Abstract
Aims
Hypertension is only controlled in approximately 35% of the patients, which could be partially due to nonadherence. Recently, bioanalytical assessment of adherence to blood pressure (BP) lowering drugs has gaining interest. Our aim was to explore possible determinants of nonadherence in treatment resistant hypertension, assessed by objective screening for antihypertensive agents in serum. The secondary aim was to study the effect of adherence on the change in BP.
Methods
This project was a substudy of SYMPATHY; an open‐label randomized‐controlled trial to assess the effect of renal denervation on BP 6 months after treatment compared to usual care in patients with resistant hypertension. Stored serum samples were screened for antihypertensive agents to assess adherence at baseline and 6 months after intervention, using liquid chromatography–tandem mass spectrometry. Office and 24‐h BP were measured on the same day as blood was sampled. Patients and physicians were unaware of adherence measurements.
Results
Ninety‐eight baseline and 83 6‐month samples were available for analysis. Sixty‐eight percent [95% confidence interval (CI) 59–78%] of the patients was nonadherent (n = 67). For every onw pill more prescribed, 0.785 [95%CI 0.529–0.891] prescribed pill was less detected in blood. A decrease of one pill in adherence between baseline and 6 months was associated with a significant rise in office systolic BP of 4 (95%CI 0.230–8.932) mmHg.
Conclusion
Objective measurement of BP lowering drugs in serum, as a tool to assess adherence, showed that nonadherence was very common in patients with apparent resistant hypertension. Furthermore, the assessment results were related to (changes in) blood pressure. Our findings provide direct and objective methodology to help the physician to understand and to improve the condition of apparent resistant hypertension.
Keywords: adherence, randomized controlled trial, resistant hypertension
What is Already Known about this Subject
Hypertension is only pharmacologically controlled in 35% of the patients.
Objective screening of BP lowering drugs showed that poor adherence is common among resistant hypertensive patients.
Non‐adherence to BP lowering medication might result in unnecessary diagnostic tests and interventions, such as renal denervation, to improve BP.
What this Study Adds
(Change in) adherence, quantified with a bioanalytical assay using serum, was strongly related to number of prescribed BP lowering drugs and (change in) office blood pressure.
Our findings provide objective methodology to help the physician to understand and to improve the condition of apparent resistant hypertension.
Introduction
Hypertension, defined as an office systolic blood pressure (SBP) > 140 mmHg and/or diastolic blood pressure (DBP) > 90 mmHg, is only controlled in approximately 35% of patients 1, 2. Worldwide, 874 million adults suffer from an office SBP of 140 mmHg or higher, which is associated with an annual death rate of 106 per 100 000 patients 3. For the pharmacological control of hypertension, adherence to blood pressure (BP) lowering drugs is essential. Poor adherence is associated with a higher residual cardiovascular risk (for the patient) and a high healthcare burden, due to greater effort to improve BP with additional diagnostic tests and interventions, such as renal denervation (RDN) 4, 5. Previous cross‐sectional studies published information on factors of nonadherence in patients with hypertension 6, 7, 8, 9, 10. However, almost all studies on adherence in hypertension are based on the self‐assessment Morrisky questionnaire; a method shown to overestimate adherence and to be potentially biased. In contrast, the results of objective adherence measurements using drug screening in urine and blood are unbiased. In general, the assessment based on screening in urine provides information on long‐term use, whilst detection in serum can be considered indicative of short‐term drug intake 11. From a pharmacological perspective, the latter is more related to BP measurements performed within the same time frame as sample collection 12.
Unbiased information on adherence is of importance in the decision‐making process of the treating physician, an objective tool can be of great importance for example to refine or define treatment‐resistant hypertension. Such a diagnostic tool can prevent new invasive treatment options and divert focus towards adherence training. In the present study, we investigated drug adherence through bioanalytical screening for antihypertensive agents in serum and explored the relation between adherence and BP levels over time.
