Abstract
Background
Pivotal clinical trials found that ticagrelor reduced ischaemic complications to a greater extent than clopidogrel, and also that the benefit gradually increased with the reduction in creatinine clearance. However, the underlying mechanisms remains poorly explored.
Methods
This was a single‐centre, prospective, randomized clinical trial involving 60 hospitalized Adenosine Diphosphate (ADP) P2Y12 receptor inhibitor‐naïve patients with chronic kidney disease (CKD) (estimated glomerular filtration rate <60 ml min–1 1.73 m–2) and non‐ST‐elevation acute coronary syndromes (NSTE‐ACS). Eligible patients were randomly assigned in a 1:1 ratio to receive ticagrelor (180 mg loading dose, then followed by 90 mg twice daily) or clopidogrel (600 mg loading dose, then followed by 75 mg once daily). The primary endpoint was the P2Y12 reactive unit (PRU) value assessed by VerifyNow at 30 days. The plasma concentrations of ticagrelor and clopidogrel and their active metabolites were measured in the first 10 patients in each group at baseline, and at 1 h, 2 h, 4 h, 8 h, 12 h and 24 h after the loading dose.
Results
Baseline characteristics were well matched between the two groups. Our results indicated a markedly lower PRU in patients treated with ticagrelor vs. clopidogrel at 30 days (32.6 ± 11.29 vs. 203.7 ± 17.92; P < 0.001) as well as at 2 h, 8 h and 24 h after the loading dose (P < 0.001). Ticagrelor and its active metabolite AR‐C124910XX showed a similar time to reach maximum concentration (Cmax) of 8 h, with the maximum concentration (C max) of 355 (242.50–522.00) ng ml–1and 63.20 (50.80–85.15) ng ml–1, respectively. Both clopidogrel and its active metabolite approached the Cmax at 2 h, with a similar C max of 8.67 (6.64–27.75) ng ml–1 vs. 8.53 (6.94–15.93) ng ml–1.
Conclusion
Ticagrelor showed much more potent platelet inhibition in comparison with clopidogrel in patients with CKD and NSTE‐ACS.
Keywords: chronic kidney disease, non‐ST‐elevation acute coronary syndromes, pharmacodynamics, ticagrelor
What is Already Known about this Subject
Platelet responsiveness to clopidogrel is poor in patients with non‐ST‐elevation acute coronary syndrome (NSTE‐ACS) and chronic kidney disease (CKD), and this is one of the independent factors of worse clinical outcomes in CKD patients compared with those with normal renal function.
Renal impairment has a minimal effect on systemic exposure to ticagrelor and its active metabolite AR‐C124910XX. A previous clinical study showed that the superiority of ticagrelor over clopidogrel on the composite ischaemic endpoint gradually increased with the reduction in creatinine clearance.
Pharmacodynamic/pharmacokinetic data underlying the difference between ticagrelor and clopidogrel in NSTE‐ACS patients with impaired renal function are scare.
What this Study Adds
Ticagrelor exerted much more potent platelet inhibition compared with clopidogrel in non‐ST‐elevation ACS patients with CKD.
Neither baseline estimated glomerular filtration rate (eGFR) nor cytochrome P450 (CYP) 2C19 genotype showed a significant impact on the inhibition of platelet aggregation. In the current study, irrespective of the eGFR or the CYP2C19 genotype, platelet inhibition was mostly strong in ticagrelor‐treated patients, and weak in clopidogrel‐treated patients.
Among patients with impaired renal function, the pharmacokinetics of ticagrelor was uninfluenced while the biotransformation of clopidogrel might have been inhibited.
Introduction
Clinical outcomes in patients receiving clopidogrel has been reported to be worse in the presence of chronic kidney disease (CKD) 1, 2. Poor platelet responsiveness to clopidogrel has been demonstrated in patients with CKD 3, 4, 5. Furthermore, renal dysfunction was found to be related to reduced expression of the transporter for clopidogrel 6, 7 and weakened activity of a series of cytochrome P450 (CYP450) genotypes, including CYP2C19, which is critical for clopidogrel metabolism 7. Ticagrelor is a new potent Adenosine Diphosphate (ADP) P2Y12 receptor inhibitor which undergoes extrarenal metabolism 8. It has been found that renal impairment has a minimal effect on systemic exposure to ticagrelor and its active metabolite AR‐C124910XX 8. Furthermore, a subgroup analysis of the PLATelet inhibition and patient Outcomes (PLATO) trial demonstrated that the superiority of ticagrelor over clopidogrel on the composite ischaemic endpoint gradually increased with the reduction in creatinine clearance 9, implying that the benefits of ticagrelor may be even more pronounced in patients with CKD. However, in spite of the above evidence, the pharmacodynamic (PD)/pharmacokinetic (PK) data underlying the difference between ticagrelor and clopidogrel in this special population were sparse. The current study aimed to compare the PD/PK characteristics of ticagrelor and clopidogrel in patients with non‐ST‐elevation acute coronary syndromes (NSTE‐ACS) and CKD, which may help to provide a deeper insight into the advantage of ticagrelor vs. clopidogrel in patients with renal dysfunction.
