ABSTRACT
Challenges with the production and suboptimal immunogenicity of malaria vaccine candidates have slowed the development of a Plasmodium falciparum multiantigen vaccine. Attempting to resolve these issues, we focused on the use of highly immunogenic merozoite surface protein 8 (MSP8) as a vaccine carrier protein. Previously, we showed that a genetic fusion of the C-terminal 19-kDa fragment of merozoite surface protein 1 (MSP119) to P. falciparum MSP8 (PfMSP8) facilitated antigen production and folding and the induction of neutralizing antibodies to conformational B cell epitopes of MSP119. Here, using the PfMSP1/8 construct, we further optimized the recombinant PfMSP8 (rPfMSP8) carrier by the introduction of two cysteine-to-serine substitutions (CΔS) to improve the yield of the monomeric product. We then sought to test the broad applicability of this approach using the transmission-blocking vaccine candidate Pfs25. The production of rPfs25-based vaccines has presented challenges. Antibodies directed against the four highly constrained epidermal growth factor (EGF)-like domains of Pfs25 block sexual-stage development in mosquitoes. The sequence encoding mature Pfs25 was codon harmonized for expression in Escherichia coli. We produced a rPfs25-PfMSP8 fusion protein [rPfs25/8(CΔS)] as well as unfused, mature rPfs25. rPfs25 was purified with a modest yield but required the incorporation of refolding protocols to obtain a proper conformation. In comparison, chimeric rPfs25/8(CΔS) was expressed and easily purified, with the Pfs25 domain bearing the proper conformation without renaturation. Both antigens were immunogenic in rabbits, inducing IgG that bound native Pfs25 and exhibited potent transmission-reducing activity. These data further demonstrate the utility of PfMSP8 as a parasite-specific carrier protein to enhance the production of complex malaria vaccine targets.
KEYWORDS: MSP8 carrier protein, malaria, subunit vaccines, transmission blocking
INTRODUCTION
While malaria remains a major global health concern, significant progress has been made in the control and treatment of the disease such that recent reports estimate a 22% decrease in the number of cases and a 50% decline in mortality rates between the years 2000 and 2015 (1). This progress has been attributed largely to the implementation of various interventions, including (i) the deployment of insecticide-treated bed nets and indoor spraying of residual insecticides, (ii) the intermittent administration of preventative treatment, (iii) the development of rapid diagnostic tests, and (iv) the routine use of artemisinin-based combination therapy. It is widely believed that the development of a highly efficacious malaria vaccine will greatly enhance these ongoing efforts to control and eventually eliminate malaria.
The most advanced malaria vaccine, RTS,S, is a subunit-based, preerythrocytic-stage vaccine intended to prevent infection and/or eliminate liver-stage parasites by inducing protective immune responses against the circumsporozoite protein (2). Efficacy in a large phase III trial in children was suboptimal, as initial protection ranged between 30% and 50% but waned over time (3–5). The limited success with this single-antigen vaccine may reflect the need to develop multiantigen formulations targeting multiple stages of the parasite life cycle. In fact, the natural immunity that develops in individuals living in areas of endemicity that prevents clinical disease may be a result of concurrent responses generated against a wide array of antigens. This concept is central in the development of whole-parasite-based vaccines, including radiation-attenuated (6–8) and genetically attenuated (8–11) sporozoite vaccines, infection-treatment preerythrocytic-stage vaccines (12–14), and chemically inactivated whole blood-stage vaccines (15, 16). Challenges facing the advancement of these whole-organism-based vaccines include safety, standardization, and scalability.
The need to target multiple parasite antigens is not reflected in the design and formulation of many current subunit malaria vaccines. In part, this has been due to difficulties in the production of candidate antigens in adequate quantities and the appropriate conformation and/or poor immunogenicity of single antigen components. As we move forward with the testing of multiantigen formulations, issues with respect to immunogenicity are further complicated due to antigenic competition. To improve immunogenicity, much effort has focused on the development of appropriate adjuvants and/or novel vaccine platforms for delivery, with significant emphasis on viral vectors and particle-based approaches in conjunction with heterologous prime-boost regimens (reviewed in references 17–21). We have had some success with the development of merozoite surface protein 8 (MSP8) as a malaria-specific carrier protein to enhance the production, folding, and immunogenicity of neutralizing B cell epitopes in the 19-kDa C-terminal domain of merozoite surface protein 1 (MSP119) (22, 23).
It has been well documented that antibodies directed against the conformational epitopes within the double epidermal growth factor (EGF)-like domains of MSP119 are highly protective in rodent and nonhuman primate models of malaria (24–31). It is therefore essential that recombinant forms of this vaccine antigen are properly folded in order to induce protective humoral responses. Although there is limited sequence variation in MSP119 of Plasmodium falciparum, the induction of sufficiently high titers of protective antibodies against this domain with larger MSP142-based constructs has not been adequate (32–38). We were able to overcome issues associated with production by fusing MSP119 to the N terminus of MSP8 (22, 23). MSP8 is a highly immunogenic, well-conserved blood-stage antigen that is readily expressed and purified by using Escherichia coli expression systems (39–41). The resulting chimeric antigens, Plasmodium yoelii MSP1/8 (PyMSP1/8) and P. falciparum MSP1/8 (PfMSP1/8), were produced in high yields and exhibited proper folding of the MSP119 domain (22, 23). Importantly, studies in mice, rabbits, and nonhuman primates showed that antibodies elicited by immunization with MSP1/8 chimeras were highly functional, as demonstrated by protection from lethal P. yoelii challenge (recombinant PyMSP1/8 [rPyMSP1/8]) in vivo (22) and the ability to inhibit P. falciparum blood-stage growth in vitro (rPfMSP1/8) (23, 42).
We sought to test the hypothesis that MSP8 is broadly useful as a malaria-specific carrier protein to facilitate the production and/or enhance the immunogenicity of targeted, protective B cell epitopes. Pfs25 is a 25-kDa surface protein of P. falciparum composed of an N-terminal signal sequence followed by four EGF-like domains and a C-terminal glycosylphosphatidylinositol (GPI) anchor (43). Its expression is first detected upon the activation of female gametes within the mosquito midgut and continues through the subsequent zygote and ookinete stages of development. It is well established that vaccine-induced antibodies generated against Pfs25 exhibit potent transmission-blocking activity. Importantly, these antibodies recognize conformational epitopes within the four EGF-like domains of the native protein (44–47).
Recombinant production of properly folded Pfs25 has been attempted by using various expression hosts, including yeast (Saccharomyces cerevisiae [48] and Pichia pastoris [49–54]), algae (55, 56), and plants (57–59), with various successes in terms of yield and scalability. Initial efforts to express and purify recombinant Pfs25 from E. coli were unsuccessful (48, 53). Recent progress with this system through the combined use of codon harmonization strategies and the optimization of purification procedures that resulted in the production of a high-quality recombinant antigen capable of eliciting potent, transmission-blocking antibodies has been reported (60). Several approaches to enhance the immunogenicity of Pfs25 produced in Pichia have also been tested, including chemical conjugation to Pseudomonas aeruginosa exoprotein A (EPA) (51, 61–63), tetanus toxoid (63), or the outer membrane protein complex (OMPC) of Neisseria meningitidis (52) as well as the expression as a fusion protein linked to the oligomerization domain of the chicken complement inhibitor C4b-binding protein (C4bp) (54). Here, we began by further optimizing the PfMSP8 expression construct, introducing modifications at two cysteine residues to minimize the formation of intrachain disulfide bonds. The modified PfMSP8(CΔS) construct was subsequently evaluated for its ability to function as a malaria-specific carrier protein to enhance the production and/or immunogenicity of the structurally complex Pfs25 transmission-blocking vaccine candidate.
RESULTS
Targeted modification of the PfMSP8 carrier protein improves yields of monomeric rPfMSP1/8.
Previous studies involving the expression and purification of rPfMSP1/8 showed that the final purified product contained monomeric and various amounts of dimeric and higher-molecular-mass material, which was resolved to a single 58-kDa antigen under reducing conditions (23). Similar results were observed with purified preparations of the rPfMSP8(ΔAsn/Asp) carrier alone (41), indicating that the intermolecular disulfide bonds responsible for aggregation involved cysteines located in the rPfMSP8(ΔAsn/Asp) carrier. This recombinant protein contains 14 cysteine residues, 12 of which are located within the two C-terminal EGF-like domains. The two remaining and potentially unpaired cysteine residues lie within the N-terminal half of the protein.
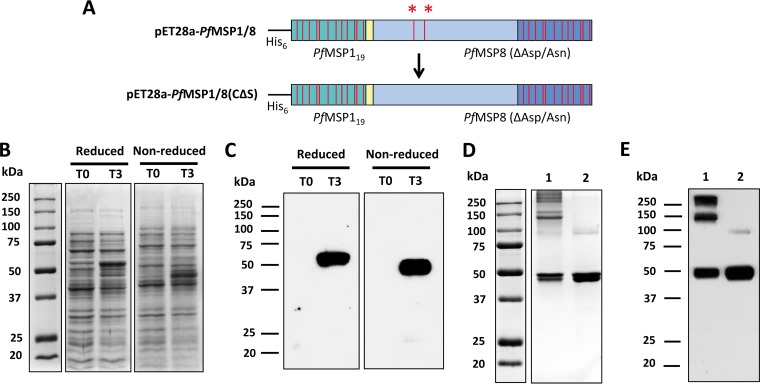
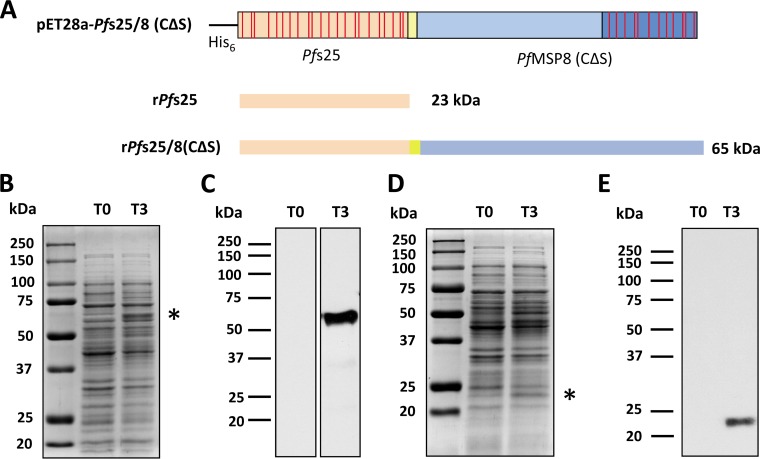
To decrease aggregation and increase the yield of monomeric PfMSP1/8, the two N-terminal cysteine codons (TGC) were replaced with serine codons (TCC) by site-directed mutagenesis (Fig. 1A). The sequence of the pET28-PfMSP1/8(CΔS) insert was verified, and the protein was expressed in E. coli SHuffle T7 Express lysY cells upon induction with isopropyl-β-d-thiogalactopyranoside (IPTG). Lysates of bacterial cultures sampled before (0-h postinduction time point [T0]) and after (T3) induction were assessed by SDS-PAGE (Fig. 1B). Coomassie-blue stained gels showed a robust induction of rPfMSP1/8(CΔS) expression, with a prominent band of ∼58 kDa being observed under reducing conditions (Fig. 1B, left) and a faster-migrating band of ∼50 kDa under nonreducing conditions (Fig. 1B, right), which is suggestive of the folding of the EGF-like domains. The identity of rPfMSP1/8(CΔS) was confirmed by an immunoblot assay using previously generated polyclonal rabbit sera raised against the rPfMSP1/8 chimeric antigen (Fig. 1C) under reducing (left) and nonreducing (right) conditions.
FIG 1.
