Abstract
The current review is meant to synthesize research presented as part of a symposium at the 2016 Neurobiology of Stress workshop in Irvine California. The focus of the symposium was “Stress and the Synapse: New Concepts and Methods” and featured the work of several junior investigators. The presentations focused on the impact of various forms of stress (altered maternal care, binge alcohol drinking, chronic social defeat, and chronic unpredictable stress) on synaptic function, neurodevelopment, and behavioral outcomes. One of the goals of the symposium was to highlight the mechanisms accounting for how the nervous system responds to stress and their impact on outcome measures with converging effects on the development of pathological behavior. Dr. Kevin Bath's presentation focused on the impact of disruptions in early maternal care and its impact on the timing of hippocampus maturation in mice, finding that this form of stress drove accelerated synaptic and behavioral maturation, and contributed to the later emergence of risk for cognitive and emotional disturbance. Dr. Scott Russo highlighted the impact of chronic social defeat stress in adolescent mice on the development and plasticity of reward circuity, with a focus on glutamatergic development in the nucleus accumbens and mesolimbic dopamine system, and the implications of these changes for disruptions in social and hedonic response, key processes disturbed in depressive pathology. Dr. Kristen Pleil described synaptic changes in the bed nuclei of the stria terminalis that underlie the behavioral consequences of allostatic load produced by repeated cycles of alcohol binge drinking and withdrawal. Dr. Eric Wohleb and Dr. Ron Duman provided new data associating decreased mammalian target of rapamycin (mTOR) signaling and neurobiological changes in the synapses in response to chronic unpredictable stress, and highlighted the potential for the novel antidepressant ketamine to rescue synaptic and behavioral effects. In aggregate, these presentations showcased how divergent perspectives provide new insights into the ways in which stress impacts circuit development and function, with implications for understanding emergence of affective pathology.
Keywords: Early-life stress, Hippocampus, Susceptibility, Resilience, Nucleus accumbens, Bed nuclei of the stria terminalis, Neuropeptide Y, Corticotropin-releasing factor, Prefrtonal cortex, Mammalian target of rapamycin, Major depressive disorder
List of abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BEC
blood alcohol content
- BNST
bed nuclei of the stria terminalis
- CRF
corticotropin-releasing factor
- CSDS
chronic social defeat stress
- CUS
chronic unpredictable stress
- D1
dopamine receptor type 1
- D2
dopamine receptor type 2
- DID
Drinking in the Dark model of binge drinking
- ELS
early-life stress
- GABA
gamma-aminobutyric acid
- GABAA
gamma-aminobutyric acid type A receptor
- GPCR
G-protein coupled receptor
- ILT
intralaminar thalamus
- mAChR
muscarinic acetylcholine receptor
- MBP
myelin basic protein
- MDD
major depressive disorder
- MSN
medium spiny neuron
- mTOR
mammalian target of rapamycin
- mTORC1
mammalian target of rapamycin complex 1
- NAc
nucleus accumbens
- NMDA
N-methyl-D-asparate
- NPY
neuropeptide Y
- P70S6K
p70 ribosomal protein S6-kinase
- PFC
prefrontal cortex
- PV
parvalbumin
- PVT
paraventricular nucleus of the thalamus
- REDD1
regulated in development and DNA damage responses 1
- RT-qPCR
real-time quantitative polymerase chain reaction
- SCVS
subchronic variable stress
- uEPSC
unitary excitatory postsynaptic current
- VGLUT
vesicular glutamate transporter
- Y1R
neuropeptide Y type 1 receptor
1. Introduction
Stress profoundly alters neural and behavioral development, drives changes in physiology and behavior, and contributes to increased morbidity and earlier mortality across nearly all species studied. A stressor may be any stimulus that disrupts, or is perceived to disrupt, selective homeostatic responses within the individual. Stressful stimuli can range from an attack by a predator or rival, diminished maternal care, an immune challenge, to a lack of available energy to run cellular processes (e.g. hunger) (Karatsoreos and McEwen, 2011, McEwen and McEwen, 2016), and organisms have a highly evolved set of responses to adapt to this wide range of challenges. The biological responses in stress adaptation involve the reallocation of metabolic resources until homeostasis can be restored, thereby enhancing the probability of survival and promoting reproductive success, the ultimate selection process driving evolutionary change.
