population Attributable Risk of Different Lifestyle Factors on Diverticulitis
| Lifestyle Factors | Low-Risk Definition | Person-Years at Low Risk, % | Multivariable RR1 (95% CI) | Population Attributable Risk (95% CI), % | |
|---|---|---|---|---|---|
|
| |||||
| Individual Risk Factor | Combined | ||||
| Total red meat intake | < 51 grams/day/2000 Kcal2 | 37% | 0.82 (0.70, 0.95) | 13 (3–22) | 50 (20–71) |
| Dietary fiber intake | 4th and 5th quintiles3 | 40% | 0.83 (0.71, 0.96) | 12 (3–20) | |
| Vigorous physical activity | 3rd and 4th quartiles4 | 29% | 0.73 (0.62, 0.86) | 21 (10–32) | |
| Body mass index | Normal body weight5 | 43% | 0.82 (0.71, 0.94) | 12 (4–20) | |
| Smoking | Never smoker | 53% | 0.87 (0.76, 0.99) | 7 (0–14) | |
CI, confidence interval; RR, relative risk.
Adjusted for age, questionnaire cycle, total energy intake (quintiles), regular use of aspirin, NSAID, and acetaminophen.
Approximately fewer than 4 servings of red meat per week.
Roughly at least 23 grams of dietary fiber intake daily.
At least two hours of jogging, running, biking, swimming, or playing tennis each week.
Normal body weight: BMI 18.5–24.9 kg/m2.
DISCUSSION
In this large, prospective study of men, dietary red meat intake, low dietary fiber intake, lack of vigorous physical activity, BMI ≥ 25 kg/m2, and smoking were independently associated with an increased risk of diverticulitis. Adherence to a low-risk lifestyle (average red meat intake less than 51 grams daily, dietary fiber intake in the highest 40% of the cohort, vigorous physical activity in the highest 50% of the cohort among participants with non-zero vigorous physical activity, BMI between 18.5 and 24.9 kg/m2, and never smoker) was linearly and inversely associated with diverticulitis risk. Men at low-risk for all 5 factors had a 73% decreased diverticulitis risk compared with men with no low-risk factor. Assuming these associations are causal, half of diverticulitis cases may have been prevented if all men adhered to a low-risk lifestyle. Our results support the enormous potential of lifestyle modification for the prevention of diverticulitis in addition to other potential health benefits.
Previous population-based studies have evaluated separately the association between various lifestyle factors and incidence of diverticulitis (8, 10–12, 14, 16, 17, 32). Evidence revealed that red meat intake, low dietary fiber intake, physical inactivity, obesity, and smoking, are associated with diverticulitis. Our work further examined the joint effects of lifestyle factors on the risk of incident diverticulitis. As far as we know, no prior study has examined the combined effects of lifestyle factors on diverticulitis. Our results indicate that lifestyle patterns may act jointly and independently to influence risk for diverticulitis.
The PAR estimate is population-specific. The generalizability of our results to women, a younger population, or other ethnic groups is limited. The HPFS is a unique population in that health professionals tend to pursue a healthier lifestyle. For example, fewer than 30% of US people aged 45 to 74 years maintain a BMI less than 25 kg/m2 (33). In contrast, 43% of HPFS participants maintain a normal BMI. US men in this age group consume roughly 19 grams of dietary fiber per day, while HPFS participants consume more than 22 grams of dietary fiber daily (34). Finally, although we do not have data about prevalence of vigorous physical activity in the general population, only 45% of US people aged 45 to 74 years met the general physical activity goal of 7.5 MET-hour per week, while more than 75% of men in HPFS cohort met this target (33). Overall, the prevalence of low-risk lifestyle factors is lower in the general population than in the HPFS. Hence, adherence to a low-risk lifestyle may have prevented an even larger absolute proportion of incident diverticulitis cases in a population that is more reflective of the general population. Finally, we did not employ a strict threshold for describing a low-risk profile to allow for more meaningful translation into public health actions. In a sensitivity analysis using an even more lenient threshold for low-risk lifestyle, more than one-fourth of diverticulitis cases could have been prevented with adherence to health lifestyle.