Targeted amino acid modifications within the PfMSP8 carrier domain of rPfMSP1/8 improve the yield of monomeric rPfMSP1/8(CΔS). (A) Schematic of the original pET28a-PfMSP1/8 construct (top) depicting two cysteine residues (red asterisks) within the PfMSP8 domain that were mutated to serine residues via site-directed mutagenesis, resulting in the construct pET28a-PfMSP1/8(CΔS) (bottom). (B) Lysates of E. coli transformed with pET28a-PfMSP1/8(CΔS), harvested before (T0) or 3 h after (T3) induction, were separated by SDS-PAGE (10% gel) under both reducing and nonreducing conditions, followed by Coomassie blue staining. (C) Immunoblot analysis of T0 and T3 lysates probed with rabbit anti-PfMSP1/8 IgG. (D and E) Purified rPfMSP1/8 (original) (lane 1) and rPfMSP1/8(CΔS) (modified) (lane 2) were separated by SDS-PAGE (10% gel) under nonreducing conditions, followed by Coomassie blue staining (3 μg/lane) (D) or immunoblot analysis (50 ng/lane) (E) of samples probed with rabbit anti-PfMSP1/8 IgG.
rPfMSP1/8(CΔS) was purified as described previously (23) under nondenaturing conditions using nickel chelate affinity chromatography, with a final yield of ∼4 mg/g (wet weight) bacterial cells. The purity and quality of the final purified product were assessed by SDS-PAGE followed by Coomassie blue staining (Fig. 1D) or immunoblot analysis using rabbit anti-rPfMSP1/8 IgG (Fig. 1E). As described above, the original rPfMSP1/8 antigen contained a mixture of monomeric and higher-molecular-mass species under nonreducing conditions (Fig. 1D and E, lane 1). However, the final purified preparation of the modified rPfMSP1/8(CΔS) antigen showed a significant decrease in aggregation and a corresponding increase in the amount of the monomeric product (Fig. 1D and E, lane 2).
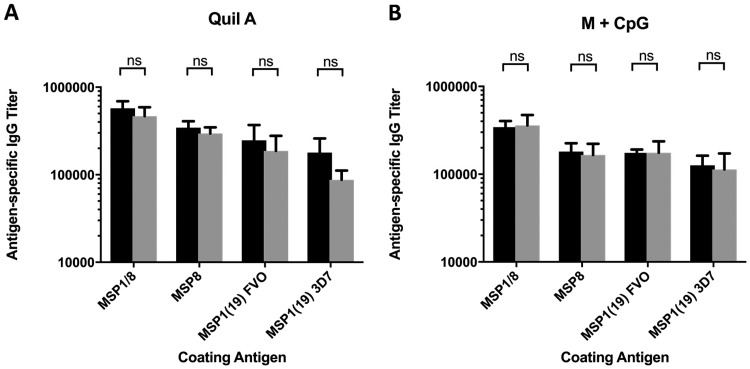
To confirm that Cys-to-Ser mutations did not affect the immunogenicity of the chimeric antigen, comparative studies were conducted in mice. Groups of CB6F1/J mice were immunized with rPfMSP1/8 or rPfMSP1/8(CΔS) adjuvanted with either Quil A or Montanide ISA 720 plus a CpG oligodeoxynucleotide (ODN). Sera collected following the third immunizations were assessed for antigen-specific antibodies against rPfMSP1/8, rPfMSP8(ΔAsn/Asp), recombinant glutathione S-transferase (rGST)-PfMSP119(FVO), and rGST-PfMSP119(3D7) by an enzyme-linked immunosorbent assay (ELISA). As shown in Fig. 2, immunization with either the rPfMSP1/8 or rPfMSP1/8(CΔS) antigen induced high and comparable antibody responses against all component domains when formulated with either adjuvant. In particular, both antigens induced comparable responses to homologous (FVO) and heterologous (3D7) PfMSP119 domains that bear merozoite-neutralizing epitopes. Combined, these data indicate that the two Cys-to-Ser substitutions in the PfMSP8 carrier protein increased the yield of monomeric rPfMSP1/8 without any significant alteration in immunogenicity.
FIG 2.
Modification of the PfMSP8 domain does not affect the immunogenicity of rPfMSP1/8(CΔS) relative to the original rPfMSP1/8 antigen. CB6F1/J mice (5/group) were immunized three times with 10 μg/dose of rPfMSP1/8 (black bars) or rPfMSP1/8(CΔS) (gray bars) formulated with Quil A (A) or Montanide plus CpG ODN (B) as an adjuvant. Sera collected following the final immunization were analyzed for antigen-specific IgG titers (means ± standard deviations) by an ELISA using plates coated with rPfMSP1/8, rPfMSP8, GST-PfMSP119(FVO), or GST-PfMSP119(3D7) antigens. ns, nonsignificant (P > 0.05).
PfMSP8 is expressed during sexual-stage development but is not a target of transmission-reducing antibodies.
The expression and potential role(s) of MSP8 through blood-stage development have been investigated and characterized for multiple species of plasmodial parasites (39–41, 64, 65). MSP8 is a target of neutralizing antibody in the P. yoelii model (39, 66), and the EGF domains of MSP8 can functionally replace those of MSP1 (67). However, anti-PfMSP8 antibodies do not inhibit the in vitro growth of P. falciparum blood-stage parasites (41), and MSP8 is nonessential for blood-stage growth (64). Transcriptomic and proteomic data indicate that PfMSP8 is also expressed in gametocytes. Based on these observations and the intention to use PfMSP8 as a fusion partner for the sexual-stage antigen Pfs25, the expression profile of PfMSP8 during sexual-stage development and the transmission-reducing activity (TRA) of PfMSP8-specific IgG were assessed.
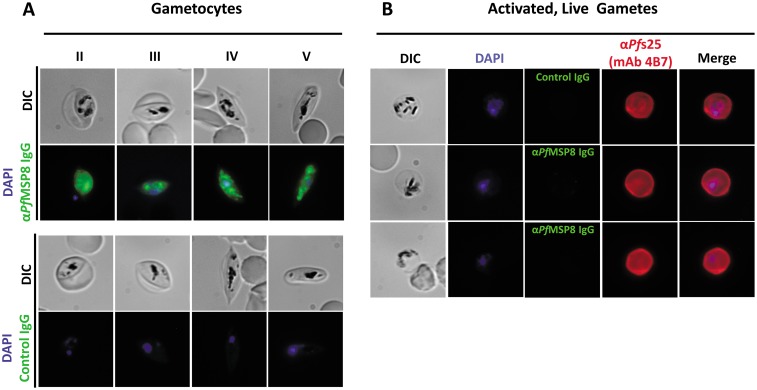
Gametocytogenesis was induced in P. falciparum parasites grown in culture as previously described (68). Following the enrichment of sexual stages, parasites were harvested and fixed for subsequent immunofluorescence assays. Fixed parasite preparations were probed with purified rabbit anti-rPfMSP8 IgG or control, nonimmune IgG. As shown in Fig. 3A, PfMSP8 expression was detected in morphologically distinct gametocyte stages (stages II to V). Fluorescence was detected throughout gametocyte development, with stronger internal and punctate staining being noted. No fluorescence staining was detected on gametocytes probed with control IgG.
FIG 3.
PfMSP8 is expressed during P. falciparum gametocyte development but is absent on the surface of activated macrogametes. (A) Detection of PfMSP8 expression in fixed and permeabilized P. falciparum gametocytes (stages II to V) by an immunofluorescence assay with rabbit anti-rPfMSP8 IgG or control IgG followed by FITC-conjugated secondary antibodies. DAPI was used to stain parasite DNA. (B) Analysis of PfMSP8 expression on activated, live P. falciparum macrogametes by an immunofluorescence assay, as described above, with rabbit anti-rPfMSP8 IgG. Samples were costained with MAb 4B7, which is specific for Pfs25 (MAb 4B7), followed by TRITC-conjugated secondary IgG. DIC, differential interference contrast.
To evaluate the expression of PfMSP8 on macrogametes, late-stage P. falciparum gametocytes were enriched, activated with xanthurenic acid, and placed at 27°C. At 24 h postactivation, live macrogametes were probed with purified rabbit anti-rPfMSP8 IgG or control, nonimmune IgG. As a positive control, macrogametes were costained with mouse monoclonal antibody (MAb) 4B7, which specifically recognizes Pfs25. Consistent with data from previous reports, macrogamete surface expression of Pfs25 was readily detected by MAb 4B7, as evidenced by the strong halo staining around the perimeter of the parasites (Fig. 3B). In comparison, the expression of PfMSP8 was not detected on the surface of live, mature macrogametes. Preparations of fixed and permeabilized macrogametes demonstrated low levels of internal PfMSP8 expression (data not shown).
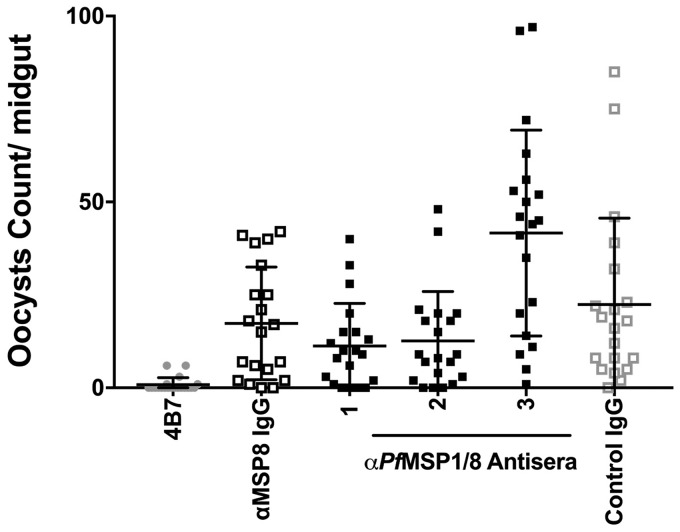
Considering the level of PfMSP8 expression in gametocytes, the potential for PfMSP8 to serve as a target of transmission-reducing antibodies was assessed. IgGs purified from high-titer rabbit antisera induced by immunization with rPfMSP8 (n = 1) (41) or rPfMSP1/8 (n = 3) (23) were tested for their ability to prevent sexual-stage development within the mosquito vector via a standard membrane feeding assay (SMFA). As shown in Fig. 4, rabbit antisera containing high titers of anti-PfMSP8 antibodies did not affect oocyst development in mosquitoes (P ≥ 0.2) (see Data Set S1 in the supplemental material). While PfMSP8 is expressed in P. falciparum gametocytes, the data indicate that PfMSP8 is not a target of transmission-reducing antibodies.
FIG 4.
PfMSP8 is not a target of transmission-reducing antibodies. The functional activity of IgG purified from rabbit antisera raised against rPfMSP8 (n = 1) or rPfMSP1/8 (n = 3) was assessed in a P. falciparum standard membrane feeding assay at a concentration of 3.75 mg/ml. The graph depicts the average number of oocytes formed in the midgut of 20 mosquitoes per IgG sample. Positive and negative controls were MAb 4B7 and adjuvant control rabbit IgG, respectively.
To facilitate the construction of expression plasmids to produce other vaccine candidates of interest fused to rPfMSP8 as a carrier protein, a modified pET-28-based vector was constructed. The PfMSP119-coding sequence of the pET28-PfMSP1/8(CΔS) plasmid was removed by restriction digestion and replaced with a short multiple-cloning sequence (MCS) containing three restriction sites (SpeI, AflII, and AvrII) (see Fig. S1A in the supplemental material). The sequence of the pET28-MCS-PfMSP8(CΔS) construct was verified, and nonfused rPfMSP8(CΔS) alone was then expressed and purified. Purity and conformation were confirmed by SDS-PAGE under reducing and nonreducing conditions, followed by Coomassie blue staining (Fig. S1B) and an immunoblot assay (Fig. S1C). The overall yield of purified rPfMSP8(CΔS) from the modified construct was comparable to that reported previously for the original rPfMSP8(ΔAsn/Asp) fusion partner (41) but with a higher proportion of the monomeric product.