On the one hand, moderate levels of stress exposure may well serve as catalysts for experience-dependent structural and functional changes associated with memory consolidation, behavioral regulation, and developmental processes (Hostinar and Gunnar, 2013, Karatsoreos and McEwen, 2011, Lupien et al., 2009, McEwen, 2004). However, chronic or excessively high levels of stress has been shown to have deleterious effects, impacting neural structure and functional plasticity of the brain and contributing to a variety of negative health outcomes (Bath et al., 2016, Chattarji et al., 2015, Liston et al., 2006, McEwen et al., 1992, Popoli et al., 2012, Radley et al., 2008, Vyas et al., 2006). Moreover, significant or prolonged stress early in life further increases susceptibility to morbidity and mortality, with increased risk for disease, decreased fecundity, and shortened lifespans for a variety of species, including humans (Anda et al., 1999, Anda et al., 2002, Brown et al., 2006, Dietz et al., 1999, Dong et al., 2003, Dube et al., 2001, Dube et al., 2002, Felitti et al., 1998, Hillis et al., 2004, Williamson et al., 2002). Stress-related psychiatric disorders per se, are estimated to affect 20% of people in the United States within their lifetime, and remain a major cause of disability leading to significant social and economic costs (Culpepper, 2010, Kessler, 2012, Kessler et al., 2005, Murray et al., 2013, Wang et al., 2003).
While elucidating the neurobiological mechanisms of vulnerability to stress-related mental disorders remains a formidable challenge, significant progress has nevertheless been made in unraveling the cellular and circuit mechanisms in a variety of experimental and pre-clinical contexts using animal models. In this review, we provide insights from several forms of stress, including social defeat, chronic unpredictable stress, binge alcohol exposure, and disruptions in maternal care, implemented across different developmental epochs (either early postnatal, adolescent or adult) on neurobiological, circuit, and behavioral outcomes related to stress-associated pathology. Moreover, we highlight the synaptic changes associated with stress as well as new approaches and techniques for probing the impact of stress on circuit development, stability, and function. The results of these studies provide novel insights into the complex effects of stress on circuit development and function, how they may contribute to the multifaceted endophenotypes that when grouped together underlie disease susceptibility, and highlight novel pathways for the treatment of stress-related mental illnesses.
2. Impact of early life stress on the timing of neurobehavioral development – Kevin Bath, Brown University
Early life stress (ELS) significantly increases the risk for the later development of affective pathology, including depression and anxiety disorders. For example, a single significant stressor experienced during childhood increases the lifetime risk of anxiety or depressive pathology by ∼30% (Anda et al., 2006). Importantly, as many as sixty-four percent of individuals will experience at least one significant stressor during childhood (Anda et al., 2006). Having three or more adverse experiences early in life more than doubles the lifetime risk of developing affective pathology (Anda et al., 2006), with females being nearly twice as likely as males to develop disorders (Breslau, 2009, Breslau et al., 1997a, Breslau et al., 1997b, Burt and Stein, 2002, De Munck et al., 2009, Felitti et al., 1998, Gater et al., 1998, Hankin, 2009, Keita, 2007, Kuehner, 2003, Olino et al., 2010, Pratchett et al., 2010, Weissman et al., 1996). The majority of these disorders are slow to develop with symptom expression increasing in late childhood and peaking during adolescence and early adulthood (Teicher et al., 2009), possibly implicating an atypical developmental process underlying the later emergence of pathology (Burt and Stein, 2002, Wacker, 2000, Wittchen et al., 1994). Despite such observations, most work to date has focused on either the proximate effects of stress on neuroanatomy, or outcome measures in adolescence and adulthood, with few studies assessing the effects of early adverse experience on developmental processes. A principle focus of this work was to assess the impact of stress on the timing of neural developmental events, and as an initial target we focused on the hippocampus, a region heavily implicated in stress-associated pathology.
