Abstract
Aims
Dual‐urate‐lowering therapy (ULT) with xanthine oxidase inhibitor and uricosuric medications is a treatment option for severe gout. Uricosuric agents can cause hyperuricosuria, a risk factor for nephrolithiasis and acute uric acid nephropathy. The aims of the present study were to simulate the relationship between suboptimal drug adherence and efficacy, and to quantify the risk of hyperuricosuria in gout patients receiving mono‐ and dual‐ULTs.
Methods
The impact of poor medication adherence was studied using two‐compartment pharmacokinetic (PK) models based on published evidence, and a semi‐mechanistic four‐compartment pharmacodynamic (PD) model. The PKPD model was used to simulate mono and dual‐ULT in gout patients with either under‐excretion (lowered clearance) or overproduction of uric acid, with suboptimal adherence modelled as either a single drug holiday of increasing duration or doses taken at random.
Results
Simulation results showed a surge in urinary uric acid occurring when dosing is restarted following missed doses. For under‐excreters taking a 20‐day drug holiday, the addition of 200 mg (or 400 mg) lesinurad to 80 mg febuxostat increased the percentage of patients experiencing hyperuricosuria from 0% to 1.4% (or 3.1%). In overproducers, restarting ULTs following drug holidays of more than 5 days leads to over 60% of patients experiencing hyperuricosuria.
Conclusions
Suboptimal medication adherence may compromise the safety and efficacy of mono‐ and dual‐ULTs, especially in patients with gout resulting from an overproduction of uric acid. Clinicians and pharmacists should consider counselling patients with respect to the risks associated with partial adherence, and offer interventions to improve adherence or tailor treatments, where appropriate.
Keywords: febuxostat, hyperuricosuria, lesinurad, pharmacodynamics, pharmacokinetics, urate‐lowering therapy
What is Already known about this Subject
Uricosuric agents, used for the treatment of gout, increase the risk of hyperuricosuria and therefore also acute kidney injury.
Adherence to urate‐lowering therapies for treating gout is among the worst of any chronic disease.
What this Study Adds
Restarting uricosuric treatment following a drug holiday increases the rate of episodic hyperuricosuria.
Suboptimal medication adherence may compromise the safety and efficacy of mono‐ and dual‐urate‐lowering therapies, especially in patient groups such as those with gout resulting from an overproduction of uric acid.
Clinicians and pharmacists should consider counselling patients with respect to the risks associated with partial adherence, and offer interventions to improve adherence or tailor treatments, where appropriate.
Introduction
Gout is a painful and disabling chronic disease which affects a large and increasing number of people and has proven difficult to treat 1. Long‐term treatment with urate‐lowering therapies (ULTs) aims to reduce serum uric acid (sUA) concentrations to below the point of saturation (approximately 6 mg dl–1). When treatment with a xanthine oxidase inhibitor (XOI) alone is unsuccessful, a uricosuric agent can be administered as a co‐treatment 2. Historically, the use of uricosuric agents for long‐term therapy has been limited owing to possible hepatotoxicity (benzbromarone) and drug–drug interactions (probenecid). However, the uric acid transporter‐1 (URAT‐1) inhibitor lesinurad has recently gained regulatory approved and is intended for long‐term therapy in combination with an XOI (such as allopurinol or febuxostat) 3.
As they increase the renal excretion of UA, uricosuric agents such as lesinurad can cause hyperuricosuria (urinary excretion of UA ≥800 mg day−1 in men; ≥750 mg day−1 in women) 4. High levels of urinary UA (uUA) can cause kidney damage, which may be acute – for example, through stone formation (nephrolithiasis) 5 or intrarenal obstruction (acute urate nephropathy) – or chronic, as in chronic (or gouty) nephropathy. Acute kidney injury can occur when UA concentrations in renal tubules reach supersaturation, which also depends on urine pH 6, 7. Chronic nephropathy is thought to result from long‐term hyperuricosuria, in which UA concentrations may be below supersaturation. The existence of chronic nephropathy remains controversial 8 but is supported by animal models and some epidemiological studies 9. The harmful effects of UA on the kidney are a possible explanation of the association, in recent clinical trials, between lesinurad and an increase in the rate of raised serum creatinine and, for higher doses, with serious renal adverse events 10.
Adherence to ULT is known to be among the lowest of any chronic disease treatment 11, 12, with 70% of patients having a drug holiday of at least 60 days over 6 years. Poor adherence to allopurinol monotherapy is associated with lower treatment success rates 13. While dual therapy increased response rates compared with monotherapy in clinical trials 14, 15, 16, an interruption in dosing (drug holiday) could result in high peaks in uUA concentration when treatment is restarted. Suboptimal implementation of the dosing regimen (e.g. late doses, skipping a dose or drug holidays) 17 may therefore increase the risk of renal adverse events caused by UA nephropathy.
The present study aimed to simulate the relationship between poor implementation of dosing and efficacy, and to quantify the risk of hyperuricosuria in gout patients receiving mono‐ and dual‐ULT.
Methods
Strategy
A semi‐mechanistic pharmacokinetic–pharmacodynamic (PKPD) model, based on previous research on the systems pharmacology of the purine metabolic pathway 18, was developed to capture the pharmacology of ULTs (Figure 1). The system comprised four compartments utilizing a zero‐order production rate (k0) governing the formation of xanthine and first‐order production rates characterizing its biotransformation to UA (k1) and the elimination of xanthine (k2) and UA (k3) into the urine. These, in turn, were parameterized in terms of volumes and clearance terms.
Figure 1.
