ABSTRACT
During infection, pathogens must obtain all inorganic nutrients, such as phosphate, from the host. Despite the essentiality of phosphate for all forms of life, how Staphylococcus aureus obtains this nutrient during infection is unknown. Differing from Escherichia coli, the paradigm for bacterial phosphate acquisition, which has two inorganic phosphate (Pi) importers, genomic analysis suggested that S. aureus possesses three distinct Pi transporters: PstSCAB, PitA, and NptA. While pitA and nptA are expressed in phosphate-replete media, expression of all three transporters is induced by phosphate limitation. The loss of a single transporter did not affect S. aureus. However, disruption of any two systems significantly reduced Pi accumulation and growth in divergent environments. These findings indicate that PstSCAB, PitA, and NptA have overlapping but nonredundant functions, thus expanding the environments in which S. aureus can successfully obtain Pi. Consistent with this idea, in a systemic mouse model of disease, loss of any one transporter did not decrease staphylococcal virulence. However, loss of NptA in conjunction with either PstSCAB or PitA significantly reduced the ability of S. aureus to cause infection. These observations suggest that Pi acquisition via NptA is particularly important for the pathogenesis of S. aureus. While our analysis suggests that NptA homologs are widely distributed among bacteria, closely related less pathogenic staphylococcal species do not possess this importer. Altogether, these observations indicate that Pi uptake by S. aureus differs from established models and that acquisition of a third transporter enhances the ability of the bacterium to cause infection.
KEYWORDS: Staphylococcus aureus, phosphate metabolism, PstSCAB, PitA, NptA, transporter, Gram positive, infection
INTRODUCTION
Staphylococcus aureus, carried asymptomatically by approximately one-third of the population, is capable of establishing infection in virtually every host tissue (1–3). The threat of staphylococcal infections is amplified by prolific resistance to a variety of antibiotics among health care-associated isolates and the spread of antibiotic resistance to community-associated strains (4, 5). These factors have led organizations such as the Centers for Disease Control and Prevention and the World Health Organization to designate S. aureus as a serious threat to human health and to call for the development of new strategies to battle S. aureus (6, 7). During infection, pathogens must obtain their nutrients from the host. Understanding how pathogens such as S. aureus obtain vital nutrients during infection has the potential to lead to the identification of novel targets for therapeutic intervention.
Phosphate is an essential nutrient for all organisms due to its critical role in signaling, metabolism, and macromolecular structure. Because it is an inorganic nutrient, invading microbes must acquire phosphate from the host. The importance of phosphate acquisition is emphasized by the observation that disruption of phosphate transporters in pathogenic Escherichia coli, Salmonella enterica serovar Typhimurium, and other Enterobacteriaceae, as well as other species, including Vibrio cholerae, Mycobacterium tuberculosis, and Streptococcus pneumoniae, compromises their ability to cause infection (8–15). However, the repertoire of phosphate importers expressed by pathogens outside the Enterobacteriaceae and their contribution to the ability of pathogens such as S. aureus to cause infection are largely unknown.
The preferred source of phosphate for most bacteria is inorganic phosphate (Pi). Bacteria are known to possess three distinct classes of Pi importers: the PstSCAB (phosphate-specific transport) system, PitA (phosphate inorganic transport), and NptA (Na-dependent phosphate transport) (16–20). Significant insight into the molecular features of the Pst and Pit systems has been gained by studying their contribution to Pi uptake in laboratory isolates of E. coli and Bacillus subtilis (16–18, 21–25). PstSCAB is an ABC family Pi transporter in which PstS is the solute-binding protein, PstA and PstC comprise the transmembrane channel, and PstB is the ATPase that energizes translocation. The Pst system is a high-affinity (Km of ∼0.4 μM) importer that transports Pi ions with high specificity (16, 18, 22). PstSCAB is the most highly upregulated target of the phosphate-responsive two-component regulatory system PhoBR and is important for Pi transport when this nutrient is scarce (16, 18, 21, 26). In E. coli, mutation of the pstSCAB genes results in dysregulated, constitutive expression of the Pi starvation-induced Pho regulon due to activation of PhoBR (26). In contrast, when Pi is in excess, it is predominantly transported by the Pit system. PitA of E. coli has a lower affinity than Pst (Km of ∼38 μM) and is constitutively expressed (16, 18, 21). Pit transporters are proton motive force (PMF) driven and translocate Pi complexed with a divalent cation, such as Mg2+, as a neutral metal-phosphate complex (MeHPO4) (25). Analysis of NptA in bacteria has largely been limited to Vibrio cholerae and Streptococcus pneumoniae (19, 20). NptA belongs to the NaPi-2 family of eukaryotic sodium-phosphate cotransporters, which have been more extensively studied in eukaryotic organisms (27). Differing from the Pit and Pst systems, NptA uses sodium to move Pi into the cell at a stoichiometry of 3 Na+ atoms to 1 Pi molecule. Transport via NaPi-2 transporters is augmented by increased Na+ concentration and increased pH (19, 28). Analysis of the nptA gene of V. cholerae expressed in E. coli cells revealed that NptA has a considerably lower affinity (Km of ∼300 μM) for Pi than either the Pit or Pst system of E. coli (19). This observation led to the suggestion that NptA is important during the establishment of infection when the need for nutrients is especially great (19). However, the contribution of NptA to bacterial pathogenesis has not been directly evaluated.
Given the importance of phosphate, we set out to identify the Pi uptake systems expressed by S. aureus and elucidate their respective contributions to pathogenesis. Our analysis revealed that S. aureus encodes three distinct Pi transporters, PstSCAB, PitA, and NptA. Expression of all three systems increases in response to Pi limitation. Analysis of a panel of single and double transporter mutants revealed that each importer is attuned to import Pi optimally in a discrete environment. While no single transporter is necessary for virulence, only NptA is sufficient to mediate full pathogenesis of S. aureus. Notably, this indicates that S. aureus differs significantly from many Enterobacteriaceae, including E. coli, which currently serves as the paradigm for bacterial Pi transport. These findings also illuminate the important contribution of NptA, a widely distributed but little-studied transporter, to bacterial pathogenesis.
RESULTS
Staphylococcus aureus encodes three putative Pi transporters.
As an initial step to identify the staphylococcal Pi transporters, the S. aureus Newman genome was analyzed via BLAST for known Pi importers. This analysis identified three potential Pi transporters: pstSCAB, pitA, and nptA (see Fig. S1A in the supplemental material). In addition to the putative Pi transport genes, each locus encoded a PhoU homolog (pstSCAB and pitA loci) or domain (nptA locus). The presence at each locus of phoU, which contributes to regulating the Pi starvation response in E. coli and other organisms (26), strengthens the presumption that the staphylococcal PstSCAB, PitA, and NptA homologs are Pi transporters. A comprehensive analysis of ∼9,000 staphylococcal genomes, representing 38 species available from the NCBI database, revealed that pstSCAB and pitA are almost universally conserved (pstSCAB is missing from only a single species, Staphylococcus microti) (Table S1). In contrast, nptA had a much more heterogeneous distribution, with only 60% (24/38) of species, including S. aureus, encoding an NptA ortholog (Table S1). Comparative genome alignments of the nptA-containing regions in S. aureus and the other staphylococcal species indicated that nptA is located in distinct genomic locations (Fig. S1B). Intriguingly, our analysis of the nptA loci suggests that following an ancestral loss event, S. aureus reacquired nptA from a staphylococcal donor (Fig. S1C and D). An expanded investigation of the distribution of NptA homologs in a variety of bacteria revealed that this Pi transporter was widely distributed among bacterial phyla (Table 1). NptA homologs were found to be particularly common among the Firmicutes, with an average copy number of 0.82 per genome, as well as in the family Enterobacteriaceae, with an average of 0.76 copy per genome (Table 1).
