Abstract
Neuroblastoma is a pediatric tumor characterized by histologic heterogeneity, and accounts for ~15% of childhood deaths from cancer. The five-year survival for patients with high-risk stage 4 disease has not improved in two decades. We used whole exome sequencing (WES) to identify mutations present in three independent high-risk stage 4 neuroblastoma tumors (COA/UAB-3, COA/UAB -6 and COA/UAB -8) and a stage 3 tumor (COA/UAB-14). Among the four tumors WES analysis identified forty-three mutations that had not been reported previously, one of which was present in two of the four tumors. WES analysis also corroborated twenty-two mutations that were reported previously. No single mutation occurred in all four tumors or in all stage 4 tumors. Three of the four tumors harbored genes with CADD scores ≥20, indicative of mutations associated with human pathologies. The average depth of coverage ranged from 39.68 to 90.27, with >99% sequences mapping to the genome. In summary, WES identified sixty-five coding mutations including forty-three mutations not reported previously in primary neuroblastoma tumors. The three stage 4 tumors contained mutations in genes encoding protein products that regulate immune function or cell adhesion and tumor cell metastasis.
Introduction
Neuroblastoma (NB) is an embryonal tumor arising from neural crest cells of the sympathetic nervous system1. It is the most common extracranial solid tumor of children, and accounts for ~15% of all childhood cancer deaths. Treatment of children with high-risk disease has been a major challenge in pediatric oncology. Patients less than 18 months of age with low risk disease attain cancer-free status with tumor resection alone or without intervention, due to spontaneous tumor regression2. In contrast, patients older than 18 months of age who have high-risk factors such as MYCN amplification, bilateral disease, and near-diploid or near-tetraploid karyotype often relapse after initial treatment and remission, with an almost uniformly fatal outcome3–6.
The new International Neuroblastoma Risk Group (INRG) Staging System has taken advantage of recent advances in medical imaging and biomolecular diagnostics to establish a consensus for risk stratification5. The criteria for classification include stage, age, histology, tumor grade and MYCN gene copy number. Criteria for high-risk NB include age greater than 18 months, stage 2 or 3 with MYCN amplification, and unfavorable histology6.
Genetic abnormalities associated with high-risk stage 4 NB include hemizygous deletions of the q arm of chromosome 11 (up to 62.5% of tumors) and of the p arm of chromosome 1 (25–35% of tumors), and MYCN amplification in ~25% of tumors3,4,7–12. Gains in the long arm of chromosome 17 (17q21–17qter) is one of the most frequent genetic alterations in NB, occurring 50–70% of all high-risk tumors3,4.
Recent advances in next-generation sequencing technology and a collaboration between The Pediatric Tumor Bank and Tumorgraft Development Initiative at Children’s of Alabama and the University of Alabama at Birmingham (COA-UAB) facilitated performing whole exome sequencing (WES) to analyze four recently acquired neuroblastoma specimens. The goals of the study were to sequence the exome of these primary tumors using Whole Exome sequencing to identify mutations, to generate CADD (Combined Annotation Dependent Depletion) scores as a measure of predicted pathogenicity of mutated gene products, and to compare WES data of the stage 3 tumor with the three stage 4 tumors.
Results
Clinical characteristics associated with primary neuroblastoma tumors in this study
Primary tumors were received from patients who underwent surgery as standard of care at Children’s of Alabama Hospital (Table 1). Tumors were obtained from patients diagnosed with intermediate (COA/UAB-14) or high-risk disease (COA/UAB-3, COA/UAB-6, COA/UAB-8). Tumors COA/UAB-3 and COA/UAB-6 were MYCN amplified. Tumor specimens COA/UAB-3, /UAB-6, and /UAB-8 were obtained from patients older than 18 months, and had high-risk characteristics that included unfavorable histology and MYCN amplification.
Table 1.
Clinical characteristics associated with four primary neuroblastoma tumors.
| Tumor ID | Tumor Type | Stage | INRG* Staging | Differentiation (Grade) | MYCN amplified | >18 months |
|---|---|---|---|---|---|---|
| COA/ UAB-3 | NB | 4 | M | Poor | Yes | Yes |
| COA/ UAB-6 | NB | 4 | M | Poor | Yes | Yes |
| COA/ UAB-8 | NB | 4 | M | Poor | No | Yes |
| COA/ UAB-14 | NB | 3 | L2 | Poor | No | No |
*INRG: International Neuroblastoma Risk Group.
WES identified 43 mutations not reported previously in four neuroblastoma tumors
WES analysis revealed that each tumor harbored between seven and twenty-five mutations (Table 2). The average of 16 mutations per tumor is consistent with previous reports of 12–18 mutations per tumor13,14. The four tumors harbored 43 mutations not previously observed in NB tumors in the dbSNP database (version 138), as well as 22 mutations reported previously to be present in other tumor types15. Those 43 mutations are in bold in Tables 2–6. In Tables 3–6, ‘p’ in the third column of each Table identifies the amino acid substitution and position; ‘c’ in this column identifies the nucleotide substitution and position. While no mutation was common to all four tumors, one of the mutations in the RHPN2 gene was present in two of the four tumors examined: the mutation in this gene (Rhophilin, Rho GTPase Binding Protein 2) was present at nucleotide 217 (G > A encoding Val73Met) in COA/UAB-3 and COA/UAB-8 tumors (Tables 3–5). RHPN2 contributes to actin cytoskeleton organization, an organelle that regulates cell motility16,17. A second mutation introducing a start site of RHPN2 gene also occurred in tumors COA/UAB-3 and COA/UAB-8. The location of the introduced start site at the intron-exon boundary suggests that this mutation is unlikely to alter the protein product in tumors COA/UAB-3 and COA/UAB-8. A genome-wide association study (GWAS) found that a region containing RHPN2 has been associated with increased susceptibility to colorectal cancer18.
Table 2.
Summary of variants (mutations) types for all mutations identified in four neuroblastoma tumors.
| Variants Types | COA/UAB-3 | COA/UAB-6 | COA/UAB-8 | COA/UAB-14 | ||||
|---|---|---|---|---|---|---|---|---|
| Not reported | Reported | Not reported | Reported | Not reported | Reported | Not reported | Reported | |
| Nonsynonymous coding1 | 12 | 3 | 6 | 7 | 4 | 1 | 13 | 8 |
| Nonsynonymous start2 | ||||||||
| Splice site acceptor3 | 1 | 1 | 1 | |||||
| Splice site donor3 | ||||||||
| Start gained4 | 2 | 1 | 1 | 1 | ||||
| Start lost4 | 1 | |||||||
| Stop gained5 | 2 | 1 | ||||||
| Stop lost5 | ||||||||
| TOATL # VARIANTS | 16 | 3 | 7 | 8 | 5 | 2 | 16 | 9 |
| TOTAL # GENES | 15 | 3 | 7 | 8 | 4 | 2 | 15 | 9 |
1Mutation of a single nucleotide, resulting in an amino acid change in the encoded protein; may affect phenotype66.
2Mutation that occurs in a coding region, at start site.
3Mutation that changes nucleotides in genomic loci where splicing takes place.
4Mutation that generates a new translation initiation codon in the 5′UTR, or that results in the loss of an initiation codon. Start site loss may result in the loss of protein product.