Methods
Study design and study population
The study was designed as a posthoc analysis as part of the SYMPATHY‐trial, in which adherence screening in serum was performed 4. The SYMPATHY‐trial is an open‐label randomized‐controlled trial in which patients were randomized to RDN plus usual care vs. usual care alone (clinicaltrials.gov number: NCT01850901) 13. Patients were included in SYMPATHY from 14 different secondary and tertiary Dutch centres, from May 2013 to December 2015. Primary endpoint in the trial was BP lowering efficacy of RDN after 6 months. In order to be eligible to participate in this study, a subject had a mean daytime systolic BP ≥ 135 mmHg, as determined with the use of ambulatory BP measurement (ABPM), while having been prescribed three or more antihypertensive agents for at least 3 months prior to inclusion or with documented intolerance to two or more of the four major classes antihypertensive drugs (ace‐converting‐enzyme/angiotensin‐receptor blocker, calcium channel blocker, β blocker, diuretic) and no possibility to take three antihypertensive drugs. The most important exclusion criteria were a treatable secondary cause of hypertension, an estimated glomerular filtration rate <20 ml min–1 1.73m–2 and an ineligible renal artery anatomy for treatment. The present posthoc analysis on adherence originated from a new research question and permission was granted to use stored blood samples of SYMPATHY patients that gave a broad consent to use their blood for future research. The storage of blood samples was optional for participating centres (appendix, Table 1). SYMPATHY and this substudy were approved by the ethical committee of UMC Utrecht.
Table 1.
Baseline characteristics of the studied population (n = 98)
| Non‐adherent | Poorly adherent | Adherent | |
|---|---|---|---|
| n = 16 | n = 52 | n = 30 | |
| Age (years) | 57 (13) | 65 (9) | 63 (11) |
| Sex male * | 7 (44) | 22 (43) | 12 (39) |
| Ethnicity Caucasian * | 14 (88) | 50 (98) | 29 (94) |
| Cardiovascular history * | 7 (44) | 24 (47) | 16 (52) |
| Diabetes Mellitus * | 4 (25) | 19 (37) | 5 (16) |
| Current smoking * | 4 (25) | 11 (22) | 4 (13) |
| Body mass index (kg m –2 ) | 28.5 (5.2) | 29.1 (5.0) | 29.1 (4.7) |
| No. of BP lowering drugs | 3.9 (1.3) | 4.0 (0.9) | 2.6 (1.7) |
| No. of classes of BP lowering drugs | 3.4 (1.0) | 3.7 (0.8) | 2.7 (1.6) |
| Office BP | |||
| Systolic (mmHg) | 184 (28) | 162 (22) | 165 (24) |
| Diastolic (mmHg) | 106 (22) | 89 (14) | 92 (13) |
| Heart rate (bpm) | 76 (11) | 69 (12) | 68 (12) |
| 24‐h ABPM | |||
| Systolic (mmHg) | 162 (17) | 155 (15) | 158 (19) |
| Diastolic (mmHg) | 96 (17) | 88 (15) | 89 (13) |
| Heart rate (bpm) | 70 (12) | 70 (11) | 68 (12) |
| Daytime ABPM | |||
| Systolic (mmHg) | 167 (17) | 158 (15) | 161 (19) |
| Diastolic (mmHg) | 100 (18) | 90 (15) | 91 (14) |
| Heart rate (bpm) | 72 (12) | 72 (11) | 70 (12) |
| Night time ABPM | |||
| Systolic (mmHg) | 149 (21) | 143 (15) | 148 (18) |
| Diastolic (mmHg) | 85 (14) | 79 (13) | 82 (13) |
| Heart rate (bpm) | 65 (12) | 65 (9) | 62 (9) |
| LDL (mmol l –1 ) | 2.8 (0.7) | 3.1 (1.2) | 3.2 (1.1) |
| eGFR ( ml min –1 1.73 m –2 ) | 91 (15) | 74 (17) | 74 (20) |
| Renal denervation * | 10 (63) | 33 (65) | 20 (65) |
Data are expressed as mean ± standard deviation, unless stated otherwise.
Data are expressed as number of patients (%).