Expression and purification of unfused rPfs25 and chimeric rPfs25/8(CΔS).
The native gene sequence encoding mature Pfs25 lacking the N-terminal signal and the C-terminal anchor sequences was codon harmonized for expression in E. coli, according to previously reported algorithms (69). As depicted in Fig. 5A, the codon-harmonized synthetic pfs25 gene was subcloned into pET28-MCS-PfMSP8(CΔS) in two configurations. In the first configuration, the pfs25 gene fragment was joined in frame with the sequence encoding the rPfMSP8(CΔS) carrier for the expression of an ∼65-kDa chimeric Pfs25/8(CΔS) antigen. Alternatively, the pfs25 gene fragment was inserted into the expression plasmid with two stop codons incorporated at the 3′ end of the Pfs25 sequence for the expression of ∼23-kDa unfused, mature Pfs25. The sequence of each construct was verified and transformed into E. coli SHuffle T7 Express lysY cells for expression. Lysates of bacterial cultures sampled before (T0) and after (T3) induction were analyzed by SDS-PAGE. Coomassie blue-stained gels demonstrated a modest induction of chimeric rPfs25/8(CΔS) (Fig. 5B) and, to a lesser degree, unfused rPfs25 (Fig. 5D). The induction of full-length rPfs25/8(CΔS) and rPfs25 was confirmed by an immunoblot assay using rabbit anti-PfMSP8 IgG or mouse anti-His MAb, respectively (Fig. 5C and E).
FIG 5.
Expression of chimeric rPfs25/8(CΔS) and unfused rPfs25. (A) Schematic depiction of expression constructs for the production of rPfs25/8(CΔS) and rPfs25. Expression plasmids for chimeric rPfs25/8(CΔS) or unfused rPfs25 were transformed into E. coli SHuffle T7 Express lysY cells. (B and C) rPfs25/8(CΔS) lysates harvested before (T0) or 3 h after (T3) induction were separated by SDS-PAGE (10% gel) under reducing conditions, followed by Coomassie blue staining (B) or immunoblot analysis (C) using rabbit-anti-rPfMSP8 IgG. The asterisk highlights rPfs25/8(CΔS) at the predicted size. (D and E) The expression of unfused rPfs25 was assessed as described above, on 12% polyacrylamide gels under reducing conditions, followed by Coomassie blue staining (D) or immunoblot analysis (E) using an anti-His MAb. The asterisk highlights rPfs25 at the predicted size.
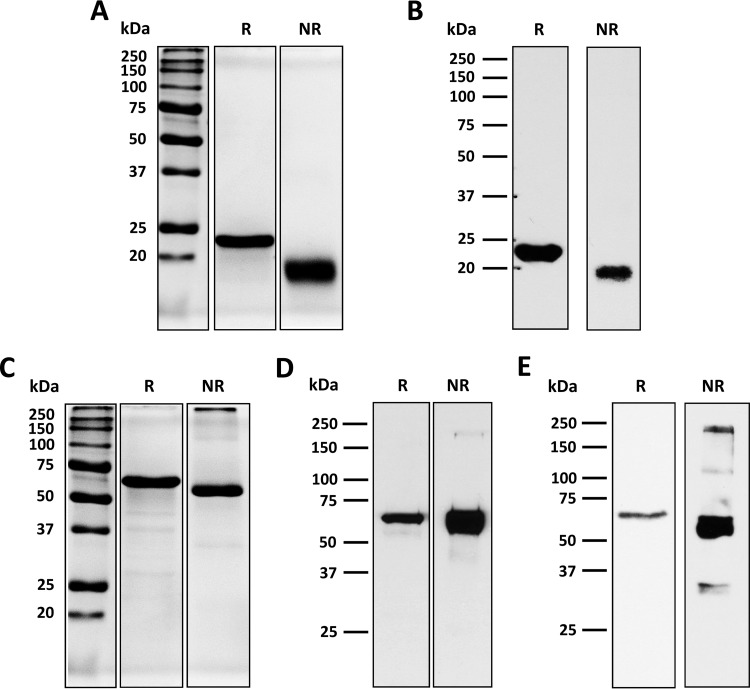
rPfs25 was solubilized from a bacterial inclusion body by resuspension in a high-pH buffer (60) containing 0.2% Sarkosyl and purified by nickel chelate affinity chromatography under nondenaturing conditions. Upon analysis, however, the purified product contained a substantial fraction of aggregated, misfolded material. Following denaturation, glutathione-catalyzed renaturation, and size exclusion chromatography, purified monomeric rPfs25 was recovered at a yield of 0.87 mg/g (wet weight) bacterial cells. rPfs25 was separated by SDS-PAGE under reducing and nonreducing conditions, followed by Coomassie blue staining and immunoblot analysis. As shown in Fig. 6A, rPfs25 migrated as a single protein band of ∼23 kDa under reducing conditions and as an ∼19-kDa product under nonreducing conditions. Immunoblot analysis confirmed the reactivity of purified rPfs25 with MAb 4B7, a Pfs25-specific transmission-blocking antibody (Fig. 6B).
FIG 6.
Purification of the rPfs25-based vaccine. (A and B) Purified rPfs25 was analyzed by SDS-PAGE (12% gel) under both reducing (R) and nonreducing (NR) conditions, followed by Coomassie blue staining (3 μg/lane) (A) or immunoblot analysis (0.2 μg/lane) (B) using anti-Pfs25 MAb 4B7. (C to E) Purified rPfs25/8(CΔS) was analyzed by SDS-PAGE (10% gel) under both reducing and nonreducing conditions, followed by Coomassie blue staining (3 μg/lane) (C) or immunoblot analysis (0.4 μg/lane) using anti-Pfs25 MAb 4B7 (D) or rabbit anti-PfMSP8 (E).
Chimeric rPfs25/8(CΔS) was similarly solubilized from a bacterial inclusion body fraction in the presence of 0.2% Sarkosyl and purified by nickel chelate affinity chromatography under nondenaturing conditions. Unlike the single rPfs25 antigen, the Pfs25 domain of purified chimeric rPfs25/8(CΔS) appeared to be properly folded and therefore did not require any denaturation/renaturation procedures. Nevertheless, size exclusion chromatography was performed to maintain consistency between protocols. The final yield of purified chimeric rPfs25/8(CΔS) was 2.5 mg/g (wet weight) bacterial cells. Purified rPfs25/8(CΔS) migrated as a predominant band of ∼65 kDa under reducing conditions, consistent with the predicted molecular mass of monomeric rPfs25/8(CΔS) (Fig. 6C). A shift in migration to a product of ∼60 kDa under nonreducing conditions was consistent with the folding of the EGF-like domains of Pfs25 and PfMSP8. Immunoblot analysis revealed similarly migrating bands that were reactive with functional anti-Pfs25 MAb 4B7 (Fig. 6D) and rabbit anti-rPfMSP8 IgG (Fig. 6E), with stronger reactivity being observed under nonreducing conditions. As such, the genetic fusion of rPf25 to the rPfMSP8 carrier promoted proper disulfide bond formation and folding and increased the product yield relative to that of unfused rPfs25.
Immunization with rPfs25 and rPfs25/8(CΔS) elicits high-titer antibodies that recognize native Pfs25.
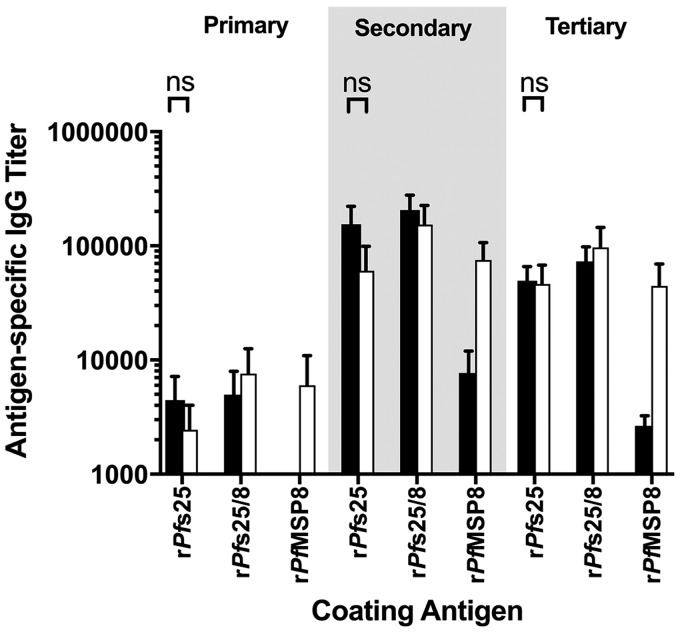
New Zealand White rabbits were immunized with rPfs25 (n = 4) or rPfs25/8(CΔS) (n = 4) formulated with Alhydrogel as an adjuvant or with the adjuvant alone (n = 1). Serum samples were collected 4 weeks following primary, secondary, and tertiary immunizations, and antibody titers against chimeric rPfs25/8(CΔS) or the individual rPfMSP8(CΔS) and rPfs25 components were determined by an ELISA. As shown in Fig. 7, primary immunization with the unfused rPfs25 antigen elicited detectible antibody titers against rPfs25 and rPfs25/8(CΔS). These responses were boosted following secondary immunization, with no additional increase in titers following a third immunization. Low but detectable titers were observed on plates coated with rPfMSP8(CΔS). This most likely reflects a response to an epitope in the common N-terminal His6 tag, as sera induced by immunization with unfused rPfs25 do not recognize native PfMSP8 by immunoblot analysis under nonreducing conditions. Titers against all three recombinant antigens were undetectable in adjuvant control serum. Similar patterns and comparable titers against rPfs25 were induced by immunization with chimeric rPfs25/8(CΔS). As expected, high titers against the rPfMSP8(CΔS) carrier were also induced. Overall, antibody responses to rPfs25 elicited by immunization with rPfs25 or chimeric rPfs25/8(CΔS) were high and comparable following the final immunization.
FIG 7.
Immunization with rPfs25 or rPfs25/8(CΔS) elicits high titers of Pfs25-specific antibodies. New Zealand White rabbits were immunized three times with rPfs25 (n = 4) (black bars), rPfs25/8(CΔS) (n = 4) (white bars), antigens formulated with Alhydrogel as an adjuvant, or the adjuvant alone. Sera collected 2 weeks following each immunization were analyzed for antigen-specific IgG titers (means ± standard deviations) by an ELISA using plates coated with rPfs25, rPfs25/8(CΔS), or rPfMSP8 antigens. The signal using adjuvant control sera was subtracted as the background. ns, nonsignificant (P > 0.05).
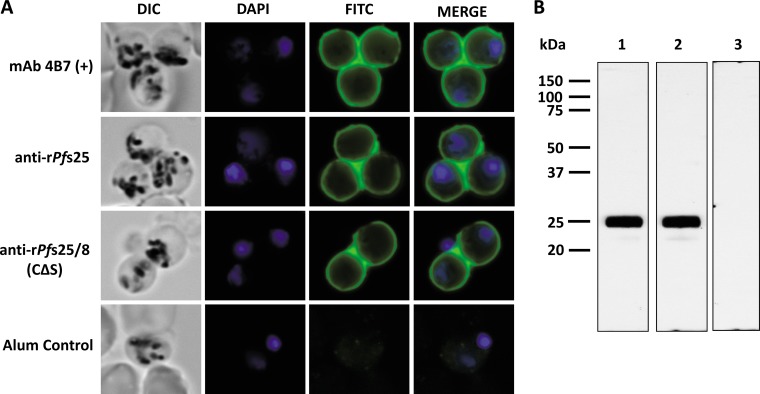
The ability of antibodies induced by immunization with rPfs25 or rPfs25/8(CΔS) to recognize native Pfs25 was evaluated by immunofluorescence and immunoblot assays. Mature, fixed P. falciparum (NF54) macrogametes were probed with pooled rabbit sera from each immunization group, obtained following the third immunization, followed by a fluorescein isothiocyanate (FITC)-conjugated secondary antibody. As shown in Fig. 8A, rabbit anti-rPfs25 and rabbit anti-rPfs25/8(CΔS) were strongly reactive with the surface of P. falciparum macrogametes. The signal and staining patterns were indistinguishable from those observed with Pfs25-specific MAb 4B7. No reactivity was detected on macrogametes probed with adjuvant control sera. The specificity of sera from each immunization group for native Pfs25 was further established by immunoblot analysis using a parasite lysate generated from purified, activated macrogametes (Fig. 8B).