5Mutation that changes the sequence of a codon to create or remove a stop codon (UAA, UAG, UGA).
Table 6.
WES identified 25 variants in COA/UAB-14. Information on each variant (mutation) including gene name, mutation location, mutation type, and known functions/pathways of normal gene product.
| Ch#+ | Gene | Mutation | Mutation Type | Known functions/pathways of normal gene product |
|---|---|---|---|---|
| 1* | CROCC | p.Lys1754Arg/c.5261 A > G | Missense | Structural component of the ciliary rootlet, a component of centrosome cohesion |
| 2 | RHOQ | p.Met80Val/c.238 A > G | Missense | Epithelial cell polarization |
| 2 | RHOQ | p.Met1?/c.1 A > G | Missense | Epithelial cell polarization |
| 2 | GPAT2 | p.Arg621Cys/c.1861C > T | Missense | Regulates glycerolipid biosynthesis |
| 4 | CRIPAK | p.Cys338Arg/c.1012 T > C | Missense | Negative regulator of estrogen receptor signaling, regulates cytoskeleton organization |
| 6 | UTRN | p.Arg297Gln/c.890 G > A | Missense | Cytoskeleton/plasma membrane anchoring |
| 8 | DOCK5 | p.Arg1627Gln/c.4880 G > A | Missense | Scaffold structure, MAP kinase pathway activation |
| 8 | PSKH2 | p.Cys3Gly/c.7 T > G | Missense | Protein serine/threonine kinase activity |
| 9 | RNF20 | p.Arg368Trp/c.1102 C > T | Missense | Epigenetic transcriptional activation and gene regulation |
| 10 | SUFU | p.Ser79Asn/c.236 G > A | Missense | Negative regulator of hedgehog signaling, negative regulator of beta-catenin signaling |
| 11 | MUC2 | p.Thr1549Asn/c.4646 C > A | Missense | Maintain gastrointestinal epithelium, epithelial cell differentiation |
| 11 | KRTAP5–7 | p.Cys120Tyr/c.359 G > A | Missense | Keratin intermediate filament protein |
| 11 | CCDC83 | Splice site acceptor | ||
| 11 | KRTAP5–7 | p.Tyr98Cys/c.293 A > G | Missense | Hair keratin formation |
| 12 | ATF7IP | p.Lys529Arg/c.1586 A > G | Missense | Modulates transcription elongation and histone methylation |
| 15 | LYSMD4 | p.Arg49Trp/c.145 C > T | Missense | LysM domain containing 4, function not well characterized |
| 17 | AATF | Start gained | Inhibits histone deacetylase HDAC1 | |
| 17 | KRTAP4–8 | p.Thr173Ser/c.518 C > G | Missense | Keratin-associated protein 4–8 |
| 17 | KRTAP4–9 | p.Asn148Thr/c.443 A > C | Missense | Keratin-associated protein 4–9 |
| 17 | GRIN2C | p.Val34Met/c.100 G > A | Missense | Involved in excitatory neurotransmission and in neuronal cell death |
| 19 | ONECUT3 | p.Ser313Arg/c.937 A > C | Missense | Transcriptional activation, cell differentiation, system development |
| 19 | MYO1F | p.Arg617Cys/c.1849C > T | Missense | Actin binding function, cell motility |
| 19 | CYP2A6 | p.Lys125Met/c.374 A > T | Missense | Drug metabolism, heme binding; steroid metabolism |
| 19 | LSM14A | Splice site acceptor | Multicellular organism development; regulation of translation | |
| x | AR | p.Gln58Leu/c.173 A > T | Missense | Androgen receptor involved in gene expression, cell proliferation and differentiation |
+Chromosome number.
*CADD score ≥ 20.
Table 3.
WES identified 19 variants in COA/UAB-3 neuroblastoma specimen. Information on each variant (mutation) including gene name, mutation location, mutation type, and known functions/pathways of normal gene product.
| Ch#+ | Gene | Mutation | Mutation type | Known functions/pathways of normal gene product |
|---|---|---|---|---|
| 1* | TCEB3 | p.Ala18Val/c.53 C > T | Missense | Activates RNA polymerase II elongation |
| 1* | TOE1 | p.Ala2Val/c.5 C > T | Missense | Inhibits cell growth and cell cycle progression |
| 1 | MAEL | p.Tyr344Asn/c.1030 T > A | Missense | Spermatogenesis |
| 1 | SELL | Start gained | Mediates adhesion | |
| 2* | WDR35 | p.Ala1018Asp/c.3053 C > A | Missense | Promotes CASP3 activation |
| 2* | COL4A4 | p.Gly645*/c.1933G > T | Nonsense | Major structural component of basement membrane |
| 3 | MUC4 | p.Ala1646Thr/c.4936 G > A | Missense | Plays a role in tumor progression; anti-adhesive properties |
| 6 | CLIC5 | p.Gln50His/c.150 G > T | Missense | Chloride ion transport |
| 6 | FOXO3 | p.Glu17Val/c.50 A > T | Missense | Apoptosis; transcriptional activator |
| 13 | ITM2B | p.Ala153Val/c.458 C > T | Missense | Processing beta-amyloids A4 precursor protein (APP) |
| 14 | RNASE4 | p.Cys85Phe/c.254 G > T | Missense | Degrades RNA |
| 14 | ADAM21 | p.Pro40Leu/c.119 C > T | Missense | Adhesion protein involved in sperm maturation; epithelial cell function |
| 17 | ACADVL | p.Phe266Leu/c.798 C > A | Missense | Mitochondrial fatty acid beta-oxidation |
| 19 | GIPR | p.His115Asn/c.343 C > A | Missense | Pathogenesis of diabetes |
| 19 | RHPN2 | p.Val73Met/c.217 G > A | Missense | Binds to and activates GTP-Rho, negatively regulates stress fiber formation and facilitates motility of many cell types including T and B cells. |
| 19 | RHPN2 | p.Arg255Gln/c.764 G > A | Missense | Binds to and activates GTP-Rho, negatively regulates stress fiber formation and facilitates motility of many cell types including T and B cells. |
| 19 | RHPN2 | Start gained | Binds to and activates GTP-Rho, negatively regulates stress fiber formation and facilitates motility of many cell types including T and B cells. | |
| 19* | STK11 | p.Arg86*/c.256 C > T | Nonsense | Tumor suppressor |
| 20 | SNX21 | p.Leu106Pro/c.317 T > C | Missense | Intracellular trafficking |
+Chromosome number.
*CADD score ≥ 20.
Table 5.