No., number; BP, blood pressure; bpm, beats per minute; ABPM, ambulatory BP measurement; LDL, low‐density lipoprotein; eGFR, estimated glomerular filtration rate
Adherence assessment
All prescribed medication, including BP lowering drugs, were listed at baseline and 6 months by their generic name, dosage and frequency. BP lowering drugs were identified according to the Anatomical Therapeutic Chemical classification system of the World Health Organization Collaborating Centre for Drug Statistics. In addition, we registered the different classes of prescribed BP lowering drugs.
Blood was collected at baseline and 6 months on the same day 24‐h ambulatory and office BP measurements were performed and stored as serum at –80°C. Serum screening for BP lowering drugs using liquid chromatography, combined with tandem mass spectrometry (LC–MS/MS) was performed as a batch at the end of the study. Patients and physicians were unaware of the adherence assessment at the time of blood sample collection.
Identification of BP lowering drugs was performed with LC–MS/MS combined with a spectra library search. First, phospholipid removal technology was employed for sample purification and enrichment. After purification, the samples were analysed using LC–MS/MS under full‐scan and data‐dependent MS/MS mode. The acquired mass spectra were compared with an in‐house library (compound library and MS/MS mass spectral library) built with automated screening software (Thermo Fisher Scientific) which contained the mass/charge of the precursor ion, retention time, product ions and the entire MS/MS spectra of 40 compounds including metabolites covering over 95% of all BP lowering drugs registered in The Netherlands. Identification was achieved by comparing full MS/MS spectra and/or mass/charge of precursor ion with confirmation by second selected reaction monitoring transitions. Furthermore, we randomly re‐sampled a batch to test the reproducibility of the method. The analysts that performed LC–MS/MS and interpret the results were unaware of the patients' BP or treatment arm.
Medication adherence was documented in three different categories: adherent (81%–100% match prescribed vs. measured), poorly adherent (1–80% match prescribed vs. measured) and completely nonadherent (0% match prescribed vs. measured) 14. Change in adherence between baseline and follow‐up was categorized as: decrease in adherence (baseline adherence higher than follow‐up adherence), stable adherence (baseline adherence equal to follow‐up adherence) and increase in adherence (baseline adherence lower than follow‐up adherence).
Physical and biochemical parameters
A detailed description of the collection of physical and biochemical outcome measures has been previously published by our group 13. In short, office and 24‐h BP measurements were performed at baseline and 6 months using recommended devices from the ESH/ESC guidelines and under standardized conditions 15. Office BP was an average of four BP measurements (two on each arm). ABPMs were considered valid when ≥70% of the recordings was successful. Information on cardiovascular history, smoking, alcohol, duration of hypertension and socio‐economic status were collected at baseline. Biochemical parameters as lipid spectrum, and creatinine (and estimated glomerular filtration rate) were assessed at baseline and 6 months follow‐up during routine patient care.
Statistical analysis
Due to the posthoc nature of the study a formal sample size calculation was not done in advance. Data were expressed as mean (standard deviation, SD), median (interquartiles) or as percentage (95% confidence interval, CI), unless stated otherwise. Paired t tests were used to compare means within individuals. To explore a relation between patient characteristics and the level of adherence we used a multivariable linear regression model with adherence as dependent variable and the following independent variables, based on literature or hypothesis: baseline office systolic BP, age, sex, duration of hypertension, education, number and type of class of BP lowering pills. A backward model was applied to minimize the chance on suppression of variables 16. To assess determinants of change in adherence, a similar multivariable linear regression analysis was applied. The treatment to which the patient was assigned in the original study (RDN or usual care) was added to this model. Univariable linear regression analysis was used to analyse the possible relationship between level of adherence and BP. A two‐sided 0.05 level of significance was used. Statistical analyses were done using SPSS version 21 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
Of the 139 patients included in SYMPATHY, 98 patients gave their consent to store blood specimens for future research purposes (Table 1). Mean age was 63 (standard deviation 11) years and 42% of the study population was male (n = 41). The prevalence of cardiovascular morbidity was 48% (n = 47). The average number of prescribed BP lowering pills was 3.6 (SD 1.4). RAS (renin–angiotensin system)‐inhibitors and diuretics were most often prescribed (Table S1). Mean office BP was 167 (SD 25) / 92 (SD 16) mmHg and mean 24‐h BP was 157 (SD 17) / 90 (SD 15) mmHg. Baseline characteristics of those with adherence measurements did not significantly differ from the original sample of139 patients in SYMPATHY (data not shown) 4.