FIG 8.
Immunization with rPfs25 or rPfs25/8(CΔS) elicits Pfs25-specific antibodies that recognize native Pfs25. (A) Recognition of native Pfs25 by pooled rabbit antisera generated against rPfs25, rPfs25/8(CΔS), or the adjuvant control was assessed by an immunofluorescence assay on activated, fixed macrogametes. Samples probed with MAb 4B7 were used as a positive control. Bound antibodies were detected with FITC-conjugated secondary IgG. (B) The purified macrogamete lysate was separated by SDS-PAGE (12% gels) under nonreducing conditions, followed by an immunoblot assay using pooled sera from rabbits immunized with rPfs25 (lane 1), rPfs25/8(CΔS) (lane 2), or the adjuvant alone (lane 3).
Immunization with rPfs25/8(CΔS) or rPfs25 induces potent transmission-reducing antibodies.
To assess the functionality of anti-Pfs25 antibodies, total IgG was purified from rabbits immunized with rPfs25 or rPfs25/8(CΔS), and the ability to reduce transmission to mosquitoes was evaluated with a standard membrane feeding assay. Potent transmission-reducing activity was demonstrated, as purified IgG derived from all rPfs25- and rPfs25/8(CΔS)-immunized rabbits showed 99 to 100% inhibition of sexual-stage development when tested at a concentration of 3.75 mg/ml (data not shown). To further evaluate whether there were any differences in the functional capacities of IgGs induced by the two constructs, a second set of assays was performed, in which IgG concentrations were normalized to an anti-Pfs25 ELISA titer of 1:2,000, a concentration expected to result in an intermediate degree of inhibition, and reevaluated. Based on the anti-Pfs25 titer, this corresponds to a dilution of the original antisera in the range of 1:12 to 1:34. As shown in Table 1 (see Data Set S1 in the supplemental material), IgG derived from all animals immunized with rPfs25 or rPfs25/8(CΔS) showed significant transmission-reducing activity (P < 0.05). However, there was no significant difference in the potency of transmission-reducing antibodies induced by immunization with rPfs25 compared to that of antibodies induced by immunization with rPfs25/8(CΔS) (P = 0.6). Combined, the data indicate that the genetic fusion of rPfs25 to the rPfMSP8(CΔS) carrier facilitated the production of a vaccine candidate that, when formulated with Alhydrogel, retained the ability to induce high titers of transmission-reducing antibodies.
TABLE 1.
Immunization with rPfs25 and rPfs25/8(CΔS) elicits antibodies with similar transmission-reducing activitiesa
| Immunization group | Rabbit | IgG level (μg/ml) | Transmission-reducing activity (expt 1 and 2) |
|||
|---|---|---|---|---|---|---|
| % inhibition | 95% CI (low) | 95% CI (high) | P value (vs alum control) | |||
| Alum control | 1 | 938 | 0 | |||
| rPfs25 | 2 | 182 | 51.3 | 14.2 | 72.5 | 0.015 |
| 3 | 156 | 48.4 | 9.9 | 71.2 | 0.015 | |
| 4 | 356 | 62.4 | 31.3 | 78.6 | 0.002 | |
| 5 | 221 | 55.4 | 19.9 | 74.3 | 0.008 | |
| rPfs25/8(CΔS) | 6 | 360 | 53.1 | 16.2 | 73.4 | 0.013 |
| 7 | 218 | 53.1 | 16.5 | 73.5 | 0.018 | |
| 8 | 579 | 54.2 | 19.8 | 73.7 | 0.005 | |
| 9 | 699 | 47.1 | 4.9 | 69.9 | 0.030 | |
Total IgG from individual rabbits immunized with rPfs25 and rPfs25/8(CΔS) was purified and tested in a standard membrane feeding assay at low total IgG concentrations, as indicated, normalized to an anti-rPfs25 titer of 2,000. The best estimates and 95% confidence intervals (CIs) of percent inhibitions and P values were calculated from two independent assays and each experiment with 20 mosquitoes per IgG sample. The P value for the rPfs25 and rPfs25/8(CΔS) groups was 0.63.
DISCUSSION
There are several requirements for the successful development of a subunit malaria vaccine. Protective, conserved antigens must be identified. The resulting vaccine formulations must be highly immunogenic and capable of eliciting strong and durable T cell and B cell responses. It must be possible to produce vaccine candidates that are of high quality and homogenous using simple, reproducible, and scalable protocols. Many obstacles have been encountered in the effort to meet these basic criteria. Polymorphisms of B cell and T cell epitopes in leading vaccine candidates such as PfAMA-1 and PfMSP142 have resulted in only modest, allele-specific protection (38, 70). Promising antigens have been shown to be poorly immunogenic due to their small size, inherent lack of immunogenic T cell epitopes, and/or complex tertiary structures that inhibit the processing and presentation of relevant epitopes (71, 72). Immunogenicity is further impacted when several targets are combined into a single formulation, as immunodominant responses to one component can inhibit productive responses to others (73–75). The production of recombinant subunit vaccines with routine heterologous expression systems has often resulted in low yields with inconsistent, misfolded products that do not elicit protective antibodies to neutralizing conformational B cell epitopes (48, 53, 76). We encountered several of these issues in P. yoelii model system studies with the production and evaluation of a simple combined formulation of MSP142 and MSP8.
Mice immunized with rPyMSP8 develop self-limiting infection when challenged with otherwise lethal P. yoelii 17XL (22, 66). When a combined vaccine formulation of rPyMSP8 and rPyMSP142 was tested, no improvement in protection was observed, as a dominant response to rPyMSP8 impeded the production of protective antibodies to rPyMSP119 (22). In an effort to overcome this antigenic competition, we genetically fused MSP119 to the N terminus of MSP8. This concept was first tested in the P. yoelii model and then applied to P. falciparum. The production and folding of the chimeric antigens were concurrently improved by codon harmonization of gene constructs for expression in E. coli. Immunization with chimeric MSP1/8 elicited a strong T cell response to epitopes within the MSP8 domain, resulting in the high-level and sustained production of growth-inhibitory, MSP119-specific antibodies (22, 23, 42). Based on these promising results, we were interested in assessing the ability of PfMSP8 to serve as a malaria-specific carrier protein for other protective but poorly immunogenic and/or difficult-to-produce vaccine targets. We selected Pfs25, a sexual-stage vaccine candidate, as a suitable antigen. Similar to PfMSP119 in nature, Pfs25 is a small (25-kDa), well-conserved protein composed of four tandem EGF-like domains that form conformational epitopes that are recognized by protective antibodies (43).
Initially, we evaluated an approach to further optimize PfMSP8 as a vaccine carrier. We modified the PfMSP8 domain of PfMSP1/8 to eliminate two unpaired cysteines and promote the production of a monomeric fusion protein. The modified PfMSP1/8(CΔS) construct was purified from E. coli in high yields similar to those achieved with the original PfMSP1/8 antigen (23), with a minimal amount of the dimeric or multimeric product. Comparative immunogenicity studies in mice demonstrated that the modifications within the PfMSP8 domain did not significantly alter the immunogenicity of the protein, as the strong antibody response against the PfMSP119 fusion partner was maintained.
The value of using PfMSP8 as a carrier for Pfs25 was first assessed by comparison of the expression and purification of two recombinant antigens, rPfs25 and rPfs25/8(CΔS). Unfused rPfs25 was successfully expressed in E. coli but remained in an insoluble aggregate. rPfs25 was solubilized from inclusion bodies and purified, but denaturation followed by refolding was required to achieve proper conformation. The final yield of the monomeric product was 0.87 mg/g bacterial cells (7.2 mg/liter), a yield comparable to that reported previously for production in E. coli (60). In comparison, the expression and purification of the chimeric rPfs25/8(CΔS) antigen under nondenaturing conditions were straightforward. The final yield of rPfs25/8(CΔS) was 2.5 mg/g bacterial cells (19.8 mg/liter). Based on the relative sizes, the final yields of the two recombinant antigens were similar with respect to the Pfs25 content. As with MSP119, these data demonstrate the benefit of using PfMSP8 as a carrier protein to enhance the folding and final yield of rPfs25, a structurally complex, disulfide bond-constrained protein. Furthermore, ongoing work to optimize production and purification indicates that our yield of bacterially produced and purified rPfs25/8(CΔS) can be increased to 35 to 40 mg/liter, comparable to the high yield of 41 mg/liter reported for Pichia-expressed rPfs25 (77). As such, we expect that this is suitable for the large-scale production of a rPfs25/8(CΔS) vaccine.
To better assess the quality and conformation of purified rPfs25 and rPfs25/8(CΔS), rabbits were immunized with Alhydrogel-based formulations. For these immunizations, the smaller unfused antigen and larger chimeric antigen were administered at the same dose of total protein (100 μg), corresponding to a 3-fold difference in the Pfs25 content. Despite this difference, both antigens elicited high and comparable titers of anti-Pfs25 antibodies, suggesting that the fusion of rPfs25 to PfMSP8(CΔS) had a dose-sparing effect. Antibodies induced by both constructs were able to recognize the native antigen on the surface of sexual-stage parasites and in a gamete lysate, verifying that a proper conformation of the Pfs25 domain was achieved in both cases. Importantly, functional analysis of purified IgG induced by each of the two antigens demonstrated transmission-reducing activity in the SMFA of nearly 100% when tested at 3.75 mg/ml total IgG. When tested at lower IgG concentrations to yield intermediate TRA, normalized based on the anti-Pfs25 titer, no differences in the transmission-reducing capacities of IgGs elicited by the two antigens were detected. Of note, an approximately 50% reduction in transmission was maintained with IgG at dilutions between 1:12 and 1:34 of the original serum level. In parallel, we demonstrated that PfMSP8 is expressed during gametocyte development, confirming previously reported transcriptomic and proteomic data. The expression of PfMSP8 in immature gametocytes appeared to be cytoplasmic and punctate; the expression of PfMSP8 was not detected on the surface of live, activated macrogametes. Consistent with this, anti-PfMSP8 IgG did not reduce parasite transmission in the SMFA.
The results of these studies with Pfs25 along with our previous results with P. yoelii and P. falciparum MSP119 support the use of PfMSP8 as a vaccine carrier protein to increase the production, folding, and/or immunogenicity of neutralizing B cell epitopes. Our genetic fusion strategy also reduces some of the variability inherent with the approaches that are currently being tested to conjugate chemically modified rPfs25 to various heterologous carrier proteins. From the perspective of a vaccine carrier, additional features of MSP8 are attractive. PfMSP8 is well conserved among P. falciparum isolates, with variability being limited primarily to small insertions and/or deletions in an ∼170-residue asparagine- and aspartic acid-rich domain, a region that is not present in the PfMSP8 fusion partner being evaluated as a vaccine carrier protein. The PfMSP8 carrier domain is highly immunogenic and contains CD4+ T cell epitopes recognized upon immunization of mice, rabbits, and monkeys as well as multiple epitopes predicted to bind to an array of human major histocompatibility complex class II (MHC-II) alleles. Immunization and challenge studies in mice showed that antibodies elicited by MSP1/8 immunization persist at protective levels (78). As a parasite-specific carrier, vaccine-induced responses have the potential to be boosted by natural P. falciparum infection.
