ABSTRACT
The reproductive system complications of genital chlamydial infection include fallopian tube fibrosis and tubal factor infertility. However, the molecular pathogenesis of these complications remains poorly understood. The induction of pathogenic epithelial-mesenchymal transition (EMT) through microRNA (miRNA) dysregulation was recently proposed as the pathogenic basis of chlamydial complications. Focusing on fibrogenesis, we investigated the hypothesis that chlamydia-induced fibrosis is caused by EMT-driven generation of myofibroblasts, the effector cells of fibrosis that produce excessive extracellular matrix (ECM) proteins. The results revealed that the targets of a major category of altered miRNAs during chlamydial infection are key components of the pathophysiological process of fibrogenesis; these target molecules include collagen types I, III, and IV, transforming growth factor β (TGF-β), TGF-β receptor 1 (TGF-βR1), connective tissue growth factor (CTGF), E-cadherin, SRY-box 7 (SOX7), and NFAT (nuclear factor of activated T cells) kinase dual-specificity tyrosine (Y) phosphorylation-regulated kinase 1a (Dyrk1a). Chlamydial induction of EMT resulted in the generation of α-smooth muscle actin (α-SMA)-positive myofibroblasts that produced ECM proteins, including collagen types I and III and fibronectin. Furthermore, the inhibition of EMT prevented the generation of myofibroblasts and production of ECM proteins during chlamydial infection. These findings may provide useful avenues for targeting EMT or specific components of the EMT pathways as a therapeutic intervention strategy to prevent chlamydia-related complications.
KEYWORDS: Chlamydia, miRNAs, epithelial-mesenchymal transition (EMT), myofibroblasts, extracellular matrix proteins, fibrosis, Chlamydia trachomatis
INTRODUCTION
Chlamydia trachomatis genital infection is a major public health concern, the most common bacterial sexually transmitted disease (STD), with complications that include pelvic inflammatory disease, fibrosis, ectopic pregnancy, and tubal factor infertility. Tubal damage caused by Chlamydia is due to inflammatory fibrosis (scarring) that may lead to tubal occlusion and infertility (1, 2). While cytokine-mediated inflammation-induced mechanisms have been proposed to generally explain chlamydia-induced fibrosis (1, 3–5), the molecular pathogenesis is less understood. Pathophysiologically, fibrosis is a dysregulated tissue repair response to injury (e.g., infection or trauma), involving excessive deposition of extracellular matrix (ECM) proteins (i.e., collagens I and III, fibronectin, fibrins, and elastin) by effector cells called myofibroblasts, resulting in a loss of normal organ/tissue function. The origin of myofibroblasts during fibrosis include activation of neighboring tissue fibroblasts, bone marrow, and certain organ-specific cells and conversion of epithelial cells into myofibroblasts by a microRNA (miRNA)-driven differentiation process called epithelial-mesenchymal transition (EMT) (6–9). EMT converts normal epithelial cells into fibroblastic (elongated) mesenchymal cells that exhibit enhanced motility and invasiveness and high resistance to senescence and apoptosis, and it represents an important phase in epithelial response to development, differentiation, chronic inflammation, fibrogenesis, and tumor progression (7, 10). Mechanistically, EMT inducers (e.g., transforming growth factor β [TGF-β]) initiate both the canonical Smad and non-Smad (Akt and extracellular signal-regulated kinases 1/2 [ERK1/2]), Wnt, and focal adhesion signaling pathways to downregulate miRNAs and transcription factors that protect epithelial integrity but upregulate fibrogenic and oncogenic miRNAs and transcription factors that initiate EMT (8, 9). The prototypical hallmark of EMT is E-cadherin loss, representing a cadherin expression switching from E- to N- or T-cadherin (8). E-cadherin maintains epithelial functional integrity due to its role in cell-cell adhesive interaction mediated through its cytoskeletal interactions with cytoplasmic catenins to provide the epithelial cobble-stone-like tissue architecture, ensure the epithelial barrier, and aggregate forms as functional tissue glands. On the other hand, the mesenchymal markers induced during EMT include N-/T-cadherin, fibronectin, and vimentin. In addition, mesenchymal cells further differentiate into myofibroblasts, producing increased ECM components, consequent fibrogenesis, and alteration of epithelial integrity and functions (7, 8). Specifically, during fibrosis, EMT converts normal epithelial parenchymal tissue architecture into nonfunctional scar tissue (11) and alters the differentiated physiological functions of epithelial cells (i.e., secretory, barrier, and transport), leading to organ failure (12–14). Thus, EMT plays a major role in most organ and tissue fibrotic diseases, such as pulmonary, renal, and hepatic fibrosis, making it a current target in therapeutic intervention against several fibrotic diseases (6, 9, 15, 16). However, the role of EMT in the tubal fibrosis associated with genital chlamydial disease (1) has not been clarified.