Adherence to BP lowering medication
At baseline, 68% (95%CI 59–78) of the patients was nonadherent to their prescribed BP lowering drugs (n = 67). Sixteen patients were completely nonadherent (16%), 51 poorly adherent (52%) and 31adherent (32%; Table 1). Overall, of the 3.6 drugs prescribed, 1.5 could be detected in the blood sample (P < 0.001; Table S2). In seven patients, more pills were detected than prescribed (Figure 1). Adherence at baseline declined significantly with the increase of number of prescribed drugs (Figure 1 and Table S3): for every one pill more prescribed, 0.785 prescribed pill was less detected in blood (B = 0.785, P < 0.001). Other important determinants for nonadherence at baseline were: higher baseline office SBP (P < 0.001) and younger age (P = 0.082; Table S3).
Figure 1.
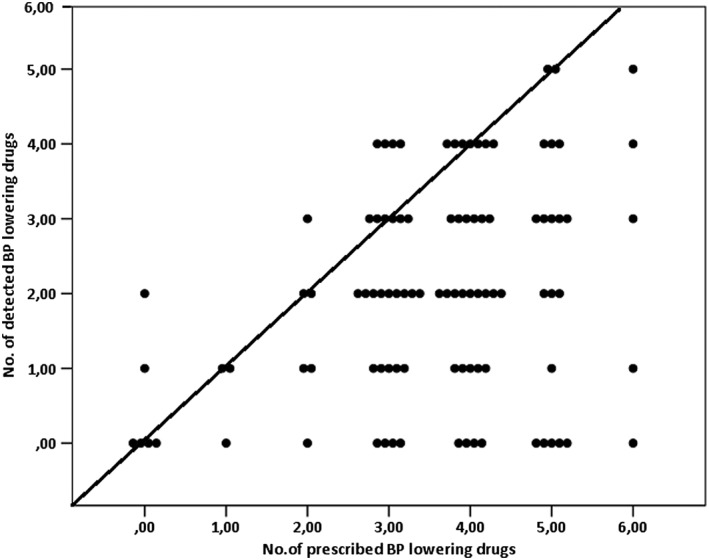
Relation between the number of prescribed and the number of detected BP lowering pills in blood at baseline with line of identity (0 = 0, 1 = 1, etc.). One dot represents one patient
Overall, the best adherence was found for RAS‐inhibitors and β‐blockers (59% and 59% of the adherent patients, respectively) and the worst for calcium antagonists (27% of the patients adherent; Figure 2). At 6 months, a similar pattern was seen in the 83 patients of whom stored samples were available (data not shown).
Figure 2.
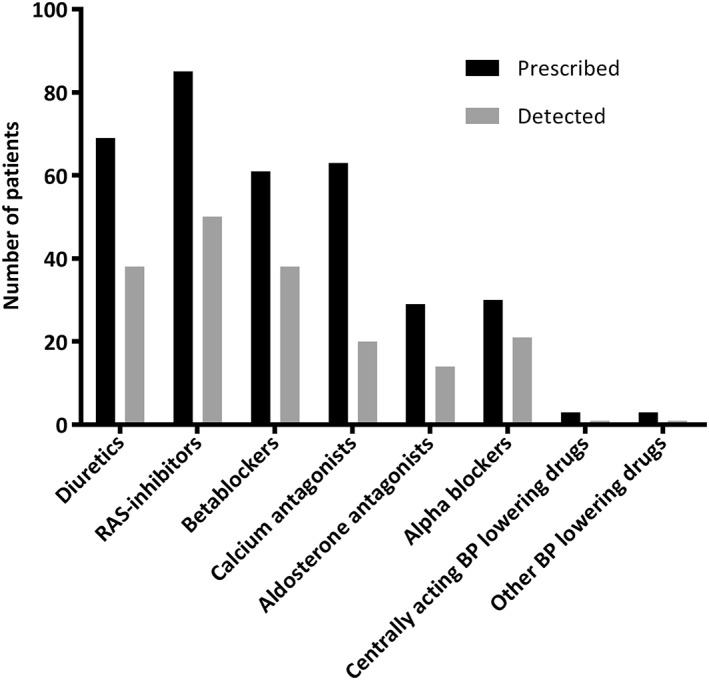
Number of patients that was prescribed to different classes of BP lowering drugs and the number of patients in which the prescribed class was detected. BP: blood pressure; RAS: renin‐angiotensin system
Using the described bioanalytical method for the identification of antihypertensive agents in serum a sensitivity of 95%and a specificity of 91% was reached. Reproducibility testing showed identical serum screening results in 49 of 52 samples (93%) of in total 147 analysed compounds.