WES identified 7 variants in COA/UAB-8. Information on each variant (mutation) including gene name, mutation location, mutation type, and known functions/pathways of normal gene product.
| Ch#+ | Gene | Mutation | Mutation type | Known functions/pathways of normal gene product |
|---|---|---|---|---|
| 2 | POTEF | p.Pro738Ala/c.2212 C > G | Missense | Involved in retina homeostasis |
| 3 | MUC4 | p.His1613Asp/c.4837 C > G | Missense | Tumor progression, cell-cell adhesion, epithelial cell proliferation and differentiation |
| 6 | KIF25 | p.Lys28Met/c.83 A > T | Missense | Negative regulator of amino acid starvation-induced autophagy |
| 8 | ATAD2 | Start gained | Estrogen-induced cell proliferation, cell cycle progression of breast cancer cells | |
| 17 | KRT31 | p.Ile37Thr/c.110 T > C | Missense | Structural component of cytoskeleton, epidermis development |
| 19 | RHPN2 | p.Val73Met/c.217 G > A | Missense | Binds to and activates GTP-Rho, negatively regulates stress fiber formation and facilitates motility of many cell types including T and B cells |
| 19 | RHPN2 | Start gained | Binds to and activates GTP-Rho, negatively regulates stress fiber formation and facilitates motility of many cell types including T and B cells |
+Chromosome number.
Genes encoding MUC4 and ADAM21 also contained mutations in two of the four tumors, but at different loci. Mucin 4 (MUC4), a transmembrane mucin expressed predominantly by normal epithelial cells, is involved in cell differentiation, inhibition of cell adhesion, and cell migration19–21. MUC4 protein is thought to contribute to tumor metastasis by limiting the adhesion of tumor cells to primary tumor sites. The mutations identified in this gene include the previously reported 4936 G > A encoding Ala1646Thr in COA/UAB-3 and a not reported mutation at nucleotide 4837 (C > G encoding His1613Asp) in COA/UAB-8.
The previously reported mutation at nucleotide 119 (C > T encoding Pro40Leu) of the ADAM21 gene was also present in two of the four tumors (COA/UAB-3 and COA/UAB-6). ADAM21 (A Disintegrin And Metallopeptidase Domain 21) contributes to cell-cell and cell-matrix adhesion and neurogenesis22,23.
Each of the three genes (RHPN2, MUC4 or ADAM21) that harbored mutations in more than one tumor has a regulatory role in cell adhesion and motility, cell functions essential to the metastatic process16,19,23–25.
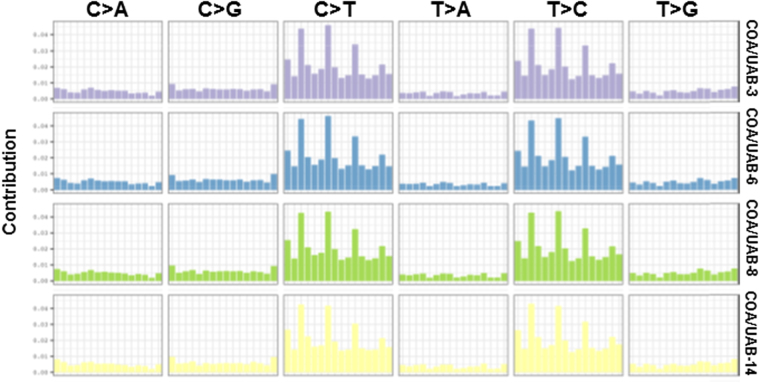
A majority of mutations were nonsynonymous coding mutations, indicating that the genes in which these mutations were present encoded proteins containing amino acid substitutions (Table 2). Additional mutations identified were those that introduced ATG start sites or the splice site acceptor sites at an intron-exon boundary. Among the type of mutations, a majority was found to be missense mutations (Tables 3–6). While some of the mutated proteins contribute to common functions, the wide range of functions affected by mutated genes was diverse as has been seen in previous studies13,14,26,27. Further, we retrieved the somatic motifs for each variant from the reference sequence, converted into a matrix to estimate the somatic mutational signature and plotted in Fig. 1. The probability bars (UAB-3: purple, UAB-6: blue, UAB-8: green and UAB-14: yellow) from the 6 substitution subtypes (C > A, C > G, C > T, T > A, T > C, or T > G) are shown in Fig. 1.
Figure 1.
Somatic mutational signature profiling. The somatic motifs for each variant were retrieved from the reference sequence and converted into a matrix. Non-negative Matrix Factorization (NMF) was used to estimate the somatic signature and then plotted. We used SomaticSignatures package to extract the somatic motifs of these samples.
Three of the four tumors harbored genes that had CADD score greater than 20
Tables 3–6 detail genes that harbor mutations, identified by WES. Three of the four tumors had genes with elevated CADD (Combined Annotation Dependent Depletion) scores, a scoring system designed to predict the potential pathogenicity of nucleotide mutations, deletions, or insertions. Scores of ≥20 for a given gene have been associated with specific human pathologies28. Genes that were mutated in any of the four tumors analyzed and that had CADD scores ≥20 are described briefly below.
COA/UAB-3
Five of the nineteen mutated genes were designated as CADD ≥ 20: TCEB3 and TOE1 on chromosome 1, WDR35 and COL4A4 on chromosome 2, and STK11 on chromosome 19. TOE1 is a target of EGR1 (Early Growth Response 1), and inhibits cell growth29. Mutations in the TOE1 gene have been associated with hepatic and pancreatic malignancies, but the sample number supporting this association is relatively small15. Mutations in the WDR35 gene have been observed in patients with Sensenbrenner syndrome, also known as cranioectodermal dysplasia30. Mutations in COL4A4 have been linked to thin basement membrane disease31. Mutations in STK11 have been associated with Peutz-Jeghers syndrome, a disease characterized by development of hamartomatous polyps in the gastrointestinal tract32. Patients with Peutz-Jeghers syndrome have a ~15-fold higher risk of developing intestinal cancer than the normal population33.
COA/UAB-6
Five of the fifteen mutated genes in this tumor were identified as CADD ≥ 20. These include EVC2 on chromosome 4, CDK5RAP2 on chromosome 9, CRY2 on chromosome 11, ATXN2 on chromosome 12, and NUP62 on chromosome 19. EVC2 (EvC ciliary complex subunit 2) contributes to growth and development of bone and skeleton, and regulates Sonic Hedgehog pathway signaling, a pathway described as essential to NB progression34–41. Mutations in the EVC2 gene have been related to Ellis-van Creveld syndrome and Weyers acrofacial dysostosis42. These syndromes are disorders of skeletal dysplasia of the teeth, nails, and bones, respectively43.
COA/UAB-14
One of the twenty-five mutated genes in this intermediate risk tumor was identified as CADD ≥ 20: CROCC on chromosome 1. The protein encoded by the CROCC (ciliary rootlet coiled-coil, Rootletin) gene is a major structural component of the ciliary rootlet, and contributes to centrosome cohesion prior to mitosis44,45.
Ingenuity Pathway Analysis (IPA) identified pathways and physiological systems, development and function, and function associated with network using proteins encoded by mutated genes
We next used Ingenuity Pathway Analysis (IPA) to identify pathways (Tables 7–10), physiological systems, and functions likely to be affected by variant proteins encoded by mutated genes (Tables 11–14). P-values indicate the greater or less likelihood that a given protein is involved in a specific pathway. P-values < 0.05 indicate a likely association between indicated proteins and pathways46. The range of p-values in Tables 11–14 reflects the likelihood that proteins of interest were related to specific functional subcategories in the broader functional categories indicated in the Table. Functions associated with networks known to include variant proteins encoded by mutated genes are listed in Tables 15–18.
Table 7.