To our knowledge, there are no proven medical means to prevent either incident or recurrent diverticulitis. Six randomized, placebo controlled clinical trials of mesalamine, as well as a meta-analysis, failed to show a benefit of this medication in the prevention of recurrent diverticulitis (35). Small trials of probiotics and rifaximin for the prevention of recurrent diverticulitis have been inconclusive. Furthermore, the benefits of elective colonic resection for the prevention of recurrent uncomplicated diverticulitis are unclear, and recent guidelines have generally tended against recommending surgical prophylaxis based on the number of diverticulitis episodes (36). Finally, NSAID, while not one of lifestyle factors per se, appeared to increase the risk of diverticulitis (26, 32). In this study, regular NSAID user had a RR of 1.42 for diverticulitis compared to participants who did not use NSAID regularly. While participants may have been prescribed NSAID for a compelling indication, NSAID avoidance may be important for certain patients that are unable to adopt other healthy behaviors. Thus, given limited alternative options for prevention, our findings suggest that lifestyle modifications, smoking cessation, and NSAID avoidance may also play a particularly critical role in patients at risk for recurrent episodes of diverticulitis.
The strengths of our study include a large, well-characterized, prospective nationwide cohort that has been regularly assessed for more than 25 years. The repeated assessments allowed us to examine lifestyle factors throughout follow-up. The detailed lifestyle information allowed us to estimate the combined effects of distinct lifestyle factors to finely adjust for potential confounders and to calculate the proportion of diverticulitis that was attributable to non-adherence to a low-risk lifestyle. We were also able to distinguish diverticulitis from diverticular bleeding and uncomplicated diverticulosis, which appear to have distinct risk factors.
There are certain limitations in this study. First, the observational nature of the study precluded us from ruling out residual confounding. Second, the study relied on self-reported questionnaires, which may be subject to measurement error. However, the accuracy of self-reported lifestyle factors and diverticulitis has been validated in this cohort (11, 15, 29). Misclassification bias, if present, tends to bias the association towards the null. Such bias is likely to be non-differential, and likely underestimates the true population effects (37). Third, we dichotomized each lifestyle factor for estimation of PAR, while the relationship between lifestyle factors and diverticulitis risk may be more complex. Nonetheless, classification of exposures into two levels yields an unbiased estimate of attributable risk when misclassification is non-differential, and the simplicity of dichotomized cut-points may help in forming discrete public health guidance (38). Furthermore, the estimated PAR was similar when we used an expanded lifestyle score to estimate PAR. Fourth, the cohort consisted only of White male health professionals. The generalizability to women, other ethnic groups, and people with different socioeconomic status is limited. Finally, the PAR estimates assume a causal relationship between lifestyle factors and diverticulitis risk. Adequate clinical trials are required to establish the causal linkage between modifiable lifestyle factors and disease incidence. However, large-scale, long-term trials assessing the effects of various lifestyle factors on diverticulitis risk would be challenging to execute. In the absence of such trials, carefully performed observational studies provide a reasonable approach for evaluating the association between lifestyle factors and risk of diverticulitis.
CONCLUSION
In summary, we found that red meat intake, low dietary fiber intake, lack of vigorous physical activity, obesity, and smoking were jointly and independently associated with increased risk of diverticulitis. An overall low-risk lifestyle was associated with a 50% lower risk of diverticulitis. Adherence to a low-risk lifestyle may be an effective strategy for preventing diverticulitis. Given the high burden of diverticulitis on the US population and the lack of alternative preventive interventions, broader adoption of a healthy, low-risk lifestyle may have a substantial impact on the health care landscape.
Supplementary Material
STUDY HIGHLIGHTS.
What is current knowledge
Diverticulitis is a common disease with high clinical and economic burden.
The combine effect of multiple lifestyle factors on incidence of diverticulitis incidence is underexplored.
What is new here
Red meat, dietary fiber, vigorous physical activity, body mass index, and smoking appear to act jointly and independently to influence the incidence of diverticulitis.
Adherence to low-risk lifestyle potentially prevents half of incidence diverticulitis.
Acknowledgments
We would like to thank the participants and staff of the Health Professionals Follow-Up Study for their valuable contributions.