Adherence and BP
Baseline office and ambulatory BP were the highest in the nonadherent group (Table 1). Low adherence to BP lowering medication was related to higher baseline BP (Table 2a). This relationship was the strongest for office BP: for every prescribed yet undetected pill, office BP increased with 4/3 mmHg (P = 0.018 and P = 0.003, respectively). Ambulatory BP increased with 2/2 mmHg (P = 0.152 and P = 0.019, respectively). The same trend was seen for change in adherence after 6 months of follow‐up. Office BP showed the largest rise after 6 months with an increase in BP of 4/2 mmHg (P = 0.038 and P = 0.044, respectively) for every prescribed yet undetected antihypertensive agent at 6 months, compared to baseline (Table 2b).
Table 2a.
Relation between baseline adherence and baseline blood pressure (BP)
| B‐coefficient | 95% CI for B‐coefficient | P‐value | |
|---|---|---|---|
| Office systolic BP | 3.563 | 0.637 to 6.490 | 0.018 |
| Office diastolic BP | 2.841 | 0.965 to 4.717 | 0.003 |
| 24‐h systolic ABPM | 1.473 | −0.551 to 3.497 | 0.152 |
| 24‐h diastolic ABPM | 2.128 | 0.362 to 3.895 | 0.019 |
Table 2b.
Relation between change in adherence and change in blood pressure
| B‐coefficient | 95% CI for B‐coefficient | P‐value | |
|---|---|---|---|
| Office systolic BP | 4.081 | 0.230 to 8.932 | 0.038 |
| Office diastolic BP | 2.362 | 0.060 to 4.663 | 0.044 |
| 24‐h systolic ABPM | 2.307 | −0.803 to 5.417 | 0.144 |
| 24‐h diastolic ABPM | 1.578 | −0.263 to 3.420 | 0.092 |
Univariable analyses of the possible relation between baseline adherence and baseline BP (2a) and the relation between change in adherence and change in BP (2b). Example: when there is one prescribed BP lowering pill not detected at baseline, office systolic BP is 3.563 mmHg higher at baseline (4a). Or, when one prescribed pill is less detected at 6 months compared to baseline, office systolic BP increases with 4.081 mmHg at 6 months compared to baseline. ABPM, ambulatory BP measurement.
Change in adherence
Overall, the number of prescribed BP lowering drugs increased significantly with 0.3 pills (P < 0.001) at 6 months. The number of detected drugs increased also with 0.3 pills (P = 0.058; Table S2). Of the 27 changes in prescribed drug classes (e.g. diuretic was started or stopped) at 6 months, 19 (70%) were not detected in blood. Notably, prescribed changes in RAS‐inhibitors and calcium antagonists were not found (Figure 3). In the 25 patients with a change in adherence overtime, drug adherence increased in13 (17%) patients and 12 (15%) patients were less adherent (Table S4).
Figure 3.