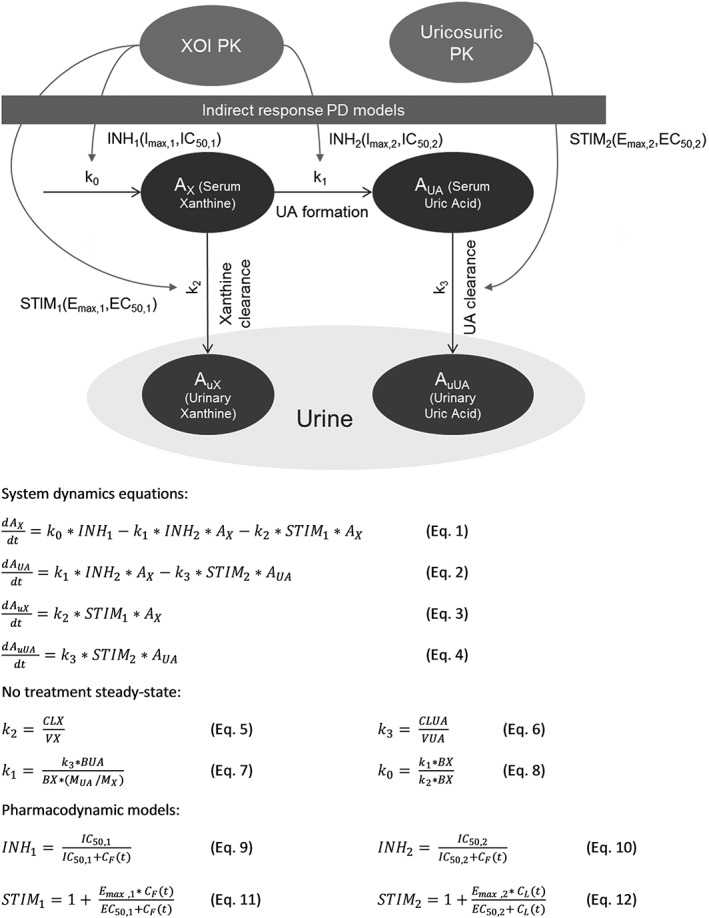
Diagrammatic and mathematical representations of the pharmacodynamics (PD) of dual‐urate‐lowering therapies. AX and AUA are the total time‐varying amounts of xanthine and uric acid in serum respectively; AuX and AuUA are the total time‐varying amounts of xanthine and uric acid in urine respectively; BUA, baseline amount of uric acid; BX, baseline amount of xanthine; CF(t) and CL(t) are the plasma concentrations of febuxostat of lesinurad, respectively; CLUA, renal clearance of uric acid; CLX, renal clearance of xanthine; EC50,1 and EC50,2 are drug concentrations corresponding to 50% of the maximum possible level of stimulation in the pharmacodynamic drug models STIM1 and STIM2 respectively; Emax,1 and Emax,2 are the maximum possible levels of stimulation in the pharmacodynamic drug models STIM1 and STIM2 respectively; IC50,1 and IC50,2 are the drug concentrations corresponding to 50% of the maximum possible level of inhibition in the pharmacodynamic drug models INH1 and INH2 respectively; INH1 and INH2 are inhibitory pharmacodynamic model drug functions; k0, k1, k2 and k3 are the rate parameters for the production of xanthine, xanthine to uric acid conversion, removal of xanthine to urine and removal of uric acid to urine, respectively; STIM1 and STIM2 are stimulatory pharmacodynamic model drug functions; VUA, volume of uric acid distribution; VX, volume of xanthine distribution
The PD model characterizes the time course of sUA, uUA, xanthine and urinary xanthine. Two inhibitory indirect response (turnover) models were used to account for the effect of multiple doses of febuxostat on k0 and k1 19. A stimulatory indirect response 20 equation acting on the k2 rate parameter was incorporated to model the increased xanthine renal clearance associated with febuxostat 21. The clearance of UA upon multiple doses of lesinurad was modelled using a stimulatory indirect response equation acting on the k3 rate parameter.
The system and drug PD model parameter estimates were obtained from the literature and other publicly available sources. As described below, some parameters values were taken directly from the literature, while others were estimated using nonlinear mixed‐effects models and clinical trials data. The parameters required to characterize the pharmacodynamic model are given in Table 1.
Table 1.
Pharmacodynamic (PD) parameters for febuxostat and lesinurad: literature and statistical estimates combined
| Model | Name | Source | Parameter estimates | BSV (SD2) | ||
|---|---|---|---|---|---|---|
| System PD parameter | BX (mg) | Estimated | θ1 | 8.94 | NE | |
| VX (dl) | Estimated | θ2 | 333 | NE | ||
| CLX (dl h–1) | Literature | θ3 | 10.57 | NE | ||
| BUA (mg) | Estimated | θ4 | 703 | NE | ||
| VUA (dl) | Estimated | θ5 | 154 | NE | ||
| CLUA (dl h–1) | Literature | θ6 | 4.11 | NE | ||
| Febuxostat PD parameter | Emax,1 | Assumed | θ7 | 3 | NE | |
| EC50,1 | Assumed | θ8 | 0.001 | NE | ||
| Imax,1 | Assumed | θ9 | 1 | NE | ||
| IC50,1 | Estimated | θ10 | 0.1320 | η3 | 0.2 | |
| Imax,2 | Assumed | θ11 | 1 | NE | ||
| IC50,2 | Estimated | θ12 | 0.00113 | η3 | 0.2 | |
| Lesinurad PD parameter a | E0 | Literature | θ13 | 6.77 | NE | |
|
|
Literature | θ14 | −2.55 | η4 | 0.346 | |
| bCrCl | Literature | θ15 | 0.564 | NE | ||
|
|
Literature | θ16 | 0.0974 | NE | ||
BSV, between‐subject variability; bCrCl, covariate effect parameter for creatinine clearance (ml min−1); BUA, baseline amount of uric acid; BX, baseline amount of xanthine; CLUA, renal clearance of uric acid; CLX, renal clearance of xanthine; E0, baseline sUA concentration; EC50,1, drug concentration corresponding to 50% of the maximum possible level of stimulation Emax,1; EC50 D, drug concentration corresponding to 50% of the maximum reduction in sUA; Emax,1, maximum possible level of stimulation for model STIM1; Emax D, maximum possible reduction in sUA; Imax,1, maximum possible level of inhibition in equation INH1; Imax,2, maximum possible level of inhibition in equation INH2; IC50,1, drug concentration corresponding to 50% of maximum possible inhibition Imax,1; IC50,2, drug concentration corresponding to 50% of maximum possible inhibition Imax,2; INH1 (acting on k0) and INH2 (acting on k1) are inhibitory pharmacodynamic model drug functions; k0, k1, k2 and k3, rate parameters for the production of xanthine, xanthine to uric acid conversion, removal of xanthine to urine and removal of uric acid to urine, respectively; NE, not estimated; SD, standard deviation; STIM1 (acting on k2), stimulatory pharmacodynamic model drug function; VUA, volume of uric acid distribution; VX, volume of xanthine distribution
Error model used: θi = θuexp(ηi)
Lesinurad: Parameters of the direct Emax model used to derive the corresponding parameters of the indirect response model in Figure 1
PK
Two‐compartment models with first‐order absorption for febuxostat and lesinurad obtained from the literature 22, 23 were used to simulate typical and individual subject drug plasma concentration–time courses. The PK parameters, covariate effects and associated between‐subject variability (BSV) are reproduced in Table 2.
