Dear Editor,
Acinetobacter baumannii is a cause of healthcare-associated infections and one of the most important pathogens found in intensive care units (ICUs). The recent dramatic spread of carbapenem-resistant A. baumannii complex (CRAB) in Korea has raised substantial concern in medical practice owing to the lack of adequate therapeutic options [1,2]. It is well-known that the selective pressure of antibiotics contributes to emergence of new resistant strains [3]. However, there is no sufficient evidence for the association between carbapenem administration and emergence of imipenem-resistant A. baumannii. The rate of imipenem resistance in A. baumannii isolates from general hospitals in Korea markedly increased from 27% in 2007 to 72% in 2010, according to data from a nationwide multicenter study conducted by the Korean Antimicrobial Resistance Monitoring System (KARMS) [4], representing major public threat in Korea. In addition to antibiotic selection pressure, other factors could affect the spread of resistant organisms, such as new antimicrobial resistance mechanisms, virulence, host factors, and the infection control policy. In this study, we aimed to elucidate the potential explanation for this abrupt increase in imipenem resistance in A. baumannii in Korea, focusing on the antibiotic selection pressure nationwide.
Carbapenem is usually considered a treatment option for extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae. The rate of cefotaxime resistance in Klebsiella pneumoniae isolates increased from 18% in 2003 to 41% in 2009, and that of Escherichia coli increased from 10% in 2004 to 21% in 2008, in Korea [4]. It is mainly attributed to the spread of ESBL producers, which could be a main driver of carbapenem usage because carbapenem is the last resort for treatment of these infections.
This study was approved by the National Health Insurance Service Ilsan Hospital Institutional Review Board, as required by the local hospital policy (NHIMC 2017-03-025), with permission from the National Health Insurance Service-National Sample Cohort (REQ0000007622). The antibiotic usage data in Korea for 12 years (from 2002 to 2013) were acquired from the National Health Insurance Service-National Sample Cohort (NHIS-NSC) database [5]. The systemic prescription of antibiotics based on Anatomical Therapeutic Chemical (ATC) classification is standardized according to the daily defined dose (DDD) per 1000 inhabitants [6]. The data were analyzed using SAS software version 9.2 (SAS Institute, Cary, NC, USA). The relationship between antibiotic use and resistance were evaluated by simple correlation analysis. The significance level was set at P<0.05.
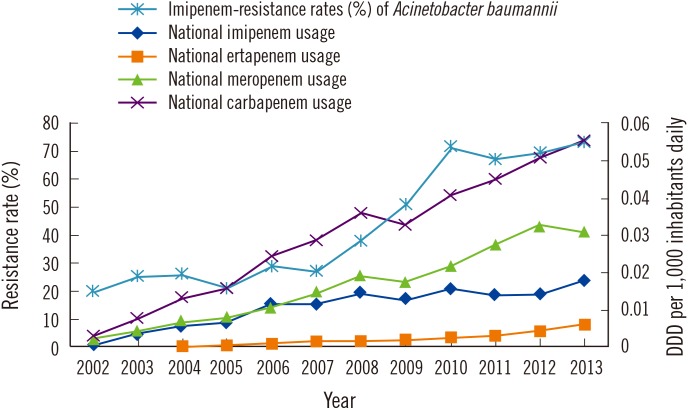
As shown in Fig. 1, the rate of imipenem resistance in A. baumannii strains isolated from general hospitals showed a good correlation with the national usage data for carbapenems (including imipenem, meropenem, ertapenem, and total carbapenems). The resistance mechanism of CRAB is primarily mediated by Ambler's class D OXA-type β-lactamases. Gurung et al [7] investigated the clonal diversity and genetic basis of antimicrobial resistance among CRAB isolates in a Korean hospital, all of which were found to be sequence type 2 but were classified into two sequence groups and nine pulso-types. blaOXA-23, which is a plasmid-borne gene encoding carbapenemase, was found to be largely responsible for resistance to carbapenems, and increased selection pressure of carbapenem might have facilitated the selection of dominant clones. There are some limitations of this study. Because most CRAB strains harbor not only blaOXA-23 but also multidrug resistance genes such as armA, blaESBL, and aminoglycosides-modifying enzyme genes [8], they may be co-selected by other classes of antibiotics that were not considered in this study. In addition, A. baumannii can survive for long periods in hospital settings, particularly on inanimate surfaces. Such environments may act as a reservoir for cross-colonization and infection outbreaks [9]. Although the KARMS data were collected from non-duplicated first isolates, the potential effect of outbreaks of A. baumannii infection within healthcare facilities could not be fully eliminated. Essentially, this represents an ecological study comparing groups rather than individuals, which is associated with some intrinsic methodological problems that limit any inference on causality [10]. Nevertheless, our data was based on nationwide surveillance and the antimicrobial prescription database collected over a long period, which could provide useful information on the correlation between carbapenem administration and resistance in A. baumannii for practical applications. The NHIS maintains and stores all the national records for healthcare utilization and prescriptions, which could provide large-scale, extensive, and stable nationwide antimicrobial prescription data.
