Abstract
Since brain's microvasculature is compromised in gliomas, intravenous injection of tumor-targeting nanoparticles containing drugs (D-NPs) and superparamagnetic iron oxide (SPIO-NPs) can deliver high payloads of drugs while allowing MRI to track drug distribution. However, therapeutic effect of D-NPs remains poorly investigated because superparamagnetic fields generated by SPIO-NPs perturb conventional MRI readouts. Because extracellular pH (pHe) is a tumor hallmark, mapping pHe is critical. Brain pHe is measured by biosensor imaging of redundant deviation in shifts (BIRDS) with lanthanide agents, by detecting paramagnetically shifted resonances of nonexchangeable protons on the agent. To test the hypothesis that BIRDS-based pHe readout remains uncompromised by presence of SPIO-NPs, we mapped pHe in glioma-bearing rats before and after SPIO-NPs infusion. While SPIO-NPs accumulation in the tumor enhanced MRI contrast, the pHe inside and outside the MRI-defined tumor boundary remained unchanged after SPIO-NPs infusion, regardless of the tumor type (9L versus RG2) or agent injection method (renal ligation versus coinfusion with probenecid). These results demonstrate that we can simultaneously and noninvasively image the specific location and the healing efficacy of D-NPs, where MRI contrast from SPIO-NPs can track their distribution and BIRDS-based pHe can map their therapeutic impact.
1. Introduction
Treatment and management of glioblastoma, the most common and malignant form of primary brain tumors, represent an unmet clinical challenge [1]. While gliomas are relatively rare compared to other forms of cancer malignancies [1], they are characterized by the worst prognosis, with a 5-year survival of less than 10% [2]. Treatments fail because gliomas are highly invasive, the blood brain barrier (BBB) prevents drugs from reaching the tumor at therapeutic doses, and systemic toxicity limits benefits from therapy [3–5]. In addition, there is a lack of reliable in vivo methods that can simultaneously and noninvasively measure the delivery and therapeutic benefits of cancer drugs. Therapy can be greatly improved by delivering high drug doses specifically to the tumor (while minimizing systemic toxicities) and by timely and quantitative monitoring of the delivery and efficacy of these drugs.
The transport and delivery of therapeutic agents into the brain parenchyma are impeded by a dense network of capillary endothelial cells, pericytes, and perivascular macrophages, which together form the BBB [6]. In the healthy brain, the BBB allows a highly selective transport of endogenous substances (e.g., nutrients) that are critical to brain function while keeping out potentially harmful toxins and drugs that are circulating in the blood [7, 8]. However, the BBB is disrupted in several pathologies including high-grade gliomas leading to increased leakiness (i.e., hyperpermeability) [9, 10]. Breakthroughs in glioma imaging and chemotherapy exploit the fact that nanoparticles (NPs) loaded with drugs (D-NPs) and MRI contrast agents like superparamagnetic iron oxide (SPIO-NPs) can extravasate from the blood through the large vascular fenestrations into the tumor [11, 12]. The combination of increased vascular permeability and poor lymphatic clearance in tumors leads to accumulation of NPs in tumors through enhanced permeation and retention (EPR) [13]. While tumors, including gliomas, generally possess larger vascular fenestrations (and hence higher permeability) compared to healthy tissue, these fenestrations are highly dependent on the location of the vessels in the tumor (i.e., fewer abnormalities in vessels on tumor periphery and higher in the tumor core), and moreover the fenestrations depend on the age/size of the tumor (i.e., larger tumors tend to have more abnormalities) [14, 15]. The pores on tumor vasculature include caveolae, vesiculo-vacuolar organelles, and fenestrations that are on the order of 10–20 nm along with larger but sporadic interendothelial cell gaps, which are significantly larger than 200 nm in diameter [16–20]. Thus NPs like the Molday ION (or SPIO-NPs; 30–50 nm hydrodynamic diameter) can extravasate passively across the BBB of the tumor niche more effectively compared to the normal neuropil. Therefore extravasation and accumulation of NPs will vary between the tumor core, tumor boundary, and healthy tissue [21–25].
Tumor-specific delivery of D-NPs can be further enhanced by coating the D-NPs with ligands that target overexpressed receptors and/or transporters in tumors [26–29]. Despite these advances in targeting of D-NPs for delivering high drug payloads to tumors, the effect of these D-NPs on the tumor microenvironment remains largely unknown. SPIO-NPs have been evaluated and approved for clinical use as MRI contrast agents [30–37]. Because the MRI contrast generated by SPIO-NPs persists for a long time, SPIO-NPs have recently been combined with D-NPs and used to simultaneously image drug delivery and biodistribution with MRI [12, 38–41]. However, the large superparamagnetic fields generated by SPIO-NPs disturb most MRI molecular readouts.
Because low extracellular pH (pHe) is a hallmark of cancer pathogenesis and promotes tumor invasion and resistance to therapy [42–48], there is need for advanced pHe mapping methods to enable monitoring of glioma invasion. Since some drugs only work in certain pH ranges, precise knowledge of pHe can aid in choosing and tailoring therapeutic regimens [49–51]. Additionally, their therapeutic efficacy may be assessed by measuring their ability to raise and normalize pHe, for example, by drugs that alter pHe directly or affect tumor's aerobic glycolysis. Many MRI methods exist for measuring and mapping pHe. Relaxation-based methods (e.g., with Gd3+) are highly dependent on the degree of tissue perfusion and local agent concentration thus making quantification of pHe difficult [52]. pHe-sensitive MRI methods based on proton exchange (i.e., between water protons and protons of amide/amine and hydroxyl moieties) such as chemical exchange saturation transfer (CEST) are also dependent on agent concentration and may additionally be complicated by magnetization transfer effects [53]. Spectroscopic methods, for example, 31P MRS with 3-aminopropyl phosphonate (3-APP), which have pHe-sensitive exchangeable protons [54, 55], suffer from low spatial resolution and significant line broadening in the presence of SPIO-NPs [56].
We previously obtained pHe maps in glioma-bearing rats with biosensor imaging of redundant deviation in shifts (BIRDS) using lanthanide agents, for example, thulium 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrakis(methylene phosphonate), TmDOTP5− [57, 58]. Since the BIRDS platform is based on direct detection of the paramagnetically shifted resonances of the nonexchangeable protons on the agents (rather than their peak amplitude or effect on water relaxation rate), the pHe readout with BIRDS is independent of agent concentration [59, 60]. The functional part of the pH sensitivity stems from the pH-sensitive exchangeable protons of the phosphonate groups on the agents. With advanced k-space sampling of ultra-fast chemical shift imaging (CSI), the spatiotemporal resolution of BIRDS has improved [61]. Previously we observed in vitro that the pH sensitivities and readout with BIRDS agents are not compromised by the presence of SPIO-NPs [62]. Here we hypothesized that BIRDS-based pHe readout in glioma-bearing rats remains uncompromised by the presence of SPIO-NPs. We compared pHe measured with TmDOTP5− by BIRDS before and after infusion of SPIO-NPs in rats bearing 9L gliosarcomas and RG2 gliomas. We used different agent administration methods (renal ligation versus coinfusion with probenecid) to inhibit the rapid clearance of the agent by the renal system. In addition, we compared the transverse relaxation rate enhancement from SPIO-NPs across brain regions. Our results suggest that we can use the MRI contrast from SPIO-NPs to track the distribution of D-NPs and then use the BIRDS-based pHe readout to map their therapeutic impact.
2. Materials and Methods
TmDOTP5− for BIRDS was purchased from Macrocyclics Inc. (Plano, TX, USA), while SPIO-NPs (Molday ION) were purchased from BioPAL Inc. (Worcester, MA, USA). The Molday ION (10 mg Fe/mL, dextran-coated, hydrodynamic diameter 30 nm, zeta potential −4.8 mV) were used without further modification or dilution to avoid altering their physical properties. Probenecid (used for temporary inhibition of renal clearance) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Fischer 344 rats (male, 200–250 g) were obtained from Yale University vendors. RG2 and 9L tumor cell lines were purchased from American Type Culture Collections (Manassas, VA, USA). All animal experiments were conducted in accordance with Yale University's approved institutional animal care and use committee (IACUC) protocols. Tumor inoculation, animal preparation, and handling were conducted as described in our previous work [57, 58]. In vivo magnetic resonance (MR) scans were conducted on a 9.4T Agilent (Santa Clara, CA, USA) or Bruker (Billerica, MA, USA) horizontal-bore spectrometer with a 1.4-cm 1H surface RF coil.
2.1. Tumor Inoculation
The RG2 and 9L tumor cell lines were cultured and grown at 37°C and 5% CO2 in DMEM media containing 10% heat-activated fetal bovine serum and 1% penicillin-streptomycin. The cells were harvested when they reached 80% confluence and suspended in serum-free media for inoculation. Rats were anesthetized with 3% isoflurane and placed on a stereotactic holder. A heating pad was used to maintain the rat at physiological temperature (36-37°C). An aliquot volume of 5 μL with RG2 cells (1,250 cells) or 9L cells (100,000 cells) was injected into the right striatum 3 mm laterally to the right of bregma and 3 mm below the dura using a 10 μL Hamilton syringe fitted with a 26-gauge beveled needle. The 5 μL volume was injected over the course of 5 minutes and the needle was left in place for an additional 5 minutes after the infusion stopped. The needle was then withdrawn slowly to prevent backflow of the cells. The cranial burr hole was sealed with bone wax. The scalp was sutured and treated with antibiotics to prevent infection. Meloxicam (1 mg/kg) was administered to prevent pain and inflammation.
2.2. Animal Preparation and Scanning
The tumor-bearing rats were scanned ~3 weeks after tumor inoculation when the tumor diameter was at least ~3 mm. The rats were anesthetized with 2% isoflurane, tracheotomized, and artificially ventilated (70% N2O/30% O2). The rats were placed on a heating pad to keep them warm during surgery. A femoral vein was cannulated with a PE-10 line for contrast agent administration (1 mmol/kg for TmDOTP5− and 14 mg Fe/kg for SPIO-NPs). A femoral artery was cannulated with a PE-50 line for monitoring animal physiology (pCO2, pO2, pH, blood pressure) throughout the experiment. The rat was then anesthetized with α-chloralose using an intraperitoneal line. To inhibit renal clearance and enhance contrast agent extravasation into the extracellular space and accumulation in the tumor, rats either received a coinfusion of TmDOTP5− and probenecid (n = 5) or underwent renal ligation and infusion of TmDOTP5− alone (n = 3). While renal ligation inhibits clearance efficiently, it is not suitable for longitudinal studies. Previously, we demonstrated that probenecid temporarily inhibited renal clearance when coinjected with the agent, thus enabling longitudinal studies and obviating the need for invasive renal surgeries [58]. Probenecid (100 mg/kg) was infused for 10 minutes (24.5 μL/min), followed by a waiting period of 20 minutes, and then coinfused slowly with TmDOTP5− over a period of 90 minutes. A water-heating blanket was used to maintain body temperature of the animals between 36 and 37°C over the course of the experiment. A rectally placed fiber optic probe was used to monitor the body temperature during the scans.