2.1. Stress and the hippocampus
In humans, depression and post-traumatic stress disorder have been associated with reductions in hippocampal volume (Bremner et al., 1995, Sheline et al., 1996, Sheline et al., 1999, Steffens et al., 2000). In animal models, stress or the mimicking of HPA axis stimulation through chronic treatment with stress hormones can induce anxiety and depressive-like symptoms and drive regressive morphological changes of cells of the hippocampus, such as dendritic atrophy in CA1 and CA3 (Magarinos et al., 1998, McEwen et al., 1992, Watanabe et al., 1992) and the suppression of dentate gyrus neurogenesis (Cameron and Gould, 1994, Gould and Tanapat, 1999, Gould et al., 1991a, Gould et al., 1991b, Gould et al., 1997). The majority of those studies focus upon morphological changes in the adolescent or adult animal. However, ELS exposure has also been associated with: reduced cell proliferation and increased cell death in the dentate gyrus of the hippocampal formation of the perinatal rat (Gould et al., 1991a, Gould et al., 1991b, Tanapat et al., 1998); simplification of dendritic arbors and enhanced dendritic turnover in CA1, CA3 and frontal regions (Brunson et al., 2001, Chen et al., 2008, Liston and Gan, 2011); decreased hippocampal synaptic density and reduced overall hippocampal volume when measured in adolescence and adulthood (Andersen and Teicher, 2004) (Frodl et al., 2010, Teicher et al., 2012, Vythilingam et al., 2002). Thus, the hippocampus undergoes significant morphological changes in response to stress and represents an attractive target for understanding the impact of stress on developmental processes and for probing possible neurobiological substrates of pathological behavior.
2.2. Stress and development
In human studies and rodent models of ELS, adverse early experiences have been associated with somatic growth restriction and reductions in brain volume, including reduced hippocampus volume observed during adolescence and adulthood (Frodl et al., 2010, Teicher et al., 2012, Vythilingam et al., 2002). Exposure to chronic early adversity and adoption is associated with diminished stature and suppressed expression of growth hormone (Nakata et al., 2009, Tuvemo et al., 1999, Tuvemo et al., 2004). Previous work in rodents has identified suppressed neurogenesis in hippocampus following ELS or exposure to stress hormones (Gould et al., 1991a, Gould et al., 1991b, Gould et al., 1991c, Tanapat et al., 1998). Such observations have led to a prevailing view that ELS serves to suppress processes of growth at the somatic as well as neural level. Importantly, recent work has indicated that ELS may have activating effects on some aspects of somatic, hormonal, and behavioral development. In fact, it has been well documented that children exposed to chronic stress early in life show accelerated gonadal development, with females showing an earlier onset of menarche (Allsworth et al., 2005, Costello et al., 2007, Foster et al., 2008, Jones et al., 1972, Moffitt et al., 1992), effects that have been mirrored in the sexual development of rodent models of poor maternal care (Cameron, 2011, Cameron et al., 2008). In rodent models, ELS has also been associated with precocious development of defensive behaviors (Takahashi, 1996, Takahashi and Kim, 1995), earlier development of fear associated learning (Moriceau and Sullivan, 2004, Sullivan et al., 2000), and what appear to be more maturate forms of fear extinction in juvenile animals (Callaghan and Richardson, 2014). These effects appear to mirror earlier maturation of fear responding and functional connectivity in humans who experienced institutional rearing (Gee et al., 2013, Gee et al., 2014). Taken together, these studies indicate that ELS, at least in some domains of somatic and neural function, may actually lead to accelerated maturation. Despite such observations, few studies have directly assessed the effects of ELS on the timing of maturation at the neural level. Here, we leverage a rodent model of ELS to test the hypothesis that stress serves to accelerate maturation of at least some regions of the brain, with a focus on the hippocampus.
2.3. Fragmented maternal care as a model of ELS
ELS was induced in a mouse model by disrupting maternal care through use of restricted access to bedding and nesting material from postnatal day 4 (P4) to postnatal day 11. In this paradigm, at P4 the dam and her litter were moved from standard housing to a cage with a wire mesh floor and ½ of a nestlet as their only source of nesting material. The limited access to nesting material leads to a fragmentation in maternal care (Bath et al., 2016, Heun-Johnson and Levitt, 2016, Rice et al., 2008), with the dam leaving the nest more frequently than is observed under control housing conditions, and dams showing elevations in basal stress hormone levels during the period of restricted bedding. The dam and pups remain in these housing conditions for 7 days, until P11, and then are returned to standard housing conditions with full nesting. The combination of maternal stress and increased departures from the nest is associated with growth restriction in the pups, an effect that persists throughout early life (Bath et al., 2016, Heun-Johnson and Levitt, 2016, Rice et al., 2008). Pups also show significant, but transient, elevations in basal stress hormone levels (Bath et al., 2016, Rice et al., 2008), and disruptions in the expression of key regulatory elements of the HPA axis (Bath et al., 2016, Rice et al., 2008). In the current studies, we collected tissue samples from the brains of control and ELS reared mice beginning prior to stress and sampling at regular intervals through early adulthood.