Table 2.
Pharmacokinetic parameters for lesinurad and febuxostat
| Parameter | Febuxostat | Lesinurad | ||
|---|---|---|---|---|
| Estimate | BSV (CV%) | Estimate | BSV (CV%) | |
| CL/F_0 (dl h −1 ) a | 49.3 | 18.3 | 69.9 | 63.4 |
| b_CrCl | 0.142 | NA | 0.322 | NA |
| b_WT | 0.155 | NA | NA | |
| Vc/F_0 (dl) b | 322 | NE | 241 | 12.2 |
| b_WT | NA | 0.511 | NA | |
| Vp/F (dl) | 222 | NE | 83 | 20.5 |
| Q/F (dl h −1 ) | 55.7 | NE | 4.48 | NE |
| Ka (h −1 ) | 13.7 | 176 | 0.69 | 121.7 |
| Tlag (h) | 0.23 | NE | 0.233 | 38.9 |
BSV, between‐subject variability; CL/F, apparent clearance; CrCl, creatinine clearance rate; CV%, percentage coefficient of variation; Ka, first‐order absorption; NA, not applicable; NE, not estimated; Q/F, intercompartmental clearance rate; Tlag, absorption time‐lag; Vc/F, volume of the central compartment; Vp/F, volume of the peripheral compartment; WT, individual body weight (kg)
Febuxostat: CL/F = CL/F_0 + b_CrCl*CrCl + b_WT*WT; Lesinurad: CL/F = CL/F_0 * (CrCl/87)^b_CrCl
Lesinurad: VC/F = VC/F_0 * (WT/70)^b_WT
PD
Parameters obtained from the literature
The mean rates of renal clearance of UA and xanthine (CLUA and CLX) in healthy volunteers, along with the BSV, were obtained using summary data from a phase I dose‐escalation study of 154 healthy volunteers receiving febuxostat 24. The reported average clearance in each group and standard deviations (see supplementary material) were used to obtain a weighted average estimate of population typical value and the BSV.
This trial also found that the CLX rate in subjects taking febuxostat, even at doses as low as 10 mg day−1, increased three‐ to fivefold from baseline. This may result from the saturation of active transport processes responsible for the reabsorption of xanthine from the renal tubules 21. A step function was assumed using a stimulatory Emax drug function (Equation 11 in Figure 1), with an EC50,1 of 0.001 mg dl−1 (a low concentration associated with the 10 mg dose) and Emax,1 of 3.
A previous PD model of lesinurad used a direct‐effect Emax model to relate the steady‐state average plasma concentration of lesinurad to the individual's sUA concentration 23. The parameters of the indirect model (Emax,2, EC50,2) were derived from those given in the published direct model ( and ) using the steady‐state equations 19 (see supplementary material). The published model includes a covariate effect of creatinine clearance on the maximum reduction in UA, . The stimulatory model drug function STIM2 is given by Equation 12 in Figure 1, while the equations used to derive Emax,2 and EC50,2 are given below.
CrCl is the individual's creatinine clearance rate and E0 is the baseline sUA concentration of trial participants used to derive the direct Emax model parameters.
Estimations using statistical modelling
All other parameters were estimated using nonlinear mixed‐effects modelling and febuxostat phase I trial summary data on daily area under the plasma concentration–time curve (AUC) and 24‐h urinary excretion of xanthine and UA 24 (see supplementary material). This was conditional on the clearance estimates and drug PD function parameters obtained directly from the literature in the previous section. A NONMEM dataset was created using the AUC and urinary data and the trial dosing schedule. Each value was an average across all individuals within a dose group and has, therefore, been replicated according to the number of subjects within the group, in order to weight by sample size.
The PKPD modelling was conducted using NONMEM 7.3 (ICON Development Solutions, Hanover, MD, USA) and the ADVAN6 routine for solving differential equations. The PD model was coded using the differential equations in Figure 1, where Equations 3 and 4 correspond directly to published data on 24‐h urinary excretion 24. However, additional sUA and serum xanthine accumulation compartments were added to compute the area under the concentration–time curve at 24‐h intervals. Parameter estimation used the first‐order algorithm, and different initial parameter estimates were tested. No random effects were included on system parameters estimated in NONMEM as the data points did not come from individual subjects. The inhibitory model drug functions INH1 and INH2 are given by Equations 9 and 10, respectively, in Figure 1.
In order to simplify the modelling procedure and make use of all available evidence, the statistical modelling was performed in two stages. The first stage used a published PKPD model of febuxostat that used an indirect inhibitory response model applied to a zero‐order rate of UA production 22. Rewriting UA production in the differential equations in our model as zero order, the literature parameter estimate of 0.0239 mg dl−1 was assumed for IC50,2 and the remaining parameters were then estimated. In the second stage, the UA production was returned to being first order, such that it was a function of changing xanthine levels, and a new parameter estimate was made of IC50,2 with all other parameters fixed.
Gout patient simulation model
We assumed that the febuxostat PD parameters estimated for healthy volunteers could be applied to gout patients with hyperuricaemia. However, systems parameters have been adjusted to be representative of a patient population. A typical patient sUA concentration was assumed to be 8.83 mg dl−1 (standard deviation 1.53) as this was the pretreatment sUA concentration for patients in the CRYSTAL (Combination Treatment Study in Subjects With Tophaceous Gout With Lesinurad and Febuxostat (NCT01510769)) trial, which compared febuxostat with lesinurad 25. We considered two phenotypes – overproducers and under‐excreters of UA 26, 27 – and modified the healthy subject system parameters accordingly. For overproducers, the amount of xanthine was scaled up, and for under‐excreters the clearance of UA was scaled down in proportion to the sUA concentration (Table 3). This assumes the same volumes of distribution of xanthine and UA for patients as for healthy subjects.
Table 3.