The number of patients in which the prescription of BP lowering classes changed at 6 months compared to baseline (e.g. at baseline no diuretics, but, at 6 months, diuretics were prescribed) and the number of patients in which the change in prescription was detected in blood. BP: blood pressure; RAS: renin–angiotensin system
Discussion
Objective assessment in serum of adherence to BP lowering drugs showed that adherence to BP lowering drugs was poor, with factors related to poor adherence being higher number of prescribed BP lowering pills, higher baseline BP and younger age. When adherence decreased overtime, office BP increased significantly. The present study has three unique features:(i) patients and physicians were unaware of the adherence assessments; (ii) BP lowering drugs were measured objectively in blood at different time‐points; and (iii) blood samples were taken synchronously with the BP measurements. This is the first study that bioanalytically confirmed compliance in using serum samples while measuring blood pressure.
Medication adherence has been subject of debate for many years and different approaches have been used to screen drug adherence 17, 18, 19, 20. The most widely used method is a questionnaire, of which the Morrisky is the best known and used in previous intervention studies with hypertensive patients 21, 22, 23, 24. Questionnaires are relatively inexpensive and noninvasive. However, they are based on the patients' self‐report of adherence, often leading to overestimation. A small study of 47 patients with apparent treatment resistant hypertension concluded that, based on this questionnaire, 26% patients were nonadherent. However, based on serum screening using a bioanalytical assay, objectively assessed nonadherence was found to be 51% 25. Furthermore, in recent studies with resistant hypertensive patients the percentage nonadherent patients (defined as taken <80% of the prescribed medication detected in urine and blood) was on average 50% 18, 26, 27. Here, we report a higher prevalence of almost 70%. The discrepancy could be related to the use of urine samples for the assessment in most of the other studies, which could have led to detection long after drug administration. As half‐lives of antihypertensive agents and the amount excreted by the kidneys (unchanged or as metabolite) vary greatly, urine screening results are more difficult to relate to short‐term drug intake and concomitant BP 11. Of note, in one study reporting 50% nonadherence, patients were asked to give informed consent for adherence measurements beforehand, which could theoretically have led to an improvement in adherence 26.
One of the perceived limitations may be that only part of the study population participated, i.e. those in the centre where storage of blood samples was possible. Nonparticipation was mostly due to logistical reasons and expected to be random, and not selective. Indeed, this notion is confirmed by the fact that baseline characteristics did not differ between these two groups of centres. A second limitation may be that included patients were analysed as one group, despite that part of the group underwent RDN. This may have affected the 6 months results. However, we adjusted these multivariable analyses, by adding intervention as independent variable. Thirdly, the study population was part of a randomized trial, including a more invasive and frequent follow‐up, which generally is associated with an increase in adherence 28. In this case underestimation of nonadherence as opposed to overestimation is expected. Further, we do not know how many participants used fixed‐dose combinations. It would be of interest to study the effect of fixed‐dose combination treatment on adherence in future trials. Finally, in seven patients, more pills were detected than prescribed. We believe that this finding resembles daily practice (instead of a detection error), as supranormal adherence has been reported to be a common finding 29. In our study, we considered these patients to be completely adherent.
Our results support that no or poor adherence is related to higher BP, which is also found in previous studies 18, 26, 27, 30. However, in contrast with earlier studies, in which no relationship was observed between change in adherence and change in BP during follow‐up 26, 27, we found that office BP increased when adherence decreased during follow‐up. As this and other studies found especially a low adherence to calcium channel blockers 8, 31, it is important to evaluate if there are alternatives for this class of BP lowering drug. Tablets with a combination of classes of BP lowering drugs are preferred, since it will increase the adherence. In conclusion, objective methodology, using a bioanalytical screening assay, to assess adherence to BP lowering drugs, provides a valuable tool to define true resistant hypertension and, when applicable, refine a treatment plan in consultation with the patient.
Competing Interests
P.J.B. declares grants from ZonMw, Nierstichting, Medtronic and personal fees from Medtronic during the conduct of the study. R.L.J., E.M.M. and M.L.B. have no competing interests to declare.