TABLE 1.
Distribution of nptA among bacteria
| Taxonomic group | No. of genomesa | No. of nptA copiesb |
|---|---|---|
| Actinobacteria | 224 | 0.10 |
| Bacteroidetes | 109 | 0.34 |
| Chlamydiae | 13 | 0.00 |
| Firmicutes | 331 | 0.82 |
| Bacilli | 193 | 0.68 |
| Staphylococcaceae | 15 | 0.40 |
| Clostridia | 127 | 1.03 |
| Others | 11 | 0.73 |
| Fusobacteria | 6 | 1.17 |
| Proteobacteria | 749 | 0.57 |
| Alphaproteobacteria | 196 | 0.56 |
| Betaproteobacteria | 131 | 0.46 |
| Deltaproteobacteria | 56 | 0.61 |
| Epsilonproteobacteria | 38 | 0.61 |
| Gammaproteobacteria | 328 | 0.61 |
| Enterobacteriaceae | 105 | 0.76 |
| Spirochaetes | 39 | 0.64 |
| Tenericutes | 59 | 0.08 |
Number of individual species' genomes in the KEGG database.
Average gene copy per genome.
Expression of three putative Pi transporters in S. aureus increases upon Pi limitation.
To facilitate studying phosphate acquisition in S. aureus, a Pi-limiting, defined medium (PFM9), based on M9 salts and a previously described defined staphylococcal growth medium (29), was created so that Pi starvation could be imposed in culture. In this medium, S. aureus growth is dependent on the addition of Pi, with maximal optical density observed in the presence of 1.58 mM Pi or higher (Fig. 1A). The reduced growth rate of S. aureus throughout the growth assay in medium containing 50 and 158 μM Pi suggested that it could be utilized to impose Pi starvation. To confirm this assumption, intracellular phosphate levels were assessed during exponential growth in PFM9 supplemented with various concentrations of Pi. Concentrations of Pi that limited the staphylococcal growth rate also resulted in reduced accumulation of intracellular phosphate (Fig. 1B). At the lowest concentration tested, S. aureus accumulated ∼7-fold less phosphate than when bacteria were grown in Pi-replete medium (5 mM Pi). In total, these results demonstrate that PFM9 can be used to impose phosphate limitation on S. aureus.
FIG 1.
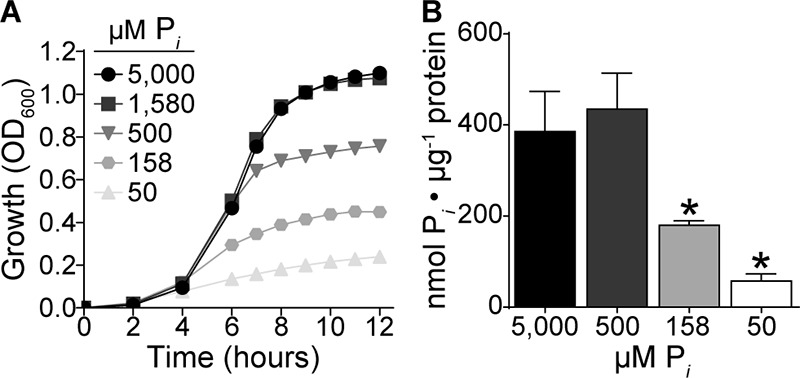
Intracellular Pi concentrations in S. aureus decrease during Pi limitation. (A) Growth of wild-type S. aureus in PFM9, pH 7.4, supplemented with various concentrations of Pi, measured by OD600. n = 3; error bars indicate SEMs and are frequently smaller than the symbols. (B) Intracellular Pi levels normalized to protein concentration in wild-type S. aureus grown in PFM9 supplemented with the indicated concentrations of Pi. n ≥ 2; error bars indicate SEMs. *, P < 0.05 via unpaired t test.
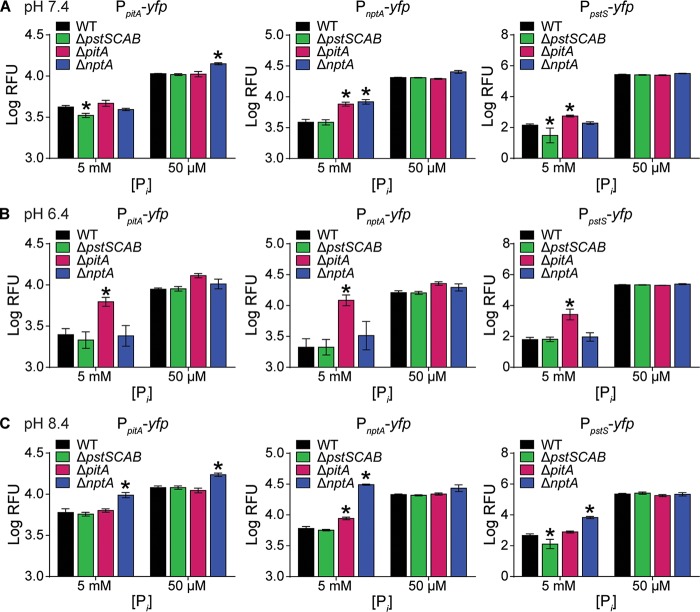
If PstSCAB, PitA, and NptA are Pi importers, their expression would be expected to increase in response to reduced Pi availability. Using transcriptional reporter fusions, we found that while pitA and nptA were expressed under Pi-replete conditions, expression of both systems increased significantly in Pi-limiting medium (Fig. 2A and B). While we observed negligible expression of pstSCAB in Pi-replete medium, the system was induced ∼6,000-fold when Pi was limiting (Fig. 2C). In total, these results support the hypothesis that PstSCAB, PitA, and NptA are Pi importers.
FIG 2.
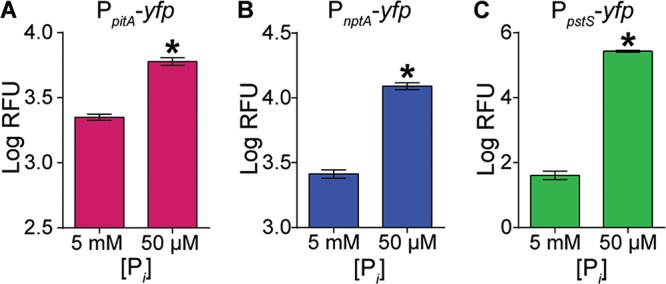
Expression of the three Pi transporters in S. aureus increases upon Pi limitation. Shown is expression of pitA (A), nptA (B), and pstSCAB (C) in wild-type S. aureus after 9 h of growth in PFM9, pH 7.4, supplemented with 5 mM (excess) or 50 μM (limiting) Pi. Expression was assessed using the reporter plasmids PpitA-yfp, PnptA-yfp, and PpstS-yfp. *, P < 0.05 compared to result with 5 mM Pi via unpaired t test. n = 3; error bars indicate SEMs. A similar pattern of expression was observed at other time points (data not shown).
PstSCAB, PitA, and NptA promote growth of S. aureus in divergent environments.
To elucidate under which conditions the putative transporters support growth of S. aureus, ΔpstSCAB, ΔpitA, and ΔnptA mutants were grown in medium supplemented with high and low concentrations of Pi. No growth defects were observed with any of the single mutants (Fig. S2), indicating that none of the transporters are essential under these conditions. As S. aureus encodes multiple putative Pi transporters, we reasoned that the systems might be able to compensate for one another in vitro. To evaluate this possibility, a series of double mutants (ΔpstSCAB ΔpitA, ΔpstSCAB ΔnptA, and ΔnptA ΔpitA mutants) was constructed. Multiple attempts to construct a ΔpstSCAB ΔnptA ΔpitA triple mutant were unsuccessful, suggesting that PstSCAB, PitA, and NptA are the only Pi transporters expressed by S. aureus under standard laboratory conditions.