Methods
Study population
The COmparison of The Pharmacodynamics and pharmacokinetics of Ticagrelor vs. clopidogrel in patients with non–ST‐elevation acute coronary syndromes and Chronic Kidney Disease (OPT‐CKD) trial was a prospective, randomized, open‐label, single‐centre study aimed at comparing the PD/PK effects of ticagrelor vs. clopidogrel among NSTE‐ACS patients with CKD against a background of aspirin therapy (clinicaltrials.gov identifier: NCT02578537). ADP P2Y12 inhibitor‐naïve patients >18 years of age, presenting with NSTE‐ACS and an estimated glomerular filtration rate (eGFR) <60 ml min–1 1.73 m–2, were enrolled in the Department of Cardiology in the General Hospital of Shenyang Military Region between October 2015 and December 2016. The eGFR was calculated according to modified glomerular filtration rate estimating equation for Chinese patients with CKD as: 175 × [Scr (mg dl–1)]‐1.234 × (age)‐0.179 × 0.79 (if female) 10. Patients were excluded if any of the following criteria were present: cardiogenic shock; thrombolytic therapy administered before randomization; active bleeding or bleeding predisposition, including retinal or vitreous haemorrhage, gastrointestinal or urinary tract haemorrhage, or a history of intracranial haemorrhage or cerebral infarction; hypersensitivity to ticagrelor or to any of its excipients; deep puncture or major surgery within the previous month; untreated or uncontrolled hypertension with a blood pressure >180/110 mmHg; a known haemoglobin level of <10 g dl–1 or platelet count <100 × 109 l–1; known moderate or severe hepatic impairment; known aminotransferase level >3× the upper limit of normal; known allergy to any of the study drugs or devices (e.g. aspirin, clopidogrel, ticagrelor, stainless steel, contrast agents); pregnancy or lactation; any condition which might interfere with study compliance, or otherwise unsuitable for study participation, as judged by the investigators; unwilling or unable to undergo a repeat platelet assay or clinical follow‐up. The study was approved by the ethics committee at the General Hospital of Shenyang Military Region, and all patients provided written informed consent before randomization.
Study design
The total duration of the study was 30 days. Eligible patients were randomized to receive ticagrelor (180 mg loading dose, followed by 90 mg twice daily) or clopidogrel (600 mg loading dose, followed by 75 mg once daily) in a 1:1 ratio. All patients were given aspirin 100 mg per day unless they were intolerant to this agent. Platelet reactive unit (PRU) value was assessed by VerifyNow (PD testing) at predosing (0 h), and at 2 h, 8 h, 24 h and 30 days after the loading dose of study drug. Planned angiography was performed over 24 h after the loading dose, followed by stent implantation, coronary artery bypass graft or medicinal therapy only, according to the angiography outcomes. Inhibition of platelet aggregation (IPA) as well as high on‐treatment platelet reactivity (HPR) at different time points were determined according to PRU value. The plasma concentrations of ticagrelor and clopidogrel and their active metabolites (PK testing) were measured in the first 10 enrolled patients in each group in the prespecified time frame of predosing (0 h), then at 1, 2, 4, 8, 12 and 24 h after the loading dose of study drug. The CYP2C19 genotype was also tested in all patients, in both groups, after enrolment. Clinical follow‐up was performed at 30 days. A flow diagram of the study is presented in Figure 1.
Figure 1.
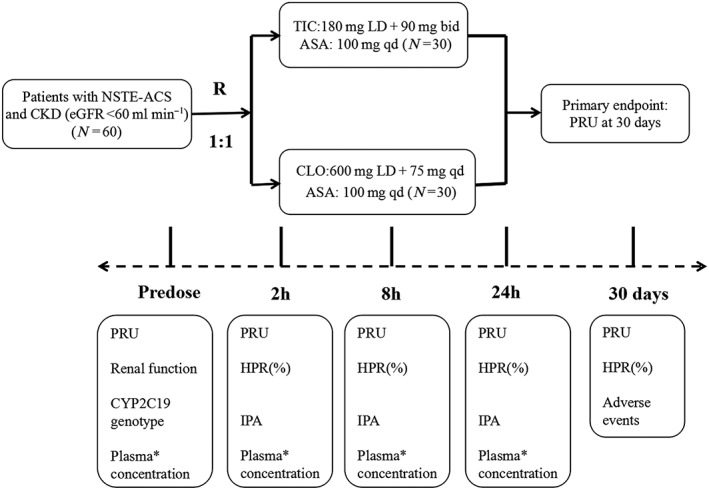
Flow chart of the COmparison of The Pharmacodynamics and pharmacokinetics of Ticagrelor vs. clopidogrel in patients with non–ST‐elevation acute coronary syndromes and Chronic Kidney Disease (OPT‐CKD) trial. Eligible patients were randomly assigned in a 1:1 ratio to receive ticagrelor or clopidogrel (R 1:1). ASA, aspirin; CKD, chronic kidney disease; CLO, clopidogrel; CYP, cytochrome P450; eGFR, estimated glomerular filtration rate; HPR, high on‐treatment platelet reactivity; IPA, inhibition of platelet aggregation; LD, loading dose; NSTE‐ACS, non‐ST‐elevation acute coronary syndromes; PRU, platelet reactive unit; qd, once daily; TIC, ticagrelor *Plasma concentrations of clopidogrel, clopidogrel active metabolite‐derivatized (CAMD), ticagrelor and its active metabolite AR‐C124910XX were assessed predose and 1, 2, 4, 8, 12 and 24 h after the loading dose
PD assessment by VerifyNow
Blood was collected from the antecubital vein into tailor‐made tubes that contained 3.2% sodium citrate (Greiner Bio‐One GmbH, Austria) for VerifyNow measurements. VerifyNow is a turbidimetric‐based optical detection system that measures platelet aggregation as an increase in light transmittance in whole blood 11. HPR was defined as a PRU >208 12, and IPA in the current study was calculated as follows, where b is baseline, and t is the time point after the loading dose: IPA (%) = 100% × .