2.3. MRI and BIRDS
In vivo transverse relaxation rate (R2) maps were obtained using a standard spin-echo sequence with 11 slices, 128 × 128 in-plane resolution, 1 mm slice thickness, field of view (FOV) 25 × 25 mm2, recycle time (TR) 6 s, and 12 different values of echo time (TE) from 10–120 ms. The transverse relaxivity (r2) of Molday ION (SPIO-NPs) was measured in vitro using the same pulse sequence using samples of varying concentrations of Molday ION (1 mg/kg to 15 mg/kg). The relaxivity was calculated from the slope of the linear fit of R2 versus concentration. Although extreme pH changes can significantly alter properties of NPs, Liu et al. showed that the zeta potentials and hydrodynamic diameters of dextran-coated SPIO-NPs are fairly stable at physiologically relevant pH and ionic concentrations [63]. They showed that, between pH 4–8 and media of different ionic strength (0–140 mM), there was no aggregation of SPIO-NPs and that the change in hydrodynamic diameter of SPIO-NPs was less than 10 nm, while the change in zeta potential was less than 10%. Nevertheless, extreme pH changes could affect the physical features of SPIO-NPs. For example, a pH less than 4 could degrade the SPIO-NPs altogether, while a pH greater than 10 could lead to significant aggregation. Because we did not modify the SPIO-NPs or change their media and pH, we do not expect property changes within the pHe range of tumors, normal tissue, and blood.
The rats were infused with TmDOTP5− and the 3D CSI acquisition was started 40 minutes after TmDOTP5− infusion in both the probenecid coinfused and renal-ligated rats. TmDOTP5− and similar lanthanide agents have previously been shown to cross the BBB and have been used to map whole brain pHe and temperature by BIRDS in healthy rodents [60, 64, 65]. We previously proposed that these agents slowly diffuse in the brain through the fenestrated vessels of circumventricular organs [66, 67]. Moreover, diffusion of these agents from blood vessels into the extracellular space is enhanced by the high concentration gradient achieved by inhibition of renal clearance using renal ligation or coinfusion of the agent with probenecid [58, 60, 64].
The 3D CSI datasets were acquired with a reduced spherical encoding of k-space, as previously described [57], with a TR of 5 ms, FOV of 25 × 25 × 25 mm3, and a nominal voxel resolution of 1 μL. A dual-banded refocused 90° Shinnar-Le Roux (SLR) pulse of 35 kHz bandwidth, 90 kHz separation, and 205 μs duration was used to selectively excite the H2/H3 and H6 protons of TmDOTP5− (i.e., on either side of water). The phase encoded gradient duration was 160 μs, the spectral width was 250 kHz, and the acquisition time was 4.1 ms. The total acquisition time for each 3D CSI dataset scan was 12 minutes. First a pHe map was acquired before the SPIO-NPs injection. Then a spin-echo dataset was obtained to determine the R2 enhancement induced by TmDOTP5−. Next, the TmDOTP5− infusion was stopped and SPIO-NPs were injected slowly (over 5 minutes). Then another spin-echo dataset was obtained 15 minutes after the infusion of SPIO-NPs to determine the additional R2 enhancement due to SPIO-NPs. Finally, infusion of remaining TmDOTP5− dose was then resumed and another pHe map was obtained after infusion of SPIO-NPs.
R 2 maps were obtained by fitting the absolute MRI intensity at different TEs to a single exponential function using Matlab (Mathworks Inc., Natick, MA, USA). R2 values from the 3 conditions (i.e., no contrast agent, after TmDOTP5− infusion, and after SPIO-NPs infusion) were compared to determine the relaxation enhancement of each contrast agent. Average R2 values were measured in regions of interest (ROIs), where 1 mm circular rings were taken from the center of mass of the tumor. The tumor edge was defined as regions 1 mm immediately outside the MRI-defined tumor core. Comparing the measured R2 against the relaxivity of Molday ION allowed the amount of SPIO-NPs in each region to be approximated.
The 3D CSI datasets were used to create maps of the H2, H3, and H6 resonances of TmDOTP5− before and after infusion of SPIO-NPs. The linewidth (LW) of the H6 resonance was measured to generate LW maps and create histograms before and after infusion of SPIO-NPs. While any of the three resonances could have been used to make the LW maps, H6 was chosen because it had the highest signal-to-noise ratio (SNR). BIRDS-based pHe maps of the brain obtained with TmDOTP5− were calculated as previously described [57, 58, 60, 62]. Briefly, the 3D CSI datasets were reconstructed to a 25 × 25 × 25 matrix using an in-house Matlab script. pHe was calculated by fitting the H2, H3, and H6 resonances (i.e., δ2, δ3, and δ6, respectively) to
| (1) |
where the coefficients a0, a1k, and a2kj were calculated from linear least-squares fit of pHe as a function of the resonances δ2, δ3, and δ6 [60]. Average pHe values before and after infusion of SPIO-NPs were determined as a function of distance from the center of mass of the tumor, similar to the procedure described above for the R2 maps.
2.4. Prussian Blue Iron Staining
Rats were sacrificed at the end of the experiments and brains were perfusion-fixed in 4% paraformaldehyde for Prussian blue iron staining to assess the distribution of SPIO-NPs. 10 μm thick coronal sections of the fixed tissue were incubated in a solution of 4% potassium ferrocyanide and 4% hydrochloric acid twice for 10 minutes and then counterstained with nuclear fast red. Regions with Fe3+ (from SPIO-NPs) were expected to stain blue due to formation of ferric ferrocyanide.
3. Results
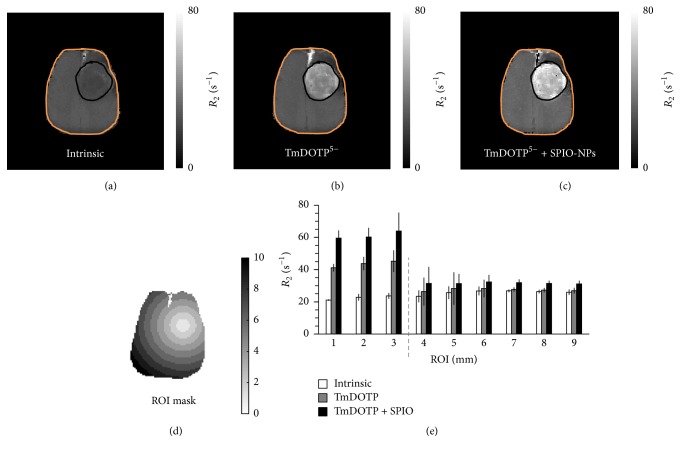
The R2 maps before any contrast agent infusion (Figure 1(a)), after TmDOTP5− infusion (Figure 1(b)), and after the infusion of SPIO-NPs (Figure 1(c)) are shown for a renal-ligated rat bearing an RG2 tumor. While tumor localization was obtained in all three MRI cases, much better delineation was observed upon enhancements by TmDOTP5− alone or TmDOTP5− + SPIO-NPs. While R2 increases were observed after infusion of TmDOTP5− (relative to the intrinsic contrast), a superior MRI contrast was observed after the infusion of SPIO-NPs. The circular ROIs that were drawn from the tumor center are shown in Figure 1(d). The R2 relaxation enhancement was ROI-dependent with higher R2 values inside the tumor and lower R2 outside the tumor (Figure 1(e)). Since R2 enhancement was dependent on the concentration of the paramagnetic agents, the observed ROI-specific R2 enhancement suggests that the extravasation (and accumulation) of both TmDOTP5− and SPIO-NPs was highest in the tumor core and lower in regions farthest from the tumor's center of mass.
Figure 1.
Transverse relaxation rate (R2) maps of an RG2 glioma-bearing rat that underwent renal ligation for TmDOTP5− infusion, (a) without any contrast agent, (b) after infusion of TmDOTP5−, and (c) after infusion of SPIO-NPs. The scale bar in (a–c) denotes R2 values from 0 to 80 s−1. Compared to the R2 map before TmDOTP5− infusion (a), the R2 enhancement was observed throughout the brain after TmDOTP5− infusion (b), but superior R2 enhancement and tumor delineation were observed following infusion of SPIO-NPs which also had cumulative effects from infusion of TmDOTP5− (c). The contrast enhancement from both TmDOTP5− and TmDOTP5− with SPIO-NPs was region-specific, with highest enhancement in the tumor core and limited enhancement outside the tumor (relative to the intrinsic contrast, (a)). The black outline in (a–c) denotes the tumor boundary, which is based on the superior MRI contrast after infusion of SPIO-NPs. The region of interest (ROI) mask based on 1 mm circular rings from the tumor center (d) was used to generate the radial R2 distribution histogram of these ROIs (e). Scale bar in (d) denotes 0 to 10 mm diameter circular ROIs (portrayed on a representative rat brain slice). The gray dashed line in (e) denotes the demarcation between tumor and nontumor regions. The amount of SPIO-NPs in the tumor was 4.3 times greater than in the healthy tissue suggesting a preferential extravasation and accumulation of SPIO-NPs in the tumor. See Figure S1 for examples of Prussian blue staining for SPIO-NPs of an RG2 glioma-bearing rat that underwent renal ligation for TmDOTP5− infusion. See Figure S2 for examples of R2 maps of an RG2 glioma-bearing rat that underwent coinfusion of probenecid and TmDOTP5−.