This work was supported by unrestricted grants from ZonMw [#837004006 to R.L.J.], Nierstichting [#CPI12.02 to R.L.J.] and from Medtronic Inc. SYMPATHY was an investigator‐driven trial. Clinicaltrial.gov : NCT01850901.
Supporting information
Table S1 Prescribed classes of blood pressure lowering medication (n = 98)
Table S2 Prescribed and detected blood pressure lowering drugs
Table S3 Determinants of adherence at baseline
Table S4 Baseline characteristics of patients with stable or change in adherence during follow‐up
de Jager, R. L. , van Maarseveen, E. M. , Bots, M. L. , Blankestijn, P. J. , and on behalf of the SYMPATHY investigators (2018) Medication adherence in patients with apparent resistant hypertension: findings from the SYMPATHY trial. Br J Clin Pharmacol, 84: 18–24. doi: 10.1111/bcp.13402.
References
- 1. Falaschetti E, Mindell J, Knott C, Poulter N. Hypertension management in England: a serial cross‐sectional study from 1994 to 2011. Lancet 2014; 383: 1912–1919. [DOI] [PubMed] [Google Scholar]
- 2. Pereira M, Lunet N, Azevedo A, Barros H. Differences in prevalence, awareness, treatment and control of hypertension between developing and developed countries. J Hypertens 2009; 27: 963–975. [DOI] [PubMed] [Google Scholar]
- 3. Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990‐2015. JAMA 2017; 317: 165–182. [DOI] [PubMed] [Google Scholar]
- 4. de Jager RL, de Beus E, Beeftink MM, Sanders MF, Vonken EJ, Voskuil M, et al Impact of medication adherence on the effect of renal denervation. The SYMPATHY trial. Hypertension 2017; 69: 678–684. [DOI] [PubMed] [Google Scholar]
- 5. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet 2016; 387: 957–967. [DOI] [PubMed] [Google Scholar]
- 6. Calderon‐Larranaga A, Diaz E, Poblador‐Plou B, Gimeno‐Feliu LA, Abad‐Diez JM, Prados‐Torres A. Non‐adherence to antihypertensive medication: the role of mental and physical comorbidity. Int J Cardiol 2016; 207: 310–316. [DOI] [PubMed] [Google Scholar]
- 7. Farah R, Zeidan RK, Chahine MN, Asmar R, Chahine R, Salameh P, et al Predictors of uncontrolled blood pressure in treated hypertensive individuals: first population‐based study in Lebanon. J Clin Hypertens (Greenwich) 2016; 18: 871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krousel‐Wood M, Joyce C, Holt E, Muntner P, Webber LS, Morisky DE, et al Predictors of decline in medication adherence: results from the cohort study of medication adherence among older adults. Hypertension 2011; 58: 804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meena J, Raghav P, Rustagi N. LBOS 03‐06 anti hypertensive treatment compliance and adverse effect profile among hypertension clinic attendees in jodhpur, INDIA. J Hypertens 2016; 34 (Suppl 1): e552. [Google Scholar]
- 10. Napolitano F, Napolitano P, Angelillo IF. Medication adherence among patients with chronic conditions in Italy. Eur J Public Health 2016; 26: 48–52. [DOI] [PubMed] [Google Scholar]
- 11. van Zwieten PA. Pharmacology of antihypertensive agents with multiple actions. Eur J Clin Pharmacol 1990; 38 (Suppl 2): S77–S81. [DOI] [PubMed] [Google Scholar]
- 12. Moffat A, Osselton D, Widdop B, Watts J. Clarke's Analysis of Drugs and Poisons, 4th edn. London: Pharmaceutical Press, 2011. [Google Scholar]
- 13. Vink EE, de Beus E, de Jager RL, Voskuil M, Spiering W, Vonken EJ, et al The effect of renal denervation added to standard pharmacologic treatment versus standard pharmacologic treatment alone in patients with resistant hypertension: rationale and design of the SYMPATHY trial. Am Heart J 2014; 167: 308–314. [DOI] [PubMed] [Google Scholar]
- 14. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005; 353: 487–497. [DOI] [PubMed] [Google Scholar]
- 15. Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31: 1281–1357. [DOI] [PubMed] [Google Scholar]
