Abstract
Backgrounds/Aims
Diagnosis and staging of hepatocellular carcinoma (HCC) is critical because of the variety of treatment methods and prognosis. [18F]fludeoxyglucose-positron emission tomography/computed tomography ([18F]FDG-PET/CT) has been suggested as a diagnostic modality in HCC. The purpose of this study is to evaluate the accuracy of FDG-PET for staging of HCC after surgical resection and histological confirmation.
Methods
We retrospectively collected data of 56 patients that underwent FDG-PET before surgical resection for HCC March 2011–May 2017, all of whom were suitable for resection by conventional HCC staging. Results of the maximal standardized uptake value (SUVmax) were compared with histological confirmation.
Results
A larger tumor size was related with higher SUVmax (≥4.9). The serum alpha-feto protein was associated with SUVmax. Recurrence rate was higher in patients with higher SUVmax and patients with lower SUVmax had a better survival rate.
Conclusions
The SUVmax correlates well with tumor size and factors associated with biological behavior of HCC such as alpha-feto protein, and it could be a beneficial modality in providing prognostic information for HCC.
Keywords: Hepatocellular carcinoma, [18F]fludeoxyglucose positron emission tomography, Standardized uptake value (SUV)
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignancies and has been ranked 7th and 5th for cancer incidence worldwide and in South Korea, respectively. HCC is the 3rd leading cause of cancer-related deaths.1 Prognosis of patients with HCC is usually poor, and predicting life expectancy is difficult because of variable factors such as portal vein thrombosis, tumor stage, alpha-fetoprotein (AFP), Child–Pugh score, and high recurrence rate of the tumor.2 Therefore, accurate staging of HCC is crucial. Accurate staging is crucial because only patients with small tumors (<5 cm) without distant metastasis would benefit from liver resection or liver transplantation as a curative treatment.3 Conventionally, dynamic computed tomography (CT), ultrasonography (USG), and magnetic resonance imaging (MRI) are used as imaging modalities in staging and diagnosis of HCC.
Positron emission tomography (PET) is an imaging modality using positron-emitting markers. The most commonly used marker in evaluating cancer patients is [18F]fludeoxyglucose (FDG), an analogue of glucose, used in processes of glucose metabolism. Glucose metabolism increases rapidly in dividing and growing cells causing an increased uptake of FDG. In some cancers, FDG-PET, especially when merged with CT, is highly sensitive in the staging of the malignancies, and can be used in management of individual patients. This modality has been established as a diagnostic tool of various cancers.4 However, diagnostic accuracy of FDGPET for evaluation of HCC is limited due to variable FDG uptake in HCC. Sensitivity of FDG-PET for detecting HCC is approximately 50%–70% and FDG uptake by HCC varies according to histological differentiation of the tumor.5 Poorly differentiated HCC reveals an increased uptake of FDG. Given that poorly differentiated HCC is more likely to metastasize, FDG-PET is useful to detect distant metastasis.6 Evidence supports a relationship between FDG-PET imaging and degree of tumor differentiation. In a study of patients that underwent surgical resection for HCC, preoperative FDG-PET imaging was related to tumor differentiation and appeared to predict tumor recurrence and survival rates.7 There have been many studies reporting clinical usefulness of FDG-PET in the primary neoplasm of another group of hepatobiliary system, cholangiocarcinoma. It is a more accurate modality for distinguishing intrahepatic duct and common bile duct cancers than CT (88.9% vs. 81.5%) and it is superior to other methods in detecting occult metastasis.8 However, few studies report accuracy and clinical aspects of FDG-PET in HCC.
In this study, we retrospectively evaluated the correlation of FDG-PET imaging, characteristics of HCC and the role of FDG-PET in diagnosis and staging of HCC after surgical resection and histological confirmation.
MATERIALS AND METHODS
Subjects
We selected and analyzed 68 patients with newly diagnosed HCC that underwent FDG-PET before surgery March 2011–May 2017 at the Chosun University Hospital. This single-center study was based on retrospective analysis. Each patient underwent conventional diagnostic work-up based on review of the system, a physical examination, serologic studies, dynamic abdomino-pelvic CT and FDG-PET at diagnosis. Fifty-six patients that underwent surgical resection and had histological confirmation of HCC were included in this study. Eight patients that had no HCC in histology were excluded (7 patients with liver cirrhosis and one patient with angiomyolipoma). Four patients were excluded due to distant metastasis confirmed during operation. In those cases, no further surgical intervention was conducted. Confirmation of diagnosis was conducted by permanent histological review after operation. Informed consent was obtained from all patients prior to enrollment and the institutional review board at Chosun University Hospital had approved the study protocol.