When growth of the double transporter mutants was assessed in PFM9 medium supplemented with high and low concentrations of Pi (buffered to pH 7.4), the ΔpstSCAB ΔpitA and ΔpstSCAB ΔnptA mutants grew similarly to the wild type in both Pi-replete and -deplete medium, suggesting that NptA and PitA, respectively, are sufficient for growth under these conditions (Fig. 3A and B). While the ΔnptA ΔpitA mutant, which presumptively relies on PstSCAB for Pi uptake, also grew similarly to the wild type in PFM9 (Fig. S3A), we observed that it formed smaller colonies on tryptic soy agar (TSA) plates (Fig. S3B). Additionally, when growth of the ΔnptA ΔpitA mutant was assessed in Pi-replete rich medium (tryptic soy broth [TSB]), this strain grew more slowly than wild-type S. aureus or the other single and double mutants (Table 2). Ectopic expression of either nptA or pitA reversed the growth rate defect of the ΔnptA ΔpitA mutant in TSB (Table 2). Together, these data suggest that Pi acquisition via the Pst system is insufficient to support robust growth of S. aureus in rich medium. When combined with the observation that pstSCAB is expressed in low- but not high-Pi medium, these data suggest that the Pst system largely contributes to Pi uptake when the availability of this nutrient is limiting.
FIG 3.
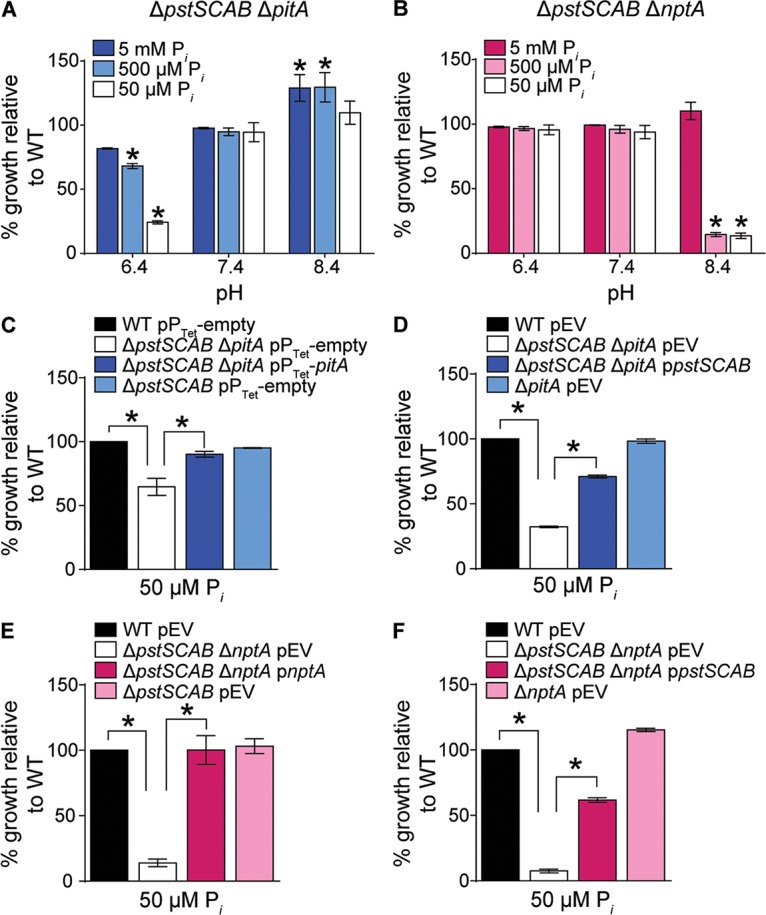
NptA and PitA promote growth of S. aureus in divergent environments. (A and B) Growth of ΔpstSCAB ΔpitA (A) and ΔpstSCAB ΔnptA (B) mutants in PFM9 adjusted to pH 6.4, 7.4, or 8.4 with different supplemental Pi concentrations. Growth was measured by assessing OD600 at 12 h. *, P < 0.05 compared to result for the wild type (WT) via two-way analysis of variance (ANOVA) with Dunnett's posttest. n = 3; error bars indicate SEMs. Similar results were obtained when growth was compared at earlier time points (data not shown). (C to F) Growth of the indicated strains measured by OD600 at 12 h and normalized to the wild type in PFM9, pH 6.4 (C and D) or 8.4 (E and F), with 50 μM supplemental Pi. pEV, empty vector. *, P < 0.05 for the indicated comparisons via one-way ANOVA with Sidak's posttest. n = 3; error bars indicate SEMs.
TABLE 2.
Doubling times of Pi transporter mutants in tryptic soy brotha
| Strain description | Doubling time, mean ± SD (min) |
|---|---|
| WT | 33.0 ± 1.7 |
| ΔpstSCAB | 31.8 ± 0.6 |
| ΔpitA | 32.8 ± 1.0 |
| ΔnptA | 30.3 ± 0.6* |
| ΔpstSCAB ΔpitA | 32.0 ± 0.3 |
| ΔpstSCAB ΔnptA | 31.8 ± 1.0 |
| ΔnptA ΔpitA | 37.5 ± 1.5* |
| WT pEV | 32.8 ± 0.6 |
| ΔpitA pEV | 32.5 ± 0.6 |
| ΔnptA ΔpitA pEV | 38.2 ± 1.1* |
| ΔnptA ΔpitA pnptA | 32.2 ± 0.6 |
| WT pPTet-empty | 39.5 ± 0.7 |
| ΔnptA pPTet-empty | 42.7 ± 2.8 |
| ΔnptA ΔpitA pPTet-empty | 48.3 ± 2.0* |
| ΔnptA ΔpitA pPTet-pitA | 40.8 ± 1.5 |
*, P < 0.05 compared to wild type via one-way ANOVA with Sidak's posttest. WT, wild type; pEV, empty vector.
In several bacteria, the efficacy of Pi transporters is differentially affected by environmental conditions. For example, the transport rate of NptA from V. cholerae is augmented under alkaline conditions and PitA from E. coli optimally imports Pi in acidic environments (19, 30). This raises the possibility that the expanded repertoire of importers expressed by S. aureus may expand the environmental niches that it can occupy. To evaluate if the S. aureus transporters enhance growth in divergent environments, PFM9 was adjusted from physiological to acidic or alkaline pH. As before, no growth defects were observed with any of the single mutants under any of the conditions tested (Fig. S2). However, the presumably NptA-dependent ΔpstSCAB ΔpitA mutant had a severe growth defect in acidic Pi-limiting medium (Fig. 3A). Conversely, the presumptively PitA-dependent ΔpstSCAB ΔnptA mutant was unable to grow in alkaline Pi-limiting medium (Fig. 3B). The growth defects of the ΔpstSCAB ΔpitA and ΔpstSCAB ΔnptA mutants could be complemented by ectopic expression of either of the deleted transporters (Fig. 3C to F; see also Fig. S4). Both pitA and nptA were expressed in Pi-limited medium regardless of pH, indicating that the growth defect is due to reduced activity of the transporter rather than reduced expression of pitA and nptA (Fig. S5). Importantly, the growth defects of the mutants were also reversed by the addition of Pi, indicating that the phenotypes are not due to a generalized growth defect in either acidic or alkaline environments (Fig. 3A and B). Cumulatively, these observations suggest that Pi transport by PitA and NptA is suboptimal in basic and acidic pHs, respectively.