PK assessment
Blood was collected from the antecubital vein into vacutainer tubes (Improvacuter®, Guangdong, China) that contained 2 mg ml–1 ethylenediamine tetra‐acetic acid (EDTA) K2. The plasma concentration of ticagrelor and its active metabolite AR‐C124910XX was determined using the high‐performance liquid chromatography–tandem mass spectrometry (HPLC‐MS/MS) system (Agilent, Santa Clara, CA, USA), as previously described by Sillén et al. 13. 25 μl 2‐bromo‐3‐methoxyacetophenone (Sigma‐Aldrich, St Louis, MO, USA) solution (500 mM) was added at the time of each blood sample collection for detection of clopidogrel and its active metabolite‐derivatized (CAMD) concentrations, determined by HPLC–MS/MS (Agilent), as previously described 14. The maximum concentration (C max) and time to reach C max (T max) were established directly from the measured plasma concentration for each patient and presented as medians with interquartile range.
Sample size calculation and statistical analysis
The primary endpoint was the PRU assessed by VerifyNow at 30 days after the loading dose. Assuming a 30‐day average PRU of 264 with a standard deviation (SD) of 60 in the clopidogrel arm, and allowing for 15% of patients being lost to follow‐up, with a two‐sided alpha of 0.05, 30 patients per group would provide at least 90% power to demonstrate an absolute reduction of 80 on PRU with ticagrelor. All analyses were by intent‐to‐treat. Categorical variables are expressed as frequencies and percentages, and compared using the χ2 or Fisher exact test. For baseline characters, continuous variables are expressed as mean ±SD and compared using the t‐test. The PRU as well as the IPA of ticagrelor vs. clopidogrel were presented as the mean ±SE and analysed by the Wilcoxon rank sum test. C max and T max were expressed as median and interquartile range (Percentiles 25 ‐Percentiles 75). The correlations between baseline eGFR and CYP2C19 genotype with 30‐day IPA were analysed by linear regression. Statistical analyses were performed using SPSS version 22.0 (SPSS, Chicago, IL, USA) and P‐value of <0.05 was considered statistically significant.
Results
Patient enrolment
Between October 2015 and December 2016, a total of 60 eligible patients were enrolled in the present study, with 23.3% and 30.0% of patients indexed for non‐ST‐elevation acute myocardial infarction in the ticagrelor and clopidogrel groups, respectively (P = 0.559). The average eGFR was 45.5 ± 12.3 ml min–1 1.73 m–2 vs. 45.8 ± 10.3 ml min–1 1.73 m–2 in the ticagrelor and clopidogrel groups, respectively (P = 0.916). The study patients also had a similar distribution of the CYP2C19 genotype, presented mainly with an intermediate metabolizer (ticagrelor vs. clopidogrel, 53.3% vs. 63.3%, P = 0.432). Other baseline characteristics were also generally matched between the two groups (Table 1).
Table 1.
Basic characteristics of the study
| Ticagrelor (n = 30) | Clopidogrel (n = 30) | P‐value | |
|---|---|---|---|
| Age, mean (SD), years | 69.7 ± 7.7 | 65.1 ± 10.9 | 0.072 |
| Male, No. (%) | 17 (56.7) | 18 (60.0) | 0.793 |
| BMI, mean (SD), kg m –2 | 23.9 ± 3.8 | 25.5 ± 3.9 | 0.114 |
| Type of NSTE‐ACS | |||
| UA | 23 (76.7) | 21 (70.0) | 0.559 |
| NSTEMI | 7 (23.3) | 9 (30.0) | 0.559 |
| Medical history, No. (%) | |||
| Current smoker | 6 (20.0) | 9 (30.0) | 0.376 |