The epithelial cell is the target of chlamydial infection at both the lower and upper reproductive tract, and recent reports have revealed that chlamydia induces EMT through dysregulation of miRNAs in the reproductive (17) or ocular (18) epithelium. These findings suggested that EMT plays a key role in the pathogenesis of chlamydia-induced fibrosis. In previous reports, we demonstrated that Chlamydia induced EMT via the fibrogenic and tumor promoters ZEB1, Snail1/2, and Tsp1, involving caspase-mediated dicer inactivation and miRNA dysregulation (17). Also, caspase inhibitors, specifically the caspase 3 or the pan-caspase inhibitor Z-VAD-FMK, prevented EMT (17) and chlamydia-induced infertility in vivo (19). Thus, caspase cleavage inactivation of dicer (20, 21) is a key upstream initiating event for miRNA-mediated, chlamydia-induced EMT that drives reproductive tissue fibrosis (2, 22), infertility (17), and possibly the cofactor role of chlamydia in human papillomavirus (HPV)-related invasive cervical carcinoma. Better knowledge of the key components of EMT and EMT inhibitors may provide targets for therapy against chlamydia complications. In the present study, we used bioinformatics to identify the targets of anti- and profibrotic miRNAs that are altered during genital chlamydial infection and investigated the role of these targets in the pathophysiological pathways of fibrogenesis. Also, using chlamydia-infected primary reproductive tract epithelial cells that undergo EMT (17), we investigated the hypothesis that Chlamydia-induced EMT generates myofibroblasts that produce the ECM proteins causing fibrosis.
RESULTS
Identification and role of targets of fibrogenic and antifibrosis miRNAs during genital chlamydial infection.
We recently reported that chlamydial genital infection caused dysregulation of miRNAs that control epithelial functional integrity and induced EMT in the reproductive epithelial tissues (17). In the present study, we continued the study but focused on the detailed bioinformatics analysis of the miRNAs that regulate fibrosis and the role of their targets in the pathophysiolocal pathway of fibrogenesis. As shown in Table 1, genital chlamydial infection that resulted in infertility (17) was associated with the downregulation of key miRNAs (i.e., mir-24, miR-29 family, miR-101-1, miR-185, and miR-221) that prevent epithelial fibrosis through biological processes mediated by their targets; however, there was upregulation of the major fibrogenic miRNAs, miR-9 and miR-199b, that promote fibrogenesis through biological processes mediated by their targets (6). Specifically, target function validation analyses using TargetScanMouse (http://www.targetscan.org/mmu_71/) and other miRNA software (6, 23) revealed that members of the miR-29 family target and actively suppress the expression of ECM proteins, especially collagen types I, III, and IV, to control fibrosis. The targets for miR-15a are TGF-β receptor 1 (TGF-βR1) and connective tissue growth factor (CTGF) (6, 24), while miRNA-27 targets Sp1, TGF-βR1, SMAD2, and Gremlin1 (25, 26) to control fibrogenesis. miR-101-1 targets fos on the tumor necrosis factor alpha (TNF-α)–TGF-β signaling pathway to prevent the production ECM and prevent fibrosis (23, 27–29). The TGF-β maturation processing protease, furin, is the direct target of miR-24 (30–32). miR-203 controls fibrosis and maintains epithelial integrity by specifically targeting SMAD3, a key intracellular mediator of TGF-β signaling, leading to EMT and production of ECM proteins during fibrosis (6, 33). In addition, miR-203 targets the EMT- and cell invasion-promoting transcription factor Snail2 (Slug) (34) and SMAD3 (33, 34) to prevent fibrosis. Furthermore, among the key downregulated miRNAs, miR-185 targets TGF-β and collagen type I to control tissue fibrosis (6, 35) and miR-221 targets fibrosis-promoting osteopontin, which is also the target of the polyphenol epigallocatechin-3-gallate (EGCG), which is found in green tea and upregulates miR-221 (36). On the other hand, miR-9 is a key fibrogenic and oncogenic miRNA that targets E-cadherin and the tumor suppressor and transcription SRY-box 7 (SOX7) of the SOX transcription factor family (8, 37). Also, miR-199b targets the NFAT (nuclear factor of activated T cells) kinase dual-specificity tyrosine (Y) phosphorylation-regulated kinase 1a (Dyrk1a) to suppress calcineurin-NFAT signaling and fibrosis (38, 39). Thus, as previously surmised (17), chlamydia downregulated miRNAs that control EMT and fibrosis to protect epithelial functional integrity but upregulated fibrogenic miRNAs that induce pathogenic EMT and profibrotic ECM proteins (6–9). These results suggested that key effector cells and molecules that drive fibrosis, such as myofibroblasts and ECM proteins, are produced during chlamydial infection in association with EMT induction. While the production of ECM proteins in the oviducts of chlamydia-infected mice has been demonstrated in mice (1, 2), the generation of myofibroblasts is yet to be shown.
TABLE 1.
Summary of miRNAs used in this study
| No. | miRNA | Expression in reproductive tract during Chlamydia infection (fold change) | Target(s)/role in fibrogenesis | Reference(s) |
|---|---|---|---|---|
| 1 | miR-15a | Downregulation (∼5) | TGF-βR1, CTGF/prevention | 6, 24 |
| 2 | miR-24 | Downregulation (∼3) | Furin/prevention | 30–32 |
| 2 | miR-27a | Downregulation (∼4) | Sp1, TGF-βR1, SMAD2, Gremlin1/prevention | 25, 26 |
| 3 | miR-29a | Downregulation (∼6) | TGF-β, collagen types I, III, and IV/prevention | 6, 23 |
| 4 | miR-29b | Downregulation (∼6) | Collagen type I, α-SMA, TGF-βR1, SMAD2, Gremlin1/prevention | 6, 49, 50 |
| 5 | miR-101-1 | Downregulation (∼2) | TGF-βR1, fos, KLF6/prevention | 23, 27, 28 |
| 6 | miR-185 | Downregulation (∼2) | TGF-β1, collagen I/prevention | 6, 35 |
| 7 | miR-203 | Downregulation (∼2) | Snail2 (Slug), SMAD3/prevention | 33, 34 |
| 8 | miR-221 | Downregulation (∼3) | Osteopontin/prevention | 36 |
| 9 | miR-9 | Upregulation (∼6) | E-cadherin/SOX7/promotion | 8 |
| 10 | miR-199b | Upregulation (∼3) | Dyrk1a, E-cadherin, claudin/promotion | 38, 39 |
Chlamydial induction of EMT results in the generation of myofibroblasts, the effector cells of fibrosis.