Funding: This work was supported by grants R01 DK101495, R01 DK084157, K24 DK098311, and UM1 CA167552 from the National Institutes of Health.
Footnotes
Role of sponsor: The study sponsors have no role in the study design, collection, analysis, and interpretation of data.
Author Contributions: Drs. Liu, Cao and Chan had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: P.H.L., Y.C., L.L.S., E.L.G., A.T.C.
Acquisition of data: L.L.S., B.R.K., I.T., A.T.C.
Analysis and interpretation of data: all coauthors
Drafting of the manuscript: P.H.L.
Critical revision of the manuscript for important intellectual content: all coauthors
Statistical analysis: P.H.L., Y.C.
Obtained funding: L.L.S., E.L.G., A.T.C.
Administrative, technical, or material support: Y.C., L.L.S., E.L.G., A.T.C.
Study supervision: Y.C., L.L.S., E.L.G., A.T.C.
Competing interests: A.T.C. previously served as a consultant for Bayer Healthcare, Aralaz Pharmaceuticals and Pfizer Inc. for work unrelated to the topic of this manuscript. This study was not funded by Bayer Healthcare, Aralez Pharmaceuticals or Pfizer Inc.
References
- 1.Painter NS, Burkitt DP. Diverticular disease of the colon: a deficiency disease of Western civilization. Br Med J. 1971;2:450–4. doi: 10.1136/bmj.2.5759.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delvaux M. Diverticular disease of the colon in Europe: epidemiology, impact on citizen health and prevention. Aliment Pharmacol Ther. 2003;18(Suppl 3):71–4. doi: 10.1046/j.0953-0673.2003.01720.x. [DOI] [PubMed] [Google Scholar]
- 3.Peery AF, Crockett SD, Barritt AS, et al. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology. 2015;149:1731–1741. e3. doi: 10.1053/j.gastro.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wheat CL, Strate LL. Trends in Hospitalization for Diverticulitis and Diverticular Bleeding in the United States From 2000 to 2010. Clin Gastroenterol Hepatol. 2016;14:96–103. e1. doi: 10.1016/j.cgh.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strate LL. Lifestyle factors and the course of diverticular disease. Dig Dis. 2012;30:35–45. doi: 10.1159/000335707. [DOI] [PubMed] [Google Scholar]
- 6.Strate LL, Keeley BR, Cao Y, et al. Western Dietary Pattern Increases, and Prudent Dietary Pattern Decreases, Risk of Incident Diverticulitis in a Prospective Cohort Study. Gastroenterology. 2017;152:1023–1030. e2. doi: 10.1053/j.gastro.2016.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aldoori WH, Giovannucci EL, Rockett HR, et al. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr. 1998;128:714–9. doi: 10.1093/jn/128.4.714. [DOI] [PubMed] [Google Scholar]
- 8.Crowe FL, Balkwill A, Cairns BJ, et al. Source of dietary fibre and diverticular disease incidence: a prospective study of UK women. Gut. 2014;63:1450–6. doi: 10.1136/gutjnl-2013-304644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowe FL, Appleby PN, Allen NE, et al. Diet and risk of diverticular disease in Oxford cohort of European Prospective Investigation into Cancer and Nutrition (EPIC): prospective study of British vegetarians and non-vegetarians. BMJ. 2011;343:d4131. doi: 10.1136/bmj.d4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao Y, Strate LL, Keeley BR, et al. Meat intake and risk of diverticulitis among men. Gut. 2017 doi: 10.1136/gutjnl-2016-313082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strate LL, Liu YL, Aldoori WH, et al. Physical activity decreases diverticular complications. Am J Gastroenterol. 2009;104:1221–30. doi: 10.1038/ajg.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams PT. Incident diverticular disease is inversely related to vigorous physical activity. Med Sci Sports Exerc. 2009;41:1042–7. doi: 10.1249/MSS.0b013e318192d02d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aldoori WH, Giovannucci EL, Rimm EB, et al. Prospective study of physical activity and the risk of symptomatic diverticular disease in men. Gut. 1995;36:276–82. doi: 10.1136/gut.36.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosemar A, Angeras U, Rosengren A. Body mass index and diverticular disease: a 28-year follow-up study in men. Dis Colon Rectum. 2008;51:450–5. doi: 10.1007/s10350-007-9172-5. [DOI] [PubMed] [Google Scholar]