It is well established that adjuvant selection, antigen dose, and choice of carrier protein can have profound effects on the immune response to targeted B cell epitopes. Indeed, these parameters have been evaluated in an effort to improve the efficacy and duration of the response generated by immunization with the Pfs25-exoprotein A conjugate (63). Similar mouse immunogenicity studies are ongoing in our laboratory with rPfs25-based formulations with various doses and adjuvants. Preliminary results suggest that the immunogenicity of rPf25 is reduced when administered in an admixture of antigens, but this antigenic competition can be eliminated by immunization with chimeric rPfs25/8(CΔS).
MATERIALS AND METHODS
Generation and purification of modified rPfMSP1/8(CΔS).
Using the QuikChange Lightning site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA) and the pET28a-PfMSP1/8 plasmid (23) as the template, codons for Cys-318 and Cys-332 (TGC) of PfMSP8 (GenBank accession no. AF325161.1) were mutated to serine codons (TCC), according to the manufacturer's protocol. The resulting construct, pET28a-PfMSP1/8(CΔS), was sequence verified and transformed into competent Escherichia coli SHuffle T7 Express lysY cells (New England BioLabs, Ipswich, MA). The expression and purification of rPfMSP1/8(CΔS) were performed according to protocols reported previously for unmodified rPfMSP1/8, at a 5-liter scale, using a BioFlo115 benchtop bioreactor (New Brunswick Scientific, Edison, NJ) with nickel chelate affinity chromatography conducted under nondenaturing conditions (23). The concentration of purified rPfMSP1/8(CΔS) was determined by using the Pierce bicinchoninic acid (BCA) protein assay (ThermoFisher Scientific, Waltham, MA). The purity of the final product was assessed by SDS-PAGE on 10% polyacrylamide gels under reducing and nonreducing conditions, followed by Coomassie blue staining. Immunoblot analysis was done by utilizing high-titer rabbit antiserum raised against the original, unmodified rPfMSP1/8 (rabbit anti-rPfMSP1/8 IgG at 0.5 μg/ml) antigen (23). Bound antibody was detected by horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (0.1 μg/ml; ThermoFisher Scientific) and the SuperSignal West Pico chemiluminescent substrate (ThermoFisher Scientific).
Generation of the pET28-MCS-PfMSP8(CΔS) expression vector.
To facilitate the cloning of target sequences fused to PfMSP8 as a carrier, the PfMSP119 sequence was removed from the pET28a-PfMSP1/8(CΔS) plasmid by digestion with BamHI and replaced with a MCS. Two short, complementary oligonucleotides containing three sequential restriction sites (Spe1, AflII, and AvrII) flanked by BamHI sites were annealed and ligated into BamHI-digested pET28a-PfMSP8(CΔS) to generate the pET28-MCS-PfMSP8(CΔS) plasmid. The sequence-verified plasmid was transformed into competent E. coli SHuffle T7 Express lysY cells (New England BioLabs) for expression, and nonfused rPfMSP8(CΔS) was purified by nickel chelate affinity chromatography and quantified by a BCA protein assay as previously described (23). The final product was assessed for purity by SDS-PAGE on 10% polyacrylamide gels under reducing and nonreducing conditions, followed by Coomassie blue staining or an immunoblot assay using a His tag-specific MAb (0.04 μg/ml; EMD Millipore, Billerica, MA) as described above.
Mouse immunizations.
Five-week-old male CB6F1/J (BALB/cJ × C57BL/6J) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed in the Animal Care Facility of the Drexel University College of Medicine (DUCOM). Protocols associated with the mouse experiments were reviewed, approved, and conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of Drexel University (protocol no. 20308). Groups of mice (n = 5) were immunized with 10 μg/dose of the original rPfMSP1/8 or the modified rPfMSP1/8(CΔS) antigen. Antigens were formulated in adjuvant, including (i) Quil A (25 μg/dose; Accurate Chemical and Scientific Corporation, Westbury, NY) or (ii) CpG ODN 1826 (25 μg/dose; Eurofins MWG Operon, Huntsville, AL) emulsified in Montanide ISA 720 VG (Seppic, Inc., Paris, France) at a ratio of 70:30 (vol/vol). Animals received three subcutaneous immunizations administered 3 weeks apart. Serum was collected 2 weeks following the final immunization for comparison of antibody responses.
Determination of antigen-specific antibody titers by an ELISA.
Antigen-specific antibody responses induced by immunization with either rPfMSP1/8 or rPfMSP1/8(CΔS) were evaluated by an ELISA as previously described (23). Briefly, plates coated with 0.25 μg/well of rPfMSP1/8, rPfMSP8, rGST-PfMSP119(3D7), or rGST-PfMSP119(FVO) were incubated with 2-fold serial dilutions of mouse immunization sera for 2 h at room temperature (RT). Bound antibodies were detected by using HRP-conjugated rabbit anti-mouse IgG (0.08 μg/ml; ThermoFisher Scientific) and ABTS [2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)] as the substrate. A405 values of between 0.1 and 1 were plotted, and titers were calculated as the reciprocal of the dilution yielding an A405 of 0.5. Statistical analysis of differences in antigen-specific IgG titers was conducted by using the nonparametric Mann-Whitney test (GraphPad Prism 7; GraphPad Software, Inc., La Jolla, CA), with probability (P) values of ≤0.05 being considered significant.
Production and purification of unfused rPfs25 and chimeric rPfs25/8. (i) Expression constructs.
The gene sequence encoding mature Pfs25 (amino acids 23 to 193) containing the four EGF-like domains was subjected to codon harmonization using previously reported algorithms (69). The goal of harmonization is to recode the native sequence to achieve comparable codon frequencies in the expression host, to increase yield of properly folded recombinant antigens. The codon-harmonized pfs25 gene sequence with additional 5′ SpeI and 3′ AflII sites was commercially synthesized by Blue Heron Biotechnology, Inc. (Bothell, WA). The synthesized pfs25 gene was subcloned into the SpeI/AflII-digested pET28-MCS-PfMSP8(CΔS) expression vector. In the first configuration, the synthetic pfs25 coding sequence was cloned in frame with PfMSP8(CΔS) to produce chimeric rPfs25/8(CΔS) (GenBank accession no. MG288685). In a second configuration, two stop codons (TGA and TAA) were incorporated upstream of the 3′ AflII site to facilitate the expression of full-length rPfs25 only, lacking the rPfMSP8(CΔS) fusion partner. The sequences of both Pfs25-based expression constructs were verified prior to expression and purification.
(ii) Unfused rPfs25 antigen.
The rPfs25 expression construct was transformed into SHuffle T7 Express lysY competent E. coli cells (New England BioLabs). For expression, 3-liter bacterial cultures were grown by using a BioFlo115 benchtop bioreactor (New Brunswick Scientific). At an optical density at 600 nm (OD600) of ∼4.0, the expression of rPfs25 was induced by the addition of IPTG (ThermoFisher Scientific) to a final concentration of 1 mM. An additional 2 liters of growth medium was added at the time of induction. At 3 h postinduction, the culture was harvested by centrifugation at 10,500 × g for 20 min at 4°C. Cells were washed once with phosphate-buffered saline (PBS) (pH 7.4), pelleted, and then stored at 80°C. For purification, pelleted cells were lysed with BugBuster HT protein extraction reagent containing recombinant lysozyme and Benzonase nuclease (EMD Millipore). Following centrifugation, the resulting pellet was solubilized by brief resuspension (20 min at RT) in high-pH solubilization buffer containing Sarkosyl (25 mM Tris base [pH 12.0], 0.2% Sarkosyl). Solubilized material was centrifuged at 7,600 × g for 20 min at 4°C and then neutralized by the addition of 3 volumes of 275 mM Tris-HCl (pH 6.0)–0.2% Sarkosyl to a final pH of 6.85. Neutralized material was subjected to nickel chelate affinity chromatography using nickel-nitrilotriacetic acid (Ni-NTA)-agarose beads (Qiagen, Valencia, CA). Eluted rPfs25 was dialyzed into renaturation buffer (50 mM Tris-HCl [pH 8.3], 100 mM NaCl, 3 mM reduced glutathione, 0.3 mM oxidized glutathione) containing 4 M urea. rPfs25 was refolded by the gradual, stepwise removal of urea by dialysis into renaturation buffer, with final dialysis into a solution containing 50 mM Tris-HCl (pH 6.85), 500 mM NaCl, 3 mM reduced glutathione, and 0.1% Sarkosyl.
To remove any high-molecular-weight protein aggregates, the rPfs25 preparation was then subjected to size exclusion chromatography on a Superdex 75 column (GE Healthcare Bio-Sciences Corp., Marlborough, MA). Fractions containing monomeric rPfs25 were pooled, and the purified antigen was concentrated by a second round of nickel chelate affinity chromatography. The concentration of the final product was determined by a BCA protein assay. Purity and conformation were assessed by SDS-PAGE on 12% polyacrylamide gels under reducing and nonreducing conditions, followed by Coomassie blue staining or immunoblot analysis using Pfs25-specific MAb 4B7 (0.2 μg/ml) (47), as described above.
(iii) Chimeric rPfs25/8(CΔS) antigen.
The expression and solubilization of chimeric rPfs25/8(CΔS) were carried out as described above for unfused rPfs25. The purification of rPfs25/8(CΔS) also involved nickel chelate affinity chromatography and size exclusion chromatography, but the glutathione-urea renaturation procedure was not required. Following quantitation by a BCA protein assay, the purity and conformation of chimeric rPfs25/8(CΔS) were assessed by SDS-PAGE on 10% acrylamide gels under reducing and nonreducing conditions, followed by Coomassie blue staining or immunoblot assays as described above, using (i) rabbit anti-PfMSP8 IgG (0.4 μg/ml) (41) or (ii) MAb 4B7 (0.2 μg/ml).
Generation of polyclonal rabbit antisera.
Polyclonal antisera were raised against Pfs25-based recombinant proteins by Lampire Biological Laboratories (Pipersville, PA). Adult New Zealand White rabbits (n = 4) were immunized with purified rPfs25 or rPfs25/8(CΔS) antigens (100 μg/dose) formulated with 2% Alhydrogel adjuvant (2.5 mg/dose; InvivoGen, San Diego, CA) in a total volume of 0.5 ml. An additional control rabbit was immunized with Alhydrogel alone. Rabbits received three subcutaneous immunizations administered at 4-week intervals. Sera were collected 2 weeks following each immunization, and antigen-specific antibody titers were determined by an ELISA as described above, using wells coated with rPfs25, rPfs25/8(CΔS), or rPfMSP8.
P. falciparum in vitro culture.
Cryopreserved P. falciparum parasites (NF54 strain) were thawed, placed into culture medium (RPMI 1640, 2 mM l-glutamine, 20 mM HEPES, 10 mg/liter hypoxanthine, 25 mM sodium bicarbonate, 20 mM glucose, 0.5% AlbuMAX II, 1× penicillin-streptomycin [pH 7.35]) containing O-positive (O+) human red blood cells (RBCs) (Interstate Blood Bank, Inc., Memphis, TN) at 2% hematocrit, and maintained at 37°C in the presence of a blood-gas mixture (5% O2–5% CO2–90% N2). To induce gametocytogenesis, new flasks were seeded at 1% parasitemia and 4% hematocrit in the above-described medium supplemented with 10% heat-inactivated human sera, as described previously (68). Cultures were fed daily for 16 days without the addition of fresh RBCs, allowing peak parasitemia to occur, followed by a crash of asexual stages and the emergence of gametocytes. To activate late-stage gametocytes (day 16), culture medium was replaced with ookinete medium (RPMI 1640, 2 mM l-glutamine, 20 mM HEPES, 10 mg/liter hypoxanthine, 25 mM sodium bicarbonate, 20 mM glucose, 0.25% AlbuMAX II, 20% heat-inactivated human serum, 1× penicillin-streptomycin, 100 μM xanthurenic acid [pH 7.35]), and the culture was placed at 27°C for 24 h (68). Activated gametes were harvested at 24 h postactivation.