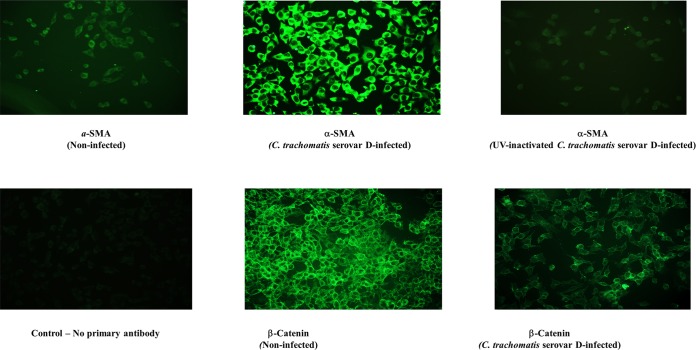
It is unknown whether myofibroblasts are generated during chlamydia-induced EMT (17). In several organ and tissue fibrosis systems, EMT contributes to fibrogenesis through the generation of myofibroblasts, the effector cells of fibrosis that produce the excess ECM components whose accumulation results in scars, a loss of normal tissue function, and chronic organ/tissue disease manifestation (7, 8, 16). Alpha smooth muscle actin (α-SMA) is a reliable marker of myofibroblasts (8), and so we investigated whether α-SMA-positive cells could be generated from chlamydia-infected primary epithelial cells. The results shown in Fig. 1 indicated that chlamydia-induced EMT (i.e., suppression of normal epithelial markers, such as β-catenin) is accompanied by the generation of α-SMA-positive cells (myofibroblasts). The results also indicated that EMT induction and generation of myofibroblasts required live chlamydiae, suggesting that it is an active, chlamydia-driven process. We next investigated the ability of these α-SMA-positive cells (myofibroblasts) to secrete ECM proteins during chlamydial infection.
FIG 1.
Chlamydia infection of epithelial cells that induces EMT results in the generation of myofibroblasts (effector cells of fibrosis). Monolayers of murine oviduct epithelial cells were infected with C. trachomatis serovar D, and after 48 h, immunofluorescence staining of the cells for epithelial and markers as well as the specific myofibroblast marker alpha smooth muscle actin (α-SMA) was performed on infected and noninfected monolayers by standard procedures. Fluoresceinated antibodies against the following markers were used: β-catenin and α-SMA. Antibodies stained both intracellular and surface antigens. Images were acquired with a 20× objective on a Nikon fluorescence microscope with the same microscope settings and exposure time. The representative slides are from at least 4 repeated experiments showing similar results.
Chlamydial induction of EMT also results in the generation of ECM proteins.
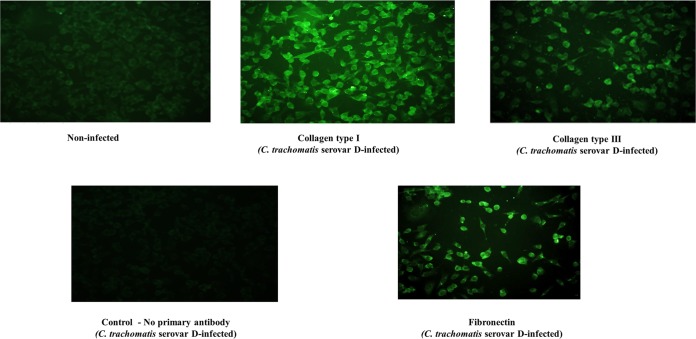
The ability of chlamydia to induce obstructive and dysfunctional epithelial fibrosis that partly replicated the human fallopian tube fibrotic occlusion disease caused by chlamydia has been demonstrated (1, 2). We tested the hypothesis that the induction of EMT and generation of myofibroblasts result in the production of excessive major components of the ECM proteins, such as collagen types I and III and fibronectin. As shown in Fig. 2, chlamydial infection of reproductive tract epithelial cells results in the production of excessive collagen types I and III and fibronectin, which are key components of the ECM proteins and the hallmark of fibrosis.
FIG 2.
Chlamydia infection results in the production of ECM proteins. Monolayers of murine oviduct epithelial cells were infected with C. trachomatis serovar D, and after 48 h, immunofluorescence staining of the cells for collagen types I and III was performed on infected and noninfected monolayers by standard procedures. Images were acquired with a 20× objective on a Nikon fluorescence microscope with the same microscope settings and exposure time. The representative slides are from at least 3 repeated experiments showing similar results.
Inhibition of EMT prevents chlamydia-induced fibrosis (myofibroblasts and ECM proteins).