FDG-PET/CT study
Patients fasted for at least 6 hours and were hydrated intravenously with normal saline (serum glucose level <180 mg/dl). Some patients were given sugar-free liquids orally for stomach expansion before tests. Patients received 0.15 mCi/kg of FDG intravenously and were advised to rest in bed for 60 minutes. PET imaging was conducted for approximately 25 minutes on a scanner using a combination of contrast-enhanced CT. Whole-body coverage was obtained from the subcranial region to the upper thigh (torso). A low-dose contrast non-enhanced CT acquisition was initiated first, followed by PET acquisition. Then, the contrast-enhanced CT scans were collected. The Discovery ST8 PET/CT instrument (GE Healthcare) was used in this test. Images were reconstructed in 3.75 mm increments. PET and CT images acquired by this combined imaging system were viewed in separate and fused modes using the Fusion software AW 4.6 version (GE Healthcare). Images were re-evaluated by an experienced, board-certified nuclear medicine doctor.
Surgical procedures
All patients underwent a scheduled operation under general anesthesia. Forty-six cases of anatomical resection and 10 cases of liver transplantation were conducted by a surgical team specializing in hepatobiliary system and liver transplantation. During resection, the abdomen was carefully explored for lymph node involvement distant to liver, extra-hepatic metastasis and peritoneal seeding. Such lesions were biopsied by frozen section histology and after confirmation of distant metastasis, no further surgical procedures were conducted.
Statistical analysis
We used independent-samples t-test to compare SUVmax results with tumor size. Pearson's correlation analysis was completed to compare SUVmax to AFP and indocyanine green retention rate at 15 minutes (ICG R15). Disease-free survival and overall survival curves were analyzed by the Kaplan Meier method. p-values<0.05 were statically significant. All data were analyzed using SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA).
RESULTS
Baseline patient characteristics
Of the 56 patients included 49 were men and 7 were women. Mean age of the patients was 61±10.7 (range, 33–85). Types of hepatitis considered the most important cause of hepatocellular carcinoma were as follow: Hepatitis B in 32 (57.1%) patients, hepatitis C in 9 (16.1%) patients and alcoholic liver disease in 13 (23.2%) patients. The location of the tumor was 46 on the right lobe of the liver and 10 on the left lobe. Mean alpha-fetoprotein (AFP) was 188.2±463.4 and ICG R15 was 15.9±9.6. Tumor size measured on preoperative CT was 3.6±2.3 cm and that confirmed on histological report was 3.7±2.7 cm. Mean SUVmax result was 4.9±2.2 cm. Types of operations conducted were as follow: lobectomy and segmentectomy of the liver in 11 (19.6%) patients and 35 (62.5%) patients respectively. Liver transplantation occurred in 10 (17.9%) patients (Table 1).
Table 1. Demographic characteristics of patients.
Values are shown as mean±standard deviation,number (%).
AFP, alpha-fetoprotein; ICG R15, indocyanine green retention rate at 15 minutes; PET SUV, positron emission tomography standard uptake value
Tumor characteristics according to the SUVmax
Mean SUVmax was 4.9±2.2, and 4.9 was used as a cut-off value for analysis. SUVmax revealed significant predictive power for tumor size (Table 2). A larger tumor size was associated with higher SUVmax result, in CT findings and histological results (p=0.026, 0.019, respectively). We compared serological factors with SUVmax and AFP, and both were significantly related to SUVmax (p=0.012) (Table 3).
Table 2. Standard uptake value (SUV) according to tumor size.
Table 3. Standard uptake value (SUV) according to alpha-fetoprotein (AFP) and indocyanine green retention rate at 15 minutes (ICG R15).