Pathways identified by IPA to be associated with proteins encoded by mutated genes in COA/UAB-3.
| Pathways affected by variant gene products | p-value | Ratio |
|---|---|---|
| ERK5 Signaling | 0.049 | 1/63 (0.016) |
| PXR/RXR Signaling | 0.0521 | 1/67 (0.015) |
| GPCR Signaling | 0.0551 | 1/71 (0.014) |
Table 10.
Pathways identified by IPA to be associated with proteins encoded by mutated genes in COA/UAB-14.
| Pathways affected by variant gene products | p-value | Ratio |
|---|---|---|
| CDP-diacylglycerol biosynthesis I | 0.0166 | 1/16 (0.062) |
| Phosphatidylglycerol biosynthesis II | 0.0187 | 1/18 (0.056) |
| Sonic hedgehog signaling | 0.0309 | 1/30 (0.0033) |
Table 11.
Physiological systems or functions identified by IPA to be associated with proteins encoded by mutated genes in COA/UAB-3.
| Systems affected by variant gene products | p-value (range) | Molecules |
|---|---|---|
| Cell-mediated immune response, immune cell trafficking | 0.000796–0.0448 | SELL, STK11, FOXO3 |
| Embryonic Development | 0.000794–0.0482 | FOXO3, MAEL, STK11, TOE1, COL4A4, GIPR, SELL, TCEB3 |
| Hematological system development and function, hematopoiesis | 0.000796–0.049 | SELL, FOXO3, STK11 |
Table 14.
Physiological systems or functions identified by IPA to be associated with proteins encoded by mutated genes in COA/UAB-14.
| Systems affected by variant gene products | p-value | Molecules |
|---|---|---|
| Nervous system development and function | 0.00105–0.049 | UTRN, AR,GRIN2C, RNF20, SUFU |
| Reproductive system development and function | 0.00105–0.048 | AR |
| Skeletal and muscular system development and function | 0.00105–0.043 | AR, UTRN, SUFU, DOCK5 |
Table 15.
Diseases and functions associated with networks identified by IPA to be affected by observed mutations in COA/UAB-3.
| ID | Molecules in network | Diseases and Functions associated with this network |
|---|---|---|
| 1 | ACAD10, ACADVL, APP, ARMC9, BOD1, C18orf21, C5orf15, C9orf41, C9orf142, CHAC2, CHID1, CLIC5, COL4A4, DDHD2, GIMAP4, HINT3, ITM2B, NENF, NME8, PUS7L, RABL3, RHPN2, RNASE11, SNX21, TBCC, TCEB3, TOE1, TOMM5, UBC, UTP23, VWA5A, WDR35, WDR88, WDR45B, ZNF720 | Cell morphology and organization, neurological disease |
| 2 | ADAM18, ADAM20, ADAM21, Adam24, ADAM29, ADAM30, Adam26a/Adam26b, ADAMTS6, ADAMTS8, Akt, CNR1, EMR2, ERK, Focal adhesion kinase, FOXO3, GIPR, GPR55, GPR126, GPRC5C, IFNB1, Insulin, KIRREL3, MAEL, Metalloprotease, MMP21, MUC4, NMUR2, PI3K (complex), PROKR1, RNASE4, SELL, SLC22A17, SRC (family), STK11, TAS1R2 | Nervous system development and function, connective tissue disorders, cell-cell signaling |
Table 18.
Diseases and functions associated with networks identified by IPA to be affected by observed mutations in COA/UAB-14.
| ID | Molecules in Network | Diseases and functions associated with this network |
|---|---|---|
| 1 | AATF, ATF7IP, C10orf90, CPPED1, CRIPAK, CROCC, CYP2A6 (includes others), Cyp2g1, CYP4 × 1, DOCK5, ESR1, GPAT2, GSTP1, KRT25, KRTAP1–3, KRTAP4–9, LOC391322, LSM14A, MAPK1, MRI1, MUC2, MYO1F, NCCRP1, PABPC4L, PCDHB14, RAC1, RHOQ, SPRED3, SUFU, SUSD1, TMEM150C, UBC, UTRN, ZCRB1, ZMAT5 | Drug metabolism, small molecule biochemistry, cancer |
| 2 | ABCD1, Akr1c19, ALOX15B, AMD1, AMHR2, Androgen-ARA55-AR-ARA70-HSP40-HSP70-HSP90, AQP8, AR, AR-HSP90, AR-HSP40-HSP70-HSP90, ATAD2, CHTF18, DISP2, DTX4, ELMO1, GRIN2C, HSD17B3, HSP90AA1, KCNG1, MAK, MAPK15, MSMB, MYL3, PATZ1, PI4K2B, PLXNC1, PSKH2, RLN1, RNF20, Scgb1b27 (includes others), SLC39A8, SMTN, TACC2, TGFB1, ZMIZ1 | Organ development, reproductive system development and function |
IPA determined that biological functions associated with proteins mutated in the stage 3 COA/UAB-14 tumor included nervous system development and function (p < 0.049), reproductive system development and function (p < 0.048), and musculoskeletal development and function (p < 0.043) (Table 14)- all early developmental processes. In contrast, IPA of genes mutated in the three stage 4 high-risk tumors (Tables 11–13) indicate the potential involvement of cellular functions more closely related to cell-mediated immune response, hematologic development and function, immune cell trafficking, and cell adhesion or motility. Detailed findings by IPA for each tumor are as follows.
Table 13.
Physiological systems or functions identified by IPA to be associated with proteins encoded by mutated genes in COA/UAB-8.
| Systems affected by variant gene products | p-value(range) | Molecules |
|---|---|---|
| Tissue development | 0.000597–0.00119 | MUC4 |
COA/UAB-3
IPA data indicated that the 19 mutations identified in this tumor were likely to involve proteins that contribute to ERK5 signaling (p = 0.049), PXR/RXR (p = 0.0521), and GPCR signaling (p = 0.0551) (Table 7). ERK5, extracellular-signal-regulated 5, is a member of the MAPK (mitogen-activated protein kinase) family. This pathway is activated by epidermal growth factors which are reported to play key roles in cell proliferation and differentiation47. The pregnane X receptor (PXR) is predominantly expressed in the liver and intestine, is usually activated by PXR in conjunction with the retinoid X receptor (RXR), and contributes to drug metabolism by inducing the family of cytochrome P450 enzymes48,49. Table 11 shows that the most affected physiological system and development and function in this tumor includes cell-mediated immune response (p < 0.0452), embryonic development (p < 0.0482), hematologic system development and function (p < 0.049), hematopoiesis (p < 0.046), and immune cell trafficking (p < 0.0448).
Nine genes in which mutations occurred in this tumor contribute to cell morphology, cellular assembly and organization, and neurological disease: ACADVL, CLIC5, COL4A4, ITM2B, RHPN2, SNX21, TCEB3, TOE1, and WDR35 (Table 15). Nine mutated genes are associated with nervous system development and function, connective tissue disorders, and cell-to-cell signaling or interaction: ADAM21, FOXO3, GIPR, MAEL, MUC4, RNASE4, SELL, STK11, and TAS1R2 (Table 15).