The R2 values in the tumor (boundary marked by black outlines in Figures 1(a)–1(c)) were 22.5, 43.4, and 61.2 s−1 before contrast agent infusion, after infusion of TmDOTP5−, and after infusion of SPIO-NPs, respectively. For the healthy/nontumor tissue (contralateral side), the R2 values were 25.8, 27.3, and 31.5 s−1 before contrast agent administration, after infusion of TmDOTP5−, and after infusion of SPIO-NPs, respectively. The measured R2 relaxivity of Molday ION at 9.4 T in vitro was 2.45 s−1 mg−1 Fe/kg. By comparing the R2 enhancement by SPIO-NPs against the relaxivity of the Molday ION, the average concentration of SPIO-NPs in the tumor (ROIs 1–3 mm) was determined to be 7.27 mg Fe/kg. In healthy/nontumor tissue (ROIs 4–9 mm), there was a 4.1 s−1 change in R2 with SPIO-NPs, which corresponds to 1.69 mg Fe/kg. Thus, the concentration of SPIO-NPs in the tumor was 4.3 times greater than in healthy/nontumor tissue, suggesting a fourfold enhanced extravasation/accumulation in the tumor.
Given the physical characteristics of Molday ION, we anticipate that the induced MRI effect is from SPIO-NPs within the extracellular milieu and calculating the concentration of SPIO-NPs in vivo should not be significantly affected by using the relaxivity measured in vitro. Girard et al. showed that the relaxivity of SPIO-NPs internalized in cells was lower than that of freely dispersed (in vitro) SPIO-NPs by as much as up to 4 times [68]. Taylor et al. also showed that the relaxivity of Molday ION internalized in cells was 4 times lower than the relaxivity in solution [69]. If we assume the relaxivity of Molday ION in vivo is 4 times lower than what was measured in vitro, then the calculated concentration of SPIO-NPs in both the tumor and healthy brain would be 4 times higher, but the relative distribution in tumor versus healthy/nontumor tissue would remain the same. For example, if we assume a 4x lower in vivo relaxivity (0.61 mg−1 s−1 in vivo versus 2.45 mg−1 s−1 in vitro), the concentration of SPIO-NPs in the tumor would be 29.16 mg Fe/kg while the concentration in the healthy tissue would be 6.72 mg Fe/kg (4.3 times lower than in the tumor). However, we expect that most of the SPIO-NPs will accumulate in the extracellular space where the microenvironment is more similar to the in vitro situation than that of SPIO-NPs internalized in cells. Moreover, we do not expect the relaxation of SPIO-NPs to change significantly over the pH range of our in vivo studies (i.e., pHe 6.8 in tumors to 7.3 in healthy/nontumor tissue). Liu et al. and others have shown that the R2 of dextran-coated SPIO-NPs was not significantly different over this pH range [63, 70]. Using different concentrations of Molday ION, Shu et al. showed that the R2 increase with increasing Molday ION dose was uniform across different brain regions [71]. Thus we expect the relaxivity of SPIO-NPs calculated in vitro to be a good approximation of the in vivo situation. Although we expect our concentration estimation to be only minimally affected, nevertheless these values should be considered “apparent” concentrations.
The preferential distribution of SPIO-NPs in tumors over healthy/nontumor tissues was tested with Prussian blue staining for Fe3+. Although the results from Prussian blue staining are not quantitative, regions that showed higher levels of SPIO-NPs were stained blue, indicating presence of Fe3+ (see Figure S1 in the Supplementary Material available online at https://doi.org/10.1155/2017/3849373). The Prussian blue stained images show an abundance of SPIO-NPs in the tumor, but very little staining on the healthy/nontumor contralateral side of the brain, supporting the enhanced accumulation of SPIO-NPs in the tumors observed with R2 mapping.
Since the R2 data shown in Figure 1 is from a renal-ligated rat, we obtained similar data from a rat that underwent coinfusion of TmDOTP5− and probenecid (Figure S2). The amount of SPIO-NPs in the tumor was 2 times higher than in nontumor tissue (i.e., 4.5 versus 2.2 mg Fe/kg). In this case, the R2 enhancement (Figure S2) was slightly lower than that observed in a renal-ligated rat (Figure 1), which is possibly due to higher TmDOTP5−/SPIO-NPs concentration buildup in a renal-ligated rat.
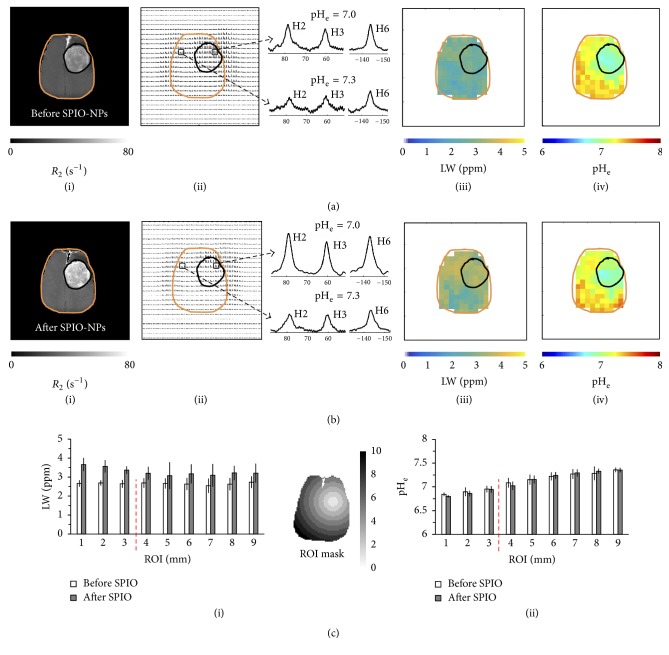
Because acidic pHe is a hallmark of tumor pathology [72, 73], we obtained brain pHe maps in glioma-bearing rat brains with BIRDS using TmDOTP5− before and after infusion of SPIO-NPs. We previously demonstrated that high concentrations of SPIO-NPs increase the LW of the TmDOTP5− proton resonances in vitro [62]. In the current work, in addition to pHe maps, we also calculated the LW of the H6 proton of TmDOTP5− in each voxel in the brain, before and after infusion of SPIO-NPs. Multimodal data (R2 maps (i), CSI maps (ii), LW maps (iii), and pHe maps (iv)) before (Figure 2(a)) and after (Figure 2(b)) infusion of SPIO-NPs for the same RG2 tumor-bearing rat shown in Figure 1, which had undergone renal ligation, were obtained. The R2 maps (Figures 2(a)(i) and 2(b)(i)) were used to delineate and localize the tumor (black outline) and brain (orange outline) boundaries on the CSI, LW, and pHe maps. The CSI maps (Figures 2(a)(ii) and 2(b)(ii)) were used to create the LW maps (Figures 2(a)(iii) and 2(b)(iii)) and pHe maps (Figures 2(a)(iv) and 2(b)(iv)). Examples of 1H spectra of TmDOTP5− protons from voxels inside and outside the tumor—illustrated in the panel between the CSI maps and LW maps—show that there is a significant intratumoral and peritumoral LW and pHe differences. The SNR was higher in the spectra after infusion of SPIO-NPs than before because TmDOTP5− infusion was resumed 15 minutes after the end of SPIO-NPs infusion. While the CSI maps (Figures 2(a)(ii) and 2(b)(ii)) show regionally varying TmDOTP5− intensities, after infusion of SPIO-NPs, there is a clear variation in the LW maps, increasing from ~2.5 ppm globally before the infusion (Figure 2(a)(iii)) to ~3.7 ppm inside the tumor and ~3.4 ppm in the healthy/nontumor contralateral side of the brain (Figure 2(b)(iii)). A detailed ROI analysis of the average LWs shows similar LWs inside and outside the tumor before infusion of SPIO-NPs (Figure 2(c)(i), white bars). However, upon infusion of SPIO-NPs (Figure 2(c)(i), gray bars), the average LW increased (from 2.6 to 3.5 ppm) in the tumor (ROIs # 1–3) and to a lesser extent (from 2.6 to 3.1 ppm) outside the tumor (ROIs # 4–9). The LW broadening in the tumor correlated with the R2 enhancement suggesting that the LW broadening was due to higher concentration of SPIO-NPs in the tumor.
Figure 2.
Multimodal data of relaxation rate (R2), chemical shift imaging (CSI), linewidth (LW), and extracellular pH (pHe) maps obtained for the same RG2 tumor-bearing rat in Figure 1, which had undergone renal ligation. ((a)(i)–(iv)) represent maps before the SPIO-NPs infusion while ((b)(i)–(iv)) represent the maps after the SPIO-NPs infusion. The R2 maps were used to delineate and localize the tumor (black outline) and brain (orange outline) boundaries on the CSI, LW, and pHe maps. R2 values inside the tumor increased significantly after infusion of SPIO-NPs. The CSI maps were used to create the LW maps and pHe maps. The LW increased after SPIO-NPs infusion especially in the tumor. The pHe values within the tumor core and also on the tumor margin were lower than in the healthy/nontumor regions. The panels between the CSI and the LW maps show examples of 1H spectra of TmDOTP5− protons from voxels inside and outside the tumor, revealing a significant intratumoral and peritumoral LW and pHe difference. A more detailed comparison was done using a region of interest (ROI) analysis of LW ((c)(i)) and pHe ((c)(ii)) maps before and after the infusion of SPIO-NPs, using the ROI mask shown. The scale bar in the mask denotes 0 to 10 mm diameter circular ROIs (portrayed on a representative rat brain slice). The red dashed line denotes the demarcation between tumor (ROIs 1–3) and tumor edge (ROI 4)/nontumor regions (ROIs 5–9). See Figure S3 for an example of multimodal data of an RG2 tumor rat that underwent coinfusion of probenecid and TmDOTP5−.
The pHe maps (Figures 2(a)(iv) and 2(b)(iv)) were obtained by fitting the chemical shifts of the H2, H3, and H6 protons of TmDOTP5− to equation (1) as previously described [60]. Although the CSI maps show regional variation of TmDOTP5− proton intensities, both before and after infusion of SPIO-NPs (Figures 2(a)(ii) and 2(b)(ii)), the pHe calculation depends only on the chemical shifts of the nonexchangeable TmDOTP5− protons and is independent of their concentration (or peak intensity) [59, 60]. The pHe maps of RG2 tumors show lower pHe within the tumor core, but also beyond the tumor boundary, which is in good agreement with previous observations of this aggressive tumor type [57, 58]. Before injection of SPIO-NPs the average pHe was 7.0 ± 0.1 within the tumor and 7.3 ± 0.1 in the healthy/nontumor tissue on the contralateral side for the RG2 tumor-bearing brain (Figure 2(a)(iv)). After injection of SPIO-NPs similar average pHe values were observed in these regions (i.e., 7.0 ± 0.1 in the tumor and 7.3 ± 0.1 in healthy/nontumor tissue; Figure 2(b)(iv)). These in vivo results are consistent with our earlier in vitro report which showed that the pH readout and sensitivities of TmDOTP5− are unaffected by the presence of paramagnetic agents like SPIO-NPs and Gd3+ agents [62]. Moreover, a detailed ROI analysis of the spatial pHe distribution shows that the average pHe increased as the ROI is positioned farther from the center of mass of the tumor (Figure 2(c)(ii)). However, no significant differences were observed between the average pHe values in each ROI before and after the SPIO-NPs infusion, indicating that BIRDS-based pHe mapping is not affected by the presence of SPIO-NPs.