Survival rates according to the SUVmax
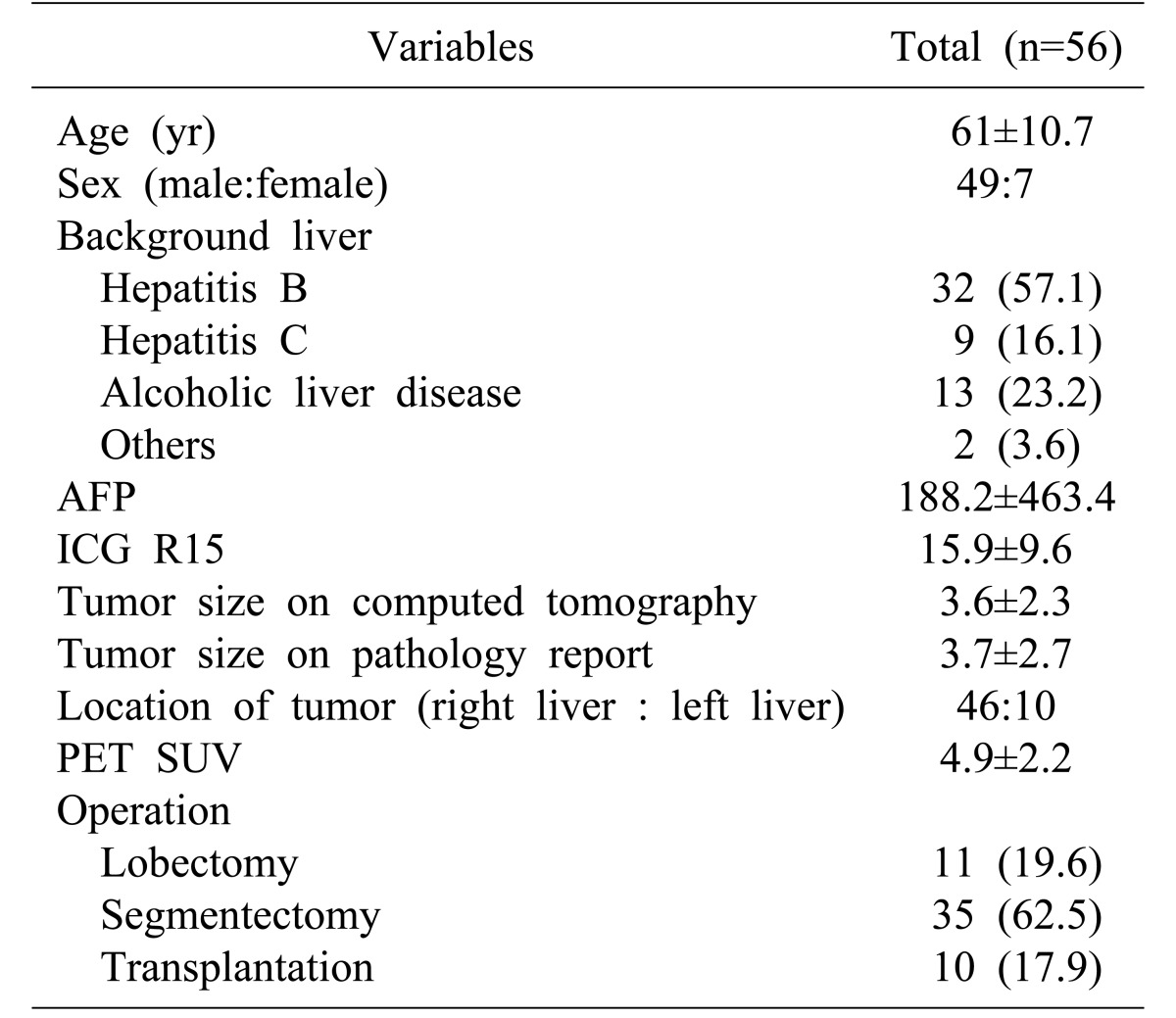
Among the 56 patients, 14 patients experienced recurrent HCC. Four patients had SUVmax<4.9 and 10 patients had SUVmax≥4.9. Disease-free survival above and below the cut-off value (SUVmax 4.9) was different. The 2-year disease-free survival was 37% for patients with SUVmax of less than 4.9 but was 42% for those with SUVmax of 4.9 or more. The result was not statistically significant (p=0.262) (Fig. 1). Nine patients died during the follow-up period. Overall survival was 81% in the group with SUVmax of 4.9 or more and 86% for those with SUVmax of less than 4.9, that was statistically insignificant (p=0.717) (Fig. 2).
Fig. 1. Disease-free survival rate in group of standard uptake value (SUV)≥4.9 and SUV<4.9.
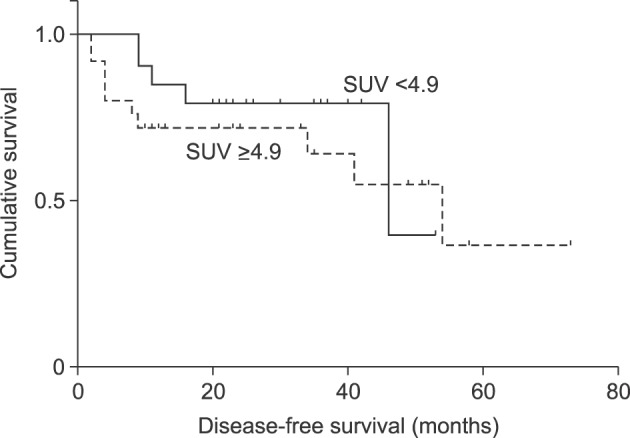
Fig. 2. Over-all survival rate in in group of standard uptake value (SUV)≥4.9 and SUV<4.9.