| Hypertension | 26 (86.7) | 28 (93.3) | 0.398 |
| Diabetes | 13 (43.3) | 17 (56.7) | 0.304 |
| Hyperlipidaemia | 17 (56.7) | 19 (63.3) | 0.605 |
| CHF | 5(16.7) | 4(13.3) | 0.718 |
| Previous | |||
| PCI | 5 (16.7) | 7 (23.3) | 0.523 |
| CABG | 2 (6.7) | 2 (6.7) | 1.000 |
| Myocardial Infarction | 6 (20.0) | 6 (20.0) | 1.000 |
| Current therapy | |||
| PCI | 15(50.0) | 9(30.0) | 0.114 |
| CABG | 6(20.0) | 4(13.3) | 0.448 |
| Medicine only | 9(30.0) | 17(56.7) | 0.037 |
| Medication | |||
| Diuretics | 10(33.3) | 11(36.7) | 0.787 |
| Nitrate | 24(80.0) | 23(76.7) | 0.754 |
| ACEI/ARB | 17(56.7) | 19(63.3) | 0.598 |
| Statin | 30(100.0) | 30(100.0) | ‐‐‐‐ |
| CYP2C19 genotype, No. (%) | |||
| Extensive | 9 (30.0) | 9 (30.0) | 1.000 |
| Intermediate | 16 (53.3) | 19 (63.3) | 0.432 |
| Poor | 5 (16.7) | 2 (6.7) | 0.421 |
| eGFR (ml min –1 1.73 m –2 ) | |||
| Admission | 45.5 ± 12.3 | 45.8 ± 10.3 | 0.916 |
| 30‐day | 40.8 ± 13.2 | 44.1 ± 20.9 | 0.518 |
| Haemoglobin, g dl –1 | 129.07 ± 18.44 | 123.53 ± 15.03 | 0.208 |
| Anaemia a | 8 (26.7) | 6 (20.0) | 0.542 |
| Platelet count, 100 × 10 9 l –1 | 218.67 ± 68.79 | 231.07 ± 61.17 | 0.464 |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CABG, coronary artery bypass graft; CHF, chronic heart failure; CYP, cytochrome P450; eGFR, estimated glomerular filtration rate; NSTE‐ACS, non‐ST‐elevation acute coronary syndromes; NSTEMI, non‐ST‐segment elevation myocardial infarction; PCI, percutaneous coronary intervention; SD, standard deviation; UA, unstable angina
Anaemia was defined as a haemoglobin level less than 120 g l–1 for men or less than 110 g l–1 for women
Platelet inhibition exerted by ticagrelor vs. clopidogrel
Baseline eGFRs were well matched in patients treated with ticagrelor or clopidogrel (P = 0.916). The PRUs at different time points within 24 h were assessed in all 60 enrolled patients. The primary endpoint of 30‐day PRU was accessed in 57 patients (three patients died during follow‐up; see Table S1) and was markedly lower in patients treated with ticagrelor compared with clopidogrel (32.6 ± 11.29 vs. 203.7 ± 17.92; P < 0.001) (Figure 2A). A significantly lower PRU in the ticagrelor group could also be observed at each time point within 24 h after the loading dose (P < 0.001 at 2 h, 8 h and 24 h) (Figure 2A). Comparable results were found when the PRU value was converted to a percentage of IPA. The mean IPA in the ticagrelor group was significantly higher than that in the clopidogrel group at 30 days, as well as at different time points after the loading dose (P < 0.001) (Figure 2B). The IPA within the ticagrelor group was marginally above 80% at 8 h and was maintained to 30 days; in the clopidogrel group, the mean IPA was only approximately 20% from 8 h after the loading dose until the end of the study (Figure 2B). HPR was also assessed (Table 2), and it was found that almost 60% of clopidogrel‐treated patients had HPR at 30 days, whereas only one case of HPR was observed in the ticagrelor group at 24 h, and none at the end of the study(0% vs. 58.6%; P < 0.001).
Figure 2.
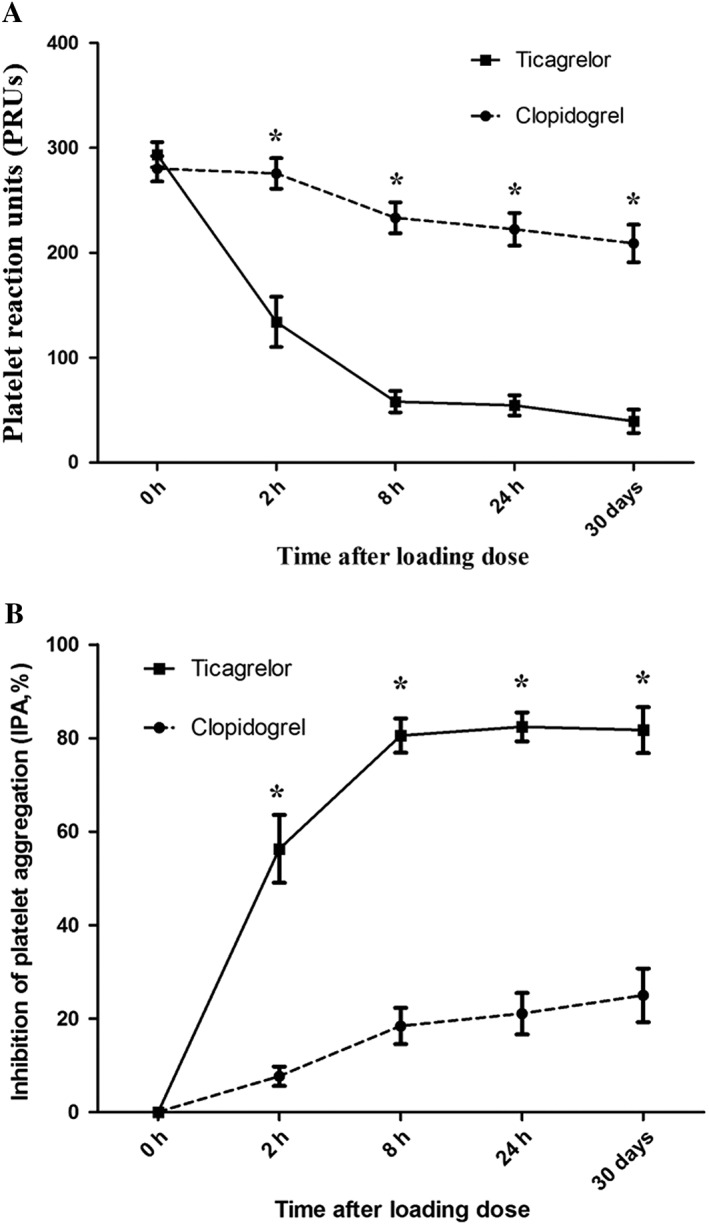
Platelet reaction units (A) and inhibition of platelet aggregation (B) by protocol time and treatment. Data are expressed as mean ± standard error *P < 0.001, ticagrelor vs. clopidogrel
Table 2.