To further interrogate the importance of each transporter in different environments, the expression of the transporters was assessed in ΔpstSCAB, ΔpitA, and ΔnptA backgrounds as a function of pH and Pi level. We reasoned that in a given environment, loss of a preferred Pi importer would result in compensatory increases in expression of the other transporters due to suboptimal Pi acquisition. At neutral pH in Pi-replete medium, nptA and pst expression increased significantly in the ΔpitA strain, suggesting that PitA may be the optimal Pi transporter under these conditions (Fig. 4A). At this pH, loss of NptA resulted in increased expression of pitA but only in Pi-limited medium. In neutral, Pi-deplete medium, a change in expression was only observed with loss of NptA, which resulted in increased expression of pitA. At acidic pH in Pi-replete medium, expression of all three transporters significantly increased in the ΔpitA strain but not the ΔpstSCAB or ΔnptA strain (Fig. 4B). This suggests that PitA is the predominant Pi transporter utilized by S. aureus under acidic conditions. In acidic, Pi-deplete medium, no increased expression of pitA, nptA, or pstSCAB was observed in any of the strains compared to that in the wild type, suggesting that all three transporters were maximally expressed. At alkaline pH in Pi-replete medium, loss of NptA resulted in significantly increased expression of pitA, pstSCAB, and nptA (Fig. 4C). Under these conditions, loss of PitA resulted in a modest increase of nptA, but not pstSCAB, expression. In alkaline, Pi-deplete medium, only the loss of NptA resulted in any increase in expression, and then only for pitA. Cumulatively, these results suggest that NptA is the preferred Pi importer under alkaline conditions. Interestingly, the fact that no significant increase in the expression of the transporters was observed in the ΔpstSCAB mutant in any media (Fig. 4) suggests that S. aureus does not primarily rely on Pst for growth under the conditions tested. Together, these data indicate that S. aureus utilizes its three Pi transporters preferentially in different environments.
FIG 4.
S. aureus differentially compensates for loss of Pi transporters depending on the environmental conditions. Shown is expression of pitA, nptA, and pstSCAB in the wild type and ΔpstSCAB, ΔpitA, and ΔnptA mutants following growth in PFM9 supplemented with 5 mM (excess) or 50 μM (limiting) Pi adjusted to pH 7.4 (A), 6.4 (B), or 8.4 (C). Expression was assessed using the reporter plasmids PpitA-yfp, PnptA-yfp, and PpstS-yfp. *, P < 0.05 compared to the value for the wild type via two-way ANOVA with Dunnett's posttest. n = 3; error bars indicate SEMs.
Pi acquisition via PstSCAB, PitA, and NptA is optimal under different conditions.
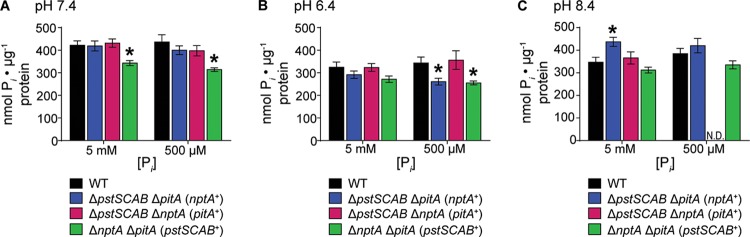
To evaluate whether loss of the putative transporters decreases Pi acquisition by S. aureus, the ability of each double mutant to accumulate Pi was assessed in replete and limiting Pi at neutral, acidic, and basic pHs. The PitA- and NptA-dependent mutants (the ΔpstSCAB ΔnptA and ΔpstSCAB ΔpitA mutants, respectively) accumulated as much Pi as wild-type bacteria at neutral pH (Fig. 5A). In contrast, the Pst-dependent ΔnptA ΔpitA mutant accumulated approximately 25% less Pi than did the wild type under both Pi-replete and -limiting conditions at neutral and acidic pHs (Fig. 5A and B). Coupled with the diminished growth rate of the ΔnptA ΔpitA mutant, this observation suggests that the Pst system is unable to satiate a rapidly growing cell's need for phosphate. In acidic Pi-limiting medium, the NptA-dependent ΔpstSCAB ΔpitA mutant contained less Pi than the wild type, suggesting that its growth defect is due to a reduced ability to acquire Pi under these conditions (Fig. 5B). The PitA-dependent ΔpstSCAB ΔnptA strain was unable to be tested in basic medium with limiting Pi because it could not grow under this condition; notably, however, the ability of this strain to grow and acquire Pi could be complemented by the addition of Pi (Fig. 5C). In total, the ability of each double mutant to acquire Pi under at least one tested growth condition highlights a role for all three systems as Pi importers. In sum, these data indicate that PstSCAB, PitA, and NptA are Pi transporters that optimally promote Pi acquisition in discrete environments.
FIG 5.
Loss of Pi transporters diminishes the ability of S. aureus to accumulate Pi. (A to C) The wild type and ΔpstSCAB ΔpitA, ΔpstSCAB ΔnptA, and ΔnptA ΔpitA mutants were grown in PFM9 in various concentrations of Pi adjusted to pH 7.4 (A), 6.4 (B), or 8.4 (C), and intracellular Pi was measured. *, P < 0.05 compared to the value for the wild type via two-way ANOVA with Dunnett's posttest. n = 5; error bars indicate SEMs. N.D., not determined.
NptA is sufficient for systemic S. aureus infection.
To evaluate the contribution of the staphylococcal Pi importers to virulence, C57BL/6 mice were infected with wild-type S. aureus and the transporter single mutants (the ΔpstSCAB, ΔpitA, and ΔnptA mutants). Similar to the growth assays, none of the single mutants had a virulence defect (Fig. S6). To determine if any transporter is sufficient for S. aureus pathogenesis, mice were infected with the transporter double mutants (the ΔpstSCAB ΔpitA, ΔpstSCAB ΔnptA, and ΔnptA ΔpitA mutants). Mice infected with the ΔpstSCAB ΔnptA and ΔnptA ΔpitA mutants lost less weight compared to mice infected with wild-type S. aureus (Fig. 6A). Significantly reduced bacterial burdens were recovered from the hearts, kidneys, and livers of mice infected with the ΔnptA ΔpitA mutant relative to those infected with wild-type S. aureus (Fig. 6B to D). Remarkably, the ΔpstSCAB ΔpitA mutant was as virulent as wild-type bacteria, indicating that Pi transport by NptA is sufficient to mediate S. aureus infection. Cumulatively, these observations highlight an important role for NptA in staphylococcal infection.
FIG 6.
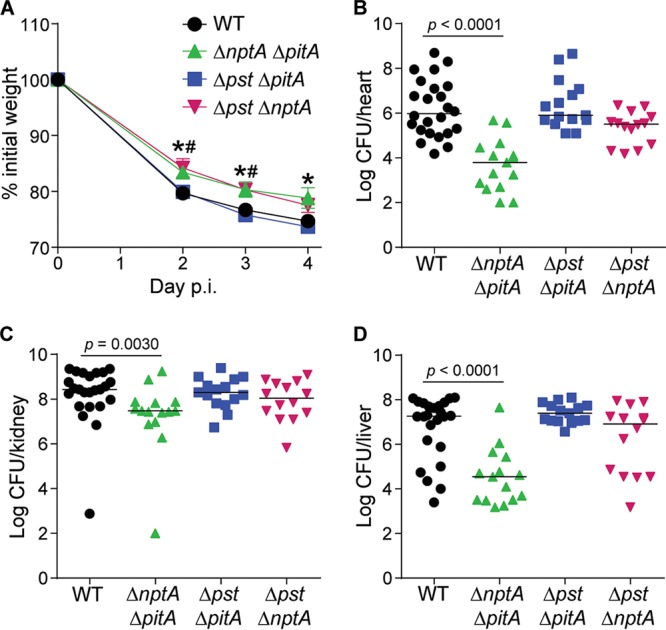
NptA but not PitA or Pst is sufficient to mediate virulence of S. aureus. Wild-type C57BL/6J mice were infected with the S. aureus wild type and ΔpstSCAB ΔpitA, ΔpstSCAB ΔnptA, and ΔnptA ΔpitA mutants. Weight loss was monitored (A) and bacterial burdens in the heart (B), kidneys (C), and liver (D) were enumerated 4 days postinfection. (A) #, P < 0.05 for the ΔpstSCAB ΔnptA mutant compared to the wild type; *, P < 0.05 for the ΔnptA ΔpitA mutant compared to the wild type via two-way ANOVA with Dunnett's posttest. Error bars indicate SEMs. (B to D) P values were determined by Mann-Whitney test; only significant P values are shown. The lines indicate medians. The data are results from three independent experiments. n ≥ 14 for each group. Δpst, ΔpstSCAB.