2.4. ELS and measures of neural maturation
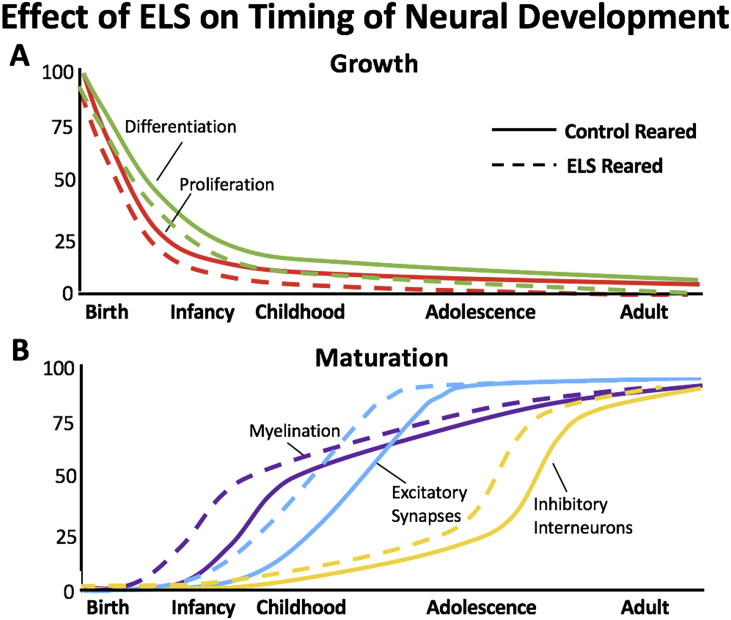
In this model, we used a combination of BrdU pulse labeling as well as real-time quantitative polymerase chain reaction (RT-qPCR) to assess markers of cell proliferation and differentiation throughout early development. Consistent with previous reports using alternate forms of stress, or pharmacological stimulation of the stress axis, maternal bedding restriction was associated with an earlier decline in marker of cell proliferation, suggesting an earlier silencing of neurogenesis. To assess the effects of ELS on maturation of the hippocampus, we tested for effects of ELS on the developmental profile of three different neuromaturational events, synaptic maturation, interneuron development, and myelination (Bath et al., 2016). At the synaptic level, previous work has shown a developmental shift in ratio of N-methyl-D-asparate (NMDA) receptor subunits NR2a and NR2b (Sheng et al., 1994). This shift occurs during the peri-adolescent period, with the expression of NR2a increasing relative to the NR2b subunit (Liu et al., 2004). Using RT-qPCR on whole hippocampus, we could visualize the shift in subunit ratio, and found that this shift occurred a full week earlier in ELS reared mice (Bath et al., 2016). During postnatal development, there is a significant increase in myelination throughout the brain. Using expression of myelin basic protein (MBP) as a marker for this process in the whole hippocampus, we observed a significant rise in MBP expression over the early postnatal period, with ELS animals showing a significant and earlier rise in MBP expression. Finally, we investigated the developmental expression of Parvalbumin (PV) in whole hippocampus, a calcium binding protein found on a subset of fast spiking interneurons. PV expression increases across early postnatal development, has been implicated in the opening and closure of sensitive periods, and has reliably been used as a marker of circuit maturation (Balmer et al., 2009, Cancedda et al., 2004, Gogolla et al., 2014, Huang et al., 1999, Inaguma et al., 1991). As with our other markers, we found an earlier expression of PV in the hippocampus of ELS mice relative to control reared animals, an effect that was confirmed by immunohistochemical detection of PV-protein in the dentate gyrus of the hippocampus (Bath et al., 2016). We provide a graphical summary of these effects in Fig. 1.
Fig. 1.
Impact of ELS on growth and maturation of the murine hippocampus. (A) Across early postnatal development there is a significant decline in rates of cell proliferation and differentiation. (B) Across this same period there is a gradual increase in makers of cellular and circuit maturation including myelination, synaptic differentiation, and interneuron integration. Across these measures, we find that ELS (dashed) leads to a more rapid silencing of neurogenesis and an earlier onset and completion of maturational processes in the hippocampus of the mouse.