For the generation of the gamete lysate, late-stage gametocytes from day 16 cultures were isolated by centrifugation in a 35% Percoll solution (GE Healthcare Bio-Sciences). Gametocytes at the Percoll interface were collected, washed, resuspended in ookinete medium, and placed at 27°C. At 24 h postactivation, the culture was overlaid onto a Percoll step gradient (35% and 50%) to further isolate activated macrogametes. The interface of the 35% and 50% layers was collected and washed three times with PBS, and the parasite pellets were stored at −80°C. For solubilization, pellets were resuspended in approximately 3 volumes of solubilization buffer (20 mM Tris-HCl [pH 8.0], 50 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.5% deoxycholate) and incubated on ice for 3 h with intermittent mixing. The lysate was clarified by centrifugation at 15,800 × g for 20 min, and solubilized material was stored at −80°C. For immunoblot analysis, the macrogamete lysate was separated by SDS-PAGE on 12% polyacrylamide gels under nonreducing conditions. Membranes were probed with pools of rabbit antisera raised against rPfs25 or rPfs25/8(CΔS) or with adjuvant control serum (1:2,000). Bound antibodies were detected by chemiluminescence as described above.
Immunofluorescence assays. (i) Analysis of fixed gametocytes.
RBCs from day 16 gametocyte cultures were recovered by centrifugation and washed 3 times with PBS. Cells were resuspended in fixation buffer (PBS containing 4% formaldehyde [Polysciences, Inc., Warrington, PA] and 0.0075% glutaraldehyde [Sigma-Aldrich, St. Louis, MO]) and gently rotated for 1 h at RT and then overnight at 4°C. The following day, fixed parasitized RBCs were permeabilized by resuspension in PBS–0.1% Triton X-100 for 5 min and then incubated with 0.1 mg/ml NaBH4 to neutralize any remaining glutaraldehyde, as described previously (79). Parasitized RBCs were blocked by resuspension in PBS–5% nonfat dried milk for 1 h at 4°C and then incubated overnight at 4°C with rabbit anti-PfMSP8 IgG (2 μg/ml) or control IgG (2 μg/ml) diluted in blocking buffer. The following day, bound IgG was detected by using FITC-conjugated goat anti-rabbit IgG (0.5 μg/ml; ThermoFisher Scientific). Parasitized RBCs were resuspended in SlowFade Gold antifade reagent containing DAPI (4′,6-diamidino-2-phenylindole; ThermoFisher Scientific) for analysis. Images were acquired by using an Olympus BX60 fluorescence microscope (Olympus America, Inc., Melville, NY) and the SensiCam QE cooled digital 12-bit charge-coupled-device (CCD) camera system (PCO-Tech, Inc., Romulus, MI) with SlideBook 5.0 software (Intelligent Imaging Innovations, Inc., Denver, CO).
(ii) Analysis of fixed and live gametes.
Activated macrogametes were fixed, blocked, and probed with pools of rabbit antisera raised against rPfs25 or rPfs25/8(CΔS), with adjuvant control rabbit sera (1:1,000), or with mouse MAb 4B7 (0.2 μg/ml) overnight at 4°C. Alternatively, live activated macrogametes were recovered by centrifugation, washed, and immediately resuspended in medium containing either rabbit anti-PfMSP8 IgG or control IgG (2 μg/ml) along with MAb 4B7 (0.2 μg/ml). The primary antibody was incubated at room temperature for 1 h with gentle rotation. Bound IgG was detected with FITC-conjugated goat anti-rabbit IgG, FITC-conjugated goat anti-mouse IgG, or tetramethylrhodamine isothiocyanate (TRITC)-labeled goat anti-mouse IgG as needed and then analyzed by fluorescence microscopy as described above.
Standard membrane feeding assay.
The TRA of IgG induced by rPfs25-containing vaccines was measured by a SMFA using cultured P. falciparum NF54 gametocytes and Anopheles stephensi mosquitoes, as described previously (80). Vaccine-induced IgG was mixed at the indicated concentrations with stage V gametocytes and fed to A. stephensi mosquitoes through a membrane-feeding apparatus. Mosquitoes were kept for 8 days prior to dissection to quantify midgut oocysts. The percent inhibition of the mean oocyst intensity was calculated relative to adjuvant control IgG. The best estimates of the percent inhibition of the mean oocyst density (percent TRA), the 95% confidence interval, and the P value (whether the observed percent TRA is significantly different from noninhibited controls) of each test sample (tested in either a single feed or two independent assays) were calculated by using a zero-inflated negative binomial model (81).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH-NIAID grant AI114292 (J.M.B.), the Intramural Program of the NIH-NIAID (C.A.L.), and the PATH Malaria Vaccine Initiative. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
J.M.B. is an inventor listed on U.S. patent no. 7,931,908, entitled “Chimeric MSP-Based Malaria Vaccine.”
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00486-17.
REFERENCES
- 1.Anonymous. 2016. World malaria report 2016. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Cohen J, Nussenzweig V, Nussenzweig R, Vekemans J, Leach A. 2010. From the circumsporozoite protein to the RTS,S/AS candidate vaccine. Hum Vaccin 6:90–96. doi: 10.4161/hv.6.1.9677. [DOI] [PubMed] [Google Scholar]
- 3.RTS,S Clinical Trials Partnership, Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmuller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Machevo S, Acacio S, Bulo H, Sigauque B, Macete E, Alonso P, Abdulla S, Salim N, Minja R, Mpina M, Ahmed S, Ali AM, Mtoro AT, Hamad AS, Mutani P, Tanner M, Tinto H, D'Alessandro U, Sorgho H, Valea I, Bihoun B, Guiraud I, Kabore B, Sombie O, Guiguemde RT, Ouedraogo JB, Hamel MJ, Kariuki S, Oneko M, Odero C, Otieno K, Awino N, McMorrow M, Muturi-Kioi V, Laserson KF, et al. 2012. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 367:2284–2295. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.RTS,S Clinical Trials Partnership. 2015. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 386:31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White MT, Verity R, Griffin JT, Asante KP, Owusu-Agyei S, Greenwood B, Drakeley C, Gesase S, Lusingu J, Ansong D, Adjei S, Agbenyega T, Ogutu B, Otieno L, Otieno W, Agnandji ST, Lell B, Kremsner P, Hoffman I, Martinson F, Kamthunzu P, Tinto H, Valea I, Sorgho H, Oneko M, Otieno K, Hamel MJ, Salim N, Mtoro A, Abdulla S, Aide P, Sacarlal J, Aponte JJ, Njuguna P, Marsh K, Bejon P, Riley EM, Ghani AC. 2015. Immunogenicity of the RTS,S/AS01 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect Dis 15:1450–1458. doi: 10.1016/S1473-3099(15)00239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, Richman A, Chakravarty S, Manoj A, Velmurugan S, Li M, Ruben AJ, Li T, Eappen AG, Stafford RE, Plummer SH, Hendel CS, Novik L, Costner PJ, Mendoza FH, Saunders JG, Nason MC, Richardson JH, Murphy J, Davidson SA, Richie TL, Sedegah M, Sutamihardja A, Fahle GA, Lyke KE, Laurens MB, Roederer M, Tewari K, Epstein JE, Sim BK, Ledgerwood JE, Graham BS, Hoffman SL, VRC 312 Study Team . 2013. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341:1359–1365. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- 7.Ishizuka AS, Lyke KE, DeZure A, Berry AA, Richie TL, Mendoza FH, Enama ME, Gordon IJ, Chang LJ, Sarwar UN, Zephir KL, Holman LA, James ER, Billingsley PF, Gunasekera A, Chakravarty S, Manoj A, Li M, Ruben AJ, Li T, Eappen AG, Stafford RE, K CN, Murshedkar T, DeCederfelt H, Plummer SH, Hendel CS, Novik L, Costner PJ, Saunders JG, Laurens MB, Plowe CV, Flynn B, Whalen WR, Todd JP, Noor J, Rao S, Sierra-Davidson K, Lynn GM, Epstein JE, Kemp MA, Fahle GA, Mikolajczak SA, Fishbaugher M, Sack BK, Kappe SH, Davidson SA, Garver LS, Bjorkstrom NK, Nason MC, et al. 2016. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med 22:614–623. doi: 10.1038/nm.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein JE, Richie TL. 2013. The whole parasite, pre-erythrocytic stage approach to malaria vaccine development: a review. Curr Opin Infect Dis 26:420–428. doi: 10.1097/QCO.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 9.Spring M, Murphy J, Nielsen R, Dowler M, Bennett JW, Zarling S, Williams J, de la Vega P, Ware L, Komisar J, Polhemus M, Richie TL, Epstein J, Tamminga C, Chuang I, Richie N, O'Neil M, Heppner DG, Healer J, O'Neill M, Smithers H, Finney OC, Mikolajczak SA, Wang R, Cowman A, Ockenhouse C, Krzych U, Kappe SH. 2013. First-in-human evaluation of genetically attenuated Plasmodium falciparum sporozoites administered by bite of Anopheles mosquitoes to adult volunteers. Vaccine 31:4975–4983. doi: 10.1016/j.vaccine.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Kublin JG, Mikolajczak SA, Sack BK, Fishbaugher ME, Seilie A, Shelton L, VonGoedert T, Firat M, Magee S, Fritzen E, Betz W, Kain HS, Dankwa DA, Steel RW, Vaughan AM, Sather DN, Murphy SC, Kappe SH. 2017. Complete attenuation of genetically engineered Plasmodium falciparum sporozoites in human subjects. Sci Transl Med 9:eaad9099. doi: 10.1126/scitranslmed.aad9099. [DOI] [PubMed] [Google Scholar]
- 11.Vaughan AM, Wang R, Kappe SH. 2010. Genetically engineered, attenuated whole-cell vaccine approaches for malaria. Hum Vaccin 6:107–113. doi: 10.4161/hv.6.1.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, van de Vegte-Bolmer M, van Schaijk B, Teelen K, Arens T, Spaarman L, de Mast Q, Roeffen W, Snounou G, Renia L, van der Ven A, Hermsen CC, Sauerwein R. 2009. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med 361:468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 13.Roestenberg M, Teirlinck AC, McCall MB, Teelen K, Makamdop KN, Wiersma J, Arens T, Beckers P, van Gemert G, van de Vegte-Bolmer M, van der Ven AJ, Luty AJ, Hermsen CC, Sauerwein RW. 2011. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 377:1770–1776. doi: 10.1016/S0140-6736(11)60360-7. [DOI] [PubMed] [Google Scholar]
- 14.Bijker EM, Bastiaens GJ, Teirlinck AC, van Gemert GJ, Graumans W, van de Vegte-Bolmer M, Siebelink-Stoter R, Arens T, Teelen K, Nahrendorf W, Remarque EJ, Roeffen W, Jansens A, Zimmerman D, Vos M, van Schaijk BC, Wiersma J, van der Ven AJ, de Mast Q, van Lieshout L, Verweij JJ, Hermsen CC, Scholzen A, Sauerwein RW. 2013. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc Natl Acad Sci U S A 110:7862–7867. doi: 10.1073/pnas.1220360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Good MF, Reiman JM, Rodriguez IB, Ito K, Yanow SK, El-Deeb IM, Batzloff MR, Stanisic DI, Engwerda C, Spithill T, Hoffman SL, Lee M, McPhun V. 1 July 2013. Cross-species malaria immunity induced by chemically attenuated parasites. J Clin Invest doi: 10.1172/JCI66634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanisic DI, Good MF. 2015. Whole organism blood stage vaccines against malaria. Vaccine 33:7469–7475. doi: 10.1016/j.vaccine.2015.09.057. [DOI] [PubMed] [Google Scholar]