Individual system parameters for healthy subject and gout patients
| Parameter | Healthy subject | Gout patient | |
|---|---|---|---|
| Under‐excreter | Overproducer | ||
| sUA (mg dl −1 ) | LN(8.83,1.53) | LN(8.83,1.53) | |
| BX (mg) | θ1 | θ1 | θ1*(BUA/θ4) |
| VX (dl) | θ2 | θ2 | θ2 |
| CLX (dl h −1 ) | θ3 | θ3 | θ3 |
| BUA (mg) | θ4 | θ5*sUA | θ5*sUA |
| VUA (dl) | θ5 | θ5 | θ5 |
| CLUA (dl h −1 ) | θ6 | θ6*(θ4/BUA) | θ6 |
BUA, baseline amount of uric acid; BX, baseline amount of xanthine; CLUA, renal clearance of uric acid; LN; Lognormal (mean, standard deviation); sUA, serum uric acid; VUA, volume of uric acid distribution; VX, volume of xanthine distribution
The model was used to simulate treatment with 120 days of ULT in a hypothetical cohort of 1000 patients with baseline characteristics corresponding to the CRYSTAL trial. The cohort was all male (95% were male in CRYSTAL) and baseline sUA, weight and age were assumed to be log‐normally distributed, with mean and standard deviations taken from CRYSTAL (study 304) 28. CrCl, calculated using the Cockcroft–Gault equation 29, overestimated the distribution of the trial participants. All estimates were reduced by 15 ml min−1, and estimates below 30 ml min−1 were excluded to obtain a better representation of the trial population CrCl. The variability of drug effects in INH1 and INH2 could not be estimated and the IC50 parameters were assumed to vary according to η3 with a coefficient of variation of 20%. Steady state was assumed following 30 days of simulated treatment and only the latter 60 days was used to derive results.
The outcomes of interest were the simulated time course of sUA and uUA concentrations, from which we estimated the proportion of patients responding (sUA below ≤5 mg dl−1 on day 120) and the proportion of patients experiencing hyperuricosuria (uUA ≥800 mg day−1 on any day). The normal range of the 24‐h volume of urine is 0.5–1 ml kg−1 h−1 but is likely to be lower in the elderly 30, 31. On this basis, a representative daily urine output for a 99 kg male of 15 dl was assumed for the purpose of estimating uUA concentrations. The soluble limit for UA is highly sensitive to urine pH, being much greater in alkaline than in acidic urine. For a given uUA concentration, the pH at which saturation would occur was estimated by fitting a linear model to literature data 32 to obtain: saturation pH = 6.36–40.96/[uUA].
Modelling adherence
The impact of poor adherence was studied for four different ULT options – namely, febuxostat 80 mg monotherapy and lesinurad 400 mg monotherapy, and febuxostat 80 mg combined with either lesinurad 200 mg or 400 mg. All are once‐daily regimens, and it was assumed that doses are taken at the same time each day. Two types of poor adherence were considered. The first was a single drug holiday of increasing duration, from 1 day to 20 days, to assess the impact on uUA burden of restarting treatment following increasing lengths of drug holiday. The second assessed the impact of poor implementation on response rates and peaks in uUA by simulating doses taken completely at random, with a probability ranging from 1 to 0.1. For all dual‐ULTs, missed doses included both drugs being missed simultaneously. A total of 30 simulations were conducted for each adherence scenario, which used random samples of the model parameter BSV, and the results were averaged over the range of simulation results.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 33, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 34, 35.
Results
The combined set of PD parameters and corresponding BSVs, which were derived or estimated from the literature, are presented in Table 1. Goodness‐of‐fit plots and visual predictive checks for the nonlinear mixed‐effects modelling are provided as supplementary material.
With perfect adherence, uUA concentrations are maintained at low levels under the combined action of febuxostat 80 mg and lesinurad 200 mg (see plots for a typical patient in Figure 2). During a simulated drug holiday of 8 days, urinary concentrations increase as sUA concentrations return towards baseline. After dosing is restarted, peaks in uUA concentrations occur; for the typical under‐excreter, the peak reached 39 mg dl−1, which exceeds the typical average concentration for a healthy person (30 mg dl−1). For the typical overproducer, the peak uUA concentration was 85 mg dl−1, which exceeds the threshold for the typical average uUA concentration of an individual with hyperuricosuria (53 mg dl−1). For the typical under‐excreter, uUA concentrations after restarting treatment following an 8‐day drug holiday could become supersaturated if the urinary pH was towards the acidic end of the normal range (pH <5.3; normal range 4.5–8.0). For the typical overproducer, peak uUA concentrations after restarting treatment are more likely to reach supersaturation at closer to the mid‐point of the normal range, at approximately 5.9.
Figure 2.