DISCUSSION
FDG-PET has increased accuracy of detecting numerous malignancies, with significant impact on treatment of cancer patients at different stages. FDG-PET, however, is inferior to conventional imaging modalities in terms of localization of malignancies. Absence of clear anatomical landmarks is a disadvantage of PET, hampering diagnostic usefulness.9 Identification of regions of normal biodistribution of FDG is usually beneficial for anatomical orientation but is neither optimal nor as accurate as CT.
FDG retention in malignant cells is dependent on intracellular glucose-6-phosphatase enzymatic activity. Normal liver cells contain high level of glucose-6-phosphatase and a small amount of hexokinase, but this ratio is reversed for HCC cells. This inconsistency enables FDG to accumulate in HCC but not in normal parenchymal cells.10 HCCs contain varying levels of this enzyme, and therefore reported sensitivity of FDG-PET/CT scans in detecting hepatocellular carcinoma ranges between 50–70%.11 Low sensitivity and variation in FDG uptake have been the main reasons for not routinely undergoing FDG-PET/CT in HCC work up. Despite accuracy in diagnosing HCC, CT and MRI cannot distinguish well differentiated HCC from poorly differentiated HCC. Since most HCCs are not biopsied, FDG PET may play a role in predicting tumor characteristics and behavior non-invasively, as the variability of FDG uptake has been related to HCC's differentiation, and proliferative activity of HCC.12
In our study, we evaluated usefulness of FDG-PET in predicting prognosis of HCC. The tumor size, a poor prognostic factor of HCC, had significant association with SUVmax of FDG-PET. A higher SUVmax resulted in a larger tumor size as determined by our study. A high SUVmax was significantly associated with serological factors such as AFP. Disease-free and overall survival rates were better in patients with SUVmax<4.9, but none of these were statistically significant.
Lin et al.13 evaluated the predictive value of FDG-PET for vascular invasion in 65 patients with HCC before liver transplantation 2010–2012. Patients with vascular invasion, associated with high risk of tumor recurrence and low survival rates in liver transplantation, exhibited significantly higher serum AFP, larger tumor size, higher SUVmax and higher ratio of tumor SUVmax to normal liver SUVmax or the normal liver SUVmean.
In our study, SUVmax was evaluated as a prognostic factor in HCC. In addition to the single value of SUVmax, ratios between the SUVmax of the tumor and the normal liver or between the SUVmax of the tumor and the mean uptake value of normal liver, SUVmean could be considered a prognostic factor.14
Boussouar et al.15 analyzed SUVs by FDG-PET-CT in native HCC patients on a waiting-list for liver transplantation. They demonstrated the ratio of the tumor SUV to normal liver SUV is associated with AFP levels, tumor size and poor differentiation, and should be integrated as a co-variable in a predictive outcome model.
There are many staging systems designed to date, including typical staging for the tumor-node-metastasis (TNM), and the Barcelona clinic liver cancer (BCLC) staging. Since many hepatocellular carcinomas are present in many cases. Additionally, because of advantages and disadvantages of each staging system, prognosis and treatment policy of the patient cannot be completely determined. These staging systems are designed based on prognostic factors; thus, finding prognostic factors in hepatocellular carcinoma patients is vital. Prognostic factors of hepatocellular carcinoma include tumor size, the number of tumors, microvascular and main vascular invasion, liver function status, systemic condition, and host factors such as AFP.16 Vauthey et al.17 analyzed prognosis factors of 557 patients with hepatocellular carcinoma that had undergone surgical resection. Results were as follows: major vessel invasion, microvascular invasion, severe liver fibrosis, cirrhosis, multiple tumor, and large size of tumor (≥5 cm). There have been few reports of prognostic factors related to tumor metabolism in hepatocellular carcinoma. Results of this study suggest that glucose metabolism activity of SUV is a critical predictor of prognosis and results of this study are significant.
There are several limitations in this study. First, this study was not a prospective study and there was selection bias related to patients' baseline clinical characteristics. Second, our study could not reveal the comparison between the groups of patients that underwent FDG-PET or only CT and MRI. Additionally, we could not compare diagnostic differences between FDG-PET and other conventional imaging modalities. This study was focused on the beneficial effect of the FDG-PET. Further data from multiple centers and with long-term follow-up after operations will be needed to validate this approach.