DISCUSSION
S. aureus is a versatile pathogen that can thrive in a multitude of environments, enabling it to infect virtually all host tissues. Despite the essential role of Pi in many cellular processes, the contribution of Pi transport to virulence remains unstudied for S. aureus and many other pathogens. In this work, we found that S. aureus expresses three Pi transporters, PstSCAB, PitA, and NptA, which optimally facilitate growth and Pi acquisition in distinct environments. While none of the transporters are essential for systemic infection, NptA, a member of a conserved but sparsely studied family of bacterial Pi transporters, is the only transporter sufficient to mediate wild-type levels of staphylococcal disease. This is particularly surprising, as PstSCAB is necessary for the pathogenesis of E. coli and other Enterobacteriaceae (8). While NptA homologs are widely distributed in bacteria, within the staphylococci, NptA is present in only ∼60% of staphylococcal species, including S. aureus. Of note, our analysis indicates that a lineage of human-associated Staphylococcus species that are closely related to but less pathogenic than S. aureus (namely, S. epidermidis, S. lugdunensis, and S. haemolyticus) underwent a shared loss of nptA but has not reacquired this gene (Fig. S1D) (31). In total, the current observations suggest that acquisition of NptA enhances the ability of S. aureus to obtain Pi and cause infection and may contribute to the heightened pathogenicity of this species.
The maintenance of proper cellular levels of phosphate via acquisition and regulation has been extensively characterized for E. coli. This has led E. coli to become the paradigm for bacterial phosphate homeostasis, a position borne out by virulence studies with many enterobacterial pathogens (8–12). However, Pi acquisition and homeostasis in S. aureus do not follow the rules of E. coli, as is the case for several other organisms, including B. subtilis, S. pneumoniae, M. tuberculosis, and V. cholerae (14, 17, 19, 20, 32). The most notable difference is that in addition to PitA and PstSCAB, S. aureus possesses a third functional Pi importer, NptA. E. coli strains lacking PitA and PstSCAB are incapable of growth on Pi as a phosphate source (33). Beyond this, our results suggest that S. aureus takes a profoundly different approach to coping with Pi limitation. Intracellular Pi concentrations of E. coli are thought to be quite stable, with E. coli experiencing only a maximal 4-fold reduction in intracellular Pi when the nutrient is limiting (21, 34). Additionally, when grown in Pi-limiting media, E. coli transiently accumulates high levels of polyphosphate, a storage form of Pi, during logarithmic growth (35, 36). In S. aureus, however, there was an approximately 7-fold reduction in the total cellular Pi concentration between S. aureus grown in Pi-replete versus -limiting media even after treatment with a polyphosphatase. This observation indicates that the dynamic range of intracellular Pi concentrations in S. aureus is larger than that of E. coli. Overall, these findings indicate that Pi acquisition and homeostasis in S. aureus differ substantially from the paradigm established by E. coli. This idea is further supported by the observation that disruption of the Pst structural genes in S. aureus does not result in constitutive expression of the Pi importers as observed in E. coli and other bacteria, including M. tuberculosis (22, 37). The presence of a phoU gene at the pst locus in S. aureus suggests that staphylococcal phosphate homeostasis is also distinct from that of the more closely related B. subtilis, which does not possess a PhoU homolog (38). Intriguingly, the presence of phoU genes/domains in addition to the pst-associated phoU further suggests that regulation of S. aureus phosphate homeostasis differs from established models. While the presence of multiple copies of phoU is not unique to S. aureus, how the presence of multiple PhoU proteins, particularly those associated with pit and nptA loci, impacts control of phosphate homeostasis is largely unknown.
In Pi-replete environments, PitA is the primary system utilized by E. coli to obtain this critical nutrient, with the PstSCAB system expressed in Pi-limiting environments (21, 22). While there are differences in how the two organisms handle Pi limitation, S. aureus utilizes a similar regulatory logic to control the expression of its Pi importers. Similar to the case with E. coli, in S. aureus, PitA appears to be the primary transporter expressed under neutral, Pi-replete conditions. In response to loss of PitA in acidic environments (Fig. 4B) or environments where the ability of PitA to transport Pi is compromised, such as in alkaline media (Fig. S5B), S. aureus induces the expression of nptA and pstSCAB. Interestingly, under both of these conditions, nptA expression reaches a value closer to its maximal induction under Pi-limiting conditions than pstSCAB expression. This suggests that when PitA is unable to supply the cell with sufficient Pi, S. aureus may selectively induce NptA before the Pst system. How S. aureus would achieve this differential regulation in response to presumptively the same stimulus is unclear. An intriguing possibility is that the expanded repertoire of PhoU-like molecules enables a more nuanced approach to controlling the expression of the S. aureus importers.
Differing from most essential nutrients, the biologically available form of Pi is strongly influenced by pH, as well as the identity and abundance of monovalent and divalent cations. Prior biochemical analysis of PitA and NptA homologs from E. coli, V. cholerae, and other bacteria revealed that these importers optimally transport Pi in distinct environments (19, 25, 30). Our analysis demonstrates that this difference in preferred substrates expands the environments in which S. aureus can satiate its need for Pi. Throughout the host, the abundance of potential counterions can change; for example, kidneys are rich in monovalent ions like Na+ and K+. Intriguingly, Na+ increases Pi transport rates of NptA from V. cholerae and rat (19, 39). As the importance of PitA and NptA is revealed in the absence of PstSCAB, the question remains, do these systems provide an advantage? In culture, when forced to rely on PstSCAB, S. aureus grows more slowly, and accumulates less Pi, in nutrient- and Pi-replete environments than wild-type bacteria. This suggests that relying on PstSCAB can be suboptimal. The virulence defect of the ΔnptA ΔpitA mutant suggests that a similar situation also occurs during systemic infection. In conjunction with the virulence defect of a strain forced to rely on PitA to obtain Pi, these results suggest that acquisition of NptA enhances the ability of S. aureus to cause infection. At the same time, the recovery of a pitA mutant from a screen for factors that contribute to the development of osteomyelitis indicates that in some host environments PitA is the preferred Pi importer (40). While the current studies do not provide a definitive rationale for the retention of the PstSCAB system, it seems unlikely that S. aureus would maintain three distinct transporters unless the bacterium encounters conditions in which each is necessary within the host. As PstSCAB is a high-affinity importer, it is tempting to speculate that it is most important for growth in environments where phosphate availability is limited, due to either reduced absolute abundance or competition with other microbes. In any case, as demonstrated by the ability of the ΔpitA and ΔnptA mutants to grow and infect mice as well as the wild type, it is clear that PstSCAB can sufficiently augment Pi uptake when either PitA or NptA is not functioning optimally. Our results suggest that the acquisition of NptA and the expression of three distinct Pi importers enhance S. aureus fitness by enabling optimal Pi acquisition in divergent environments.