HPR assessed at different time points after the loading dose of clopidogrel or ticagrelor
| Ticagrelor (n = 30) | Clopidogrel (n = 30) | P | |
|---|---|---|---|
| 2 h after loading | 11 (36.7) | 22 (81.5) | 0.001 |
| 8 h after loading | 1 (3.3) | 19 (65.5) | <0.001 |
| 24 h after loading | 1 (3.3) | 16 (57.1) | <0.001 |
| 30 days after loading | 0 (0)a | 17 (58.6)b | <0.001 |
HPR, high on‐treatment platelet reactivity, defined as platelet reaction unit value above 208
n = 28
n = 29
The impact of baseline eGFR and CYP2C19 genotype on the inhibition of platelet aggregation
Among patients treated with ticagrelor, the IPA was mostly maintained above 80%, irrespective of whether the eGFR level was almost 60 ml min–1 1.73 m–2 or declined to less than 30 ml min–1 1.73 m–2, showing no correlation with renal function (R 2 = 0.063; P = 0.179) (Figure 3A); a similar relationship was found in the clopidogrel group, with IPA mostly below 40% (R 2 = 0.077; P = 0.138) (Figure 3A). A poor IPA was also found in clopidogrel‐treated patients, with a variety of CYP2C19 genotypes, and therefore revealed no significant correlation (R 2 = 0.037; P = 0.312) (Figure 3B).
Figure 3.
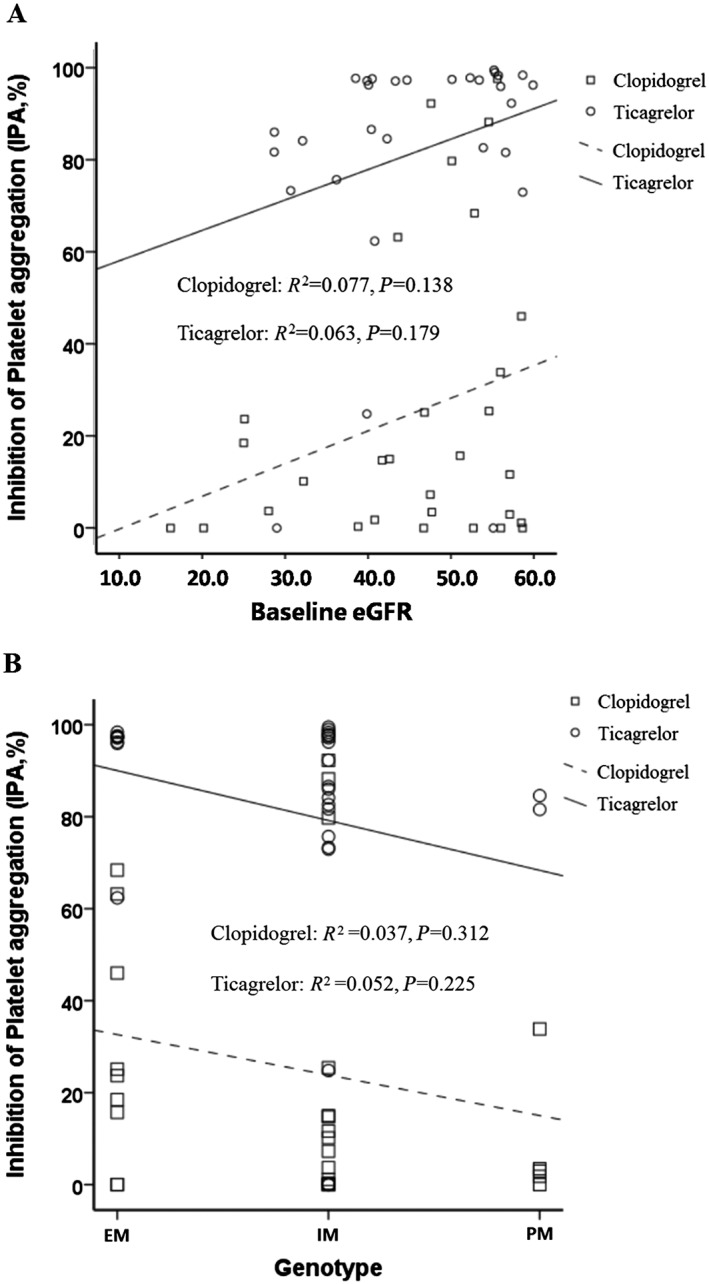
Correlation between baseline estimated glomerular filtration rate (eGFR) (A) and cytochrome B450 2C19 genotype (B) and 30‐day inhibition of platelet aggregation in non‐ST‐elevation acute coronary syndromes patients with chronic kidney disease. EM, extensive metabolizer; IM, intermediate metabolizer; PM, poor metabolizer
The pharmacokinetics of the clopidogrel and ticagrelor in patients with impaired renal function
The PK characteristics of clopidogrel and ticagrelor were studied in a subgroup of the first 10 enrolled patients in each group. Both clopidogrel and CAMD approached C max at 2 h [time to C max, 2.00 (2.00–4.00) h vs. 2.00 (1.75–4.00) h] and showed a similar C max of 8.67 (6.64–27.75) ng ml–1 vs. 8.53 (6.94–15.93) ng ml–1 (Figure 4A,B). It also showed a similar T max [8.00 (2.00–10.00) vs. 8.00 (5.00–12.00)] between ticagrelor and its active metabolite AR‐C124019XX with a C max of 355 (242.50–522.00) ng ml–1 and 63.20 (50.80–85.15) ng ml–1, respectively (Figure 4C,D). In addition, the relationship between C max and 24 h IPA was also analysed. Among patients with PK data, 24 h IPA was 92.11 ± 3.18 in the ticagrelor group and 18.51 ± 6.29 in the clopidogrel group. We found no significant correlation between C max and 24 h IPA in either ticagrelor group (ticagrelor: R 2 = 0.187, P = 0.245; AR‐C124910XX: R 2 = 0.045, P = 0.572), or clopidogrel group (clopidogrel: R 2 = 0.014, P = 0.741; CAMD: R 2 = 0.062, P = 0.487).