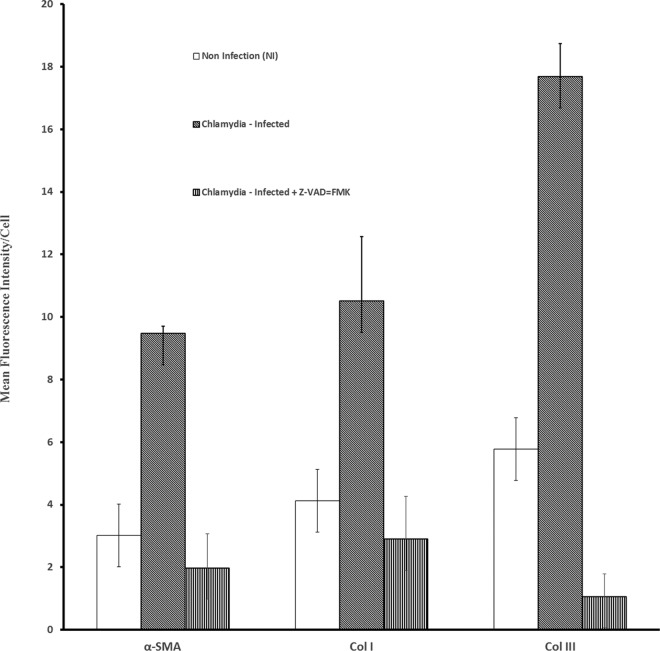
In confirmation of the general hypothesis that EMT drives chlamydial complications, we previously showed that inhibition of EMT, using the pan-caspase inhibitor Z-VAD-fmk, protected chlamydia-infected mice from infertility (17, 19). We therefore tested the hypothesis that the inhibition of EMT would also prevent myofibroblast generation and ECM protein production during chlamydial infection. As shown in Fig. 3, chlamydial infection caused significant upregulation of α-SMA expression (P > 0.000018), collagen type I (P > 0.00081), and collagen type III (P > 0.000001), indicating the generation of myofibroblasts and concurrent production of ECM components; however, treatment of chlamydia-infected epithelial cells with the EMT/caspase inhibitor substantially reduced the generation of myofibroblasts (P > 0.0000065) and the production of ECM proteins, specifically collagen types I (P > 0.000818) and III (P > 0.0000001). The results indicated that fibrosis can be controlled by inhibiting EMT.
FIG 3.
EMT inhibitor suppressed myofibroblast generation and ECM protein production. Monolayers of murine oviduct epithelial cells were infected with C. trachomatis serovar D in the presence or absence of 50 mM Z-VAD-fmk. Z-FA-fmk was used in control cultures. After 48 h, immunofluorescence staining of the cells for collagen types I and III and α-SMA was performed on infected and noninfected monolayers by standard procedures. Quantification of fluorescence was performed by scanning fluorescent-stained cells with a 20× objective on a Nikon fluorescence microscope using NIS-Elements imaging software version 3.20 (Nikon Instruments Inc., Melville, NY). Images were acquired for experimental and control cultures with the same microscope settings, exposure time, and background. The mean fluorescence intensity per cell and standard deviations were calculated from 6 scanned fields per slide. Plotted data were derived from 4 independent experiments. Chlamydial infection caused significant upregulation of α-SMA expression (P > 0.000018), collagen type I (P > 0.00081), and collagen type III (P > 0.000001); however, treatment of chlamydia-infected epithelial cells with the EMT/caspase inhibitor substantially reduced the generation of myofibroblasts (P > 0.002) and the production of collagen types I (P > 0.000818) and III (P > 0.0000001).
DISCUSSION
We recently demonstrated that Chlamydia induced EMT in vitro and in vivo, marked by the suppression of normal epithelial cell markers but upregulation of mesenchymal cell markers of pathological EMT (17). Chlamydial induction of EMT involved caspase-mediated dicer inactivation and miRNA dysregulation (17). Also, caspase inhibitors, specifically the caspase 3 or the pan-caspase inhibitor Z-VAD-FMK, prevented EMT (17) and chlamydia-induced infertility in vivo (19). In the present study, we focused on the role of EMT in the etiology of chlamydia-induced fibrosis. The detailed bioinformatics analyses revealed that a major category of miRNAs dysregulated by chlamydia during genital chlamydial infection is involved in the regulation of fibrogenesis and EMT. The targets of fibrosis-related miRNAs are directly involved in the pathophysiological pathways of fibrogenesis (6, 8, 16). Thus, these target molecules include the ECM proteins (i.e., fibronectin and collagen types I, III, and IV), TGF-β, TGF-βR1, connective tissue growth factor, E-cadherin, SRY-box 7 (SOX7), and NFAT kinase dual-specificity tyrosine (Y) phosphorylation-regulated kinase 1a (Dyrk1a). The key role of the miR-29 family in organ fibrosis is also underscored in this study, which revealed the targets to include the ECM proteins, TGF-β, and TGF-βR1. Since TGF-β and the receptors and the ECM proteins are major inducers or promoters of EMT and fibrosis, they are targets of most antifibrotic miRNAs. We further demonstrated that chlamydial induction of EMT resulted in the generation of myofibroblasts, the effector cells of fibrosis that produce ECM proteins, including fibronectin and collagen types I and III. This finding is corroborated by previous reports showing that chlamydia induced obstructive reproductive tract fibrosis that partly replicated human fallopian tube fibrotic occlusion disease (1, 2). The finding that EMT induction and generation of myofibroblasts required live chlamydiae suggested that chlamydia-induced EMT is an active, chlamydia-driven host-parasite pathobiological interaction. Furthermore, the inhibition of chlamydia-induced EMT prevented the generation of myofibroblasts and production of ECM proteins, which could at least partly explain the mechanism by which the EMT inhibitor prevented chlamydia-induced infertility in vivo (19). These findings would suggest that in addition to modulating miRNA expression (40), targeting EMT or specific components of the EMT pathways as a therapeutic intervention strategy may prevent chlamydia-related complications. Among other strategies, the use of small-molecule inhibitors, such as antagomirs, small interfering RNA (siRNA) or antisense oligonucleotide, miRNA sponges, or antibodies and chemical inhibitors, represents a potentially viable therapeutic strategy for the clinical management of fibrosis (8, 9).