In S. aureus and S. pneumoniae, NptA functions as a Pi importer. While PstSCAB and PitA are the only Pi importers expressed by E. coli, it does possess an NptA homolog, YjbB. However, overexpression studies suggest that in E. coli YjbB functions as a Pi exporter (41). While the physiological role of YjbB remains unclear, this observation suggests that the NptA homologs possessed by other bacteria may function as either importers or exporters. While transporters are typically unidirectional, in E. coli and Metallosphaera sedula, Pit family transporters have been demonstrated to mediate efflux of Pi (42, 43). Intriguingly, in these cases, the PitA homologs are thought to protect the bacteria from metal toxicity due to their ability to export a neutral phosphate-metal complex. Given the widespread distribution of NptA, particularly within the Enterobacteriaceae and other pathogenic organisms, it seems likely that homologs will be found to contribute to Pi acquisition by other virulent species. However, due to the ability of NptA and PitA homologs to function as Pi efflux pumps, the specific role of each system to the lifestyle of any given bacterium will need to be directly evaluated.
As the efficacy of current antibiotic therapies continues to decrease, infection has once again become a significant threat to human health. As both over- and underaccumulation of phosphate are detrimental to bacteria (21, 44), phosphate homeostasis represents a regulatory network primed for therapeutic disruption. Thus, continued investigations into this aspect of staphylococcal physiology will not only expand our understanding of how bacteria obtain phosphate and control cellular levels of this essential nutrient but also provide critical insight that may facilitate the development of new approaches for combating infection.
MATERIALS AND METHODS
Ethics statement.
All experiments involving animals were approved by the Institutional Animal Care and Use Committee of the University of Illinois at Urbana-Champaign (IACUC license number 15059) and performed according to NIH guidelines, the Animal Welfare Act, and U.S. federal law.
Bioinformatics analysis of distribution of inorganic phosphate transporter loci.
Inorganic phosphate transporters (PstSCAB, PitA, and NptA) and associated phosphate regulatory proteins (PhoU and PhoBR) of interest were identified from the literature. An initial BLASTP search using default parameters was completed with characterized representatives against the S. aureus strain Newman genome sequence to identify possible homologs. A detailed analysis was carried out using genomes retrieved from GenBank for ∼9,000 staphylococcal genomes, representing 38 species from NCBI and several outgroups (see Data Set S1 in the supplemental material). Predicted coding sequences were searched using HMMRSCAN (--cut_tc) with TIGRFAM, PFAM, and custom hidden Markov models for 13 universally conserved proteins in addition to the Pi transporters (Table S2) (45, 46). Representatives were manually checked to validate ortholog predictions, and tBLASTn was used to confirm the absence of NptA using default search parameters. Universally conserved proteins were individually aligned with MUSCLE, concatenated, and used to generate a phylogenetic tree with RAxML on the CIPRES website using default parameters (47, 48). The distribution and genomic context of the Pi transporters were examined and mapped to the phylogeny and aligned with MAUVE (49). Predicted gains and losses of nptA were inferred based on parsimony.
Subsequently, the Kyoto Encyclopedia of Genes and Genomes (KEGG) orthology assignments for each of the NptA gene family (KEGG accession numbers K03324 and K14683) were retrieved for all Bacteria on 17 July 2017 (50). The results were compiled for one strain of each named species and counted to provide the average number of gene copies for phyla, classes, and families of interest.
Bacterial strains and cloning.
S. aureus strain Newman and its derivatives were used for all experiments. S. aureus was routinely grown in tryptic soy broth (TSB) and on tryptic soy agar (TSA) plates, while E. coli was routinely cultivated in Luria broth (LB) and on Luria agar plates. Both species were grown at 37°C; all strains were stored in media containing 30% glycerol at −80°C. As needed to maintain plasmids, 100 μg/ml of ampicillin or 10 μg/ml of chloramphenicol was added to the growth medium. Anhydrotetracycline was added at 200 ng/ml for gene induction where indicated.
S. aureus ΔpstSCAB, ΔnptA, and ΔpstSCAB ΔnptA mutants were generated by amplifying the 5′ and 3′ flanking regions (∼1 kb up- and downstream) of pstSCAB or nptA using the indicated primers (Table S3). Fragments were cloned into the pKOR1 knockout vector (51) via site-specific recombination using the Gateway BP Clonase II enzyme mix (Thermo Fisher Scientific). The deletions were constructed via allelic exchange as previously described (51). The S. aureus USA300 (JE2) pitA::erm allele was obtained from the Nebraska Transposon Mutant Library (52) and was transduced via Phi85 phage into Newman, ΔpstSCAB, and ΔnptA backgrounds. All mutant strains were confirmed to be hemolytic when grown on TSA blood agar plates. For complementation studies, pstSCAB and nptA were cloned into the pOS1 vector (53) under the control of their native promoters using the indicated primers (Table S3). pitA was cloned under the control of an anhydrotetracycline-inducible promoter into the pRMC2 vector (54) using the indicated primers (Table S3). To create the reporter constructs, the promoters of each transporter were cloned into the pAH5 vector (55) via the indicated primers (Table S3). All PCR-generated constructs were verified by sequencing.
Growth medium, phosphate growth assays, and expression analysis.
Phosphate-free M9-based medium (PFM9) was based on a chemically defined medium reported by Richardson et al. (29). PFM9 salts consisted of 104 mM NaCl, 19 mM NH4Cl, 22 mM KCl, 12.4 mM Tris base, and a 70 mM concentration of either morpholinepropanesulfonic acid (MOPS; for pH 6.4), HEPES (for pH 7.4), or Tris (for pH 8.4). PFM9 salts were supplemented with trace amino acids (0.06 g/liter of alanine, 0.07 g/liter of arginine, 0.09 g/liter of aspartate, 0.02 g/liter of cysteine, 0.1 g/liter of glutamate, 0.05 g/liter of glycine, 0.03 g/liter of histidine, 0.03 g/liter of isoleucine, 0.09 g/liter of leucine, 0.01 g/liter of lysine, 0.07 g/liter of methionine, 0.04 g/liter of phenylalanine, 0.06 g/liter of proline, 0.03 g/liter of serine, 0.03 g/liter of threonine, 0.01 g/liter of tryptophan, 0.05 g/liter of tyrosine, and 0.08 g/liter of valine), trace vitamins (0.2 μg/liter of biotin, 0.2 mg/liter of nicotinic acid, 0.2 mg/liter of pyridoxine hydrochloride, 0.2 mg/liter of thiamine hydrochloride, 0.1 mg/liter of riboflavin, and 0.6 mg/liter of calcium pantothenate), 0.5% glucose, 2 mM MgSO4, 1 mM CaCl2, 6.2 mM β-mercaptoethanol, 1 μM FeSO4, 1 μM ZnSO4, and 1 μM MnCl2 to constitute PFM9 medium. Phosphate levels in the media were adjusted using a combination of NaH2PO4 and Na2HPO4.
For phosphate limitation growth assays, bacteria were inoculated into 5 ml of TSB for 8 h and then back-diluted 1:10 into 5 ml of PFM9 plus 70 mM MOPS plus 158 μM Pi, pH 6.40, for 12 h. Overnight cultures were normalized with PFM9 plus 70 mM MOPS plus 158 μM Pi, pH 6.40, and inoculated at 1:100 into a 96-well round-bottom plate containing 100 μl/well of PFM9. Plates were incubated at 37°C with shaking at 180 rpm. Bacterial growth was monitored by measuring the optical density at 600 nm (OD600). Expression was determined by measuring the fluorescence (excitation/emission wavelengths, 505/535 nm), normalizing to the OD600, and then subtracting the relative fluorescence units (RFU) of empty vector controls.
Phosphate accumulation assays.
Bacteria were grown as for the phosphate growth assays at the indicated pH and Pi concentration and then harvested at similar optical densities (OD600 = 0.2 to 0.25). Cells were washed once and then lysed in TE buffer (10 mM Tris [pH 8.0], 1 mM EDTA) by mechanical disruption. Lysates were centrifuged to remove particulate matter. To digest any polyphosphates, lysates were diluted 1:5 into polyphosphatase reaction buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 2 mM MgCl2) and subsequently treated with yeast exopolyphosphatase for 30 min at 37°C. Pi monomers were then measured with BIOMOL Green reagent according to the manufacturer's instructions. Protein concentration was measured in untreated lysates using the Pierce bicinchoninic acid (BCA) assay kit. Recombinant His6-tagged Saccharomyces cerevisiae exopolyphosphatase was produced in E. coli and purified as previously described (56).