Similar R2, CSI, LW, and pHe maps as those shown in Figure 2 were obtained from rats, bearing RG2 tumors, that underwent coinfusion of TmDOTP5− and probenecid (Figure S3). The results show that these distributions were similar to those observed in renal-ligated rats. Generally, the LWs increased after infusion of SPIO-NPs in all regions of the brain, but higher LW increases were observed inside the tumor. Before infusion of SPIO-NPs, the pHe was 6.85 ± 0.03 in the tumor and the pHe was 7.15 ± 0.06 in healthy/nontumor tissue (Figure S3 (A)(iv)). After infusion of SPIO-NPs, the pHe was 6.86 ± 0.07 in the tumor and the pHe was 7.17 ± 0.06 in healthy/nontumor tissue (Figure S3 (B)(iv)). The pHe of the tumor edge (ROI 4) was also relatively acidified (pH 6.98 ± 0.13 before and 6.90 ± 0.09 after infusion of SPIO-NPs) compared to healthy/nontumor tissue farthest from the tumor core (ROIs 5–9).
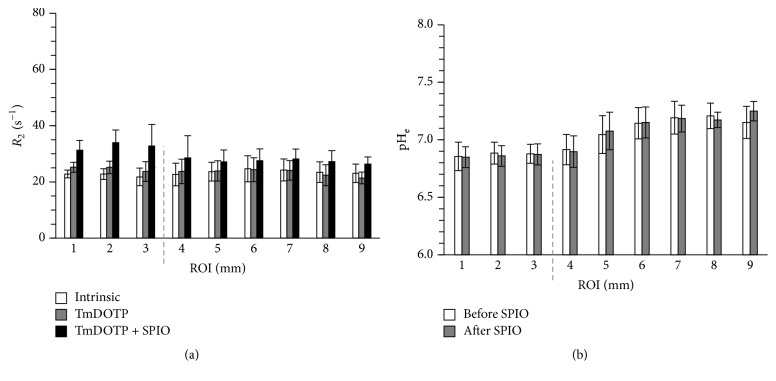
Figure 3 shows the ROI analysis for R2 and pHe before and after infusion of SPIO-NPs for all RG2 tumor-bearing rats that underwent coinfusion of TmDOTP5− and probenecid (n = 5). The R2 enhancement was region-dependent (Figure 3(a); Table 1). Small R2 enhancement was observed after infusion of TmDOTP5−, where R2 increased by 2.2 s−1 in the tumor, 1.1 s−1 in the tumor edge, and no significant increase in the healthy/nontumor tissue. The tumor edge was defined as a circular ROI just 1 mm outside of the MRI defined tumor core. It is important to identify and analyze the tumor edge because after radiation therapy, it becomes edematous and harbors most therapy-resistant cells. Additionally, greater R2 enhancement was observed upon infusion of SPIO-NPs (i.e., R2 increase of 10.2 s−1 in tumor, 5.9 s−1 in tumor edge, and 4.1 s−1 in healthy/nontumor tissue). In contrast to the R2 measurements, the average pHe values were not affected by the SPIO-NPs infusion (Figure 3(b); Table 2). However, pHe varied across regions; pHe was lowest (6.9 ± 0.1) in the tumor and highest (7.2 ± 0.1) in the healthy/nontumor tissue farthest from the tumor. Low pHe (6.9 ± 0.1) was also measured on the tumor edge. While the pHe of the tumor edge in RG2 gliomas was acidic relative to healthy/nontumor tissue, the R2 enhancement between the tumor edge and the healthy/nontumor tissue were similar, suggesting that the vasculature in the tumor margin was still intact despite the acidic transformation of their microenvironment. Future experiments should look at the vascularization inside, around, and far beyond the tumor boundary, for example, with dynamic contrast enhanced MRI and with epidermal growth factor receptor staining.
Figure 3.
Region of interest (ROI) analysis for the average relaxation rate (R2) and extracellular pH (pHe) before and after infusion of SPIO-NPs for all RG2 tumor-bearing rats that underwent coinfusion of TmDOTP5− and probenecid (n = 5). The ROI analysis is based on concentric 1 mm circular rings drawn from the center of mass of the tumor. (a) Average R2 values in different ROIs for intrinsic contrast, after infusion of TmDOTP5−, and after infusion of TmDOTP5− with SPIO-NPs. The average R2 enhancement was highest inside the tumor (ROIs 1–3) compared to tumor edge (ROI 4) and nontumor regions (ROIs 5–9). The dashed line represents the tumor edge. Small R2 enhancement was observed after infusion of TmDOTP5−. However, much higher R2 enhancement was observed upon infusion of SPIO-NPs. See Table 1 for details. (b) The average pHe values in different ROIs before and after infusion of SPIO-NPs. The pHe values measured before and after infusion of SPIO-NPs were similar, both inside and outside the tumor. The pHe was lowest in the tumor and highest in the healthy/nontumor tissue farthest from the tumor. Low pHe was also measured on the tumor margin. See Table 2 for details.
Table 1.
Regional analysis for relaxation rate (R2) for all RG2 tumor-bearing rats that underwent coinfusion of TmDOTP5− and probenecid (n = 5). See Figure 3(a) for details. R2 was measured inside the MRI-defined tumor core (see Figure S2), at the tumor edge (regions 1 mm outside the tumor boundary), and in the healthy/nontumor tissue before and after the infusion of SPIO-NPs. Data shown are mean and standard deviation (SD).
| R 2 | Intrinsic | TmDOTP5− | TmDOTP5− + SPIO-NPs | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Tumor core | 22.5 | 2.1 | 24.7 | 2.5 | 32.7 | 5.2 |
| Tumor's edge | 22.7 | 4.0 | 23.7 | 4.4 | 28.6 | 7.9 |
| Nontumor tissue | 23.8 | 3.8 | 23.2 | 3.4 | 27.3 | 3.7 |
Table 2.
Regional analysis for extracellular pH (pHe) imaging before and after infusion of SPIO-NPs for all RG2 tumor-bearing rats that underwent coinfusion of TmDOTP5− and probenecid (n = 5). See Figure 3(b) for details. The pHe was measured inside the MRI-defined tumor core (see Figure S2), at the tumor edge (regions 1 mm outside the tumor boundary), and in the healthy/nontumor tissue before and after the infusion of SPIO-NPs. Data shown are mean and standard deviation (SD).
| pHe | Before SPIO-NPs | After SPIO-NPs | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Tumor core | 6.9 | 0.1 | 6.9 | 0.1 |
| Tumor's edge | 6.9 | 0.1 | 6.9 | 0.1 |
| Nontumor tissue | 7.2 | 0.1 | 7.2 | 0.1 |
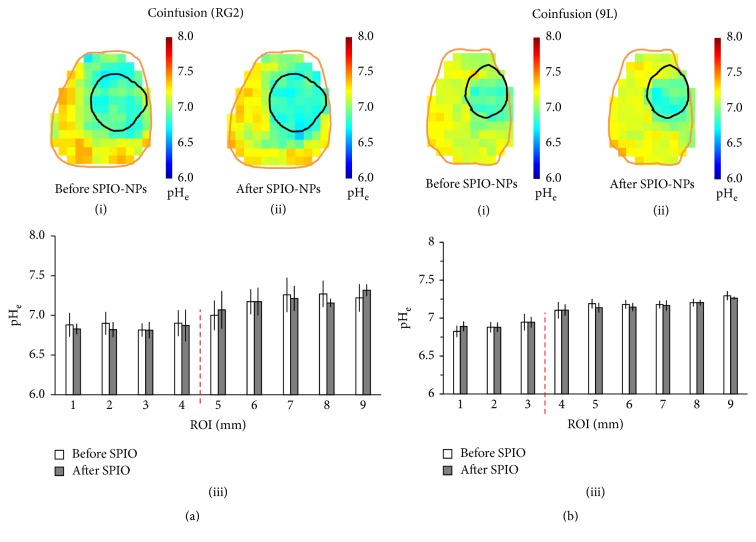
In addition to measurements obtained in the aggressive RG2 glioma, we also acquired pHe maps before and after infusion of SPIO-NPs in rats bearing the less aggressive 9L gliosarcoma (n = 4) using coinfusion of TmDOTP5− and probenecid (Figure 4). The pHe maps of the aggressive RG2 tumor (Figure 4(a)(i) before versus Figure 4(a)(ii) after infusion of SPIO-NPs) showed a lower pHe within the tumor region, but the acidification was diffuse and occurred also beyond the MRI-defined tumor boundary (see also Figures 2 and S3). Previously, it was reported that the diffuse acidification of pHe beyond the RG2 tumor boundary correlated with increased expression of the proliferation marker Ki-67 [57]. In contrast, the pHe maps of the less aggressive 9L gliosarcoma showed lower pHe only within the MRI-defined tumor core (Figure 4(b)(i) before versus Figure 4(b)(ii) after SPIO-NPs). A detailed ROI analysis of the pHe maps shows that, for the RG2, the pHe slowly increases with the distance from the tumor core (Figure 4(a)(iii)), whereas for the 9L tumor the pHe is highest outside the tumor boundary and is distance-independent (Figure 4(b)(iii)). Moreover, the regional pHe trends observed with BIRDS were not dependent on the type of infusion method, that is, coinfusion of TmDOTP5− and probenecid versus infusion of TmDOTP5− after renal ligation (Figure S4).
Figure 4.
Comparison of pHe maps for (a) RG2 glioma and (b) 9L gliosarcoma before and after infusion of SPIO-NPs in rats that underwent coinfusion of TmDOTP5− and probenecid. In both (a) and (b), (i) and (ii) represent the pHe maps before and after SPIO-NPs infusion, respectively, while (iii) depicts a detailed ROI analysis. See Figure 1 for details of the ROI mask. In (a), the pHe inside the more aggressive RG2 glioma was typically lower than in the healthy/nontumor tissue, but diffuse acidification was observed well-beyond the MRI-defined tumor boundary. Thus the pHe slowly increased as the distance from the tumor core increased. In (b), the pHe inside the less aggressive 9L gliosarcoma was also lower than in the healthy/nontumor tissue, but the acidification did not extend beyond the MRI-defined tumor boundary, before and after infusion of SPIO-NPs. See Figure S4 for a comparison of regional pHe dependence on the method used for inhibition of renal clearance (renal ligation versus probenecid).