Figure 4.
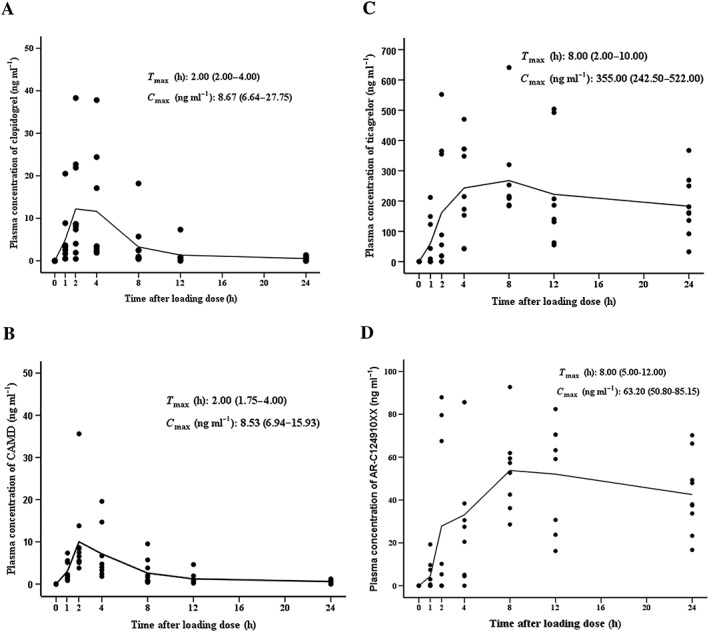
Overview of clopidogrel and ticagrelor pharmacokinetics on day 1. Plasma concentrations of clopidogrel (A), clopidogrel active metabolite‐derivatized (CAMD) (B), ticagrelor (C) and its active metabolite AR‐C124910XX (D) were measured before and 1–24 h after the administration of a loading dose of 600 mg clopidogrel or 180 mg ticagrelor. Maximum concentration (C max) and time to reach C max (T max) were established directly from the measured plasma concentration of each patient and presented as medians with interquartile range. Individual data are also shown, and the pharmacokinetic trend was demonstrated via the connecting curves of mean values
Discussion
The current study compared the PD/PK characteristics between ticagrelor and clopidogrel in NSTE‐ACS patients complicated with CKD (eGFR <60 ml min–1). Our data showed that ticagrelor exerted a much more potent platelet inhibitory effect than clopidogrel. In addition, irrespective of the eGFR level and the genotype, platelet aggregation was more potently inhibited in patients treated with ticagrelor compared with clopidogrel. Furthermore, this preliminary PK result implied that renal dysfunction significantly suppressed the biotransformation of clopidogrel, but showed no impact on the generation of the ticagrelor active metabolite.
Renal dysfunction has been reported to interfere significantly with the expression and activities of critical enzymes, CYP2C19 and paraoxonase 1, which mediate the biotransformation of clopidogrel 6, 15. Furthermore, a number of lines of evidence have strongly suggested that platelet responsiveness to clopidogrel is markedly suppressed in patients with CKD 3, 4, which might contribute to the worse clinical outcomes found in a population with impaired renal function 16. By contrast, ticagrelor, a new potent ADP P2Y12 inhibitor, is a direct‐acting agent, with no need for biotransformation in vivo 7, which therefore might overcome the adverse impact of renal dysfunction. In fact, a subgroup analysis of the PLATO trial demonstrated that the superiority of ticagrelor over clopidogrel gradually increased with the decline of creatinine clearance 8. However, there have been sparse PD and PK data to gain a deeper insight into the difference between ticagrelor and clopidogrel in patients with impaired renal function.
The current study was the first to compare the efficacy of platelet inhibition between ticagrelor and clopidogrel in NSTE‐ACS patients with moderate to severe renal dysfunction. Beginning with an equal baseline PRU in the two groups, we found a markedly lower PRU in patients on ticagrelor vs. clopidogrel at the primary endpoint of 30 days, as well as at 2 h, 8 h and 24 h after the loading dose, which clearly demonstrated a much more potent effect of ticagrelor on platelet inhibition among patients with CKD. More importantly, the current results on IPA (stable at around 80% from 8 h after the loading dose until the end of study at 30 days) in the ticagrelor group was similar to those found in the HOUYI study 17, a subgroup analysis that recruited 60 hospitalized Chinese patients with ACS (no special limitation on eGFR) from five centres and showed that the ticagrelor IPA approached 68.4% at 8 h, and increased and stabilized at about 80% from 24 h after the loading dose until the end of the study at the sixth week. In addition, the HPR rate in the ticagrelor group in the current study (3.3% at 24 h and 0% at 30 days) was also similar to that reported previously in patients with no limitation on eGFR 18. According to the above evidence, we might conclude that the inhibitory effect of ticagrelor on platelets remains consistent in patients with renal dysfunction compared with those with normal renal function.
In contrast to the finding in the ticagrelor group, we found renal dysfunction to have a clear effect on clopidogrel action in our study patients. We noted that the IPA rose to only about 25% at 30 days, with HPR observed in 58.6% of patients. Clopidogrel resistance due to the loss‐of‐function CYP2C19 genotype would have only partially explained the present results, as only 6.7% of patients in the clopidogrel group were found to be poor metabolizers. Moreover, clopidogrel was previously reported to reach a 50–70% inhibition of ADP‐induced platelet aggregation in Chinese patients undergoing a percutaneous coronary intervention who were extensive or intermediate metabolizer of CYP2C19 19. Our results thus implied that the inhibitory effect of clopidogrel on platelets might be downregulated by renal dysfunction. Notably, however, neither eGFR nor CYP2C19 genotype had an impact on the 30‐day IPA according to the correlation analysis in both the clopidogrel and ticagrelor groups, for completely different reasons. In patients on clopidogrel, it was the prevalent poor platelet inhibition (mostly at around 20%) that eliminated the linear correlation, which suggested that, among patients with moderate to severe CKD, even an eGFR of almost 60 ml min–1 or being an extensive metabolizer of CYP2C19 might not help to improve the responsiveness to clopidogrel. By contrast, the 30‐day IPA in patients on ticagrelor was generally at a high level of nearly 80%, which therefore indicated independence of renal function and the CYP2C19 genotype.