COA/UAB-6
WES identified fifteen mutations in this tumor (Table 4). IPA analysis demonstrated the likely involvement of the corresponding mutant gene products as components of the following pathways: eicosanoids (p = 0.000989), prostanoid (p = 0.0067), and protein kinase A signaling (p = 0.0253) pathways (Table 8). The eicosanoid pathway is involved in inflammation and immune-related functions, including cyclooxygenase synthesis and metabolism50. Prostanoids are the subclass of eicosanoids to which prostaglandins belong. Protein kinase A signaling pathway involves classic endocrine signaling and function to mediate the effect of cAMP51. Key physiological systems, functions and development affected by these pathways include cell-mediated immune response (p < 0.00224), hematological system development and function (p < 0.00224), immune cell trafficking (p < 0.0478), and nervous system development and function (p < 0.05) (Table 12).
Table 4.
WES identified 15 variants in COA/UAB-6. Information on each variant (mutation) including gene name, mutation location, mutation type, and known functions/pathways of normal gene product.
| Ch#+ | Gene | Mutation | Mutation type | Known functions/pathways of normal gene product |
|---|---|---|---|---|
| 1 | OR2T33 | p.Ser87Asn/c.260 G > A | Missense | G-protein receptor activity, olfactory activity |
| 3 | C3orf36 | p.Pro26Gln/c.77 C > A | Missense | Uncharacterized protein |
| 4* | EVC2 | p.Ser270*/c.809 C > A | Nonsense | Hedgehog pathway; bone formation |
| 6 | PTPRK | p.Ala27Thr/c.79 G > A | Missense | Cell adhesion and growth, tumor cell invasion and metastasis |
| 9* | CDK5RAP2 | Splice site donor | Mitotic spindle orientation, spindle checkpoint activation | |
| 9 | PTGES | p.Val37Met/c.109 G > A | Missense | Prostaglandin metabolism |
| 11* | CRY2 | p.Gly326Arg/c.976 G > A | Missense | Circadian rhythm |
| 12 | NPFF | p.Gln28Lys/c.82 C > A | Missense | Modulates morphine-induced effects |
| 12* | ATXN2 | p.Ala1032Thr/c.3094 G > A | Missense | Negative regulator of EGFR trafficking |
| 14 | ADAM21 | p.Pro40Leu/c.119 C > T | Missense | Membrane-bound cell surface adhesion molecule, sperm maturation |
| 14 | AHNAK2 | p.Leu3217Pro/c.9650 T > C | Missense | Activity may be calcium-dependent |
| 16 | POLR3E | p.Ser543Arg/c.1629 C > A | Missense | RNA transcription; DNA-dependent RNA polymerase |
| 18 | ARHGAP28 | p.Lys134Asn/c.402 G > T | Missense | GTPase activator |
| 19* | NUP62 | p.Asp365Tyr/c.1093 G > T | Missense | Key component of the nuclear pore complex, nucleocytoplasmic transport |
| X | CYSLTR1 | p.Leu7Met/c.19 C > A | Missense | Receptor for cystenyl leukotrienes, bronchoconstriction |
+Chromosome number.
*CADD score ≥ 20.
Table 8.
Pathways identified by IPA to be associated with proteins encoded by mutated genes in COA/UAB-6.
| Pathways affected by variant gene products | p-value | Ratio |
|---|---|---|
| Eicosanoid signaling | 0.000989 | 2/63 (0.032) |
| Prostanoid biosynthesis | 0.0067 | 1/9 (0.111) |
| Protein kinase A signaling | 0.0253 | 1/386 (0.003) |
Table 12.
Physiological systems or functions identified by IPA to be associated with proteins encoded by mutated genes in COA/UAB-6.
| Systems affected by variant gene products | p-value (range) | Molecules |
|---|---|---|
| Cell-mediated immune response, immune cell trafficking | 0.000149–0.0478 | CYSLTR1, PTGES |
| Hematological system development and function | 0.00149–0.00224 | CYSLTR1, PTGES, NPFF |
| Nervous system development and function | 0.00149–0.05 | CYSLTR1, PTGES, ATXN2, CRY2, NPFF |
Ten genes that harbor mutations are involved in carbohydrate metabolism and tissue morphology: AHNAK2, ARHGAP28, ATXN2, CYSLTR1, EVC2, NPFF, NUP62, POLR3E, PTGES, and PTPRK (Table 16). Three genes are involved in developmental and neurological disorders: C3orf36, CDK5RAP2, CRY2 (Table 16).
Table 16.
Diseases and functions associated with networks identified by IPA to be affected by observed mutations in COA/UAB-6.
| ID | Molecules in network | Diseases and functions associated with this network |
|---|---|---|
| 1 | AHNAK2, ARFGAP2, ARHGAP28, ATXN2, BMP2, BPHL, C11orf73, C4orf27, CEBPB, CYSLTR1, ERK, EVC2, FOXP3, GH1, GPR126, GPR137, GPR146, GPR160, GPRC5C, HNF4A, LRRC40, NFkB (complex), NMUR1, NMUR2, NPFF, NUP62, Orm, POLR3E, PTGES, PTPRK, Srrm1, TMEM176A, UTP3, VN1R1, ZNF71 | Carbohydrate metabolism, small molecule biochemistry, tissue morphology |
| 2 | APP, ARMC9, BOD1, C18orf21, C3orf36, C5orf15, C9orf41, C9orf142, CDK5RAP2, CHAC2, CHID1, CRY2, DDHD2, EEPD1, GIMAP4, HEATR5A, HINT2, HINT3, LRRC42, MMGT1, NENF, NME8, PUS7L, PYROXD1, RAB43, RABL3, RNASE11, TBCC, TOMM5, UBC, UTP23, VWA5A, WDR88, WDR45B, ZNF720 | Developmental disorders, neurological diseases |
COA/UAB-8
WES identified seven mutations in this tumor (Table 5). IPA indicated that mutated proteins were associated with Rho A signaling (p = 0.0359), a primary regulator of cell motility (Table 9), including T and B cell motility associated with immune response52–55. Tissue development (p < 0.00119) is identified as a key physiological system or function likely to be affected by these mutated gene products (Table 13). Six genes, ATAD2, KIF25, KRT31, MUC4, POTEF, RHPN2 in which mutations occurred are involved in DNA replication, recombination and repair, and in nucleic acid metabolism (Table 17).
Table 9.
Pathways identified by IPA to be associated with proteins encoded by mutated genes in COA/UAB-8.
| Pathways affected by variant gene products | p-value | Ratio |
|---|---|---|
| RhoA signaling | 0.0359 | 1/122 (0.008) |
Table 17.
Diseases and functions associated with networks identified by IPA to be affected by observed mutations in COA/UAB-8.
| ID | Molecules in network | Diseases and functions associated with this network |
|---|---|---|
| 1 | ABCA2, ATAD1, ATAD2, ATP6V1F, C1orf123, CDKN1A, CGGBP1, CSNK1G3, DSE, FMR1, HMGXB3, KIF25, KRT31, MAGEA2/MAGEA2B, MIS18BP1, MUC4, MYH8, NPRL2, NSA2, NSF, POTEE/POTEF, RAB24, RAB6C/WTH3DI, RHPN2, RNASEH2B, SLC30A5, TOR3A, TTLL5, UBC, UQCR11, WDR13, WDR73, ZNF84, ZNF135, ZNF629 | DNA replication, recombination and repair, nucleic acid metabolism |
COA/UAB-14
WES identified twenty-five mutations in this tumor (Table 6). IPA predicted that the mutated gene products contributed to CDP-diacylglycerol biosynthesis I (p = 0.0166), phosphatidylglycerol biosynthesis II (p = 0.0187), and sonic hedgehog signaling (p = 0.0309) pathways (Table 10). The sonic hedgehog pathway involvement is consistent with previous reports that this pathway is important for NB cell proliferation and progression39–41. Key physiological systems related to mutated gene products include nervous system development and function (p < 0.049), reproductive system development and function (p < 0.048), and skeletal and muscular system development and function (p < 0.043) (Table 14).