4. Discussion
Elevated aerobic glycolysis in gliomas leads to elevated lactic acid and proton production, which upon extrusion from the intracellular compartment results in acidification of the extracellular milieu [44]. Additionally, because the BBB is disrupted in gliomas, NPs loaded with imaging agents (e.g., SPIO-NPs) selectively permeate into and accumulate within tumors. In the present study, a region-specific R2 enhancement from extravasation of SPIO-NPs was observed, with higher R2 increases inside the tumor and smaller R2 increases outside the tumor. Although SPIO-NPs affected MRI contrast in all tissues, excellent SPIO-induced MRI contrast delineated the glioma boundary due to greater extravasation of SPIO-NPs from the vasculature into the tumor relative to healthy/nontumor tissue. We also measured pHe with BIRDS using TmDOTP5− before and after infusion of SPIO-NPs in rats bearing 9L and RG2 brain tumors. The results demonstrate that the pHe readout was unaffected by the presence of SPIO-NPs, because the intratumoral-peritumoral pHe gradients were essentially identical before and after the infusion of SPIO-NPs, despite slight variations in LWs of the proton peaks for TmDOTP5−. The measured pHe was lowest inside the tumor and increased with the distance from the center of mass of the tumor in the more aggressive RG2 tumors. However, in the less aggressive 9L tumors, pHe was notably higher immediately outside the tumor boundary. We envisage coinjection of BIRDS agents (e.g., TmDOTP5−) and NPs containing drugs and SPIO, as a new methodology that can deliver high drug payloads to the tumor, image drug distribution, and track tumor location/size (by MRI), and at the same time monitor pHe response to therapy (by BIRDS) [74].
The brain's microvasculature is either degraded or immature in several neuropathologies, including glioblastomas. Breakthroughs in glioma imaging and therapy exploit the fact that NPs, containing either SPIO (for MRI) or drugs (for therapy), can extravasate through the leaky microvasculature. The SPIO-NPs extravasate into the tumor to generate superior MRI contrast while tumor-targeted D-NPs safely deliver high payloads of drugs to the tumor [74].
In the present study, the highest R2 enhancement (from TmDOTP5− and SPIO-NPs) occurred in the tumor and was lowest in healthy/nontumor tissue farthest from the tumor. Because the R2 enhancement comes entirely from the infused agents, this region-specific enhancement suggests a corresponding spatial variation in vascular permeability and consequent extravasation. In addition to the enhanced extravasation, the chaotic vascular architecture in tumors contributes to poor clearance leading to increased retention of SPIO-NPs in the interstitial space of the tumor core. By using the R2 enhancement and the relaxivity of Molday ION (SPIO-NPs), we calculated that the amount of SPIO-NPs in the tumor was 2 to 4 times higher than in healthy/nontumor tissue. The EPR in tumors has been widely utilized to preferentially deliver high amounts of imaging agents and D-NPs, both passively and actively [75, 76].
High-grade solid brain tumors tend to develop necrotic cores due to a combination of poor vascularization and inadequate perfusion [77–79]. Because gliomas like RG2 are very aggressive, they rapidly invade to induce severe neurological problems. As a consequence the rodent reaches terminal situations before the tumor cores are able to become necrotic. For example, these rodent brain tumors grow within a few weeks, whereas in the human brain gliomas develop necrotic foci after many months, if not longer. Tumor necrosis has very likely not yet occurred in these rodent tumors at the time points of our experiments. The observed higher R2 in the center relative to the periphery suggests higher permeation and accumulation of SPIO-NPs in the center of the tumor due to greater extent of BBB disruption within the tumor niche. Prior studies support these observations. Beaumont et al. did not observe any necrosis in their RG2 rat gliomas at similar time points as our experiments [16]. Their staining results also showed that the BBB was significantly disrupted at the center of the tumor in RG2 tumors. While the vasculature at the primary tumor site/core is leaky, the blood vessels at the tumor infiltration sites (i.e., periphery) are often immature, which may slow the extravasation of SPIO-NPs out of the blood into these new tumor sites. Uehara et al. also showed that necrosis of tumor cores is minimal or absent in RG2 tumors at time points less than 4 weeks following inoculation [80]. Therefore based on the information regarding RG2 tumor growth from prior work in this and other laboratories, we expect the tumor cores to be non-necrotic, and thus higher R2 increase from the SPIO-NPs would be observed in the tumor core. Additionally, because gliomas including RG2 are known to have an increased presence of macrophages relative to healthy brain tissue, the higher amount of SPIO-NPs in the tumor could be due in part to macrophage phagocytosis [16].
4.1. Superparamagnetic Iron Oxide Nanoparticles in Cancer Theranostics
Owing to their strong superparamagnetic properties, tunable size, shape, coating, and magnetic susceptibility, SPIO-NPs have gained utility as therapeutic agents in alternating magnetic field hyperthermia [81–85], as MRI contrast agents for cell tracking [86–89], and for imaging tumor location/size as well as drug delivery [40, 74, 90]. Drug delivery imaging with SPIO-NPs is often accomplished by coencapsulating drugs and SPIO into a given nanocarrier platform (e.g., micelles or liposomes). In liposomes, for example, SPIO-NPs and hydrophilic drugs can be encapsulated inside the nanocarrier, whereas hydrophobic drugs can be incorporated on the nanocarrier membrane. Recent advances also involve coating the surface of SPIO-NPs itself with drugs [11, 12]. Entry and accumulation of these drug-containing and SPIO-containing NPs into the tumor have been achieved by passive targeting, whereby the NPs are small enough to extravasate through leaky tumor vasculature, but large enough not to cross the intact vessels in healthy/nontumor tissue. However, better and more selective targeting is achieved when the NPs are coated with ligands that are specific to receptors and/or transporters that are overexpressed on tumor cells and vasculature. Examples of such targets include transferrin receptors, epidermal growth factor receptors, folate receptors, vascular endothelial growth factor receptors, monocarboxylate transporters, and glucose transporters [29, 91–96]. In all these cases, the delivery and biodistribution of D-NPs are visualized and quantified through signal attenuation (negative contrast) of the R2-weighted MRI resulting from the strong superparamagnetic fields generated by SPIO-NPs. Because both the drugs and SPIO-NPs are contained in the same nanocarrier, the location and distribution of the SPIO-NPs, as observed by MRI, reflect the biodistribution of D-NPs. By quantifying the SPIO-induced MRI contrast attenuation, it is possible to quantify the D-NPs delivered to the tumor.
Currently, measurement of tumor size is the only FDA-approved method to assess the response to therapy noninvasively. Because changes in tumor size following treatment may take up to a month to manifest, this method is not ideal for aggressive brain cancers, especially when the treatment is later found not to have been effective. Thus a clear need exists for methods that can provide prompt assessment of therapeutic efficacy so that treatment can be altered quickly if desired. Recently, it was shown that quantitative monitoring of the tumor microenvironment following a pharmacologic challenge provides a better way to monitor therapeutic efficacy [97]. Because acidification of pHe promotes drug resistance, degradation of the extracellular matrix, angiogenesis, tumor invasion, and metastasis, drugs that raise (or neutralize) pHe by targeting the acid-generating glycolysis in tumors have demonstrated significant inhibition of tumor growth and enhanced apoptosis [45, 46, 48, 72, 98, 99]. Additionally, drugs that directly raise tumor pHe (e.g., bicarbonate treatment) inhibit tumor invasion and metastasis [100, 101]. Because bicarbonate and drugs that inhibit glycolysis elevate pHe in a few days, methods that quantitatively measure tumor pHe longitudinally may provide an effective evaluation of their therapeutic efficacy and allow for prompt modification of therapy if the initial treatment is not working. A recent study has reported that temozolomide, which is an alkylating agent and is adjuvant chemotherapy used to clinically treat glioblastomas, arrests glioma growth and normalizes intratumoral pHe [102].
4.2. Combining Drug Delivery Imaging with pHe Imaging to Assess Therapy
Given the significant relaxation enhancement of the nonexchangeable protons on the TmDOTP5− agent [60, 103, 104] due to pseudocontact interactions with unpaired Tm3+ electrons, we hypothesized that BIRDS-based pHe readout of TmDOTP5− will remain uncompromised by SPIO-NPs. Although SPIO-NPs altered MRI contrast in all tissues, SPIO-based MRI contrast clearly demarcated the tumor boundary due to greater extravasation of NPs through leaky blood vessels. Nonetheless, the quality of BIRDS-based pHe readout with TmDOTP5−, for both intratumoral and peritumoral regions, was unaffected by the presence of the SPIO-NPs, since the pHe maps obtained before and after the infusion of SPIO-NPs were very similar.
While separate infusions of TmDOTP5− and SPIO-NPs were employed in the present study, future studies might assess the possibility of combining them [74]. Conjugating several monomers of the pHe-sensitive agent on the surface of the NPs could possibly enhance the sensitivity of BIRDS to monitor the immediate environment of D-NPs and prolong their lifetime to enable multiple monitoring sessions at various treatment time points. Ordinarily, BIRDS agents have fast renal clearance owing to their small size and thus renal inhibition is necessary for accumulation [57, 58, 60, 105]. However, if conjugated to NPs, the BIRDS agents lifetime might increase significantly (i.e., to several days, which is the case for SPIO-NPs), thus allowing their use without inhibition of renal clearance and obviating the need for repeated infusions [106]. Towards this goal, it has been previously demonstrated in vitro that encapsulation of BIRDS agents in liposomal nanoparticles resulted in an MR signal amplification without impeding the local pH readout [62].
5. Summary
The treatment of brain gliomas is hampered in part by a limited availability of reliable in vivo methodologies that can simultaneously and noninvasively measure glioma invasion, drug delivery, and its therapeutic benefits. In this study, we demonstrated superb MRI contrast enhancement and tumor delineation with SPIO-NPs and quantitative imaging of intratumoral-peritumoral pHe gradients using BIRDS in rat models of brain gliomas. Furthermore, we demonstrated that both the intratumoral and peritumoral pHe readouts, measured with BIRDS using TmDOTP5−, are not compromised by the presence of SPIO-NPs. Thus, we propose a new cancer imaging protocol that can target high drug payloads (via D-NPs) to tumors and image the drug delivery (via SPIO-NPs), concurrently map tumor location and size (by MRI), and at the same time monitor therapeutic efficacy through drug-induced changes in pHe (by BIRDS) [74].