MATERIALS AND METHODS
Chlamydia trachomatis stock, animal infection, and assessment of infertility.
Stocks of C. trachomatis serovars D and L were propagated in HeLa cells, and the titers of purified elementary bodies (EBs) were determined as inclusion-forming units (IFU) per milliliter by standard procedure (41). Mice were infected intravaginally with 1 × 105 IFU of C. trachomatis serovar L2 or serovar D per mouse while under the long-acting anesthetic sodium pentobarbital (30 μg/kg [of body weight]; Sigma-Aldrich, St. Louis, MO) approximately 5 days after intramuscular administration of 2.5 μg of medroxyprogesterone acetate (Depo Provera; Pfizer Inc., New York, NY). These conditions are a key factor to a successful mouse model of chlamydial genital infection using the human chlamydial strains (42). Control mice were sham infected with phosphate-buffered saline (PBS). The course of the infection was monitored by tissue culture isolation of chlamydiae from cervicovaginal swabs and enumeration of chlamydial inclusions by the immunofluorescence method (41); infertility was assessed by mating of mice with proven fertile males, as previously described (43). In addition to fertility assessment, animals were visually and microscopically inspected and scored for uni- and bilateral hydrosalpinx or cysts, inflammation, and the presence of other abnormalities in the reproductive system (43, 44). All animal protocols were approved by the CDC Institutional Animal Care and Use Committee (IACUC).
Immunohistochemistry.
The murine oviduct epithelial cell line (C57epi.1) that supports the growth of chlamydiae (45) was kindly provided by Raymond Johnson, Yale University, New Haven, CT. EMT markers (E- and T-cadherin, β-catenin, fibronectin, and Snail1/2), myofibroblasts (alpha smooth muscle actin [α-SMA]), and ECM proteins (collagen types I and III) were analyzed by immunofluorescence staining of C57epi.1 cells after C. trachomatis D infection (multiplicity of infection [MOI] = 1) or mock infection of monolayers at 48 h. In preliminary studies (unpublished data), we established that there was no difference in the abilities of C. trachomatis (serovars D and L) and Chlamydia muridarum to induce EMT or cause dysregulation of miRNAs. So we chose to use C. trachomatis serovar D in these experiments because it is a more common human agent than serovar L. The pan-caspase inhibitor, Z-VAD-FMK, and the control Z-FA-FMK were used in culture at 50 μM. Quantification of fluorescence was performed by scanning fluorescence-stained cells with a 20× objective on a Nikon fluorescence microscope using the NIS-Elements imaging software version 3.20 (Nikon Instruments Inc., Melville, NY). Where quantitation was performed, the mean fluorescence intensity per cell and standard deviations were calculated from 6 scanned fields per slide.
MicroRNA analysis by microarray and real-time PCR.
Quantitative miRNA microarray analysis was performed with the Signosis miRNA Array IV service, according to the company's standard procedure (Signosis, Inc., Sunnyvale, CA), as previously described (17, 19). Briefly, total RNA isolated from homogenized oviduct tissues was annealed to a biotin-UTP-labeled oligonucleotide probe mixture corresponding to 540 randomly selected miRNAs which have been reported to play a role in fibrosis according to aliterature search (46). After hybridization, the miRNA expression arrays were detected by streptavidin-horseradish peroxidase (HRP) chemiluminescence. The chemiluminescent signals were acquired using the Alpha Innotech FluorChem FC2 imaging system. With the array assay, the expression of 540 miRNAs was profiled in the samples. Quantitative real-time PCR was used to validate the microarray data for selected miRNAs based on a minimum of a 2-fold increase or decrease among the experimental groups observed in the microarrays. There was a focus on miRNAs showing a decrease or increase in the oviducts from infected infertile mice compared to noninfected mice. For real-time miRNA PCR, the miRNA-specific oligonucleotide mix was used instead of the random oligomix. The ligated products were eluted and mixed with miRNA-specific qPCR primers and SYBR miRNA PCR buffer mix. The real-time PCR was conducted on the ABI 7700 system using TaqMan small-RNA assays (Applied Biosystems) and included 35 PCR cycles of 95°C for 15 s and 50 s. Using the small RNA U6 as an internal (endogenous) control, the relative expression of miRNAs was normalized to U6 miRNA expression. Real-time PCR data were analyzed using the threshold cycle (ΔΔCT) method by the standard procedure (46). Information on miRNA target mRNAs and genes was obtained from several Web-accessible miRNA database search programs, including those found at http://www.microrna.org, http://www.miRBase.org, and http://www.targetscan.org and in published reports (6, 47). Experiments were repeated 4 times, with at least 6 oviducts in each group. Results presented are for differentially expressed miRNAs with at least 2-fold down- or upregulation from oviducts harvested between 1 and 12 weeks (80 days) postinfection, relative to those in noninfected mice. Mock-infected mice were 100% fertile, with at least 10 embryos per mouse, whereas chlamydia-infected mice were infertile, with fewer than one embryo per mouse, as previously reported (17, 19).