2.5. ELS and cognitive and affective outcomes
To test if the observed shift in gene expression, and histological markers of maturation were associated with changes in earlier behavioral development, we took advantage of a recently identified developmental decline in contextual freezing in a fear conditioning paradigm (Pattwell et al., 2011). In that task, we found that ELS reared mice showed the anticipated decrease in freezing behavior, but that the decline in freezing occurred a full week earlier than was observed in control reared mice, occurring at P22 instead of P28. Together, our genetic, histological, and behavioral measures lead us to conclude that ELS drives an acceleration in the maturation of the hippocampus. To test if the accelerated maturation of this region was associated with the development of behavioral endophenotypes indicative of pathology, we carried out a battery of cognitive and affective tests in control and ELS reared mice. Interestingly, we found that female but not male mice reared under ELS conditions develop elevations in anxiety and depressive-like outcomes in early adulthood, while adult male but not female mice have impairments in spatial abilities (Naninck et al., 2015). In more recent work, both males and females were shown to have impaired performance on spatial learning at P21, however, the effects resolve in females by early adulthood but not in males (Bath et al. in revision). Thus, ELS leads to similar profiles in gross measures of accelerated hippocampus maturation in males and females, but different behavioral outcomes. Future studies will be critical to understand the contribution of changes in ontogenetic development to pathology and the unique consequences of ELS on the timing of neural development, sex differences in risk for select developmental events (e.g. neurogenesis, myelination), and the consequences of ELS on the development and connectivity between brain regions.
2.6. Conclusions and new research directions
In summary, the studies presented here represent an initial demonstration that ELS in the form of disrupted maternal care, leads to an acceleration in hippocampus maturation at the behavioral and neural level. These findings reveal important effects of ELS on processes of growth and maturation and identify potential mechanisms through which stress might disrupt the timing of regional maturation, with implications for pathological development. The current studies support a framework in which ELS may drive an earlier maturation of at least some brain structures, potentially in the spirit of supporting earlier development in the face of an adverse early environment. In the context of an evolutionary framework, we interpret these findings to suggest that ELS may serve as an environmental signal of an inhospitable environment, recruiting earlier maturation of select structures of the brain to drive earlier development of centers supporting reproduction. This hypothesis is borne out of observations in humans and rodents showing that ELS accelerated the onset of cycling and expression of reproductive behaviors. However, such an interpretation is still highly speculative and will require significant additional data at the neural and behavioral level to support. If true, later emerging pathological outcomes could represent a negative byproduct of a shift in investment in regional brain development to support more proximate goals. Regardless, future studies investigating ELS effects on developmental processes will be required and may provide clues with regard to adaptive changes in circuit development in response to stress that may ultimately contribute to increased risk for pathological outcomes.
3. Plasticity of reward circuitry and stress disorders – Scott Russo, Icahn School of Medicine at Mount Sinai
The behavioral symptoms of depression are extensive, covering emotional, motivational, cognitive and physiological domains. Large subsets of patients with these disorders exhibit deficits in several aspects of reward. Prominent among these reward deficits is social avoidance and anhedonia, which are thought to result from major disruptions in reward circuit function. The best-characterized reward circuit in the brain is made up of dopaminergic neurons in the ventral tegmental area that project to the nucleus accumbens (NAc), a sub-division of the ventral striatum. The primary neuronal subtype within the NAc is the medium spiny neuron (MSN), which can be further sub-divided into dopamine receptor 1- (D1) and dopamine receptor 2- (D2) containing phenotypes. MSNs are crucial for recognition of rewarding versus aversive stimuli in the environment. The primary brain reward centers are inter-connected in complex ways. In addition to the dense dopaminergic input to the NAc, there is also dense glutamatergic innervation of NAc from the prefrontal cortex (PFC), ventral subiculum, amygdala, and intralaminar thalamus (ILT), among many other regions. Recent evidence suggests that distinct glutamatergic inputs may serve different roles in regulating reward and aversion (Britt et al., 2012, Stuber et al., 2011), which make them ideal candidates for modulation of stress and depression.