- 17.Reed SG, Orr MT, Fox CB. 2013. Key roles of adjuvants in modern vaccines. Nat Med 19:1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 18.Sebastian S, Gilbert SC. 2016. Recombinant modified vaccinia virus Ankara-based malaria vaccines. Expert Rev Vaccines 15:91–103. doi: 10.1586/14760584.2016.1106319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollingdale MR, Sedegah M, Limbach K. 2017. Development of replication-deficient adenovirus malaria vaccines. Expert Rev Vaccines 16:261–271. doi: 10.1080/14760584.2016.1228454. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Narum DL, Fleury S, Jennings G, Yadava A. 2015. Particle-based platforms for malaria vaccines. Vaccine 33:7518–7524. doi: 10.1016/j.vaccine.2015.09.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ewer KJ, Sierra-Davidson K, Salman AM, Illingworth JJ, Draper SJ, Biswas S, Hill AV. 2015. Progress with viral vectored malaria vaccines: a multi-stage approach involving “unnatural immunity.” Vaccine 33:7444–7451. doi: 10.1016/j.vaccine.2015.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Q, Lynch MM, Romero M, Burns JM Jr. 2007. Enhanced protection against malaria by a chimeric merozoite surface protein vaccine. Infect Immun 75:1349–1358. doi: 10.1128/IAI.01467-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alaro JR, Partridge A, Miura K, Diouf A, Lopez AM, Angov E, Long CA, Burns JM Jr. 2013. A chimeric Plasmodium falciparum merozoite surface protein vaccine induces high titers of parasite growth inhibitory antibodies. Infect Immun 81:3843–3854. doi: 10.1128/IAI.00522-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daly TM, Long CA. 1995. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J Immunol 155:236–243. [PubMed] [Google Scholar]
- 25.Hirunpetcharat C, Tian JH, Kaslow DC, van Rooijen N, Kumar S, Berzofsky JA, Miller LH, Good MF. 1997. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J Immunol 159:3400–3411. [PubMed] [Google Scholar]
- 26.Blackman MJ, Heidrich HG, Donachie S, McBride JS, Holder AA. 1990. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med 172:379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burns JM Jr, Parke LA, Daly TM, Cavacini LA, Weidanz WP, Long CA. 1989. A protective monoclonal antibody recognizes a variant-specific epitope in the precursor of the major merozoite surface antigen of the rodent malarial parasite Plasmodium yoelii. J Immunol 142:2835–2840. [PubMed] [Google Scholar]
- 28.Chappel JA, Holder AA. 1993. Monoclonal antibodies that inhibit Plasmodium falciparum invasion in vitro recognise the first growth factor-like domain of merozoite surface protein-1. Mol Biochem Parasitol 60:303–311. doi: 10.1016/0166-6851(93)90141-J. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Yadava A, Keister DB, Tian JH, Ohl M, Perdue-Greenfield KA, Miller LH, Kaslow DC. 1995. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol Med 1:325–332. [PMC free article] [PubMed] [Google Scholar]
- 30.O'Donnell RA, de Koning-Ward TF, Burt RA, Bockarie M, Reeder JC, Cowman AF, Crabb BS. 2001. Antibodies against merozoite surface protein (MSP)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J Exp Med 193:1403–1412. doi: 10.1084/jem.193.12.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egan AF, Morris J, Barnish G, Allen S, Greenwood BM, Kaslow DC, Holder AA, Riley EM. 1996. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J Infect Dis 173:765–769. doi: 10.1093/infdis/173.3.765. [DOI] [PubMed] [Google Scholar]
- 32.Ockenhouse CF, Angov E, Kester KE, Diggs C, Soisson L, Cummings JF, Stewart AV, Palmer DR, Mahajan B, Krzych U, Tornieporth N, Delchambre M, Vanhandenhove M, Ofori-Anyinam O, Cohen J, Lyon JA, Heppner DG, MSP-1 Working Group. 2006. Phase I safety and immunogenicity trial of FMP1/AS02A, a Plasmodium falciparum MSP-1 asexual blood stage vaccine. Vaccine 24:3009–3017. doi: 10.1016/j.vaccine.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 33.Stoute JA, Gombe J, Withers MR, Siangla J, McKinney D, Onyango M, Cummings JF, Milman J, Tucker K, Soisson L, Stewart VA, Lyon JA, Angov E, Leach A, Cohen J, Kester KE, Ockenhouse CF, Holland CA, Diggs CL, Wittes J, Heppner DG Jr, MSP-1 Malaria Vaccine Working Group. 2007. Phase 1 randomized double-blind safety and immunogenicity trial of Plasmodium falciparum malaria merozoite surface protein FMP1 vaccine, adjuvanted with AS02A, in adults in western Kenya. Vaccine 25:176–184. doi: 10.1016/j.vaccine.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 34.Thera MA, Doumbo OK, Coulibaly D, Diallo DA, Sagara I, Dicko A, Diemert DJ, Heppner DG Jr, Stewart VA, Angov E, Soisson L, Leach A, Tucker K, Lyke KE, Plowe CV, Mali FMP1 Working Group . 2006. Safety and allele-specific immunogenicity of a malaria vaccine in Malian adults: results of a phase I randomized trial. PLoS Clin Trials 1:e34. doi: 10.1371/journal.pctr.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Withers MR, McKinney D, Ogutu BR, Waitumbi JN, Milman JB, Apollo OJ, Allen OG, Tucker K, Soisson LA, Diggs C, Leach A, Wittes J, Dubovsky F, Stewart VA, Remich SA, Cohen J, Ballou WR, Holland CA, Lyon JA, Angov E, Stoute JA, Martin SK, Heppner DG Jr, MSP-1 Malaria Vaccine Working Group. 2006. Safety and reactogenicity of an MSP-1 malaria vaccine candidate: a randomized phase Ib dose-escalation trial in Kenyan children. PLoS Clin Trials 1:e32. doi: 10.1371/journal.pctr.0010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellis RD, Martin LB, Shaffer D, Long CA, Miura K, Fay MP, Narum DL, Zhu D, Mullen GE, Mahanty S, Miller LH, Durbin AP. 2010. Phase 1 trial of the Plasmodium falciparum blood stage vaccine MSP1(42)-C1/Alhydrogel with and without CPG 7909 in malaria naive adults. PLoS One 5:e8787. doi: 10.1371/journal.pone.0008787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otsyula N, Angov E, Bergmann-Leitner E, Koech M, Khan F, Bennett J, Otieno L, Cummings J, Andagalu B, Tosh D, Waitumbi J, Richie N, Shi M, Miller L, Otieno W, Otieno GA, Ware L, House B, Godeaux O, Dubois MC, Ogutu B, Ballou WR, Soisson L, Diggs C, Cohen J, Polhemus M, Heppner DG Jr, Ockenhouse CF, Spring MD. 2013. Results from tandem phase 1 studies evaluating the safety, reactogenicity and immunogenicity of the vaccine candidate antigen Plasmodium falciparum FVO merozoite surface protein-1 (MSP1(42)) administered intramuscularly with adjuvant system AS01. Malar J 12:29. doi: 10.1186/1475-2875-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogutu BR, Apollo OJ, McKinney D, Okoth W, Siangla J, Dubovsky F, Tucker K, Waitumbi JN, Diggs C, Wittes J, Malkin E, Leach A, Soisson LA, Milman JB, Otieno L, Holland CA, Polhemus M, Remich SA, Ockenhouse CF, Cohen J, Ballou WR, Martin SK, Angov E, Stewart VA, Lyon JA, Heppner DG, Withers MR, MSP-1 Malaria Vaccine Working Group. 2009. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PLoS One 4:e4708. doi: 10.1371/journal.pone.0004708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burns JM Jr, Belk CC, Dunn PD. 2000. A protective glycosylphosphatidylinositol-anchored membrane protein of Plasmodium yoelii trophozoites and merozoites contains two epidermal growth factor-like domains. Infect Immun 68:6189–6195. doi: 10.1128/IAI.68.11.6189-6195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Black CG, Wu T, Wang L, Hibbs AR, Coppel RL. 2001. Merozoite surface protein 8 of Plasmodium falciparum contains two epidermal growth factor-like domains. Mol Biochem Parasitol 114:217–226. doi: 10.1016/S0166-6851(01)00265-1. [DOI] [PubMed] [Google Scholar]
- 41.Alaro JR, Angov E, Lopez AM, Zhou H, Long CA, Burns JM Jr. 2012. Evaluation of the immunogenicity and vaccine potential of recombinant Plasmodium falciparum merozoite surface protein 8. Infect Immun 80:2473–2484. doi: 10.1128/IAI.00211-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burns JM Jr, Miura K, Sullivan J, Long CA, Barnwell JW. 2016. Immunogenicity of a chimeric Plasmodium falciparum merozoite surface protein vaccine in Aotus monkeys. Malar J 15:159. doi: 10.1186/s12936-016-1226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaslow DC, Quakyi IA, Syin C, Raum MG, Keister DB, Coligan JE, McCutchan TF, Miller LH. 1988. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333:74–76. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- 44.Sharma B. 2008. Structure and mechanism of a transmission blocking vaccine candidate protein Pfs25 from P. falciparum: a molecular modeling and docking study. In Silico Biol 8:193–206. [PubMed] [Google Scholar]
- 45.Stowers AW, Keister DB, Muratova O, Kaslow DC. 2000. A region of Plasmodium falciparum antigen Pfs25 that is the target of highly potent transmission-blocking antibodies. Infect Immun 68:5530–5538. doi: 10.1128/IAI.68.10.5530-5538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Amerongen A, Sauerwein RW, Beckers PJ, Meloen RH, Meuwissen JH. 1989. Identification of a peptide sequence of the 25 kD surface protein of Plasmodium falciparum recognized by transmission-blocking monoclonal antibodies: implications for synthetic vaccine development. Parasite Immunol 11:425–428. doi: 10.1111/j.1365-3024.1989.tb00679.x. [DOI] [PubMed] [Google Scholar]
- 47.Barr PJ, Green KM, Gibson HL, Bathurst IC, Quakyi IA, Kaslow DC. 1991. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med 174:1203–1208. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaslow DC, Bathurst IC, Lensen T, Ponnudurai T, Barr PJ, Keister DB. 1994. Saccharomyces cerevisiae recombinant Pfs25 adsorbed to alum elicits antibodies that block transmission of Plasmodium falciparum. Infect Immun 62:5576–5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zou L, Miles AP, Wang J, Stowers AW. 2003. Expression of malaria transmission-blocking vaccine antigen Pfs25 in Pichia pastoris for use in human clinical trials. Vaccine 21:1650–1657. doi: 10.1016/S0264-410X(02)00701-6. [DOI] [PubMed] [Google Scholar]