Statistical analysis.
The data derived from different experiments were analyzed and compared by performing a one- or two-tailed t test, and the relationship between different experimental groupings was assessed by analysis of variance (ANOVA). Statistical significance was judged at a P value of <0.05.
Ethics statement.
All animal protocols were approved by the CDC IACUC under protocol 2605IGIMOUC-A1. The CDC IACUC is guided by title 9, chapter I, subchapter A—animal welfare (USDA regulations [48]).
ACKNOWLEDGMENTS
We thank Chryssa Kanellopoulou of the National Institutes of Health (NIH), Bethesda, MD, for providing us anti-mouse Dicer antisera for comparison with the commercially available anti-Dicer antibodies.
The conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
Funding was received from the Centers for Disease Control and Prevention (CDC) and PHS grants 1SC1AII03041-01A1 (NIGMS), 1SC2HD086066-01A1 (NICHD), 5R01AI041231 (NIAID), and 1 C06 RR18386 (NCRR) from the NIH.
REFERENCES
- 1.Hafner L. 2015. Pathogenesis of fallopian tube damage caused by Chlamydia trachomatis infections. Contraception 92:108–115. doi: 10.1016/j.contraception.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Shah A, Schripsema J, Imtiaz M, Sigar I, Kasimos J, Matos P, Inouye S, Ramsey K. 2005. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis 32:49–56. doi: 10.1097/01.olq.0000148299.14513.11. [DOI] [PubMed] [Google Scholar]
- 3.Darville T, Hiltke T. 2010. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J Infect Dis 201:S114–S125. doi: 10.1086/652397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imtiaz M, Distelhorst J, Schripsema J, Sigar I, Kasimos J, Lacy S, Ramsey K. 2007. A role for matrix metalloproteinase-9 in pathogenesis of urogenital Chlamydia muridarum infection in mice. Microbes Infect 9:1561–1566. doi: 10.1016/j.micinf.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ault K, Kelly K, Ruther P, Izzo A, Izzo L, Sigar I, Ramsey K. 2002. Chlamydia trachomatis enhances the expression of matrix metalloproteinases in an in vitro model of the human fallopian tube infection. Am J Obstet Gynecol 187:1377–1388. doi: 10.1067/mob.2002.126850. [DOI] [PubMed] [Google Scholar]
- 6.Zou X, Liu T, Gong Z, Hu C, Zhang Z. 2017. MicroRNAs-mediated epithelial-mesenchymal transition in fibrotic diseases. Eur J Pharmacol 796:190–206. doi: 10.1016/j.ejphar.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Lamouille S, Derynck R. 2014. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee K, Nelson C. 2012. New insights into the regulation of epithelial-mesenchymal transition and tissue fibrosis. Int Rev Cell Mol Biol 294:171–221. doi: 10.1016/B978-0-12-394305-7.00004-5. [DOI] [PubMed] [Google Scholar]
- 9.Kothari A, Mi Z, Zapf M, Kuo P. 2014. Novel clinical therapeutics targeting the epithelial to mesenchymal transition. Clin Transl Med 3:35–48. doi: 10.1186/s40169-014-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan J, Gumireddy K, Li A, Huang Q. 2013. Regulation of mesenchymal phenotype by microRNAs in cancer. Curr Cancer Drug Targets 13:930–934. doi: 10.2174/15680096113136660098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sweetwyne M, Murphy-Ullrich J. 2012. Thrombospondin1 in tissue repair and fibrosis: TGF-β-dependent and independent mechanisms. Matrix Biol 31:178–186. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boini K, Xia M, Xiong J, Li C, Payne L, Li P. 2012. Implication of CD38 gene in podocyte epithelial-to-mesenchymal transition and glomerular sclerosis. J Cell Mol Med 16:1674–1685. doi: 10.1111/j.1582-4934.2011.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Lin H, Kong S, Wang S, Wang H, Wang H, Armant D. 2013. Physiological and molecular determinants of embryo implantation. Mol Aspects 34:939–980. doi: 10.1016/j.mam.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Q, Zhang Y, Elad D, Jaffa A, Cao Y, Ye X, Duan E. 2013. Navigating the site for embryo implantation: biomechanical and molecular regulation of intrauterine embryo distribution. Mol Aspects Med 34:1024–1042. doi: 10.1016/j.mam.