The NAc is an established stress-responsive brain region that undergoes remodeling of excitatory synapses following stress (Russo et al., 2012, Russo and Nestler, 2013). This remodeling is thought to alter synaptic connectivity within reward centers resulting in depression-like behavioral responses. To understand the role of such glutamatergic plasticity in stress-related disorders, we have recently employed a chronic social defeat stress (CSDS) model, consisting of repeated subordinations of an experimental C57BL/6 mouse by an aggressive CD-1 mouse. Social subordination stress is a well-established risk factor in the development of depressive and anxiety disorders (Copeland et al., 2013). As in humans, chronic social subordinations in mice leads to a spectrum of depression-like behaviors, such as social avoidance and anhedonia, in a subset of mice termed susceptible, whereas another subset of resilient mice resist the development of such behaviors (Christoffel et al., 2011b). Recent work demonstrates increased density of dendritic spines and excitatory postsynaptic currents on NAc MSNs following CSDS that correlates with social avoidance (Christoffel et al., 2011a, Golden et al., 2013). However, as mentioned above there are multiple glutamatergic inputs to NAc, which can uniquely control reward-related behavior (Britt et al., 2012, Stuber et al., 2011), and it is unclear from these studies whether there are unique presynaptic alterations of glutamatergic inputs to the NAc or whether there are adaptations on specific spines on MSNs in the NAc. Lastly, the role of these synaptic changes in mediating the effects of chronic stress on behavioral adaptations, such as anhedonia and social aversion are not well defined. Here we summarize evidence that CSDS changes synaptic transmission in a circuit, cell and spine type specific fashion in susceptible versus resilient mice. Our discussion will be focused exclusively on inputs to the NAc, however it is becoming clear that interactions between connections outside the NAc are important regulators of stress susceptibility (Hultman et al., 2016, Kumar et al., 2014).
3.1. Glutamatergic stress circuits in the NAc
Previous work has extensively characterized the effects of stress on synaptic plasticity at glutamate synapses throughout cortical hippocampal, striatal and amygdala regions (Christoffel et al., 2011b, Duman et al., 2016, McEwen and Morrison, 2013, Radley et al., 2015). These studies have largely found that while stress causes loss of dendritic spines and dendritic atrophy in the cortex and hippocampus, the NAc and amygdala undergo hypertrophy of dendrites and addition of dendritic spines. However, as mentioned above, these regions are highly interconnected and send direct projections to one another, which makes circuit based analysis crucial. Glutamatergic inputs to the NAc exhibit distinct molecular and electrophysiological properties thought to contribute to their differential roles in regulating striatal-dependent behaviors. For example, vesicular glutamate transporter 2 (VGLUT2) is enriched within ILT and ventral subiculum projecting neurons in the NAc, whereas vesicular glutamate transporter 1 (VGLUT1) is enriched in PFC projecting neurons in the NAc. Interestingly, Previous studies demonstrated distinct release properties of VGLUT1 versus VGLUT2 synapses as well as differential effects on postsynaptic responses of cholinergic interneurons in dorsal striatum (Ding et al., 2010). In addition, previous work suggests that specific glutamate inputs play distinct roles in the expression of reward-related behavior (Bubser and Deutch, 1999, Covington et al., 2010, Smith et al., 2011, Wall et al., 2013). Recently, using CSDS to model depression-like behaviors, it was shown that susceptible mice exhibit increased glutamatergic synaptic transmission within the ILT-NAc pathway measure by an increase in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/NMDA currents whereas there was no effect on PFC-NAc pathway. A parallel study utilizing optogenetic stimulation to mimic long-term depression in the subiculum-NAc pathway found that enhanced transmission at subiculum synapses in the NAc also promoted susceptibility (Bagot et al., 2015). Together these data suggest that specific forms of plasticity at different glutamate inputs to NAc serve distinct functions in striatal transmission and differentially regulate depression-like behavioral responses following CSDS.
3.2. Stress-induced plasticity is cell and spine-type specific in NAc
As mentioned above, stress increases the number of dendritic spines as well as glutamatergic transmission onto MSNs. However, the NAc contains both D1 and D2 MSNs, which play distinct roles in stress-induced depression-like behavior. A recent study by Francis et al. (2014) shows that CSDS increases synaptic transmission on D2 neurons of susceptible mice, while D1 neurons are more excitable in resilient mice. Using cell specific optogenetic techniques, they confirmed that activation of D1 neurons can promote resilience, while activation of D2 neurons promotes susceptibility. Thus, an important question remains whether there are any functional changes in the strength of excitatory signaling within D1 versus D2 MSNs at the level of single spines. Using two-photon preparations coupled to glutamate uncaging at single spines, we found that resilient mice exhibit increased unitary excitatory postsynaptic currents (uEPSCs) at mushroom spines on D1 neurons and decreased uEPSCs on D2 mushroom spines compared to control and susceptible mice. These results are in line with recent evidence that increased D1 activity is associated with resilience (Khibnik et al., 2016). While we did not observe any significant changes in amplitude of uEPSCs in susceptible mice, these studies were performed at a holding potential of −70 mV, which only enables measurement of AMPA currents. Previously published work at the whole cell level suggests that the size or amplitude of AMPA currents do not differ between susceptible and control mice under these conditions (Francis et al., 2014) and that synaptic adaptations in susceptible mice are reflected only in the frequency of synaptic events. Future studies will be needed to more clearly define synaptic transmission at NAc MSN spines in susceptible mice to determine whether alterations in transmission might also be caused by greater presynaptic release or greater frequency and amplitude of postsynaptic NMDA currents.