Animal infections.
Mouse infections were performed essentially as previously described, with minor modifications (57, 58). Briefly, the wild type and the six single and double mutant strains of S. aureus were grown in TSB for 3 h on a roller drum and then washed and resuspended in phosphate-free, carbonate-buffered saline and diluted to an approximate density of 108 CFU/ml. Nine-week-old female C57BL/6J mice were injected retro-orbitally with 107 CFU in 100 μl of buffer. The infection was allowed to proceed for 96 h before the mice were sacrificed. Livers, hearts, and kidneys were removed, the organs were homogenized, and bacterial burdens were determined by plating serial dilutions.
Supplementary Material
ACKNOWLEDGMENTS
J.L.K. acknowledges the American Society for Microbiology's 2016 Scientific Writing and Publishing Institute and its participants for excellent guidance and support in the preparation of the manuscript. We thank James Morrissey for providing the vector for producing yeast exopolyphosphatase.
This work was supported by a Basil O'Connor award from the March of Dimes and National Institutes of Health grants K22 AI104805 and R01 AI118880 to T.E.K.-F. P.H.D. is supported by an investigator award from the Roy J. Carver Charitable Trust (15-4501). This work was also supported in part by a James R. Beck Graduate Research Fellowship in Microbiology awarded to J.L.K.
The funding agencies had no role in study design, data collection and interpretation, or the decision to submit the work for publication. This work does not represent the views of the March of Dimes or National Institutes of Health.
J.L.K., J.N.R., K.P.G., P.K.P.S., P.H.D., and T.E.K.-F. performed the research. J.L.K., P.H.D., and T.E.K.-F. designed the experiments, analyzed the data, and wrote the paper.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00631-17.
REFERENCES
- 1.Noble WC, Valkenburg HA, Wolters CH. 1967. Carriage of Staphylococcus aureus in random samples of a normal population. J Hyg (Lond) 65:567–573. doi: 10.1017/S002217240004609X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kluytmans JA, Wertheim HF. 2005. Nasal carriage of Staphylococcus aureus and prevention of nosocomial infections. Infection 33:3–8. doi: 10.1007/s15010-005-4012-9. [DOI] [PubMed] [Google Scholar]
- 3.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 4.Chambers HF, Deleo FR. 2009. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mera RM, Suaya JA, Amrine-Madsen H, Hogea CS, Miller LA, Lu EP, Sahm DF, O'Hara P, Acosta CJ. 2011. Increasing role of Staphylococcus aureus and community-acquired methicillin-resistant Staphylococcus aureus infections in the United States: a 10-year trend of replacement and expansion. Microb Drug Resist 17:321–328. doi: 10.1089/mdr.2010.0193. [DOI] [PubMed] [Google Scholar]
- 6.CDC. 2013. Antibiotic resistance threats in the United States. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 7.WHO. 2014. Antimicrobial resistance: global report on surveillance. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 8.Lamarche MG, Wanner BL, Crepin S, Harel J. 2008. The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol Rev 32:461–473. doi: 10.1111/j.1574-6976.2008.00101.x. [DOI] [PubMed] [Google Scholar]
- 9.Burall LS, Harro JM, Li X, Lockatell CV, Himpsl SD, Hebel JR, Johnson DE, Mobley HL. 2004. Proteus mirabilis genes that contribute to pathogenesis of urinary tract infection: identification of 25 signature-tagged mutants attenuated at least 100-fold. Infect Immun 72:2922–2938. doi: 10.1128/IAI.72.5.2922-2938.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valdivia RH, Falkow S. 1997. Fluorescence-based isolation of bacterial genes expressed within host cells. Science 277:2007–2011. doi: 10.1126/science.277.5334.2007. [DOI] [PubMed] [Google Scholar]
- 11.Lathem WW, Crosby SD, Miller VL, Goldman WE. 2005. Progression of primary pneumonic plague: a mouse model of infection, pathology, and bacterial transcriptional activity. Proc Natl Acad Sci U S A 102:17786–17791. doi: 10.1073/pnas.0506840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darwin AJ, Miller VL. 1999. Identification of Yersinia enterocolitica genes affecting survival in an animal host using signature-tagged transposon mutagenesis. Mol Microbiol 32:51–62. doi: 10.1046/j.1365-2958.1999.01324.x. [DOI] [PubMed] [Google Scholar]
- 13.Merrell DS, Hava DL, Camilli A. 2002. Identification of novel factors involved in colonization and acid tolerance of Vibrio cholerae. Mol Microbiol 43:1471–1491. doi: 10.1046/j.1365-2958.2002.02857.x. [DOI] [PubMed] [Google Scholar]
- 14.Peirs P, Lefevre P, Boarbi S, Wang XM, Denis O, Braibant M, Pethe K, Locht C, Huygen K, Content J. 2005. Mycobacterium tuberculosis with disruption in genes encoding the phosphate binding proteins PstS1 and PstS2 is deficient in phosphate uptake and demonstrates reduced in vivo virulence. Infect Immun 73:1898–1902. doi: 10.1128/IAI.73.3.1898-1902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hava DL, Camilli A. 2002. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol Microbiol 45:1389–1406. doi: 10.1046/j.1365-2958.2002.03106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg H, Gerdes RG, Chegwidden K. 1977. Two systems for the uptake of phosphate in Escherichia coli. J Bacteriol 131:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi Y, Kobayashi Y, Hulett FM. 1997. The pst operon of Bacillus subtilis has a phosphate-regulated promoter and is involved in phosphate transport but not in regulation of the pho regulon. J Bacteriol 179:2534–2539. doi: 10.1128/jb.179.8.2534-2539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willsky GR, Malamy MH. 1980. Characterization of two genetically separable inorganic phosphate transport systems in Escherichia coli. J Bacteriol 144:356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lebens M, Lundquist P, Soderlund L, Todorovic M, Carlin NI. 2002. The nptA gene of Vibrio cholerae encodes a functional sodium-dependent phosphate cotransporter homologous to the type II cotransporters of eukaryotes. J Bacteriol 184:4466–4474. doi: 10.1128/JB.184.16.4466-4474.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng JJ, Sinha D, Wayne KJ, Winkler ME. 2016. Physiological roles of the dual phosphate transporter systems in low and high phosphate conditions and in capsule maintenance of Streptococcus pneumoniae D39. Front Cell Infect Microbiol 6:63. doi: 10.3389/fcimb.2016.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wanner BL. 1996. Phosphorus assimilation and control of the phosphate regulon, p 1357–1381. In Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (ed), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed, vol 1 ASM Press, Washington, DC. [Google Scholar]
- 22.Rao NN, Torriani A. 1990. Molecular aspects of phosphate transport in Escherichia coli. Mol Microbiol 4:1083–1090. doi: 10.1111/j.1365-2958.1990.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 23.Harris RM, Webb DC, Howitt SM, Cox GB. 2001. Characterization of PitA and PitB from Escherichia coli. J Bacteriol 183:5008–5014. doi: 10.1128/JB.183.17.5008-5014.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allenby NE, O'Connor N, Pragai Z, Carter NM, Miethke M, Engelmann S, Hecker M, Wipat A, Ward AC, Harwood CR. 2004. Post-transcriptional regulation of the Bacillus subtilis pst operon encoding a phosphate-specific ABC transporter. Microbiology 150:2619–2628. doi: 10.1099/mic.0.27126-0. [DOI] [PubMed] [Google Scholar]