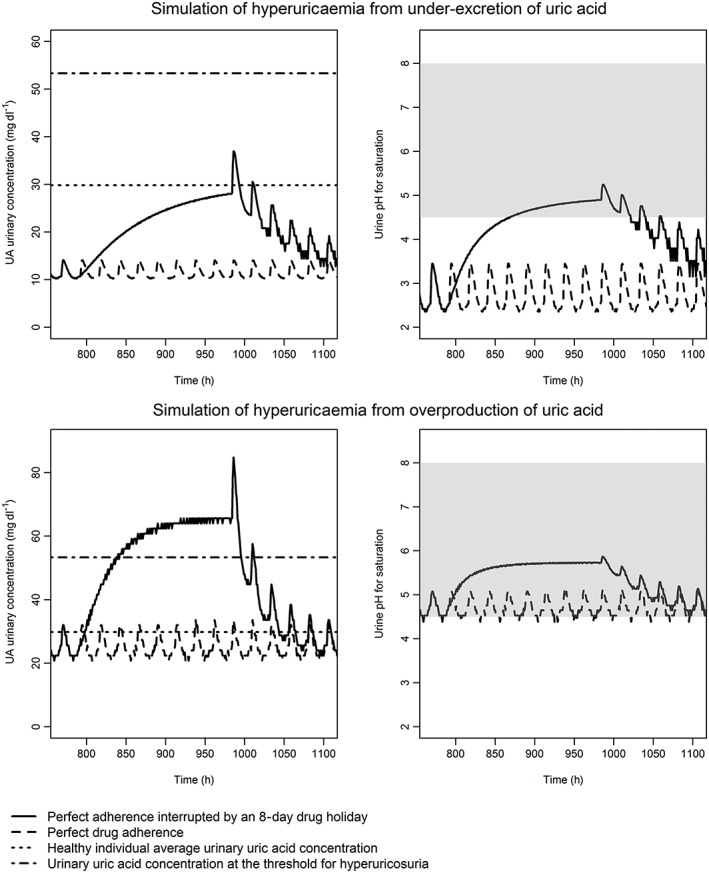
Simulated urinary uric acid (uUA) concentration and estimated pH for uric acid supersaturation, assuming a daily volume of urine of 15 dl. The simulated uUA concentration over time (left‐hand panels) and the estimated pH at which this concentration would become supersaturated (right‐hand panels). Imperfect adherence is modelled as an 8‐day drug holiday (beginning on day 33). The shaded area represents the normal range for urine pH. The upper plots are the central estimates from the pharmacokinetic–pharmacodynamic model for a gout patient with hyperuricaemia from a reduced rate of uric acid clearance, and the lower plots for hyperuricaemia due to overproduction of xanthine. The urate lowering therapies used in these simulations were febuxostat 80 mg and lesinurad 200 mg, both once daily
Across the population, increasing the length of a drug holiday increases the proportion of patients whose daily amount of UA excreted exceeds the threshold for hyperuricosuria upon restarting treatment (Figure 3). The proportion of patients with hyperuricosuria increases with increasing doses of lesinurad and is greatest for lesinurad 400 mg monotherapy. For under‐excreters taking a 20‐day drug holiday, the addition of 200 mg (or 400 mg) lesinurad to 80 mg febuxostat increased the percentage of patients experiencing hyperuricosuria from 0% to 1.4% (or 3.1%). In overproducers, restarting ULTs following drug holidays of more than 5 days led to over 60% of patients experiencing hyperuricosuria. In both patient groups, 1‐ or 2‐day drug holidays were well tolerated compared with longer holidays, with only moderate increases in the rates of hyperuricosuria.
Figure 3.
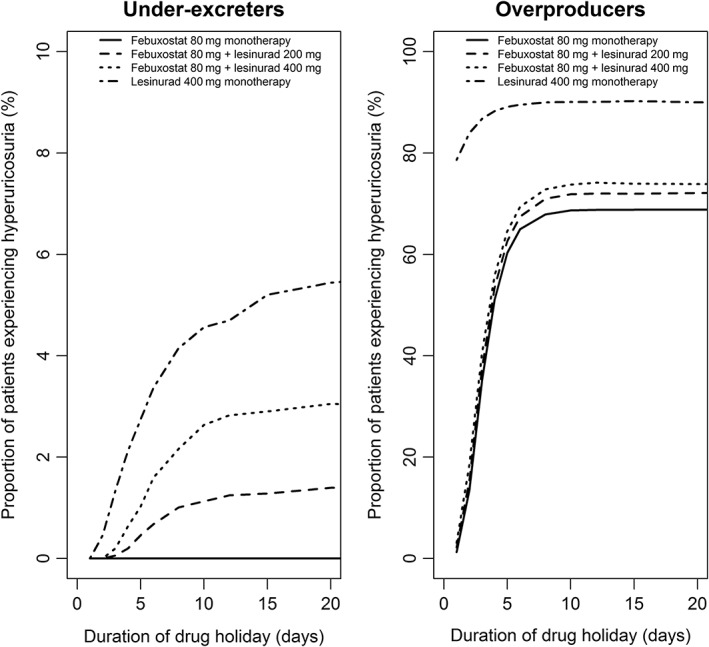
Proportion of simulated patients with 1‐day hyperuricosuria following a single drug holiday taking place after 1 month of perfect adherence
With perfect adherence, the proportion of patients treated to target (sUA ≤5 mg dl−1 on day 120) was greater than was observed in the CRYSTAL trial (Figure 4). However, success rates fell rapidly as an increasing proportion of doses were missed at random. For daily doses of febuxostat 80 mg, febuxostat 80 mg with lesinurad 200 mg, febuxostat 80 mg with lesinurad 400 mg and lesinurad 400 mg monotherapy, the success rates at 100% of doses taken in under‐excreters were 87.2%, 94.5%, 96.0% and 15.4%, respectively. At 50% of doses taken at random, these success rates fell to 27.2%, 42.6%, 47.3% and 7.4%, respectively. The corresponding plots for overproducers are provided in the supplementary material.
Figure 4.
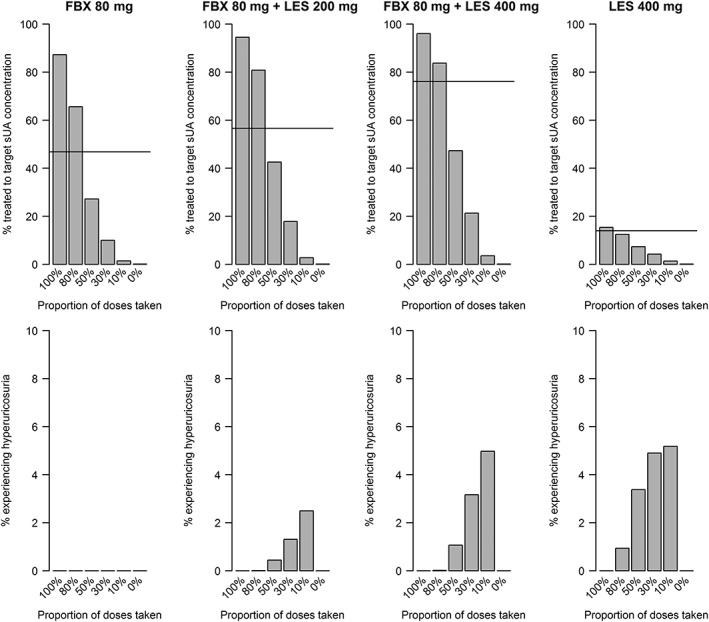
Treatment success rates (top row) and the proportion of patients experiencing 1‐day hyperuricosuria during 2 months of urate‐lowering therapy (bottom row). Horizontal lines provide the reference response rates for this treatment arm from the CRYSTAL trial comparing febuxostat and lesinurad, and Study 303 25 for lesinurad 400 mg monotherapy. Results are for under‐excreters of uric acid only; for overproducers, see the supplementary material. FBX: febuxostat; LES, lesinurad; sUA, serum uric acid
Increasing the proportion of doses missed at random resulted in higher rates of hyperuricosuria due to randomly occurring drug holidays, especially in the presence of a uricosuric agent (Figure 4). The baseline daily uUA excreted in under‐excreters was below healthy baseline levels and none of the simulated cohort showed hyperuricosuria in the absence of ULT. For dual‐ULT with a uricosuric agent, however, randomly occurring drug holidays resulted in increasing rates of hyperuricosuria. For example, at 30% of doses taken, for febuxostat 80 mg with lesinurad 200 mg, febuxostat 80 mg with lesinurad 400 mg and lesinurad 400 mg monotherapy, the rates of hyperuricosuria were 1.3%, 3.2% and 4.9%, respectively.