Fig. 1. Correlation of national carbapenem usage with rate of imipenem resistance in Acinetobacter baumannii (IMP-R-ABA) strains isolated from general hospitals in Korea from 2002 to 2013. Correlation of imipenem usage with IMP-R-ABA (correlation coefficient r=0.93, P<0.0001); correlation of meropenem usage with IMP-R-ABA (r=0.93, P<0.0001); correlation of ertapenem usage with IMP-R-ABA (r=0.92, P=0.0002); correlation of total carbapenem usage with IMP-R-ABA (r=0.94, P<0.0001).
In conclusion, a correlation between carbapenem use and resistance of A. baumannii to imipenem was observed, which can offer insight into the increasing rate of imipenem-resistance in A. baumannii in Korea. These data can be used to develop national antibiotic usage management policies to reduce the spread of antibiotic resistance.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: This study was supported by grants from the National Health Insurance Service Ilsan Hospital (2016-20-001).
References
- 1.Lee Y, Kim YA, Song W, Lee H, Lee HS, Jang SJ, et al. Recent trends in antimicrobial resistance in intensive care units in Korea. Korean J Nosocomial Infect Control. 2014;19:29–36. [Google Scholar]
- 2.Jasemi S, Douraghi M, Adibhesami H, Zeraati H, Rahbar M, Boroumand MA, et al. Trend of extensively drug-resistant Acinetobacter baumannii and the remaining therapeutic options: a multi-center study in Tehran, Iran over a 3-year period. Lett Appl Microbiol. 2016;63:466–472. doi: 10.1111/lam.12669. [DOI] [PubMed] [Google Scholar]
- 3.Levy SB. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10(12 Suppl):S122–S1S9. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 4.KCDC. Korean Antimicrobial Resistance Monitoring System 2013 annual report. [Accessed on June 18 2017]. cdc.go.kr/CDC/cms/cmsFileDownload.jsp?fid=136&cid=21224&fieldname.
- 5.Lee J, Lee JS, Park S, Shin SA, Kim KW. Cohort profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46:e15. doi: 10.1093/ije/dyv319. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Collaborating Centre for Drug Statistics and Methodology. Definitions and general considerations. [last updated December 20, 2016]. [accessed June 18, 2017]. http://www.whocc.no/ddd/definition_and_general_considera/#DDDs.
- 7.Gurung M, Rho JS, Lee YC, Kim HS, Moon SY, Yu BH, et al. Emergence and spread of carbapenem-resistant Acinetobacter baumannii sequence type 191 in a Korean hospital. Infect Genet Evol. 2013;19:219–222. doi: 10.1016/j.meegid.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Kim YH. Conditional probability analysis of multidrug resistance in Gram-negative bacilli isolated from tertiary medical institutions in South Korea during 1999-2009. J Microbiol. 2016;54:50–56. doi: 10.1007/s12275-016-5579-9. [DOI] [PubMed] [Google Scholar]
- 9.Aliramezani A, Douraghi M, Hajihasani A, Mohammadzadeh M, Rahbar M. Clonal relatedness and biofilm formation of OXA-23-producing carbapenem resistant Acinetobacter baumannii isolates from hospital environment. Microb Pathog. 2016;99:204–208. doi: 10.1016/j.micpath.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 10.Morgenstern H. Ecologic studies in epidemiology: concepts, principles, and methods. Annu Rev Public Health. 1995;16:61–81. doi: 10.1146/annurev.pu.16.050195.000425. [DOI] [PubMed] [Google Scholar]