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Li M, Luan F, Zhao Y, Hao H, Zhou Y, Han W, Fu X. 2016. Epithelial-mesenchymal transition: an emerging target in tissue fibrosis. Exp Biol Med (Maywood) 241:1–13. doi: 10.1177/1535370215597194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vettori S, Gay S, Distler O. 2012. Role of microRNAs in fibrosis. Open Rheumatol J 6:130–139. doi: 10.2174/1874312901206010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igietseme J, Omosun Y, Stuchlik O, Reed M, Partin J, He Q, Joseph K, Ellerson D, Bollweg B, George Z, Eko F, Bandea C, Liu H, Yang G, Shieh W, Pohl J, Karem K, Black CM. 2015. Role of epithelial-mesenchyme transition in Chlamydia pathogenesis. PLoS One 10:e0145198. doi: 10.1371/journal.pone.0145198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajić J, Inic-Kanada A, Stein E, Dinić S, Schuerer N, Uskoković A, Ghasemian E, Mihailović M, Vidaković M, Grdović N, Barisani-Asenbauer T. 2017. Chlamydia trachomatis infection is associated with E-cadherin promoter methylation, downregulation of E-cadherin expression, and increased expression of fibronectin and α-SMA—implications for epithelial-mesenchymal transition. Front Cell Infect Microbiol 7:253. doi: 10.3389/fcimb.2017.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Igietseme JU, Omosun Y, Partin J, Goldstein J, He Q, Joseph K, Ellerson D, Ansari U, Eko FO, Bandea C, Zhong G, Black CM. 2013. Prevention of Chlamydia-induced infertility by inhibition of local caspase activity. J Infect Dis 207:1095–1104. doi: 10.1093/infdis/jit009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa A, Shi Y, Kage-Nakadai E, Mitani S, Xue D. 2010. Caspase-dependent conversion of dicer ribonuclease into a death-promoting deoxyribonuclease. Science 328:327–334. doi: 10.1126/science.1182374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghodgaonkar M, Shah R, Kandan-Kulangara F, Affar E-B, Qi H, Wiemer E, Shah G. 2009. Abrogation of DNA vector-based RNAi during apoptosis in mammalian cells due to caspase-mediated cleavage and inactivation of Dicer-1. Cell Death Differ 16:858–868. doi: 10.1038/cdd.2009.15. [DOI] [PubMed] [Google Scholar]
- 22.Debattista J, Timms P, Allan J. 2003. Immunopathogenesis of Chlamydia trachomatis infections in women. Fertil Steril 79:1273–1287. doi: 10.1016/S0015-0282(03)00396-0. [DOI] [PubMed] [Google Scholar]
- 23.Xiao L, He H, Ma L, Da M, Cheng S, Duan Y, Wang Q, Wu H, Song X, Duan W, Tian Z, Hou Y. 2017. Effects of miR-29a and miR-101a expression on myocardial interstitial collagen generation after aerobic exercise in myocardial-infarcted rats. Arch Med Res 48:27–34. doi: 10.1016/j.arcmed.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Rawal S, Munasinghe P, Nagesh P, Lew J, Jones G, Williams M, Davis P, Bunton D, Galvin I, Manning P, Lamberts R, Katare R. 2017. Down-regulation of miR-15a/b accelerates fibrotic remodelling in the Type 2 diabetic human and mouse heart. Clin Sci (Lond) 131:847–863. doi: 10.1042/CS20160916. [DOI] [PubMed] [Google Scholar]
- 25.Ye H, Ling S, Castillo A, Thomas B, Long B, Qian J, Perez-Polo J, Ye Y, Chen X, Birnbaum Y. 2013. Nebivolol induces distinct changes in profibrosis microRNA expression compared with atenolol, in salt-sensitive hypertensive rats. Hypertension 61:1008–1013. doi: 10.1161/HYPERTENSIONAHA.111.00892. [DOI] [PubMed] [Google Scholar]
- 26.Church R, Ali I, Tate M, Lavin D, Krishnakumar A, Kok H, Hombrebueno J, Dunne P, Bingham V, Goldschmeding R, Martin F, Brazil D. 2017. Gremlin1 plays a key role in kidney development and renal fibrosis. Am J Physiol Renal Physiol 312:F1141–F1157. doi: 10.1152/ajprenal.00344.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tu X, Zhang H, Zhang J, Zhao S, Zheng X, Zhang Z, Zhu J, Chen J, Dong L, Zang Y, Zhang J. 2014. MicroRNA-101 suppresses liver fibrosis by targeting the TGFβ signalling pathway. J Pathol 234:46–59. doi: 10.1002/path.4373. [DOI] [PubMed] [Google Scholar]
- 28.Pan Z, Sun X, Shan H, Wang N, Wang J, Ren J, Feng S, Xie L, Lu C, Yuan Y, Zhang Y, Wang Y, Lu Y, Yang B. 2012. MicroRNA-101 inhibited postinfarct cardiac fibrosis and improved left ventricular compliance via the FBJ osteosarcoma oncogene/transforming growth factor-β1 pathway. Circulation 126:840–850. doi: 10.1161/CIRCULATIONAHA.112.094524. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan D, Ferris M, Nguyen H, Abboud E, Brody A. 2009. TNF-alpha induces TGF-beta1 expression in lung fibroblasts at the transcriptional level via AP-1 activation. J Cell Mol Med 13:1866–1876. doi: 10.1111/j.1582-4934.2008.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Z, Lu S, Xu M, Liu P, Ren R, Ma W. 2017. Role of miR-24, furin, and transforming growth factor-β1 signal pathway in fibrosis after cardiac infarction. Med Sci Monit 23:65–70. doi: 10.12659/MSM.898641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luna C, Li G, Qiu J, Epstein D, Gonzalez P. 2011. MicroRNA-24 regulates the processing of latent TGFβ1 during cyclic mechanical stress in human trabecular meshwork cells through direct targeting of FURIN. J Cell Physiol 226:1407–1414. doi: 10.1002/jcp.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Huang W, Xu R, Nie Y, Cao X, Meng J, Xu X, Hu S, Zheng Z. 2012. MicroRNA-24 regulates cardiac fibrosis after myocardial infarction. J Cell Mol Med 16:2150–2160. doi: 10.1111/j.1582-4934.2012.