Discussion
The use of uricosuric agents, either as monotherapy or in combination with an XOI, results in transient increases in uUA concentrations when dosing is restarted after a drug holiday. As a result, supersaturation of UA in urine can occur at pH values within the normal expected range, and therefore precipitation of UA in the renal tubules is more likely to occur during routine clinical practice. This effect is likely to be greater following a drug holiday from dual‐ULTs than when starting treatment for the first time, where, as per the regulatory approval of lesinurad, patients must already have been taking an XOI. Specifically, our simulations indicated that peak uUA concentrations reach the threshold for supersaturation at a urinary pH of 5.3 for under‐excreters and of 5.9 for overproducers, so that crystal formation may occur for a urinary pH at or below this level.
Increasing the length of a drug holiday increased the proportion of patients whose daily amount of UA excreted exceeded the threshold for hyperuricosuria. The increase was more rapid for patients with overproduction, suggesting poorer drug forgiveness in this population. Treatment outcomes deteriorated rapidly as an increasing proportion of doses were missed at random. For under‐excreters taking febuxostat 80 mg with lesinurad 200 mg, treatment‐to‐target rates fell by more than 50% when adherence reduced from 100% to 50%.
Approximately 90% of gout patients have hyperuricaemia caused by the renal under‐excretion of UA 27. In these cases, unless sUA concentrations are very high, or urinary volume is also lowered, uUA concentrations are likely to be lower than in healthy subjects. However, in simulations of drug holidays, after restarting dual‐ULT, under‐excreters had uUA concentrations raised to above the baseline levels for healthy subjects, and a small proportion exceeded the threshold for hyperuricosuria. For these patients to be at an increased risk of kidney damage, either a very low urinary output volume or a low urine pH (although still within the typical pH range) would probably be required. Urine pH is itself a primary predictor of nephrolithiasis as the solubility of UA is highly sensitive to small changes in pH 32.
Genetic disorders or a high‐purine diet can be the cause of an overproduction of UA in the remaining 10% of gout patients 36. Hyperuricosuria is a defining feature of UA overproduction 26, putting these patients at an increased risk of kidney injury without treatment. Our simulations suggest that in the case of very good medication adherence (≥80% of doses taken), dual‐ULT would result in sustained reductions in sUA concentrations and also, therefore, uUA excreted. Regular drug holidays, however, would result in episodes in which uUA output was raised above its already high baseline. For this reason, uricosuric agents may not be appropriate for patients with hyperuricaemia due to UA overproduction 37, but no cautions are provided in the label for lesinurad 38.
To our knowledge, the present study was the first to investigate the relationship between medication adherence and the efficacy and safety of dual‐ULT therapy for the treatment of gout. This was especially timely, given the recent approval of lesinurad for use in combination with an XOI in patients who have not responded on an XOI alone 39. Our analysis benefited from having used a semi‐mechanistic PD model which provides a level of complexity capable of capturing the nonsteady‐state system dynamics. The effects of treatments were investigated in two distinct patient subgroups, the cause of hyperuricaemia being either an overproduction or under‐excretion of UA. When comparing our simulation results with the findings from clinical trials, all of our perfect adherence simulations produced higher treatment success rates than had been reported in trials. Mathematical models such as this could be used to anticipate the problems resulting from suboptimal adherence, and potentially to help to identify the properties of more forgiving uricosuric agents.
The main limitation of the study was our reliance on different sources of data from different populations. This limited our ability fully to quantify the variability and co‐dependencies; nonetheless, we consider the model to be representative of existing dual‐ULTs. We assumed that the nonrenal clearance of UA, which is responsible for around a third of total excretion 40, was negligible. Nevertheless, the contribution of nonrenal clearance relative to renal clearance will be lower in scenarios where a uricosuric agent is taken. Finally, the analysis focused on the XOI febuxostat but allopurinol is by far the most commonly prescribed ULT. However, we have no reason to believe that these findings do not extend to other XOIs (allopurinol) and uricosuric agents (probenecid and benzbromarone).
With the currently available ULTs, a large proportion of patients do not achieve sustained reductions in sUA to below saturation concentrations. The potential reasons for treatment failure include poor implementation of the treatment regimen (adherence), under‐dosing, variation in treatment response and the underlying cause of hyperuricaemia 41. Persistence with ULTs is known to be among the lowest of any chronic disease treatment 11, 12 and previous studies have provided evidence both for long 42 and short 43 drug holidays. The present study showed that renal safety may also be compromised by suboptimal medication adherence and highlights the need to improve adherence and adapt treatments to poorly adherent populations. This could include instructions on drug labelling 44, indicating a number of doses which can be missed based on the forgiveness of the drug to missed doses 45. Such measures may improve the safety profile of future uricosuric agents, which for lesinurad may have influenced reimbursement decisions 46.
If gout patients adhere well to dual‐ULT, then it appears to offer a means of further reducing sUA concentrations with a negligible increase in uUA output. However, regular drug holidays, which are commonplace among gout patients using ULTs, result in much lower rates of long‐term treatment success and increased rates of hyperuricosuria when treatment is restarted. This has the potential to increase the risk of kidney damage in all patients, but especially those with hyperuricaemia due to overproduction of UA. Further research is needed into the impact of adherence patterns on treatment success rates and kidney safety in order better to understand how dual‐ULT could be used optimally in the treatment of hyperuricaemia in patients with gout. However, at present, counselling patients with respect to the risks associated with poor adherence should be advised.
Competing Interests
S.M. and E.S. are, or were, employees of Pfizer. The other authors have no competing interests to declare.