Supplementary Material
Figure S1. Prussian Blue Staining for iron (SPIO-NPs) distribution.
Figure S2. Effect of TmDOTP5- and SPIO-NPs infusion on the transverse relaxation rate (R2) in Probenecid-infused glioma-bearing animals.
Figure S3. Extracellular pH (pHe) and TmDOTP5- linewidths (LW) measured before and after SPIO-NPs infusion in Probenecid-infused glioma-bearing animals.
Figure S4. Comparison of Extracellular pH (pHe) in 9L tumor-bearing animals that underwent renal ligation or Probenecid co-infusion to inhibit renal clearance.
Acknowledgments
This paper is supported by NIH Grants (R01 EB-023366, R01 EB-011968, and R01 CA-140102).
Abbreviations
- 3-APP:
3-Aminopropyl phosphonate
- BBB:
Blood brain barrier
- BIRDS:
Biosensor imaging of redundant deviation in shifts
- CEST:
Chemical exchange saturation transfer
- CSI:
Chemical shift imaging
- EPR:
Enhanced permeation and retention
- FOV:
Field of view
- LW:
Linewidth
- MR:
Magnetic resonance
- NPs:
Nanoparticles
- pHe:
Extracellular pH
- ROI:
Region of interest
- SD:
Standard deviation
- SNR:
Signal-to-noise ratio
- SLR:
Shinnar-Le Roux
- SPIO:
Superparamagnetic iron oxide
- TmDOTP5-:
Thulium 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrakis methylene phosphonate.
Contributor Information
Samuel Maritim, Email: samuel.maritim@yale.edu.
Fahmeed Hyder, Email: fahmeed.hyder@yale.edu.
Disclosure
Parts of this work have previously been presented as a published abstract in BRAIN & BRAIN PET 2017 Poster Viewing Session III.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
Samuel Maritim and Fahmeed Hyder designed research. Samuel Maritim, Daniel Coman, Yuegao Huang, Jyotsna U. Rao, John J. Walsh, and Fahmeed Hyder performed research. Samuel Maritim, Daniel Coman, Yuegao Huang, Jyotsna U. Rao, and Fahmeed Hyder analyzed data. Samuel Maritim, Daniel Coman, Yuegao Huang, Jyotsna U. Rao, and Fahmeed Hyder wrote the paper.
References
- 1.Ferlay J., Shin H. R., Bray F., Forman D., Mathers C., Parkin D. M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Ohgaki H. Epidemiology of brain tumors. Methods in Molecular Biology. 2009;472:323–342. doi: 10.1007/978-1-60327-492-0_14. [DOI] [PubMed] [Google Scholar]
- 3.Bradford H. F. Glutamate, GABA and epilepsy. Progress in Neurobiology. 1995;47(6):477–511. doi: 10.1016/0301-0082(95)00030-5. [DOI] [PubMed] [Google Scholar]
- 4.Pardridge W. M. Drug targeting to the brain. Pharmaceutical Research. 2007;24(9):1733–1744. doi: 10.1007/s11095-007-9324-2. [DOI] [PubMed] [Google Scholar]
- 5.Pardridge W. M. Blood-brain barrier delivery. Drug Discovery Therapy. 2007;12(1-2):54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Wolburg H., Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38(6):323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 7.Demeule M., Regina A., Jodoin J., et al. Drug transport to the brain: Key roles for the efflux pump P-glycoprotein in the blood-brain barrier. Vascular Pharmacology. 2002;38(6):339–348. doi: 10.1016/S1537-1891(02)00201-X. [DOI] [PubMed] [Google Scholar]
- 8.Risau W., Wolburg H. Development of the blood-brain barrier. Trends in Neurosciences. 1990;13(5):174–178. doi: 10.1016/0166-2236(90)90043-A. [DOI] [PubMed] [Google Scholar]
- 9.Lee S.-W., Woo J. K., Park J. A., Yoon K. C., Kwon Y.-W., Kim K.-W. Blood-brain barrier interfaces and brain tumors. Archives of Pharmacal Research. 2006;29(4):265–275. doi: 10.1007/BF02968569. [DOI] [PubMed] [Google Scholar]
- 10.Huber J. D., Egleton R. D., Davis T. P. Molecular physiology and pathophysiology of tight junctions in the blood -brain barrier. Trends in Neurosciences. 2001;24(12):719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- 11.Liang P.-C., Chen Y.-C., Chiang C.-F., et al. Doxorubicin-modified magnetic nanoparticles as a drug delivery system for magnetic resonance imaging-monitoring magnet-enhancing tumor chemotherapy. International Journal of Nanomedicine. 2016;11:2021–2037. doi: 10.2147/IJN.S94139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling Y., Wei K., Zou F., Zhong S. Temozolomide loaded PLGA-based superparamagnetic nanoparticles for magnetic resonance imaging and treatment of malignant glioma. International Journal of Pharmaceutics. 2012;430(1-2):266–275. doi: 10.1016/j.ijpharm.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 13.Sarin H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. Journal of Angiogenesis Research. 2010;2(1, article no. 14) doi: 10.1186/2040-2384-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox D. J., Pilkington G. J., Lantos P. L. The fine structure of blood vessels in ethylnitrosourea-induced tumours of the rat nervous system: with special reference to the breakdown of the blood-brain barrier. Journal of Experimental Pathology. 1976;57:419–430. [PMC free article] [PubMed] [Google Scholar]
- 15.Schlageter K. E., Molnar P., Lapin G. D., Groothuis D. R. Microvessel organization and structure in experimental brain tumors: Microvessel populations with distinctive structural and functional properties. Microvascular Research. 1999;58(3):312–328. doi: 10.1006/mvre.1999.2188. [DOI] [PubMed] [Google Scholar]
- 16.Beaumont M., Lemasson B., Farion R., Segebarth C., Rémy C., Barbier E. L. Characterization of tumor angiogenesis in rat brain using iron-based vessel size index MRI in combination with gadolinium-based dynamic contrast-enhanced MRI. Journal of Cerebral Blood Flow & Metabolism. 2009;29(10):1714–1726. doi: 10.1038/jcbfm.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng D., Nagy J. A., Dvorak A. M., Dvorak H. F. Different pathways of macromolecule extravasation from hyperpermeable tumor vessels. Microvascular Research. 2000;59(1):24–37. doi: 10.1006/mvre.1999.2207. [DOI] [PubMed] [Google Scholar]
- 18.Feng D., Nagy J. A., Dvorak H. F., Dvorak A. M. Ultrastructural studies define soluble macromolecular, particulate, and cellular transendothelial cell pathways in venules, lymphatic vessels, and tumor-associated microvessels in man and animals. Microscopy Research and Technique. 2002;57(5):289–326. doi: 10.1002/jemt.10087. [DOI] [PubMed] [Google Scholar]
- 19.Hashizume H., Baluk P., Morikawa S., et al. Openings between defective endothelial cells explain tumor vessel leakiness. The American Journal of Pathology. 2000;156(4):1363–1380. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vick N. A., Bigner D. D. Microvascular abnormalities in virally-induced canine brain tumors. Structural bases for altered blood-brain barrier function. Journal of the Neurological Sciences. 1972;17(1):29–39. doi: 10.1016/0022-510X(72)90019-6. [DOI] [PubMed] [Google Scholar]
- 21.Blasberg R. G., Groothuis D. R. Chemotherapy of Brain-Tumors - Physiological and Pharmacokinetic Considerations. Seminars in Oncology. 1986;13:70–82. [PubMed] [Google Scholar]
- 22.Groothuis D. R., Fischer J. M., Lapin G., Vick N. A., Bigner D. D. Comparative Permeability of Different Glioma Models to Horseradish-Peroxidase. Cancer Treat Rep. 1982;65:13–18. [PubMed] [Google Scholar]
- 23.Tator C. H. Retention of tritiated methotrexate in a transplantable mouse glioma. Cancer Research. 1976;36:3058–3066. [PubMed] [Google Scholar]
- 24.Monsky W. L., Mouta Carreira C., Tsuzuki Y., Gohongi T., Fukumura D., Jain R. K. Role of host microenvironment in angiogenesis and microvascular functions in human breast cancer xenografts: mammary fat pad versus cranial tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8:1008–1013. [PubMed] [Google Scholar]
- 25.Yuan F., Salehi H. A., Boucher Y., Vasthare U. S., Tuma R. F., Jain R. K. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Research. 1994;54:4564–4568. [PubMed] [Google Scholar]
- 26.De Boer A. G., Gaillard P. J. Strategies to improve drug delivery across the blood-brain barrier. Clinical Pharmacokinetics. 2007;46(7):553–576. doi: 10.2165/00003088-200746070-00002. [DOI] [PubMed] [Google Scholar]
- 27.de Boer A. G., Gaillard P. J. Drug targeting to the brain. Annual Review of Pharmacology and Toxicology. 2007;47:323–355. doi: 10.1146/annurev.pharmtox.47.120505.105237. [DOI] [PubMed] [Google Scholar]
- 28.Kievit F. M., Veiseh O., Fang C., et al. Chlorotoxin labeled magnetic nanovectors for targeted gene delivery to glioma. ACS Nano. 2010;4(8):4587–4594. doi: 10.1021/nn1008512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weitman S. D., Frazier K. M., Kamen B. A. The folate receptor in central nervous system malignancies of childhood. Journal of Neuro-Oncology. 1994;21(2):107–112. doi: 10.1007/BF01052894. [DOI] [PubMed] [Google Scholar]
- 30.Howarth S. P. S., Tang T. Y., Trivedi R., et al. Utility of USPIO-enhanced MR imaging to identify inflammation and the fibrous cap: A comparison of symptomatic and asymptomatic individuals. European Journal of Radiology. 2009;70(3):555–560. doi: 10.1016/j.ejrad.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 31.Motomura K., Ishitobi M., Komoike Y., et al. SPIO-enhanced magnetic resonance imaging for the detection of metastases in sentinel nodes localized by computed tomography lymphography in patients with breast cancer. Annals of Surgical Oncology. 2011;18(12):3422–3429. doi: 10.1245/s10434-011-1710-7. [DOI] [PubMed] [Google Scholar]
- 32.Polakova K., Mocikova I., Purova D., et al. Magnetic resonance cholangiopancreatography (MRCP) using new negative per-oral contrast agent based on superparamagnetic iron oxide nanoparticles for extrahepatic biliary duct visualization in liver cirrhosis. Biomedical Papers. 2016;160(4):512–517. doi: 10.5507/bp.2016.046. [DOI] [PubMed] [Google Scholar]