Fourteen of the mutated genes in this tumor are involved in metabolism (endogenous molecules as well as drugs), small molecule biochemistry, and cancer: AATF, ATF71P, CRIPAK, CROCC, CYP2A6, DOCK5, GPAT2, KRTAP4–9, LSM14A, MUC2, MYO1F, RHOQ, SUFU, and UTRN (Table 18). Proteins encoded by four genes are involved in organ morphology, reproductive system development and function (AR, GRIN2C, PSKH2, RNF20) (Table 18).
In summary, WES identified a total of sixty-five mutations in one stage 3 and three stage 4 NB tumors. No affected gene or associated cell function was common to all four tumors. The three stage 4 tumors each had mutations in genes encoding aspects of immune function or response. Genes encoding proteins of diverse function were affected, possibly reflecting the phenotypic heterogeneity that has been observed by other methods of analysis for this tumor type.
Discussion
In our current study, we performed WES analysis of specimens from four primary NB tumors. Three of the four tumors were designated stage 4 and high-risk. Two of the four had amplified MYCN. WES identified 43 mutations not reported previously in these tumors. No single mutation was common to all four tumors. Two of those mutations and one of the previously reported mutations in RHPN2, was identical in tumors COA/UAB-3 and COA/UAB-8 (RHPN2, p.Val73Met/c.217 G > A; p.Arg255Gln/c.764 G > A). Each of the stage 4 tumors harbored mutations in genes encoding proteins that directly affect immune function. The mutation frequency in our study was similar to those reported in other studies13,14,26,27. Of note, we used patient-derived white blood cells as the control to exclude germline mutations which may not contribute to the pathologic process.
Among successful utilization of WES to identify mutations in NB, a recent paper by Pugh et al. described genetic variations of 240 high-risk NB specimens, and identified genes with significant somatic mutation frequencies (mutation frequencies of < 9.2%) including ALK, PTPN11, ATRX, MYCN and NRAS which percentages regarded as too low to be identified in a study in which fewer than hundreds of tumors were analyzed14,26,56,57. ALK has been reported as a major familial NB predisposition gene among high risk NB patients58. ALK is also a known oncogene in other tumor types such as anaplastic large cell lymphoma59. While we observed no ALK mutations in our study, this finding is consistent with the low percent of tumors affected (9.2%)14. PTPN11 (mutation frequency of 2.9%), is a member of the protein tyrosine phosphatase family, and alterations of this gene may contribute to NB transformation14. We did not detect this mutation in any of 4 NB sequenced. Pugh, et al. also identified mutations in the MYCN gene. While MYCN amplification is a well-documented prognostic indicator for poor outcome in NB, the functional significance of mutations in this gene remains elusive.
Another recent study by Lasorsa et al. identified and discussed somatic mutations that may affect cancer progression in NB26. WES analysis of 17 high-risk tumors identified 22 mutated genes implicated in cancer progression. In this study, authors also found similar low rates of mutations reported by us and others13,14,26,27. Interestingly, Lasorsa et al. proposed that CHD9 and PTK2 (FAK) comprise driver genes associated with aggressive NB, although only 2–4% of tumor specimens examined harbored mutations in these genes. The authors proposed further that loss of CHD9, chromatin related mesenchymal modulator, leads to NB tumor progression as seen in other cancer types. The somatic mutations found in PTK2 localized adjacent to two functional phosphorylation sites (Tyr576 and Tyr861), mutations that activate FAK protein. FAK activation regulates invasive and migratory properties by altering cytoskeletal function and cell adhesion60–62. Similarly, we found a mutation in RHPN2 in two of the three stage 4 tumors. RHPN2 regulates cell invasion and migration by activating RhoA, a master regulator of cell motility16. Work is ongoing to evaluate whether RHPN2 supports NB cell metastasis, and to examine the hypothesis that inhibition of tumor cell motility comprises a therapeutic approach in high-risk NB. Determining functional correlations for the mutations identified is the priority for the next study to strengthen current findings. Further, we acknowledge that our sample number is too small to designate any mutation as a NB driver mutation, which is considered as a limitation of the current study.
In summary, we identified sixty-five mutations among four NB tumors using WES, a sequencing method to identify genetic aberrations. Current work focuses on comparing expression profiles and phenotypes of these NB tumors with WES analyses. If genomic characteristics of NB tumors reflect tumor cell phenotype and sensitivity or resistance to specific therapeutic regimens, the observed genomic diversity suggests that personalized approaches to therapy may be necessary to improve clinical outcome for patients with high-risk stage 4 NB.
Methods
Ethics Statement: Human Subjects
This study included human subjects. All procedures were approved by the University of Alabama at Birmingham Institutional Review Board (IRB) in accordance with the guiding ethical principles of the IRB respect for persons, beneficence and justice, as embodied in the Belmont Report. Written informed consent and assent were obtained from all participants.
DNA isolation
Genomic DNA was isolated from primary tumors and white blood cells from each patient using a DNA/RNA extraction kit (EpiCentre, Madison, WI, USA). Purified DNA concentration and quality was determined by ND-1000 spectrophotometer using NanoDrop 3.0.1 (Coleman Technologies, Inc., Wilmington, DE, USA); 260/280 ratios for all DNA preparations ranged from 1.72 to 2.00 (Table S1). DNA samples were submitted for whole exome sequencing by the Heflin Center at UAB (Birmingham, AL, USA). DNA extracted from white blood cells of each patient from whom tumor specimens were received served as controls.
Whole exome sequencing on Illumina Platforms
Exome capture was performed using the Agilent SureSelect all Human exon v3 capture kit (Agilent SureSelect Human All Exon 50 Mb for target enrichment) by the Heflin Center at the University of Alabama at Birmingham. Briefly, high molecular weight DNA was isolated, and quality checked by electrophoresis using 1% agarose gel to ensure intact high molecular weight DNA. DNA was randomly fragmented using a Covaris S2 sonicator to produce ~200 bp fragments. Fragmented DNA was blunt ended, phosphorylated, and A-tagged to facilitate linker addition. DNA was selected using biotin labeled RNA capture molecules complementary to each exon. Following purification of the exonic sequences by streptavidin-magnetic bead separation, DNA was amplified with primers that introduce a 6-nucleotide index so that samples could be combined in a given lane for sequence analysis. The exonic libraries were run on the HiSeq. 2000 next generation sequencer from Illumina (Illumina, San Diego, CA, USA) with paired end 2 × 100 bp reads.