Although there was no control group with normal renal function, the PK results in the current trial might also provide preliminary evidence demonstrating a difference in the impact of renal dysfunction on the metabolism of ticagrelor and of clopidogrel by comparing our results about the PK of ticagrelor and clopidogrel in the patients of impaired kidney function with those of nomal kidney function in the previous reported trials. We found that the difference in the C max of ticagrelor vs. its active metabolite AR‐C124910XX was comparable with that reported in patients with no special limitation on eGFR 16, 20, suggesting that the metabolism of ticagrelor underwent no obvious change in patients with impaired renal function. Conversely, the activation of clopidogrel in the present study was found to deviate from that in published trials in patients with an unlimited eGFR. Previous studies have reported the C max values of CAMD (or H3, H4) to be significantly higher than those of clopidogrel 14, 21, 22. However, our results showed the C max of CAMD to be similar to that of clopidogrel, implying that the biotransformation of clopidogrel might be inhibited to some degree in patients with moderate to severe CKD. Further studies comparing the PK characteristics in patients with different levels of eGFR are needed to illustrate better the role of renal function in the metabolism of clopidogrel, as well as ticagrelor. Ticagrelor has also been described to approach C max and/or IPA 2 h after the loading dose in previous studies 11, 20. However, the PD and PK characteristics of ticagrelor in these trials were evaluated in patients with stable coronary artery disease, whereas patients enrolled in the current study presented with NSTE‐ACS. Ethnic differences might be another potential factor involved in the metabolism of ticagrelor. Another relevant trial 17 enrolled a similar population of Chinese ACS patients to that in the present study (52.6% NSTE‐ACS, 47.4% ST‐elevation myocardial infarction) and found that IPA within the ticagrelor group approached 68.4% at 8 h, and further increased to 78.0% at 24 h (the significance of the difference in IPA at 8 h vs. 24 h was not shown). In addition, as PK data were assessed in only 10 patients in the present trial, the small number of observations and the outlier (a value of ticagrelor concentration around 660 ng ml−1 at 8 h) might also have contributed to this discrepancy and made the difference.
Limitations
The first limitation of the present study was that it had a small sample size for determining PD differences between ticagrelor and clopidogrel, so had insufficient power to detect differences in clinical outcomes (Table S1). Secondly, There were too few sampling time points – e.g. omitting the time points of 1 h and 4 h after the loading dose. In addition, carrying out another platelet reactivity test (e.g. the vasodilator‐stimulated phosphoprotein (VASP) index) and analysing the strength of the correlation between the results of different testing methods would have further reinforced the conclusions obtained with the VerifyNow assay. Finally, as the PK data were assessed in only 10 patients, the conclusion reached regarding the biotransformation of clopidogrel was suitable only for hypothesis generating and requires further confirmation by a larger PK study.
Conclusion
Ticagrelor exerted a much more potent inhibitory effect on platelet aggregation than clopidogrel in NSTE‐ACS patients with moderate to severe CKD.
Competing Interests
There are no competing interests to declare.
The study was supported by National Key Research and Development programme of China (grant number: 2016YFC1301300), the Natural Science Foundation of Liaoning Province (grant number: 201 602 777) and a grant of external sponsored research grant from AstraZeneca Co. Ltd.
Supporting information
Table S1 Clinical outcomes during the 30‐day follow‐up
Wang, H. , Qi, J. , Li, Y. , Tang, Y. , Li, C. , Li, J. , and Han, Y. (2018) Pharmacodynamics and pharmacokinetics of ticagrelor vs. clopidogrel in patients with acute coronary syndromes and chronic kidney disease. Br J Clin Pharmacol, 84: 88–96. doi: 10.1111/bcp.13436.