- 33.Sigovan M., Gasper W., Alley H. F., Owens C. D., Saloner D. USPIO-enhanced MR angiography of arteriovenous fistulas in patients with renal failure. Radiology. 2012;265(2):584–590. doi: 10.1148/radiol.12112694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang T., Howarth S. P. S., Miller S. R., et al. Assessment of inflammatory burden contralateral to the symptomatic carotid stenosis using high-resolution ultrasmall, superparamagnetic iron oxide-enhanced MRI. Stroke. 2006;37(9):2266–2270. doi: 10.1161/01.STR.0000236063.47539.99. [DOI] [PubMed] [Google Scholar]
- 35.Tang T. Y., Patterson A. J., Miller S. R., et al. Temporal dependence of in vivo USPIO-enhanced MRI signal changes in human carotid atheromatous plaques. Neuroradiology. 2009;51(7):457–465. doi: 10.1007/s00234-009-0523-x. [DOI] [PubMed] [Google Scholar]
- 36.Tourdias T., Roggerone S., Filippi M., et al. Assessment of disease activity in multiple sclerosis phenotypes with combined gadolinium- and superparamagnetic iron oxide-enhanced MR imaging. Radiology. 2012;264(1):225–233. doi: 10.1148/radiol.12111416/-/dc1. [DOI] [PubMed] [Google Scholar]
- 37.Wagner M., Wagner S., Schnorr J., et al. Coronary MR angiography using citrate-coated very small superparamagnetic iron oxide particles as blood-pool contrast agent: Initial experience in humans. Journal of Magnetic Resonance Imaging. 2011;34(4):816–823. doi: 10.1002/jmri.22683. [DOI] [PubMed] [Google Scholar]
- 38.Sun C., Veiseh O., Gunn J., et al. In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small. 2008;4(3):372–379. doi: 10.1002/smll.200700784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun C., Fang C., Stephen Z., et al. Tumor-targeted drug delivery and MRI contrast enhancement by chlorotoxin-conjugated iron oxide nanoparticles. Nanomedicine. 2008;3(4):495–505. doi: 10.2217/17435889.3.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strohbehn G., Coman D., Han L., et al. Imaging the delivery of brain-penetrating PLGA nanoparticles in the brain using magnetic resonance. Journal of Neuro-Oncology. 2015;121(3):441–449. doi: 10.1007/s11060-014-1658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li W., Zhang Z., Gordon A. C., et al. SPIO-labeled yttrium microspheres for MR imaging quantification of transcatheter intrahepatic delivery in a rodent model. Radiology. 2016;278(2):405–412. doi: 10.1148/radiol.2015150315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stubbs M., McSheehy P. M. J., Griffiths J. R., Bashford C. L. Causes and consequences of tumour acidity and implications for treatment. Molecular Medicine Today. 2000;6(1):15–19. doi: 10.1016/s1357-4310(99)01615-9. [DOI] [PubMed] [Google Scholar]
- 43.Cardone R. A., Casavola V., Reshkin S. J. The role of disturbed pH dynamics and the NA+/H+ exchanger in metastasis. Nature Reviews Cancer. 2005;5(10):786–795. doi: 10.1038/nrc1713. [DOI] [PubMed] [Google Scholar]
- 44.Gatenby R. A., Gawlinski E. T., Gmitro A. F., Kaylor B., Gillies R. J. Acid-mediated tumor invasion: a multidisciplinary study. Cancer Research. 2006;66(10):5216–5223. doi: 10.1158/0008-5472.CAN-05-4193. [DOI] [PubMed] [Google Scholar]
- 45.Hashim A. I., Zhang X., Wojtkowiak J. W., Martinez G. V., Gillies R. J. Imaging pH and metastasis. NMR in Biomedicine. 2011;24(6):582–591. doi: 10.1002/nbm.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez-Zaguilan R., Seftor E. A., Seftor R. E., Chu Y. W., Gillies R. J., Hendrix M. J. Acidic pH enhances the invasive behavior of human melanoma cells. Clinical & Experimental Metastasis. 1996;14:176–186. doi: 10.1007/BF00121214. [DOI] [PubMed] [Google Scholar]
- 47.Gatenby R. A., Gillies R. J. Why do cancers have high aerobic glycolysis? Nature Reviews Cancer. 2004;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 48.Barar J., Omidi Y. Dysregulated pH in tumor microenvironment checkmates cancer therapy. Bioimpacts. 2013;3(4):149–162. doi: 10.5681/bi.2013.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wike-Hooley J. L., Haveman J., Reinhold H. S. The relevance of tumour pH to the treatment of malignant disease. Radiotherapy & Oncology. 1984;2(4):343–366. doi: 10.1016/S0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]
- 50.De Milito A., Canese R., Marino M. L., et al. PH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. International Journal of Cancer. 2010;127(1):207–219. doi: 10.1002/ijc.25009. [DOI] [PubMed] [Google Scholar]
- 51.McSheehy P. M. J., Stubbs M., Griffiths J. R. Role of pH in tumor-trapping of the anticancer drug 5-fluorouracil. Advances in Enzyme Regulation. 2000;40:63–80. doi: 10.1016/S0065-2571(99)00034-5. [DOI] [PubMed] [Google Scholar]
- 52.Gillies R. J., Raghunand N., Garcia-Martin M. L., Gatenby R. A. pH imaging. A review of pH measurement methods and applications in cancers. IEEE engineering in medicine and biology magazine : the quarterly magazine of the Engineering in Medicine & Biology Society. 2004;23:57–64. doi: 10.1109/memb.2004.1360409. [DOI] [PubMed] [Google Scholar]
- 53.Ward K. M., Aletras A. H., Balaban R. S. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST) Journal of Magnetic Resonance. 2000;143(1):79–87. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 54.Garcia-Martin M. L., Herigault G., Remy C., et al. Mapping extracellular pH in rat brain gliomas in vivo by 1H magnetic resonance spectroscopic imaging: comparison with maps of metabolites. Cancer Research. 2001;61:6524–6531. [PubMed] [Google Scholar]
- 55.Gillies R. J., Liu Z., Bhujwalla Z. 31P-MRS measurements of extracellular pH of tumors using 3-aminopropylphosphonate. American Journal of Physiology-Cell Physiology. 1994;267:C195–C203. doi: 10.1152/ajpcell.1994.267.1.C195. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Z., Hancock B., Leen S., et al. Compatibility of superparamagnetic iron oxide nanoparticle labeling for 1H MRI cell tracking with 31P MRS for bioenergetic measurements. NMR in Biomedicine. 2010;23(10):1166–1172. doi: 10.1002/nbm.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coman D., Huang Y., Rao J. U., et al. Imaging the intratumoral-peritumoral extracellular pH gradient of gliomas. NMR in Biomedicine. 2016;29(3):309–319. doi: 10.1002/nbm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang Y., Coman D., Herman P., Rao J. U., Maritim S., Hyder F. Towards longitudinal mapping of extracellular pH in gliomas. NMR in Biomedicine. 2016;29(10):1364–1372. doi: 10.1002/nbm.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coman D., Trubel H. K., Hyder F. Brain temperature by Biosensor Imaging of Redundant Deviation in Shifts (BIRDS): Comparison between TmDOTP5- and TmDOTMA- NMR in Biomedicine. 2010;23(3):277–285. doi: 10.1002/nbm.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coman D., Trubel H. K., Rycyna R. E., Hyder F. Brain temperature and pH measured by 1H chemical shift imaging of a thulium agent. NMR in Biomedicine. 2009;22(2):229–239. doi: 10.1002/nbm.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coman D., De Graaf R. A., Rothman D. L., Hyder F. In vivo three-dimensional molecular imaging with Biosensor Imaging of Redundant Deviation in Shifts (BIRDS) at high spatiotemporal resolution. NMR in Biomedicine. 2013;26(11):1589–1595. doi: 10.1002/nbm.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maritim S., Huang Y., Coman D., Hyder F. Characterization of a lanthanide complex encapsulated with MRI contrast agents into liposomes for biosensor imaging of redundant deviation in shifts (BIRDS) Journal of Biological Inorganic Chemistry. 2014;19(8):1385–1398. doi: 10.1007/s00775-014-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu G., Hong R. Y., Guo L., Li Y. G., Li H. Z. Preparation, characterization and MRI application of carboxymethyl dextran coated magnetic nanoparticles. Applied Surface Science. 2011;257(15):6711–6717. doi: 10.1016/j.apsusc.2011.02.110. [DOI] [Google Scholar]
- 64.Coman D., Kiefer G. E., Rothman D. L., Sherry A. D., Hyder F. A lanthanide complex with dual biosensing properties: CEST (chemical exchange saturation transfer) and BIRDS (biosensor imaging of redundant deviation in shifts) with europium DOTA-tetraglycinate. NMR in Biomedicine. 2011;24(10):1216–1225. doi: 10.1002/nbm.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trübel H. K. F., Maciejewski P. K., Farber J. H., Hyder F. Brain temperature measured by 1H-NMR in conjunction with a lanthanide complex. Journal of Applied Physiology. 2003;94(4):1641–1649. doi: 10.1152/japplphysiol.00841.2002. [DOI] [PubMed] [Google Scholar]
- 66.Brightman M. W. The Intracerebral Movement of Proteins Injected into Blood and Cerebrospinal Fluid of Mice. Progress in Brain Research. 1968;29(C):19–40. doi: 10.1016/S0079-6123(08)64147-3. [DOI] [PubMed] [Google Scholar]
- 67.Johnson A. K., Gross P. M. Sensory circumventricular organs and brain homeostatic pathways. The FASEB Journal. 1993;J7:678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 68.Girard O. M., Ramirez R., McCarty S., Mattrey R. F. Toward absolute quantification of iron oxide nanoparticles as well as cell internalized fraction using multiparametric MRI. Contrast Media & Molecular Imaging. 2012;7:411–417. doi: 10.1002/cmmi.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taylor A., Herrmann A., Moss D., et al. Assessing the efficacy of nano- and micro-sized magnetic particles as contrast agents for MRI cell tracking. PLoS ONE. 2014;9(6) doi: 10.1371/journal.pone.0100259.e100259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crayton S. H., Tsourkas A. PH-titratable superparamagnetic iron oxide for improved nanoparticle accumulation in acidic tumor microenvironments. ACS Nano. 2011;5(12):9592–9601. doi: 10.1021/nn202863x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shu C. Y., Sanganahalli B. G., Coman D., Herman P., Rothman D. L., Hyder F. Quantitative β mapping for calibrated fMRI. NeuroImage. 2016;126:219–228. doi: 10.1016/j.neuroimage.2015.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kallinowski F., Vaupel P. Ph distributions in spontaneous and isotransplanted rat tumours. British Journal of Cancer. 1988;58(3):314–321. doi: 10.1038/bjc.1988.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tannock I. F., Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Research. 1989;49:4373–4384. [PubMed] [Google Scholar]