- 50.Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, Fay MP, Narum D, Rausch K, Miles AP, Aebig J, Orcutt A, Muratova O, Song G, Lambert L, Zhu D, Miura K, Long C, Saul A, Miller LH, Durbin AP. 2008. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with Montanide ISA 51. PLoS One 3:e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimp RL Jr, Rowe C, Reiter K, Chen B, Nguyen V, Aebig J, Rausch KM, Kumar K, Wu Y, Jin AJ, Jones DS, Narum DL. 2013. Development of a Pfs25-EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine 31:2954–2962. doi: 10.1016/j.vaccine.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Y, Przysiecki C, Flanagan E, Bello-Irizarry SN, Ionescu R, Muratova O, Dobrescu G, Lambert L, Keister D, Rippeon Y, Long CA, Shi L, Caulfield M, Shaw A, Saul A, Shiver J, Miller LH. 2006. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proc Natl Acad Sci U S A 103:18243–18248. doi: 10.1073/pnas.0608545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee SM, Wu CK, Plieskatt J, McAdams DH, Miura K, Ockenhouse C, King CR. 2016. Assessment of Pfs25 expressed from multiple soluble expression platforms for use as transmission-blocking vaccine candidates. Malar J 15:405. doi: 10.1186/s12936-016-1464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, Leneghan DB, Miura K, Nikolaeva D, Brian IJ, Dicks MD, Fyfe AJ, Zakutansky SE, de Cassan S, Long CA, Draper SJ, Hill AV, Hill F, Biswas S. 2016. Enhancing immunogenicity and transmission-blocking activity of malaria vaccines by fusing Pfs25 to IMX313 multimerization technology. Sci Rep 6:18848. doi: 10.1038/srep18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gregory JA, Li F, Tomosada LM, Cox CJ, Topol AB, Vinetz JM, Mayfield S. 2012. Algae-produced Pfs25 elicits antibodies that inhibit malaria transmission. PLoS One 7:e37179. doi: 10.1371/journal.pone.0037179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patra KP, Li F, Carter D, Gregory JA, Baga S, Reed SG, Mayfield SP, Vinetz JM. 2015. Alga-produced malaria transmission-blocking vaccine candidate Pfs25 formulated with a human use-compatible potent adjuvant induces high-affinity antibodies that block Plasmodium falciparum infection of mosquitoes. Infect Immun 83:1799–1808. doi: 10.1128/IAI.02980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farrance CE, Chichester JA, Musiychuk K, Shamloul M, Rhee A, Manceva SD, Jones RM, Mamedov T, Sharma S, Mett V, Streatfield SJ, Roeffen W, van de Vegte-Bolmer M, Sauerwein RW, Wu Y, Muratova O, Miller L, Duffy P, Sinden R, Yusibov V. 2011. Antibodies to plant-produced Plasmodium falciparum sexual stage protein Pfs25 exhibit transmission blocking activity. Hum Vaccin 7(Suppl):191–198. doi: 10.4161/hv.7.0.14588. [DOI] [PubMed] [Google Scholar]
- 58.Jones RM, Chichester JA, Mett V, Jaje J, Tottey S, Manceva S, Casta LJ, Gibbs SK, Musiychuk K, Shamloul M, Norikane J, Mett V, Streatfield SJ, van de Vegte-Bolmer M, Roeffen W, Sauerwein RW, Yusibov V. 2013. A plant-produced Pfs25 VLP malaria vaccine candidate induces persistent transmission blocking antibodies against Plasmodium falciparum in immunized mice. PLoS One 8:e79538. doi: 10.1371/journal.pone.0079538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones RM, Chichester JA, Manceva S, Gibbs SK, Musiychuk K, Shamloul M, Norikane J, Streatfield SJ, van de Vegte-Bolmer M, Roeffen W, Sauerwein RW, Yusibov V. 2015. A novel plant-produced Pfs25 fusion subunit vaccine induces long-lasting transmission blocking antibody responses. Hum Vaccin Immunother 11:124–132. doi: 10.4161/hv.34366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar R, Angov E, Kumar N. 2014. Potent malaria transmission-blocking antibody responses elicited by Plasmodium falciparum Pfs25 expressed in Escherichia coli after successful protein refolding. Infect Immun 82:1453–1459. doi: 10.1128/IAI.01438-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kubler-Kielb J, Majadly F, Wu Y, Narum DL, Guo C, Miller LH, Shiloach J, Robbins JB, Schneerson R. 2007. Long-lasting and transmission-blocking activity of antibodies to Plasmodium falciparum elicited in mice by protein conjugates of Pfs25. Proc Natl Acad Sci U S A 104:293–298. doi: 10.1073/pnas.0609885104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Talaat KR, Ellis RD, Hurd J, Hentrich A, Gabriel E, Hynes NA, Rausch KM, Zhu D, Muratova O, Herrera R, Anderson C, Jones D, Aebig J, Brockley S, MacDonald NJ, Wang X, Fay MP, Healy SA, Durbin AP, Narum DL, Wu Y, Duffy PE. 2016. Safety and immunogenicity of Pfs25-EPA/Alhydrogel, a transmission blocking vaccine against Plasmodium falciparum: an open label study in malaria naive adults. PLoS One 11:e0163144. doi: 10.1371/journal.pone.0163144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Radtke AJ, Anderson CF, Riteau N, Rausch K, Scaria P, Kelnhofer ER, Howard RF, Sher A, Germain RN, Duffy P. 2017. Adjuvant and carrier protein-dependent T-cell priming promotes a robust antibody response against the Plasmodium falciparum Pfs25 vaccine candidate. Sci Rep 7:40312. doi: 10.1038/srep40312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Drew DR, Sanders PR, Crabb BS. 2005. Plasmodium falciparum merozoite surface protein 8 is a ring-stage membrane protein that localizes to the parasitophorous vacuole of infected erythrocytes. Infect Immun 73:3912–3922. doi: 10.1128/IAI.73.7.3912-3922.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi Q, Cernetich-Ott A, Lynch MM, Burns JM Jr. 2006. Expression, localization, and erythrocyte binding activity of Plasmodium yoelii merozoite surface protein-8. Mol Biochem Parasitol 149:231–241. doi: 10.1016/j.molbiopara.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 66.Shi Q, Cernetich A, Daly TM, Galvan G, Vaidya AB, Bergman LW, Burns JM Jr. 2005. Alteration in host cell tropism limits the efficacy of immunization with a surface protein of malaria merozoites. Infect Immun 73:6363–6371. doi: 10.1128/IAI.73.10.6363-6371.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Drew DR, O'Donnell RA, Smith BJ, Crabb BS. 2004. A common cross-species function for the double epidermal growth factor-like modules of the highly divergent plasmodium surface proteins MSP-1 and MSP-8. J Biol Chem 279:20147–20153. doi: 10.1074/jbc.M401114200. [DOI] [PubMed] [Google Scholar]
- 68.Delves MJ, Ruecker A, Straschil U, Lelievre J, Marques S, Lopez-Barragan MJ, Herreros E, Sinden RE. 2013. Male and female Plasmodium falciparum mature gametocytes show different responses to antimalarial drugs. Antimicrob Agents Chemother 57:3268–3274. doi: 10.1128/AAC.00325-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Angov E, Hillier CJ, Kincaid RL, Lyon JA. 2008. Heterologous protein expression is enhanced by harmonizing the codon usage frequencies of the target gene with those of the expression host. PLoS One 3:e2189. doi: 10.1371/journal.pone.0002189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thera MA, Doumbo OK, Coulibaly D, Laurens MB, Ouattara A, Kone AK, Guindo AB, Traore K, Traore I, Kouriba B, Diallo DA, Diarra I, Daou M, Dolo A, Tolo Y, Sissoko MS, Niangaly A, Sissoko M, Takala-Harrison S, Lyke KE, Wu Y, Blackwelder WC, Godeaux O, Vekemans J, Dubois MC, Ballou WR, Cohen J, Thompson D, Dube T, Soisson L, Diggs CL, House B, Lanar DE, Dutta S, Heppner DG Jr, Plowe CV. 2011. A field trial to assess a blood-stage malaria vaccine. N Engl J Med 365:1004–1013. doi: 10.1056/NEJMoa1008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Egan A, Waterfall M, Pinder M, Holder A, Riley E. 1997. Characterization of human T- and B-cell epitopes in the C terminus of Plasmodium falciparum merozoite surface protein 1: evidence for poor T-cell recognition of polypeptides with numerous disulfide bonds. Infect Immun 65:3024–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hensmann M, Li C, Moss C, Lindo V, Greer F, Watts C, Ogun SA, Holder AA, Langhorne J. 2004. Disulfide bonds in merozoite surface protein 1 of the malaria parasite impede efficient antigen processing and affect the in vivo antibody response. Eur J Immunol 34:639–648. doi: 10.1002/eji.200324514. [DOI] [PubMed] [Google Scholar]
- 73.Pichyangkul S, Tongtawe P, Kum-Arb U, Yongvanitchit K, Gettayacamin M, Hollingdale MR, Limsalakpetch A, Stewart VA, Lanar DE, Dutta S, Angov E, Ware LA, Bergmann-Leitner ES, House B, Voss G, Dubois MC, Cohen JD, Fukuda MM, Heppner DG, Miller RS. 2009. Evaluation of the safety and immunogenicity of Plasmodium falciparum apical membrane antigen 1, merozoite surface protein 1 or RTS,S vaccines with adjuvant system AS02A administered alone or concurrently in rhesus monkeys. Vaccine 28:452–462. doi: 10.1016/j.vaccine.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 74.Forbes EK, Biswas S, Collins KA, Gilbert SC, Hill AV, Draper SJ. 2011. Combining liver- and blood-stage malaria viral-vectored vaccines: investigating mechanisms of CD8+ T cell interference. J Immunol 187:3738–3750. doi: 10.4049/jimmunol.1003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McConkey SJ, Reece WH, Moorthy VS, Webster D, Dunachie S, Butcher G, Vuola JM, Blanchard TJ, Gothard P, Watkins K, Hannan CM, Everaere S, Brown K, Kester KE, Cummings J, Williams J, Heppner DG, Pathan A, Flanagan K, Arulanantham N, Roberts MT, Roy M, Smith GL, Schneider J, Peto T, Sinden RE, Gilbert SC, Hill AV. 2003. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat Med 9:729–735. doi: 10.1038/nm881. [DOI] [PubMed] [Google Scholar]
- 76.Mehlin C, Boni E, Buckner FS, Engel L, Feist T, Gelb MH, Haji L, Kim D, Liu C, Mueller N, Myler PJ, Reddy JT, Sampson JN, Subramanian E, Van Voorhis WC, Worthey E, Zucker F, Hol WG. 2006. Heterologous expression of proteins from Plasmodium falciparum: results from 1000 genes. Mol Biochem Parasitol 148:144–160. doi: 10.1016/j.molbiopara.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 77.Tsai CW, Duggan PF, Shimp RL Jr, Miller LH, Narum DL. 2006. Overproduction of Pichia pastoris or Plasmodium falciparum protein disulfide isomerase affects expression, folding and O-linked glycosylation of a malaria vaccine candidate expressed in P. pastoris. J Biotechnol 121:458–470. doi: 10.1016/j.jbiotec.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 78.Alaro JR, Lynch MM, Burns JM Jr. 2010. Protective immune responses elicited by immunization with a chimeric blood-stage malaria vaccine persist but are not boosted by Plasmodium yoelii challenge infection. Vaccine 28:6876–6884. doi: 10.1016/j.vaccine.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tonkin CJ, van Dooren GG, Spurck TP, Struck NS, Good RT, Handman E, Cowman AF, McFadden GI. 2004. Localization of organellar proteins in Plasmodium falciparum using a novel set of transfection vectors and a new immunofluorescence fixation method. Mol Biochem Parasitol 137:13–21. doi: 10.1016/j.molbiopara.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 80.Miura K, Takashima E, Deng B, Tullo G, Diouf A, Moretz SE, Nikolaeva D, Diakite M, Fairhurst RM, Fay MP, Long CA, Tsuboi T. 2013. Functional comparison of Plasmodium falciparum transmission-blocking vaccine candidates by the standard membrane-feeding assay. Infect Immun 81:4377–4382. doi: 10.1128/IAI.01056-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miura K, Swihart BJ, Deng B, Zhou L, Pham TP, Diouf A, Burton T, Fay MP, Long CA. 2016. Transmission-blocking activity is determined by transmission-reducing activity and number of control oocysts in Plasmodium falciparum standard membrane-feeding assay. Vaccine 34:4145–4151. doi: 10.1016/j.vaccine.2016.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.