This study was funded by the MRC Network of Hubs for Trial Methodological Research (HTMR), reference number MR/L004933/1‐ Q25, and the MRC North‐West HTMR, reference number MR/K025635/1. We acknowledge the support of the Super Computing Wales project, which is part‐funded by the European Union's Convergence European Regional Development Fund, administered by the Welsh Government.
Contributors
D.H.‐M., E.S., S.M., S.L. and D.A.H. contributed substantially to the study conception or design, or the acquisition, analysis or interpretation of the data. D.H.‐M. drafted the manuscript and E.S., S.M., S.L. and D.A.H. revised it critically for important intellectual content. D.H.‐M., E.S., S.M., S.L. and D.A.H. gave final approval of the version to be published. D.A.H. agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supporting information
Appendix A Study information and data tables for TMX‐99‐001
Table A1 TMX‐99‐001 Study participants
Table A2 TMX‐99‐001 24‐h area under the concentration–time curve of serum uric acid
Table A3 TMX‐99‐001 24‐h area under the concentration–time curve of serum xanthine
Table A4 TMX‐99‐001 24‐h renal clearance of serum uric acid
Table A5 TMX‐99‐001 24‐h renal clearance of serum xanthine
Table A6 TMX‐99‐001 24‐h total amount of uric acid excreted in urine
Table A7 TMX‐99‐001 24‐h total amount of xanthine excreted in urine
Appendix B Literature pharmacokinetic data and goodness of fit of febuxostat regression modelling
Table B1 Summary of pharmacokinetic and pharmacodynamic parameters from Study C02–009
Figure B1 Visual predictive checks for febuxostat model fitting to phase I data
Appendix C Derivation of lesinurad indirect response model parameters
Table C1 Food and Drug Administration‐reported pharmacodynamic model parameter estimates
Appendix D Simulation model results in overproducers of uric acid
Figure D1 Treatment success rates (top row) and the proportion of patients experiencing 1‐day hyperuricosuria in 2 months of urate‐lowering therapy (bottom row)
Hill‐McManus, D. , Soto, E. , Marshall, S. , Lane, S. , and Hughes, D. (2018) Impact of non‐adherence on the safety and efficacy of uric acid‐lowering therapies in the treatment of gout. Br J Clin Pharmacol, 84: 142–152. doi: 10.1111/bcp.13427.
References
- 1. Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet 2016; 6736: 1–14. [DOI] [PubMed] [Google Scholar]
- 2. Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castaneda‐Sanabria J, et al 2016 updated EULAR evidence‐based recommendations for the management of gout. Ann Rheum Dis 2017; 76: 29–42. [DOI] [PubMed] [Google Scholar]
- 3. Miner J, Tan PK, Hyndman D, Liu S, Iverson C, Nanavati P, et al Lesinurad, a novel, oral compound for gout, acts to decrease serum uric acid through inhibition of urate transporters in the kidney. Arthritis Res Ther 2016; 18: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pak CYC, Poindexter JR, Peterson RD, Koska J, Sakhaee K. Biochemical distinction between hyperuricosuric calcium urolithiasis and gouty diathesis. Urology 2002; 60: 789–794. [DOI] [PubMed] [Google Scholar]
- 5. Bluestone R, Klinenberg J, Lee IK. Benzbromarone as a long‐term uricosuric agent. Adv Exp Med Biol 1980; 122A: 283–286. [DOI] [PubMed] [Google Scholar]
- 6. Maalouf NM, Cameron MA, Moe OW, Sakhaee K. Novel insights into the pathogenesis of uric acid nephrolithiasis. Curr Opin Nephrol Hypertens 2004; 13: 181–189. [DOI] [PubMed] [Google Scholar]
- 7. Hahn K, Kanbay M, Lanaspa MA, Johnson RJ, Ejaz AA. Serum uric acid and acute kidney injury: a mini review. J Adv Res 2017; 8: 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moe OW. Posing the question again: does chronic uric acid nephropathy exist? J Am Soc Nephrol 2010; 21: 395–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bellomo G. Uric acid and chronic kidney disease: a time to act? World J Nephrol 2013; 2: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. EMA . Zurampic assessment report. vol. EMA/6459/2. 2015. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003932/WC500203069.pdf (last accessed 12 July 2016).
- 11. Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy 2008; 28: 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Vera MA, Marcotte G, Rai S, Galo JS, Bhole V. Medication adherence in gout: a systematic review. Arthritis Care Res 2014; 66: 1551–1559. [DOI] [PubMed] [Google Scholar]
- 13. Halpern R, Mody RR, Fuldeore MJ, Patel PA, Mikuls TR. Impact of noncompliance with urate‐lowering drug on serum urate and gout‐related healthcare costs: administrative claims analysis. Curr Med Res Opin 2009; 25: 1711–1719. [DOI] [PubMed] [Google Scholar]
- 14. Bardin T, Keenan RT, Khanna PP, Kopicko J, Fung M, Bhakta N, et al Lesinurad in combination with allopurinol: a randomised, double‐blind, placebo‐controlled study in patients with gout with inadequate response to standard of care (the multinational CLEAR 2 study). Ann Rheum Dis 2017; 76: 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dalbeth N, Jones G, Terkeltaub R, Khanna D, Kopicko J, Bhakta N, et al SAT0329: Lesinurad, a novel selective uric acid reabsorption inhibitor, in combination with Febuxostat, in patients with Tophaceous gout: the CRYSTAL phase III clinical trial. 2015; 74: 778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saag KG, Fitz‐Patrick D, Kopicko J, Fung M, Bhakta N, Adler S, et al Lesinurad combined with allopurinol: randomized, double‐blind, placebo‐controlled study in gout subjects with inadequate response to standard of care allopurinol (a US‐based study). Arthritis Rheumatol 2017; 69: 203–212. [DOI] [PubMed] [Google Scholar]
- 17. Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, et al A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 2012; 73: 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dua P, Demin O, Sokolov V, Yakovleva T, Demin O, Van Der Graaf P. A systems pharmacology model of purine metabolism to explore novel options for the treatment of gout (poster). Syst Pharmacol Drug Discov Devel 2014. Available at http://insysbio.ru/sites/default/files/2014_Gout_PDua_poster.pdf. [Google Scholar]
- 19. Gabrielsson J, Weiner D. Pharmacokinetic and Pharmacodynamic Data Analysis, 5th edn. Apotekarsocieteten, Stockholm, Sweden: 2016. [Google Scholar]
- 20. Sharma A, Jusko WJ. Characteristics of indirect pharmacodynamic models and applications to clinical drug responses. Br J Clin Pharmacol 1998; 45: 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khosravan R, Grabowski BA, Wu J‐T, Joseph‐Ridge N, Vernillet L. Pharmacokinetics, pharmacodynamics and safety of febuxostat, a non‐purine selective inhibitor of xanthine oxidase, in a dose escalation study in healthy subjects. Clin Pharmacokinet 2006; 45: 821–841. [DOI] [PubMed] [Google Scholar]
- 22. Centre for Drug Evaluation and Research . Clinical pharmacology and biopharmaceutics review (NDA 21‐856). 2008. Available at https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/021856s000_ClinPharmR_P1.pdf (last accessed 10 July 2016).