- 74.Hyder F., Hoque S. M. Brain tumor diagnostics and therapeutics with superparamagnetic ferrite nanoparticles. Contrast Media & Molecular Imaging. doi: 10.1155/2017/6387217. In press.6387217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barbaro D., Di Bari L., Gandin V., et al. Glucose-coated superparamagnetic iron oxide nanoparticles prepared by metal vapour synthesis are electively internalized in a pancreatic adenocarcinoma cell line expressing GLUT1 transporter. PLoS ONE. 2015;10(4) doi: 10.1371/journal.pone.0123159.e0123159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shan X. H., Wang P., Xiong F., et al. MRI of High-Glucose Metabolism Tumors: a Study in Cells and Mice with 2-DG-Modified Superparamagnetic Iron Oxide Nanoparticles. Molecular Imaging and Biology. 2016;18(1):24–33. doi: 10.1007/s11307-015-0874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brown J. M., Giaccia A. J. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Research. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 78.Chaplin D. J., Olive P. L., Durand R. E. Intermittent blood flow in a murine tumor: radiobiological effects. Cancer Research. 1987;47:597–601. [PubMed] [Google Scholar]
- 79.Padera T. P., Stoll B. R., Tooredman J. B., Capen D., Di Tomaso E., Jain R. K. Cancer cells compress intratumour vessels. Nature. 2004;427, article 695 doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- 80.Uehara H., Miyagawa T., Tjuvajev J., et al. Imaging experimental brain tumors with 1-aminocyclopentane carboxylic acid and alpha-aminoisobutyric acid: comparison to fluorodeoxyglucose and diethylenetriaminepentaacetic acid in morphologically defined tumor regions. Journal of Cerebral Blood Flow & Metabolism. 1997;17:1239–1253. doi: 10.1097/00004647-199711000-00013. [DOI] [PubMed] [Google Scholar]
- 81.Hoque S. M., Huang Y., Cocco E., et al. Improved specific loss power on cancer cells by hyperthermia and MRI contrast of hydrophilic Fex Co1-x Fe2 O4 nanoensembles. Contrast Media & Molecular Imaging. 2016;11:514–526. doi: 10.1002/cmmi.1713. [DOI] [PubMed] [Google Scholar]
- 82.Jordan A., Maier-Hauff K. Magnetic nanoparticles for intracranial thermotherapy. Journal of Nanoscience and Nanotechnology. 2007;7:4604–4606. [PubMed] [Google Scholar]
- 83.Thiesen B., Jordan A. Clinical applications of magnetic nanoparticles for hyperthermia. International Journal of Hyperthermia. 2008;24(6):467–474. doi: 10.1080/02656730802104757. [DOI] [PubMed] [Google Scholar]
- 84.van Landeghem F. K. H., Maier-Hauff K., Jordan A., et al. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials. 2009;30(1):52–57. doi: 10.1016/j.biomaterials.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 85.Maier-Hauff K., Rothe R., Scholz R. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: results of a feasibility study on patients with glioblastoma multiforme. Journal of Neuro-Oncology. 2007;81(1):53–60. doi: 10.1007/s11060-006-9195-0. [DOI] [PubMed] [Google Scholar]
- 86.Bulte J. W. M., Kraitchman D. L. Monitoring cell therapy using iron oxide MR contrast agents. Current Pharmaceutical Biotechnology. 2004;5(6):567–584. doi: 10.2174/1389201043376526. [DOI] [PubMed] [Google Scholar]
- 87.Daldrup-Link H. E., Meier R., Rudelius M., et al. In vivo tracking of genetically engineered, anti-HER2/neu directed natural killer cells to HER2/neu positive mammary tumors with magnetic resonance imaging. European Radiology. 2005;15(1):4–13. doi: 10.1007/s00330-004-2526-7. [DOI] [PubMed] [Google Scholar]
- 88.Daldrup-Link H. E., Rudelius M., Piontek G., et al. Migration of iron oxide-labeled human hematopoietic progenitor cells in a mouse model: In vivo monitoring with 1.5-T ME imaging equipment. Radiology. 2005;234(1):197–205. doi: 10.1148/radiol.2341031236. [DOI] [PubMed] [Google Scholar]
- 89.Kostura L., Kraitchman D. L., Mackay A. M., Pittenger M. F., Bulte J. M. W. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR in Biomedicine. 2004;17(7):513–517. doi: 10.1002/nbm.925. [DOI] [PubMed] [Google Scholar]
- 90.Fan C. H., Ting C. Y., Lin H. J., et al. SPIO-conjugated, doxorubicin-loaded microbubbles for concurrent MRI and focused-ultrasound enhanced brain-tumor drug delivery. Biomaterials. 2013;34(14):3706–3715. doi: 10.1016/j.biomaterials.2013.01.099. [DOI] [PubMed] [Google Scholar]
- 91.Zhang P., Hu L., Yin Q., Feng L., Li Y. Transferrin-modified c[RGDfK]-paclitaxel loaded hybrid micelle for sequential blood-brain barrier penetration and glioma targeting therapy. Molecular Pharmaceutics. 2012;9(6):1590–1598. doi: 10.1021/mp200600t. [DOI] [PubMed] [Google Scholar]
- 92.Zhang P., Hu L., Yin Q., Zhang Z., Feng L., Li Y. Transferrin-conjugated polyphosphoester hybrid micelle loading paclitaxel for brain-targeting delivery: Synthesis, preparation and in vivo evaluation. Journal of Controlled Release. 2012;159(3):429–434. doi: 10.1016/j.jconrel.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 93.Zhen Z., Tang W., Chen H., et al. RGD-modified apoferritin nanoparticles for efficient drug delivery to tumors. ACS Nano. 2013;7(6):4830–4837. doi: 10.1021/nn305791q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhen Z., Tang W., Guo C., et al. Ferritin nanocages to encapsulate and deliver photosensitizers for efficient photodynamic therapy against cancer. ACS Nano. 2013;7(8):6988–6996. doi: 10.1021/nn402199g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qin L., Wang C.-Z., Fan H.-J., et al. A dual-targeting liposome conjugated with transferrin and arginine-glycine-aspartic acid peptide for glioma-targeting therapy. Oncology Letters. 2014;8(5):2000–2006. doi: 10.3892/ol.2014.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rao J. U., Engelke U. F. H., Sweep F. C. G. J., et al. Genotype-specific differences in the tumor metabolite profile of pheochromocytoma and paraganglioma using untargeted and targeted metabolomics. The Journal of Clinical Endocrinology & Metabolism. 2015;100(2):E214–E222. doi: 10.1210/jc.2014-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Inai T., Mancuso M., Hashizume H., et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. The American journal of pathology. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gillies R. J., Gatenby R. A. Adaptive landscapes and emergent phenotypes: Why do cancers have high glycolysis? Journal of Bioenergetics and Biomembranes. 2007;39(3):251–257. doi: 10.1007/s10863-007-9085-y. [DOI] [PubMed] [Google Scholar]
- 99.Gillies R. J., Robey I., Gatenby R. A. Causes and consequences of increased glucose metabolism of cancers. Journal of Nuclear Medicine. 2008;49(supplement 2):24S–42S. doi: 10.2967/jnumed.107.047258. [DOI] [PubMed] [Google Scholar]
- 100.Robey I. F., Baggett B. K., Kirkpatrick N. D., et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Research. 2009;69(6):2260–2268. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ibrahim-Hashim A., Abrahams D., Enriquez-Navas P. M., Luddy K., Gatenby R. A., Gillies R. J. Tris-base buffer: a promising new inhibitor for cancer progression and metastasis. Cancer Medicine. 2017;6:1720–1729. doi: 10.1002/cam4.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rao J. U., Coman D., Walsh J. J., Ali M. M., Huang Y., Hyder F. Temozolomide arrests glioma growth and normalizes intratumoral extracellular pH. Scientific Reports. 2017;7, article 7865 doi: 10.1038/s41598-017-07609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chu K. ‐. C., Xu Y., Balschi J. A., Springer C. S. Bulk magnetic susceptibility shifts in nmr studies of compartmentalized samples: use of paramagnetic reagents. Magnetic Resonance in Medicine. 1990;13(2):239–262. doi: 10.1002/mrm.1910130207. [DOI] [PubMed] [Google Scholar]
- 104.Sherry A. D., Caravan P., Lenkinski R. E. Primer on gadolinium chemistry. Journal of Magnetic Resonance Imaging. 2009;30(6):1240–1248. doi: 10.1002/jmri.21966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ledneva E., Karie S., Launay-Vacher V., Janus N., Deray G. Renal safety of gadolinium- Based contrast media in patients with chronic renal insufficiency. Radiology. 2009;250(3):618–628. doi: 10.1148/radiol.2503080253. [DOI] [PubMed] [Google Scholar]
- 106.Zhou J., Patel T. R., Sirianni R. W., et al. Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma. Proceedings of the National Acadamy of Sciences of the United States of America. 2013;110(29):11751–11756. doi: 10.1073/pnas.1304504110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Prussian Blue Staining for iron (SPIO-NPs) distribution.
Figure S2. Effect of TmDOTP5- and SPIO-NPs infusion on the transverse relaxation rate (R2) in Probenecid-infused glioma-bearing animals.
Figure S3. Extracellular pH (pHe) and TmDOTP5- linewidths (LW) measured before and after SPIO-NPs infusion in Probenecid-infused glioma-bearing animals.
Figure S4. Comparison of Extracellular pH (pHe) in 9L tumor-bearing animals that underwent renal ligation or Probenecid co-infusion to inhibit renal clearance.