- 25.van Veen HW, Abee T, Kortstee GJ, Konings WN, Zehnder AJ. 1994. Translocation of metal phosphate via the phosphate inorganic transport system of Escherichia coli. Biochemistry 33:1766–1770. doi: 10.1021/bi00173a020. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh YJ, Wanner BL. 2010. Global regulation by the seven-component Pi signaling system. Curr Opin Microbiol 13:198–203. doi: 10.1016/j.mib.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werner A, Kinne RK. 2001. Evolution of the Na-P(i) cotransport systems. Am J Physiol Regul Integr Comp Physiol 280:R301–R312. [DOI] [PubMed] [Google Scholar]
- 28.Busch AE, Biber J, Murer H, Lang F. 1996. Electrophysiological insights of type I and II Na/Pi transporters. Kidney Int 49:986–987. doi: 10.1038/ki.1996.140. [DOI] [PubMed] [Google Scholar]
- 29.Richardson AR, Dunman PM, Fang FC. 2006. The nitrosative stress response of Staphylococcus aureus is required for resistance to innate immunity. Mol Microbiol 61:927–939. doi: 10.1111/j.1365-2958.2006.05290.x. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg H, Russell LM, Jacomb PA, Chegwidden K. 1982. Phosphate exchange in the pit transport system in Escherichia coli. J Bacteriol 149:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenstein R, Gotz F. 2013. What distinguishes highly pathogenic staphylococci from medium- and non-pathogenic? Curr Top Microbiol Immunol 358:33–89. [DOI] [PubMed] [Google Scholar]
- 32.Tischler AD, Leistikow RL, Ramakrishnan P, Voskuil MI, McKinney JD. 2016. Mycobacterium tuberculosis phosphate uptake system component PstA2 is not required for gene regulation or virulence. PLoS One 11:e0161467. doi: 10.1371/journal.pone.0161467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sprague GF Jr, Bell RM, Cronan JE Jr. 1975. A mutant of Escherichia coli auxotrophic for organic phosphates: evidence for two defects in inorganic phosphate transport. Mol Gen Genet 143:71–77. doi: 10.1007/BF00269422. [DOI] [PubMed] [Google Scholar]
- 34.Rao NN, Roberts MF, Torriani A, Yashphe J. 1993. Effect of glpT and glpD mutations on expression of the phoA gene in Escherichia coli. J Bacteriol 175:74–79. doi: 10.1128/jb.175.1.74-79.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kornberg A, Rao NN, Ault-Riche D. 1999. Inorganic polyphosphate: a molecule of many functions. Annu Rev Biochem 68:89–125. doi: 10.1146/annurev.biochem.68.1.89. [DOI] [PubMed] [Google Scholar]
- 36.Rao NN, Liu S, Kornberg A. 1998. Inorganic polyphosphate in Escherichia coli: the phosphate regulon and the stringent response. J Bacteriol 180:2186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tischler AD, Leistikow RL, Kirksey MA, Voskuil MI, McKinney JD. 2013. Mycobacterium tuberculosis requires phosphate-responsive gene regulation to resist host immunity. Infect Immun 81:317–328. doi: 10.1128/IAI.01136-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takemaru K, Mizuno M, Kobayashi Y. 1996. A Bacillus subtilis gene cluster similar to the Escherichia coli phosphate-specific transport (pst) operon: evidence for a tandemly arranged pstB gene. Microbiology 142(Part 8):2017–2020. [DOI] [PubMed] [Google Scholar]
- 39.Hartmann CM, Wagner CA, Busch AE, Markovich D, Biber J, Lang F, Murer H. 1995. Transport characteristics of a murine renal Na/Pi-cotransporter. Pflugers Arch 430:830–836. doi: 10.1007/BF00386183. [DOI] [PubMed] [Google Scholar]
- 40.Wilde AD, Snyder DJ, Putnam NE, Valentino MD, Hammer ND, Lonergan ZR, Hinger SA, Aysanoa EE, Blanchard C, Dunman PM, Wasserman GA, Chen J, Shopsin B, Gilmore MS, Skaar EP, Cassat JE. 2015. Bacterial hypoxic responses revealed as critical determinants of the host-pathogen outcome by TnSeq analysis of Staphylococcus aureus invasive infection. PLoS Pathog 11:e1005341. doi: 10.1371/journal.ppat.1005341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Motomura K, Hirota R, Ohnaka N, Okada M, Ikeda T, Morohoshi T, Ohtake H, Kuroda A. 2011. Overproduction of YjbB reduces the level of polyphosphate in Escherichia coli: a hypothetical role of YjbB in phosphate export and polyphosphate accumulation. FEMS Microbiol Lett 320:25–32. doi: 10.1111/j.1574-6968.2011.02285.x. [DOI] [PubMed] [Google Scholar]
- 42.Grillo-Puertas M, Schurig-Briccio LA, Rodriguez-Montelongo L, Rintoul MR, Rapisarda VA. 2014. Copper tolerance mediated by polyphosphate degradation and low-affinity inorganic phosphate transport system in Escherichia coli. BMC Microbiol 14:72. doi: 10.1186/1471-2180-14-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarthy S, Ai C, Wheaton G, Tevatia R, Eckrich V, Kelly R, Blum P. 2014. Role of an archaeal PitA transporter in the copper and arsenic resistance of Metallosphaera sedula, an extreme thermoacidophile. J Bacteriol 196:3562–3570. doi: 10.1128/JB.01707-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morohoshi T, Maruo T, Shirai Y, Kato J, Ikeda T, Takiguchi N, Ohtake H, Kuroda A. 2002. Accumulation of inorganic polyphosphate in phoU mutants of Escherichia coli and Synechocystis sp. strain PCC6803. Appl Environ Microbiol 68:4107–4110. doi: 10.1128/AEM.68.8.4107-4110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eddy SR. 2011. Accelerated profile HMM searches. PLoS Comput Biol 7:e1002195. doi: 10.1371/journal.pcbi.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haft DH, Selengut JD, White O. 2003. The TIGRFAMs database of protein families. Nucleic Acids Res 31:371–373. doi: 10.1093/nar/gkg128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, 14 November 2010. [Google Scholar]
- 49.Darling AE, Mau B, Perna NT. 2010. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One 5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanehisa M, Goto S. 2000. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bae T, Schneewind O. 2006. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55:58–63. doi: 10.1016/j.plasmid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4:e00537-12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneewind O, Mihaylova-Petkov D, Model P. 1993. Cell wall sorting signals in surface proteins of gram-positive bacteria. EMBO J 12:4803–4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corrigan RM, Foster TJ. 2009. An improved tetracycline-inducible expression vector for Staphylococcus aureus. Plasmid 61:126–129. doi: 10.1016/j.plasmid.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Malone CL, Boles BR, Lauderdale KJ, Thoendel M, Kavanaugh JS, Horswill AR. 2009. Fluorescent reporters for Staphylococcus aureus. J Microbiol Methods 77:251–260. doi: 10.1016/j.mimet.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi SH, Smith SA, Morrissey JH. 2011. Polyphosphate is a cofactor for the activation of factor XI by thrombin. Blood 118:6963–6970. doi: 10.1182/blood-2011-07-368811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Radin JN, Kelliher JL, Parraga Solorzano PK, Kehl-Fie TE. 2016. The two-component system ArlRS and alterations in metabolism enable Staphylococcus aureus to resist calprotectin-induced manganese starvation. PLoS Pathog 12:e1006040. doi: 10.1371/journal.ppat.1006040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garcia YM, Barwinska-Sendra A, Tarrant E, Skaar EP, Waldron KJ, Kehl-Fie TE. 2017. A superoxide dismutase capable of functioning with iron or manganese promotes the resistance of Staphylococcus aureus to calprotectin and nutritional immunity. PLoS Pathog 13:e1006125. doi: 10.1371/journal.ppat.1006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.