WES analysis, depth of coverage
Whole exome sequencing (WES) statistics showed that WES performed at the Heflin Center at UAB had a depth of coverage of 39.68 to 90.27, indicating that each base was sequenced a minimum of 39 and a maximum of 90 times. More than 99% of DNA sequences of tumors and corresponding WBCs mapped to specific genomic regions. Over 84% of reads were properly paired, indicating that both forward and reverse reads were correctly oriented. Percent duplication ranged from 9.12% to 34.34%. These parameters indicate the reliability of the WES data presented (Table S2)63. Table S3 shows allele fractions of variants not reported previously in each tumor.
Somatic mutation signature profiling
The SomaticSignatures package was used to extract the somatic motifs of these samples. In brief, the somatic motifs for each variant were retrieved from the reference sequence and converted into a matrix64. Non-negative Matrix Factorization (NMF) was used to estimate the somatic signature and then plotted.
Data analysis and statistics
To call variants (SNPs, INDELs), the raw fastq reads from the exome capture was aligned to UCSC’s high19 reference genome using Burrow-Wheeler Aligner (BWA)65. Variants were identified using Broad’s Genome Analysis Toolkit (GATK) and following Broad’s Best Practices for Variant Detection protocol66,67. Briefly, the aligned file from BWA was realigned and recalibrated using GATK. Following base recalibration, MuTect was used to identify somatic point mutations between the tumor and normal sample68. Once the variants were identified, SnpEff was then used for annotation69.
Electronic supplementary material
Acknowledgements
We are thankful for the support received from the Department of Pediatrics, University of Alabama at Birmingham and from the Children’s of Alabama for “Pediatric Tumor Bank and Tumorgraft” initiative. We are also grateful to Ribbons of Hope Foundation Alabama for their support of pediatric cancer research. The whole exome sequencing and the analysis were performed by UAB-Heflin Center, Genomic Core (Comprehensive Cancer Center: CA13148, CFAR: AI027767). This work was supported by the Department of Pediatrics, University of Alabama at Birmingham/Children’s of Alabama.
Author Contributions
Conceived experiments: J.G.P., E.A.B., R.G.W., S.L.C., K.J.Y. Coordinated specimen acquisition and performed experiments: A.L.M., P.L.G. Performed clinical resection: E.A.B. Analyzed data: D.K.C. Performed clinical and histological analysis of patients and/or patients’ tissues: D.R.K., L.N.C., R.D., S.L.C. Wrote, revised the paper: A.L.M., J.G.P., E.A.B., D.K.C., S.L.C., K.J.Y.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-17162-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maris JM. Recent advances in neuroblastoma. The New England journal of medicine. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis CU, Shohet JM. Neuroblastoma: molecular pathogenesis and therapy. Annual review of medicine. 2015;66:49–63. doi: 10.1146/annurev-med-011514-023121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bown N. Neuroblastoma tumour genetics: clinical and biological aspects. Journal of clinical pathology. 2001;54:897–910. doi: 10.1136/jcp.54.12.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang M, Weiss WA. Neuroblastoma and MYCN. Cold Spring Harbor perspectives in medicine. 2013;3:a014415. doi: 10.1101/cshperspect.a014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monclair T, et al. TheInternational Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:298–303. doi: 10.1200/JCO.2008.16.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nature reviews. Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 7.Okawa ER, et al. Expression and sequence analysis of candidates for the 1p36.31 tumor suppressor gene deleted in neuroblastomas. Oncogene. 2008;27:803–810. doi: 10.1038/sj.onc.1210675. [DOI] [PubMed] [Google Scholar]
- 8.Stigliani S, et al. High genomic instability predicts survival in metastatic high-risk neuroblastoma. Neoplasia. 2012;14:823–832. doi: 10.1593/neo.121114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attiyeh EF, et al. Chromosome 1p and 11q deletions and outcome in neuroblastoma. The New England journal of medicine. 2005;353:2243–2253. doi: 10.1056/NEJMoa052399. [DOI] [PubMed] [Google Scholar]
- 10.Spitz R, Hero B, Simon T, Berthold F. Loss in chromosome 11q identifies tumors with increased risk for metastatic relapses in localized and 4S neuroblastoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:3368–3373. doi: 10.1158/1078-0432.CCR-05-2495. [DOI] [PubMed] [Google Scholar]
- 11.Caren H, Fransson S, Ejeskar K, Kogner P, Martinsson T. Genetic and epigenetic changes in the common 1p36 deletion in neuroblastoma tumours. British journal of cancer. 2007;97:1416–1424. doi: 10.1038/sj.bjc.6604032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Look AT, et al. Clinical relevance of tumor cell ploidy and N-myc gene amplification in childhood neuroblastoma: a Pediatric Oncology Group study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1991;9:581–591. doi: 10.1200/JCO.1991.9.4.581. [DOI] [PubMed] [Google Scholar]
- 13.Molenaar JJ, et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature. 2012;483:589–593. doi: 10.1038/nature10910. [DOI] [PubMed] [Google Scholar]
- 14.Pugh TJ, et al. The genetic landscape of high-risk neuroblastoma. Nature genetics. 2013;45:279–284. doi: 10.1038/ng.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forbes SA, et al. COSMIC: somatic cancer genetics at high-resolution. Nucleic acids research. 2017;45:D777–D783. doi: 10.1093/nar/gkw1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danussi C, et al. RHPN2 drives mesenchymal transformation in malignant glioma by triggering RhoA activation. Cancer Res. 2013;73:5140–5150. doi: 10.1158/0008-5472.CAN-13-1168-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peck JW, Oberst M, Bouker KB, Bowden E, Burbelo PD. The RhoA-binding protein, rhophilin-2, regulates actin cytoskeleton organization. The Journal of biological chemistry. 2002;277:43924–43932. doi: 10.1074/jbc.M203569200. [DOI] [PubMed] [Google Scholar]
- 18.Kniffin, C. Colorectal Cancer, Susceptibility to, 10, http://omim.org/entry/612591 (2009).