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu D, Hu Y, Xu W, Yu H, Yang N, Ni S, Fu R. 6 June 2017. miR-203 inhibits the expression of collagen-related genes and the proliferation of hepatic stellate cells through a SMAD3-dependent mechanism. Mol Med Rep doi: 10.3892/mmr.2017.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang L, Liang H, Wang Y, Gao S, Yin K, Liu Z, Zheng X, Lv Y, Wang L, Zhang C-Y, Chen X, Xu G, Zhang W, Zou X. 2016. Mir-203 suppresses tumor cell proliferation, migration and invasion by targeting Slug in gastric cancer. Protein Cell 7:383–387. doi: 10.1007/s13238-016-0259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao K, Luo X, Wang X, Gao Z. 2017. MicroRNA-185 regulates transforming growth factor-β1 and collagen-1 in hypertrophic scar fibroblasts. Mol Med Rep 15:1489–1496. doi: 10.3892/mmr.2017.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arffa M, Zapf M, Kothari A, Chang V, Gupta G, Ding X, Al-Gayyar M, Syn W, Elsherbiny NNM, Kuo P, Mi Z. 2016. Epigallocatechin-3-gallate upregulates miR-221 to inhibit osteopontin-dependent hepatic fibrosis. PLoS One 11:e0167435. doi: 10.1371/journal.pone.0167435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han L, Wang W, Ding W, Zhang L. 7 March 2017. MiR-9 is involved in TGF-β1-induced lung cancer cell invasion and adhesion by targeting SOX7. J Cell Mol Med doi: 10.1111/jcmm.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.da Costa Martins P, Salic K, Gladka M, Armand A, Leptidis S, el Azzouzi H, Hansen A, Coenen-de Roo C, Bierhuizen M, van der Nagel R, van Kuik J, de Weger R, de Bruin A, Condorelli G, Arbones M, Eschenhagen T, De Windt L. 2010. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat Cell Biol 12:1220–1227. doi: 10.1038/ncb2126. [DOI] [PubMed] [Google Scholar]
- 39.Preusse M, Theis F, Mueller N. 2016. miTALOS v2: analyzing tissue specific microRNA function. PLoS One 11:e0151771. doi: 10.1371/journal.pone.0151771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grange C, Collino F, Tapparo M, Camussi G. 2014. Oncogenic micro-RNAs and renal cell carcinoma. Front Oncol 4:49. doi: 10.3389/fonc.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Igietseme JU, He Q, Joseph K, Eko F, Lyn D, Ananaba G, Campbell A, Bandea C, Black CM. 2009. Role of T lymphocytes in the pathogenesis of Chlamydia disease. J Infect Dis 200:926–934. doi: 10.1086/605411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuffrey M, Taylor-Robinson D. 1981. Progesterone as a key factor in the development of a mouse model for genital-tract infection with Chlamydia trachomatis. FEMS Microbiol Lett 12:111–115. doi: 10.1111/j.1574-6968.1981.tb07622.x. [DOI] [Google Scholar]
- 43.Tuffrey M, Alexander F, Woods C, Taylor-Robinson D. 1992. Genetic susceptibility to chlamydial salpingitis and subsequent infertility in mice. J Reprod Fertil 95:31–38. [DOI] [PubMed] [Google Scholar]
- 44.Pal S, Hui W, Peterson EM, de la Maza LM. 1998. Factors influencing the induction of infertility in a mouse model of Chlamydia trachomatis ascending genital infection. J Med Microbiol 47:599–605. doi: 10.1099/00222615-47-7-599. [DOI] [PubMed] [Google Scholar]
- 45.Johnson R. 2004. Murine oviduct epithelial cell cytokine responses to Chlamydia muridarum infection include interleukin-12–p70 secretion. Infect Immun 72:3951–3960. doi: 10.1128/IAI.72.7.3951-3960.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang CC, Zhang QY, Liu Z, Clynes RA, Suciu-Foca N, Vlad G. 2012. Downregulation of inflammatory microRNAs by Ig-like transcript 3 is essential for the differentiation of human CD8(+) T suppressor cells. J Immunol 188:3042–3052. doi: 10.4049/jimmunol.1102899. [DOI] [PubMed] [Google Scholar]
- 47.Nagaraja A, Andreu-Vieyra C, Franco H, Ma L, Chen R, Han D, Zhu H, Agno J, Gunaratne P, DeMayo FJ, Matzuk MM. 2008. Deletion of Dicer in somatic cells of the female reproductive tract causes sterility. Mol Endocrinol 22:2336–2352. doi: 10.1210/me.2008-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Federal Register. 1989. Final rules: animal welfare; 9 CFR parts 1, 2, and 3. Fed Regist 54:36112–36163. https://www.nal.usda.gov/awic/final-rules-animal-welfare-9-cfr-parts-1-2-and-3. [Google Scholar]
- 49.Zeng X, Huang C, Senavirathna L, Wang P, Liu L. 2017. miR-27b inhibits fibroblast activation via targeting TGF-β signaling. BMC Cell Biol 18:9. doi: 10.1186/s12860-016-0123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Graham J, Williams C, Yang Z. 2014. MicroRNA-29b targets gremlin 1 to modulate fibrotic responses in pulmonary cells. J Cell Biochem 115:1539–1548. doi: 10.1002/jcb.24809. [DOI] [PubMed] [Google Scholar]