- 23. Centre for Drug Evaluation and Research . Clinical pharmacology and biopharmaceutics review (application number: 207988Orig1s000). 2014. Available at https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207988Orig1s000SumR.pdf (last accessed 12 July 2016).
- 24. TAP Pharmaceutical Products Inc . TMX‐99‐001 study report 2004: obtained via the EMA on 16/06/16.
- 25. Ardea Biosciences . Lesinurad in combination with a xanthine oxidase inhibitor for treatment of hyperuricemia associated with gout. Briefing Document for the Arthritis Advisory Committee. 2015. Available at http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/ (last accessed 20 August 2016).
- 26. Pittman JR, Bross MH. Diagnosis and management of gout. Am Fam Physician 1999; 59: 1799–1806 1810. [PubMed] [Google Scholar]
- 27. Choi HK, Mount DB, Reginato AM, American College of Physicians , American Physiological Society . Pathogenesis of gout. Ann Intern Med 2005; 143: 499–516. [DOI] [PubMed] [Google Scholar]
- 28. FDA . Lesinurad for the proposed indication of treatment of hyperuricemia associated with gout in combination with a xanthine oxidase inhibitor (FDA briefing package) (NDA 207988). 2015. Available at https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM467942.pdf (last accessed 11 June 2016).
- 29. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41. [DOI] [PubMed] [Google Scholar]
- 30. Tissot W, Amundsen CL, Diokno AC, Webster GD, Coats AC. Bladder diary measurements in asymptomatic males: frequency, volume per void, and 24‐hour volume. Neurourol Urodyn 2008; 27: 198–204. [DOI] [PubMed] [Google Scholar]
- 31. Parsons M, Tissot W, Cardozo L, Diokno A, Anundsen CL, Coats AC. Normative bladder diary measurements: night versus day. Neurourol Urodyn 2007; 26: 465–476. [DOI] [PubMed] [Google Scholar]
- 32. Mehta TH, Goldfarb DS. Uric acid stones and hyperuricosuria. Adv Chronic Kidney Dis 2012; 19: 413–418. [DOI] [PubMed] [Google Scholar]
- 33. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH, et al The IUPHAR / BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 2015; 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Doherty M. New insights into the epidemiology of gout. Rheumatology 2009; 49: 613–614. [DOI] [PubMed] [Google Scholar]
- 37. Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res 2012; 64: 1431–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. FDA . Zurampic label. 2015. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207988lbl.pdf (last accessed 2 August 2016).
- 39. Center for Drug Evaluation and Research . Lesinurad approval package. Application number: 207988Orig1s000. 2015. Available at https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207988Orig1s000Approv.pdf (last accessed 9 July 2016).
- 40. Ichida K, Matsuo H, Takada T, Nakayama A, Murakami K, Shimizu T, et al Decreased extra‐renal urate excretion is a common cause of hyperuricemia. Nat Commun 2012; 3: 764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stamp LK, Merriman TR, Barclay ML, Singh JA, Roberts RL, Wright DF, et al Impaired response or insufficient dosage? Examining the potential causes of “inadequate response” to allopurinol in the treatment of gout. Semin Arthritis Rheum 2014; 44: 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harrold LR, Andrade SE, Briesacher B, Raebel MA, Fouayzi H, Yood RA, et al The dynamics of chronic gout treatment: medication gaps and return to therapy. Am J Med 2010; 123: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Klerk E, van der Heijde D, Landewe R, van der Tempel H, Urquhart J, van der Linden S. Patient compliance in rheumatoid arthritis, polymyalgia rheumatica, and gout. J Rheumatol 2003; 30: 44–54. [PubMed] [Google Scholar]
- 44. Levy G, Zamacona MK, Jusko WJ. Developing compliance instructions for drug labeling. Clin Pharmacol Ther 2000; 68: 586–591. [DOI] [PubMed] [Google Scholar]
- 45. Assawasuwannakit P, Braund R, Duffull SB. Quantification of the forgiveness of drugs to imperfect adherence. CPT Pharmacometrics Syst Pharmacol 2015; 4: 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. National Institute for Health and Care Excellence . Single Technology Appraisal: Lesinurad for treating chronic hyperuricaemia in people with gout [ID761] Committee Papers. 2016. Available at https://www.nice.org.uk/guidance/gid-tag510/documents/committee-papers (last accessed 10 July 2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A Study information and data tables for TMX‐99‐001
Table A1 TMX‐99‐001 Study participants
Table A2 TMX‐99‐001 24‐h area under the concentration–time curve of serum uric acid
Table A3 TMX‐99‐001 24‐h area under the concentration–time curve of serum xanthine
Table A4 TMX‐99‐001 24‐h renal clearance of serum uric acid
Table A5 TMX‐99‐001 24‐h renal clearance of serum xanthine
Table A6 TMX‐99‐001 24‐h total amount of uric acid excreted in urine
Table A7 TMX‐99‐001 24‐h total amount of xanthine excreted in urine
Appendix B Literature pharmacokinetic data and goodness of fit of febuxostat regression modelling
Table B1 Summary of pharmacokinetic and pharmacodynamic parameters from Study C02–009
Figure B1 Visual predictive checks for febuxostat model fitting to phase I data
Appendix C Derivation of lesinurad indirect response model parameters
Table C1 Food and Drug Administration‐reported pharmacodynamic model parameter estimates
Appendix D Simulation model results in overproducers of uric acid
Figure D1 Treatment success rates (top row) and the proportion of patients experiencing 1‐day hyperuricosuria in 2 months of urate‐lowering therapy (bottom row)