Clinical Trial Registration: NCT02578537
References
- 1. Best PJ, Steinhub SR, Berger PB, Dasgupta A, Brennan DM, Szczech LA, et al The efficacy and safety of short‐ and long‐term dual antiplatelet therapy in patients with mild or moderate chronic kidney disease: results from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. Am Heart J 2008; 155: 687–693. [DOI] [PubMed] [Google Scholar]
- 2. Dasgupta A, Steinhubl SR, Bhatt DL, Berger PB, Shao M, Mak KH, et al Clinical outcomes of patients with diabetic nephropathy randomized to clopidogrel plus aspirin versus aspirin alone (a post hoc analysis of the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance [CHARISMA] trial). Am J Cardiol 2009; 103: 1359–1363. [DOI] [PubMed] [Google Scholar]
- 3. Park SH, Kim W, Park CS, Kang WY, Hwang SH, Kim W. A comparison of clopidogrel responsiveness in patients with versus without chronic renal failure. Am J Cardiol 2009; 104: 1292–1295. [DOI] [PubMed] [Google Scholar]
- 4. Angiolillo DJ, Bernardo E, Capodanno D, Vivas D, Sabaté M, Ferreiro JL, et al Impact of chronic kidney disease on platelet function profiles in diabetes mellitus patients with coronary artery disease taking dual antiplatelet therapy. J Am Coll Cardiol 2010; 55: 1139–1146. [DOI] [PubMed] [Google Scholar]
- 5. Guo LZ, Kim MH, Shim CH, Choi SY, Serebruany VL. Impact of renal impairment on platelet reactivity and clinical outcomes during chronic dual antiplatelet therapy following coronary stenting. Eur Heart J Cardiovasc Pharmacother 2016; 2: 145–151. [DOI] [PubMed] [Google Scholar]
- 6. Geisler T, Grass D, Bigalke B, Stellos K, Drosch T, Dietz K, et al The residual platelet aggregation after deployment of intracoronary stent (PREDICT) score. J Thromb Haemost 2008; 6: 54–61. [DOI] [PubMed] [Google Scholar]
- 7. Dreisbach AW. The influence of chronic renal failure on drug metabolism and transport. Clin Pharmacol Ther 2009; 86: 553–556. [DOI] [PubMed] [Google Scholar]
- 8. Husted S, van Giezen JJ. Ticagrelor: the first reversibly binding oral P2Y receptor antagonist. Cardiovasc Ther 2009; 27: 259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. James S, Budaj A, Aylward P, Buck KK, Cannon CP, Cornel JH, et al Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the platelet inhibition and patient outcomes (PLATO) trial. Circulation 2010; 122: 1056–1067. [DOI] [PubMed] [Google Scholar]
- 10. Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, et al Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006; 17: 2937–2944. [DOI] [PubMed] [Google Scholar]
- 11. Price MJ, Coleman JL, Steinhubl SR, Wong GB, Cannon CP, Teirstein PS. Onset and offset of platelet inhibition after high‐dose clopidogrel loading and standard daily therapy measured by a point‐of‐care assay in healthy volunteers. Am J Cardiol 2006; 98: 681–684. [DOI] [PubMed] [Google Scholar]
- 12. Angiolillo DJ, Curzen N, Gurbel P, Vaitkus P, Lipkin F, Li W, et al Pharmacodynamic evaluation of switching from ticagrelor to prasugrel in patients with stable coronary artery disease: results of the SWAP‐2 study (switching anti platelet‐2). J Am Coll Cardiol 2014; 63: 1500–1509. [DOI] [PubMed] [Google Scholar]
- 13. Sillén H, Cook M, Davis P. Determination of ticagrelor and two metabolites in plasma samples by liquid chromatography and mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2010; 878: 2299–2306. [DOI] [PubMed] [Google Scholar]
- 14. Peer CJ, Spencer SD, Van Den Berg DA, Pacanowski MA, Horenstein RB, Figg WD. A sensitive and rapid ultra HPLCMS/MS method for the simultaneous detection of clopidogrel and its derivatized active thiol metabolite in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2012; 880: 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gugliucci A, Kinugasa E, Kotani K, Caccavello R, Kimura S. Serum paraoxonase 1 (PON1) lactonase activity is lower in end‐stage renal disease patients than in healthy control subjects and increases after hemodialysis. Clin Chem Lab Med 2011; 49: 61–67. [DOI] [PubMed] [Google Scholar]
- 16. Dohi T, Kasai T, Miyauchi K, Takasu K, Kajimoto K, Kubota N, et al Prognostic impact of chronic kidney disease on 10‐year clinical outcomes among patients with acute coronary syndrome. J Cardiol 2012; 60: 438–442. [DOI] [PubMed] [Google Scholar]
- 17. Chen Y, Dong W, Wan Z, Li Z, Cong H, Hong T, et al Ticagrelor versus clopidogrel in Chinese patients with acute coronary syndrome: a pharmacodynamic analysis. Int J Cardiol 2015; 201: 545–546. [DOI] [PubMed] [Google Scholar]
- 18. Franchi F, Rollini F, Cho JR, Bhatti M, DeGroat C, Ferrante E, et al Impact of escalating loading dose regimens of ticagrelor in patients with ST‐segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of a prospective randomized pharmacokinetic and pharmacodynamic investigation. JACC Cardiovasc Interv 2015; 8: 1457–1467. [DOI] [PubMed] [Google Scholar]
- 19. Tang XF, Wang J, Zhang JH, Meng XM, Xu B, Qiao SB, et al Effect of the CYP2C19 2 and 3 genotypes, ABCB1 C3435T and PON1 Q192R alleles on the pharmacodynamics and adverse clinical events of clopidogrel in Chinese people after percutaneous coronary intervention. Eur J Clin Pharmacol 2013; 69: 1103–1112. [DOI] [PubMed] [Google Scholar]
- 20. Price MJ, Clavijo L, Angiolillo DJ, Carlson G, Caplan R, Teng R, et al A randomised trial of the pharmacodynamic and pharmacokinetic effects of ticagrelor compared with clopidogrel in Hispanic patients with stable coronary artery disease. J Thromb Thrombolysis 2015; 39: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karaźniewicz‐Łada M, Danielak D, Burchardt P, Kruszyna L, Komosa A, Lesiak M, et al Clinical pharmacokinetics of clopidogrel and its metabolites in patients with cardiovascular diseases. Clin Pharmacokinet 2014; 53: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Danielak D, Karaźniewicz‐Łada M, Wiśniewska K, Bergus P, Burchardt P, Komosa A, et al Impact of CYP3A4*1G allele on clinical pharmacokinetics and pharmacodynamics of clopidogrel. Eur J Drug Metab Pharmacokinet 2017; 42: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Clinical outcomes during the 30‐day follow‐up