References
- 1.Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trojan J, Schroeder O, Raedle J, Baum RP, Herrmann G, Jacobi V, et al. Fluorine-18 FDG positron emission tomography for imaging of hepatocellular carcinoma. Am J Gastroenterol. 1999;94:3314–3319. doi: 10.1111/j.1572-0241.1999.01544.x. [DOI] [PubMed] [Google Scholar]
- 3.Cho E, Jun CH, Kim BS, Son DJ, Choi WS, Choi SK. 18F-FDG PET CT as a prognostic factor in hepatocellular carcinoma. Turk J Gastroenterol. 2015;26:344–350. doi: 10.5152/tjg.2015.0152. [DOI] [PubMed] [Google Scholar]
- 4.Asman Y, Evenson AR, Even-Sapir E, Shibolet O. [18F]fludeoxyglucose positron emission tomography and computed tomography as a prognostic tool before liver transplantation, resection, and loco-ablative therapies for hepatocellular carcinoma. Liver Transpl. 2015;21:572–580. doi: 10.1002/lt.24083. [DOI] [PubMed] [Google Scholar]
- 5.Kim MJ, Kim YS, Cho YH, Jang HY, Song JY, Lee SH, et al. Use of 18F-FDG PET to predict tumor progression and survival in patients with intermediate hepatocellular carcinoma treated by transarterial chemoembolization. Korean J Intern Med. 2015;30:308–315. doi: 10.3904/kjim.2015.30.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murakami K. FDG-PET for hepatobiliary and pancreatic cancer: Advances and current limitations. World J Clin Oncol. 2011;2:229–236. doi: 10.5306/wjco.v2.i5.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seo S, Hatano E, Higashi T, Hara T, Tada M, Tamaki N, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res. 2007;13:427–433. doi: 10.1158/1078-0432.CCR-06-1357. [DOI] [PubMed] [Google Scholar]
- 8.Moon CM, Bang S, Chung JB, Park SW, Song SY, Yun M, et al. Usefulness of 18F-fluorodeoxyglucose positron emission tomography in differential diagnosis and staging of cholangiocarcinomas. J Gastroenterol Hepatol. 2008;23:759–765. doi: 10.1111/j.1440-1746.2007.05173.x. [DOI] [PubMed] [Google Scholar]
- 9.Bar-Shalom R, Yefremov N, Guralnik L, Gaitini D, Frenkel A, Kuten A, et al. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med. 2003;44:1200–1209. [PubMed] [Google Scholar]
- 10.Wang XY, Chen D, Zhang XS, Chen ZF, Hu AB. Value of 18F-FDG-PET/CT in the detection of recurrent hepatocellular carcinoma after hepatectomy or radiofrequency ablation: a comparative study with contrast-enhanced ultrasound. J Dig Dis. 2013;14:433–438. doi: 10.1111/1751-2980.12064. [DOI] [PubMed] [Google Scholar]
- 11.Khan MA, Combs CS, Brunt EM, Lowe VJ, Wolverson MK, Solomon H, et al. Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J Hepatol. 2000;32:792–797. doi: 10.1016/s0168-8278(00)80248-2. [DOI] [PubMed] [Google Scholar]
- 12.Abuodeh Y, Naghavi AO, Ahmed KA, Venkat PS, Kim Y, Kis B, et al. Prognostic value of pre-treatment F-18-FDG PET-CT in patients with hepatocellular carcinoma undergoing radioembolization. World J Gastroenterol. 2016;22:10406–10414. doi: 10.3748/wjg.v22.i47.10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin CY, Liao CW, Chu LY, Yen KY, Jeng LB, Hsu CN, et al. Predictive value of 18F-FDG PET/CT for vascular invasion in patients with hepatocellular carcinoma before liver transplantation. Clin Nucl Med. 2017;42:e183–e187. doi: 10.1097/RLU.0000000000001545. [DOI] [PubMed] [Google Scholar]
- 14.Yang SH, Suh KS, Lee HW, Cho EH, Cho JY, Cho YB, et al. The role of (18)F-FDG-PET imaging for the selection of liver transplantation candidates among hepatocellular carcinoma patients. Liver Transpl. 2006;12:1655–1660. doi: 10.1002/lt.20861. [DOI] [PubMed] [Google Scholar]
- 15.Boussouar S, Itti E, Lin SJ, Decaens T, Evangelista E, Chiaradia M, et al. Functional imaging of hepatocellular carcinoma using diffusion-weighted MRI and (18)F-FDG PET/CT in patients on waiting-list for liver transplantation. Cancer Imaging. 2016;16:4. doi: 10.1186/s40644-016-0062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai TJ, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, et al. Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery. 2000;127:603–608. doi: 10.1067/msy.2000.105498. [DOI] [PubMed] [Google Scholar]
- 17.Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–1536. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]