3.3. Sex differences in synaptic plasticity
Much of the work discussed above has been performed in male mice only despite the fact that females often show distinct or more pronounced forms of plasticity within brain regions compared to males. For example, Wissman et al. (2011) showed that chronic cocaine more robustly increases synaptic transmission and dendritic spine formation in female mice, which they suggest may also play a role in their enhanced behavioral response to cocaine. In our social stress studies, we have only recently been successful at adapting the social defeat model in C57BL/6 mice to females. Thus, we have relied more heavily on use of a subchronic variable stress (SCVS, consisting of 6 d of alternating stressors) protocol that selectively induces depression and anxiety-like behavior in female mice (Hodes et al., 2015, LaPlant et al., 2009). While males eventually succumb to chronic variable stress, they require up to 21 days to develop symptoms. Following SCVS, we find that females exhibit increases in the number of VGLUT2 positive similar to susceptible male mice in the social defeat model (Hodes et al., 2016). Importantly VGLUT2 does not change in resilient male mice following CSDS or SCVS. While these studies suggest that similar circuit mechanisms may drive depression-like behavior in both sexes, further work is needed to determine the specific circuits that undergo plasticity following stress as well as to test their functional role in regulating depression-like behavior.
3.4. Conclusions and new research directions
Stress-induced synaptic plasticity within NAc microcircuitry is extremely complex. With the availability of new circuit specific tools described above we are gaining a level of precision and detail regarding cell types and circuits mediating stress-induced depression-like behavior not possible in earlier investigations of glutamate plasticity in stress phenotypes. In addition, by using circuit specific optogenetic approaches, we can now show directly whether changes in glutamate transmission can alter behavioral stress susceptibility. Furthermore, plasticity is occurring on both the macro (circuit) and micro (spine) level. The added precision and functional nature of two-photon glutamate uncaging studies on specific NAc MSN subtypes have allowed us to identify altered excitatory synaptic responses within resilient mice that may reflect an active, adaptive coping response to limit stress experience. Thus, it seems likely that there is a change in the postsynaptic machinery within D1 versus D2 neurons that alters synaptic strength—a hypothesis that we can now test.
Despite much progress, future work into the circuit basis of depression is clearly needed. For example, much of the work so far has focused on single monosynaptic inputs to the NAc. We know that these brain regions are connected in complex ways and a systems level analysis may help us to better understand how these brain regions are working together to control depression-like behavior. Furthermore, in vivo tools to measure neural activity are becoming increasingly accessible. It is now possible to perform multi-structure analysis of specific cell types and circuits utilizing photometric calcium imaging or single unit opto-tagging to measure changes in neural activity longitudinally during the course of stress exposure. The importance of such longitudinal analysis is that we may be able to identify patterns of activity that predict susceptibility or resilience to stress. Ultimately, we need to confirm results from rodent studies in human patients using state-of-art non-invasive imaging tools so that we can build a relevant circuit map of depression and antidepressant treatment responses.