- 15.Strate LL, Liu YL, Aldoori WH, et al. Obesity increases the risks of diverticulitis and diverticular bleeding. Gastroenterology. 2009;136:115–122. e1. doi: 10.1053/j.gastro.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hjern F, Wolk A, Hakansson N. Smoking and the risk of diverticular disease in women. Br J Surg. 2011;98:997–1002. doi: 10.1002/bjs.7477. [DOI] [PubMed] [Google Scholar]
- 17.Humes DJ, Fleming KM, Spiller RC, et al. Concurrent drug use and the risk of perforated colonic diverticular disease: a population-based case-control study. Gut. 2011;60:219–24. doi: 10.1136/gut.2010.217281. [DOI] [PubMed] [Google Scholar]
- 18.Strate LL, Modi R, Cohen E, et al. Diverticular disease as a chronic illness: evolving epidemiologic and clinical insights. Am J Gastroenterol. 2012;107:1486–93. doi: 10.1038/ajg.2012.194. [DOI] [PubMed] [Google Scholar]
- 19.FAQ of the Health Professionals Follow-Up Study Website. [cited 08/01/2017]; Available from: https://content.sph.harvard.edu/hpfs/hpfs_faq.htm.
- 20.Rimm EB, Giovannucci EL, Stampfer MJ. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 21.Prosky L, Asp NG, Furda I, et al. Determination of total dietary fiber in foods and food products: collaborative study. J Assoc Off Anal Chem. 1985;68:677–9. [PubMed] [Google Scholar]
- 22.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 23.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–6. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Nishihara R, Morikawa T, Kuchiba A, et al. A prospective study of duration of smoking cessation and colorectal cancer risk by epigenetics-related tumor classification. Am J Epidemiol. 2013;178:84–100. doi: 10.1093/aje/kws431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strate LL, Liu YL, Huang ES, et al. Use of aspirin or nonsteroidal anti-inflammatory drugs increases risk for diverticulitis and diverticular bleeding. Gastroenterology. 2011;140:1427–33. doi: 10.1053/j.gastro.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7. Washington, DC: U.S. Government Printing Office; Dec, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 29.Strate LL, Liu YL, Syngal S, et al. Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA. 2008;300:907–14. doi: 10.1001/jama.300.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control. 2007;18:571–9. doi: 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 31.Cao Y, Nishihara R, Wu K, et al. Population-wide Impact of Long-term Use of Aspirin and the Risk for Cancer. JAMA Oncol. 2016;2:762–9. doi: 10.1001/jamaoncol.2015.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aldoori WH, Giovannucci EL, Rimm EB. Use of acetaminophen and nonsteroidal anti-inflammatory drugs: a prospective study and the risk of symptomatic diverticular disease in men. Arch Fam Med. 1998;7:255–260. doi: 10.1001/archfami.7.3.255. [DOI] [PubMed] [Google Scholar]
- 33.Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat. 2014;10:1–161. [PubMed] [Google Scholar]
- 34.U.S. Department of Agriculture, Agricultural Research Service, Beltsville Human Nutrition Research Center, Food Surveys Research Group (Beltsville, MD) and U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics (Hyattsville, MD) [accessed 02/04/17];What We Eat in America, NHANES 2013–2014 Data. Available from: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/1314/tables_41-56_2013-2014.pdf.
- 35.Strate LL, Peery AF, Neumann I. American Gastroenterological Association Institute Technical Review on the Management of Acute Diverticulitis. Gastroenterology. 2015;149:1950–1976. e12. doi: 10.1053/j.gastro.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284–94. doi: 10.1097/DCR.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 37.Chiuve SE, Fung TT, Rexrode KM, et al. Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA. 2011;306:62–9. doi: 10.1001/jama.2011.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wacholder S, Benichou J, Heineman EF, et al. Attributable risk: advantages of a broad definition of exposure. Am J Epidemiol. 1994;140:303–9. doi: 10.1093/oxfordjournals.aje.a117252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.