- 16. Field A. Choosing a method In: Discovering statistics using IBM SPSS Statistics. London: SAGE, 2013; 323. [Google Scholar]
- 17. Ayoade A, Oladipo I. Evaluation of the correlation between self‐report and electronic monitoring of adherence to hypertension therapy. Blood Press 2012; 21: 161–166. [DOI] [PubMed] [Google Scholar]
- 18. Jung O, Gechter JL, Wunder C, Paulke A, Bartel C, Geiger H, et al Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens 2013; 31: 766–774. [DOI] [PubMed] [Google Scholar]
- 19. Morisky DE, Ang A, Krousel‐Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008; 10: 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20. Zeller A, Schroeder K, Peters TJ. An adherence self‐report questionnaire facilitated the differentiation between nonadherence and nonresponse to antihypertensive treatment. J Clin Epidemiol 2008; 61: 282–288. [DOI] [PubMed] [Google Scholar]
- 21. Azizi M, Sapoval M, Gosse P, Monge M, Bobrie G, Delsart P, et al Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open‐label, randomised controlled trial. Lancet 2015; 385: 1957–1965. [DOI] [PubMed] [Google Scholar]
- 22. Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, et al A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370: 1393–1401. [DOI] [PubMed] [Google Scholar]
- 23. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment‐resistant hypertension (the Symplicity HTN‐2 trial): a randomised controlled trial. Lancet 2010; 376: 1903–1909. [DOI] [PubMed] [Google Scholar]
- 24. Kario K, Ogawa H, Okumura K, Okura T, Saito S, Ueno T, et al SYMPLICITY HTN‐Japan ‐ first randomized controlled trial of catheter‐based renal denervation in Asian patients. Circ J 2015; 79: 1222–1229. [DOI] [PubMed] [Google Scholar]
- 25. Pandey A, Raza F, Velasco A, Brinker S, Ayers C, Das SR, et al Comparison of Morisky medication adherence scale with therapeutic drug monitoring in apparent treatment‐resistant hypertension. J Am Soc Hypertens 2015; 9: 420–426. [DOI] [PubMed] [Google Scholar]
- 26. Ewen S, Meyer MR, Cremers B, Laufs U, Helfer AG, Linz D, et al Blood pressure reductions following catheter‐based renal denervation are not related to improvements in adherence to antihypertensive drugs measured by urine/plasma toxicological analysis. Clin Res Cardiol 2015; 104: 1097–1105. [DOI] [PubMed] [Google Scholar]
- 27. Schmieder RE, Ott C, Schmid A, Friedrich S, Kistner I, Ditting T, et al Adherence to antihypertensive medication in treatment‐resistant hypertension undergoing renal denervation. J Am Heart Assoc 2016; 5: pii: e002343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cramer JA, Scheyer RD, Mattson RH. Compliance declines between clinic visits. Arch Intern Med 1990; 150: 1509–1510. [PubMed] [Google Scholar]
- 29. Rudd P, Byyny RL, Zachary V, LoVerde ME, Mitchell WD, Titus C, et al Pill count measures of compliance in a drug trial: variability and suitability. Am J Hypertens 1988; 1: 309–312. [DOI] [PubMed] [Google Scholar]
- 30. Tomaszewski M, White C, Patel P, Masca N, Damani R, Hepworth J, et al High rates of non‐adherence to antihypertensive treatment revealed by high‐performance liquid chromatography‐tandem mass spectrometry (HP LC‐MS/MS) urine analysis. Heart 2014; 100: 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reuter H, Markhof A, Scholz S, Wegmann C, Seck C, Adler C, et al Long‐term medication adherence in patients with ST‐elevation myocardial infarction and primary percutaneous coronary intervention. Eur J Prev Cardiol 2015; 22: 890–898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Prescribed classes of blood pressure lowering medication (n = 98)
Table S2 Prescribed and detected blood pressure lowering drugs
Table S3 Determinants of adherence at baseline
Table S4 Baseline characteristics of patients with stable or change in adherence during follow‐up