- 19.Carraway KL, Theodoropoulos G, Kozloski GA, Carothers Carraway CA. Muc4/MUC4 functions and regulation in cancer. Future oncology. 2009;5:1631–1640. doi: 10.2217/fon.09.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CY, et al. MUC4 gene polymorphisms associate with endometriosis development and endometriosis-related infertility. BMC medicine. 2011;9:19. doi: 10.1186/1741-7015-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaturvedi P, Singh AP, Batra SK. Structure, evolution, and biology of the MUC4 mucin. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22:966–981. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang P, Baker KA, Hagg T. A disintegrin and metalloprotease 21 (ADAM21) is associated with neurogenesis and axonal growth in developing and adult rodent CNS. The Journal of comparative neurology. 2005;490:163–179. doi: 10.1002/cne.20659. [DOI] [PubMed] [Google Scholar]
- 23.Yang P, Baker KA, Hagg T. The ADAMs family: coordinators of nervous system development, plasticity and repair. Progress in neurobiology. 2006;79:73–94. doi: 10.1016/j.pneurobio.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nature reviews. Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 25.Steeg PS. Targeting metastasis. Nature reviews. Cancer. 2016;16:201–218. doi: 10.1038/nrc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lasorsa VA, et al. Exome and deep sequencing of clinically aggressive neuroblastoma reveal somatic mutations that affect key pathways involved in cancer progression. Oncotarget. 2016;7:21840–21852. doi: 10.18632/oncotarget.8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sausen M, et al. Integrated genomic analyses identify ARID1A and ARID1B alterations in the childhood cancer neuroblastoma. Nature genetics. 2013;45:12–17. doi: 10.1038/ng.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kircher M, et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nature genetics. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Belle I, Wu JX, Sperandio S, Mercola D, Adamson ED. In vivo cloning and characterization of a new growth suppressor protein TOE1 as a direct target gene of Egr1. The Journal of biological chemistry. 2003;278:14306–14312. doi: 10.1074/jbc.M210502200. [DOI] [PubMed] [Google Scholar]
- 30.Gilissen C, et al. Exome sequencing identifies WDR35 variants involved in Sensenbrenner syndrome. American journal of human genetics. 2010;87:418–423. doi: 10.1016/j.ajhg.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badenas C, et al. Mutations in theCOL4A4 and COL4A3 genes cause familial benign hematuria. Journal of the American Society of Nephrology: JASN. 2002;13:1248–1254. doi: 10.1681/ASN.V1351248. [DOI] [PubMed] [Google Scholar]
- 32.Jenne DE, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nature genetics. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- 33.Schumacher V, et al. STK11 genotyping and cancer risk in Peutz-Jeghers syndrome. Journal of medical genetics. 2005;42:428–435. doi: 10.1136/jmg.2004.026294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badri MK, et al. Expression of Evc2 in craniofacial tissues and craniofacial bone defects in Evc2 knockout mouse. Archives of oral biology. 2016;68:142–152. doi: 10.1016/j.archoralbio.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Badri MK, et al. Ellis Van Creveld2 is Required for Postnatal Craniofacial Bone Development. Anatomical record. 2016;299:1110–1120. doi: 10.1002/ar.23353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blair HJ, et al. Evc2 is a positive modulator of Hedgehog signalling that interacts with Evc at the cilia membrane and is also found in the nucleus. BMC biology. 2011;9:14. doi: 10.1186/1741-7007-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dorn KV, Hughes CE, Rohatgi R. A Smoothened-Evc2 complex transduces the Hedgehog signal at primary cilia. Developmental cell. 2012;23:823–835. doi: 10.1016/j.devcel.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang C, Chen W, Chen Y, Jiang J. Smoothened transduces Hedgehog signal by forming a complex with Evc/Evc2. Cell research. 2012;22:1593–1604. doi: 10.1038/cr.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mao L, et al. A critical role of Sonic Hedgehog signaling in maintaining the tumorigenicity of neuroblastoma cells. Cancer science. 2009;100:1848–1855. doi: 10.1111/j.1349-7006.2009.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Souzaki R, et al. Hedgehog signaling pathway in neuroblastoma differentiation. Journal of pediatric surgery. 2010;45:2299–2304. doi: 10.1016/j.jpedsurg.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 41.Xu L, et al. Sonic Hedgehog pathway is essential for neuroblastoma cell proliferation and tumor growth. Molecular and cellular biochemistry. 2012;364:235–241. doi: 10.1007/s11010-011-1222-6. [DOI] [PubMed] [Google Scholar]
- 42.D’Asdia MC, et al. Novel and recurrent EVC and EVC2 mutations in Ellis-van Creveld syndrome and Weyers acrofacial dyostosis. European journal of medical genetics. 2013;56:80–87. doi: 10.1016/j.ejmg.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz-Perez VL, Goodship JA. Ellis-van Creveld syndrome and Weyers acrodental dysostosis are caused by cilia-mediated diminished response to hedgehog ligands. American journal of medical genetics. Part C, Seminars in medical genetics. 2009;151C:341–351. doi: 10.1002/ajmg.c.30226. [DOI] [PubMed] [Google Scholar]
- 44.Bahe S, Stierhof YD, Wilkinson CJ, Leiss F, Nigg EA. Rootletin forms centriole-associated filaments and functions in centrosome cohesion. The Journal of cell biology. 2005;171:27–33. doi: 10.1083/jcb.200504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang J, et al. Rootletin, a novel coiled-coil protein, is a structural component of the ciliary rootlet. The Journal of cell biology. 2002;159:431–440. doi: 10.1083/jcb.200207153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kramer A, Green J, Pollard J, Jr., Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30:523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kato Y, et al. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature. 1998;395:713–716. doi: 10.1038/27234. [DOI] [PubMed] [Google Scholar]
- 48.Jones SA, et al. The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Molecular endocrinology. 2000;14:27–39. doi: 10.1210/mend.14.1.0409. [DOI] [PubMed] [Google Scholar]
- 49.Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocrine reviews. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 50.Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nature reviews. Immunology. 2015;15:511–523. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenberg D, et al. Role of the PKA-regulated transcription factor CREB in development and tumorigenesis of endocrine tissues. Annals of the New York Academy of Sciences. 2002;968:65–74. doi: 10.1111/j.1749-6632.2002.tb04327.x. [DOI] [PubMed] [Google Scholar]
- 52.Heasman SJ, Carlin LM, Cox S, Ng T, Ridley AJ. Coordinated RhoA signaling at the leading edge and uropod is required for T cell transendothelial migration. The Journal of cell biology. 2010;190:553–563. doi: 10.1083/jcb.201002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Infante E, Ridley A. J. Roles of Rho GTPases in leucocyte and leukaemia cell transendothelial migration. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2013;368:20130013. doi: 10.1098/rstb.2013.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saci A, Carpenter CL. RhoA GTPase regulates B cell receptor signaling. Molecular cell. 2005;17:205–214. doi: 10.1016/j.molcel.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 55.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nature reviews. Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 56.Parsons DW, et al. Diagnostic Yield of Clinical Tumor and Germline Whole-Exome Sequencing for Children With Solid Tumors. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2015.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schramm A, et al. Mutational dynamics between primary and relapse neuroblastomas. Nat Genet. 2015;47:872–877. doi: 10.1038/ng.3349. [DOI] [PubMed] [Google Scholar]
- 58.Mosse YP, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455:930–935. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nature reviews. Cancer. 2008;8:11–23. doi: 10.1038/nrc2291. [DOI] [PubMed] [Google Scholar]
- 60.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nature reviews. Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 61.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nature reviews. Molecular cell biology. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 62.Taliaferro-Smith L, et al. FAK activation is required for IGF1R-mediated regulation of EMT, migration, and invasion in mesenchymal triple negative breast cancer cells. Oncotarget. 2015;6:4757–4772. doi: 10.18632/oncotarget.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sims D, Sudbery I, Ilott NE, Heger A, Ponting CP. Sequencing depth and coverage: key considerations in genomic analyses. Nature reviews. Genetics. 2014;15:121–132. doi: 10.1038/nrg3642. [DOI] [PubMed] [Google Scholar]
- 64.Gehring JS, Fischer B, Lawrence M, Huber W. SomaticSignatures: inferring mutational signatures from single-nucleotide variants. Bioinformatics. 2015;31:3673–3675. doi: 10.1093/bioinformatics/btv408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DePristo MA, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nature genetics. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McKenna A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome research. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cibulskis K, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31:213–219. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cingolani P, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strainw1118; iso-2; iso-3. Fly (Austin) 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.