4. Stressing thalamo-limbic circuits with repeated drinking – Kristen Pleil, Weill Cornell Medical College
Excessive alcohol consumption is a leading cause of preventable death and disease burden, costing the U.S. government an estimated $249 billion per year. It leads to increased risk for many chronic negative health conditions, including cancers, cardiovascular disease, diabetes, and neuropsychiatric diseases such as alcohol dependence and mood disorders (Fan et al., 2008, George et al., 1990, van de Wiel and de Lange, 2008). Epidemiologic research shows that the two most important factors determining the negative health impact of alcohol use are the cumulative volume consumed and the pattern of this consumption over one's lifetime. In particular, a repetitive, cyclic pattern of binge alcohol drinking to intoxication, defined as acutely reaching a blood ethanol content (BEC) of at least 80 mg/dl, and withdrawal is hypothesized to contribute to alcohol dependence by causing aberrant adaptations in brain regions that underlie stress responses and reward-seeking, leading to a state of anxiety or negative affect that drives subsequent drinking (Breese et al., 2005, Koob, 2003, Koob, 2008, Sinha et al., 2008). As such, individuals with an alcohol use disorder are at a substantially increased risk for having a comorbid mood disorder such as anxiety or depression, but the underlying mechanisms of these diseases remain elusive and current pharmacotherapies are fairly ineffective at treating people without causing devastating off-target effects. We have focused our research efforts on identifying the brain circuits that drive binge drinking behavior and the crucial molecular mechanisms of neuropeptides that tune plasticity within those circuits, as these may be essential to finding effective targets for the treatment for alcoholism and mood disorders.
4.1. Mouse models of binge alcohol drinking
Many studies over the past several decades have used dependence-inducing models of chronic alcohol exposure in rodents to characterize adaptations in neural circuitry, neuronal activity, and molecular signaling in alcohol dependence. More recently, a focus has been placed on identifying the key adaptations that occur during repeated binge drinking prior to the development of alcohol addiction and/or mood disorders that might precipitate their emergence or trigger other neuroadaptations that maintain disease states. Specifically, these paradigms use limited or intermittent access to alcohol within or across days in order to produce binge-like alcohol drinking to intoxication upon each exposure, which is followed by withdrawal when alcohol access is removed. Repeated cycles of this “roller coaster” of BEC closely model human binge drinking behavior (Rhodes et al., 2005, Thiele et al., 2014) and capitalize on the epidemiological evidence that large volume, cyclic alcohol intake maximizes the potential negative behavioral and health effects of alcohol consumption.
A model that is now standard in our lab and others’ is the Drinking in the Dark (DID) model of binge drinking (Pleil et al., 2015b, Thiele et al., 2014, Thiele and Navarro, 2014). In this paradigm, mice are given access to 20% alcohol for 2 h, beginning 3 h into the dark phase of the light-dark cycle, for three consecutive days, followed by 4 h of access on the fourth day. Mice binge drink to intoxication, particularly on the fourth day, achieving high BEC levels (≥80 mg/dl) (Pleil et al., 2015b, Rhodes et al., 2005) prior to withdrawal when alcohol access is removed. As few as three cycles of DID with three-day protracted abstinence periods between cycles lead to long-lasting increases in subsequent voluntary alcohol consumption prior to the development of alcohol dependence (Cox et al., 2013). We and others have used this paradigm to identify key neural circuits and neuropeptide signaling mechanisms involved in binge drinking, determine at what point(s) during chronic alcohol exposure these mechanisms begin to diverge from a “normal” pattern, and evaluate whether these changes persist into alcohol dependence and chronic anxiety states (Lowery et al., 2010, Lowery-Gionta et al., 2012, Pleil et al., 2012, Pleil et al., 2015a, Pleil et al., 2015b). These may be key changes that occur early on during exposure to confer increased risk for the development of neuropsychiatric disease, providing especially useful targets for prevention and treatment.
4.2. The roles of extrahypothalamic corticotropin-releasing factor (CRF) and neuropeptide Y (NPY) in binge drinking
Endogenous “stress” and “anti-stress” neuropeptide systems have long been described to play important opposing roles in many behaviors and physiological functions, including alcohol drinking and anxiety behavior, via signaling at their G protein-coupled receptors (GPCRs) located throughout the brain. In particular, the stress neuropeptide CRF and the anti-stress NPY produce divergent behavioral phenotypes, and peptide levels and polymorphisms in the genes coding both of these peptides and their receptors are associated with alcohol drinking behavior, stress responsivity, and the susceptibility to the development of alcohol addiction and anxiety states in humans, monkeys, and rodents (Bale and Vale, 2004, Hayes et al., 2005, Heilig, 2004, Heilig and Koob, 2007, Heilig and Thorsell, 2002, Lindell et al., 2008, Muller et al., 2003, Shekhar et al., 2005, Yehuda et al., 2005). Both peptides are recruited during binge alcohol drinking and altered in rodent models of alcohol dependence (Funk et al., 2006, Heilig and Koob, 2007, Heilig and Thorsell, 2002, Lowery-Gionta et al., 2012, Zhang et al.,), and a large literature indicates that pharmacological or genetic manipulations of CRF, NPY, and their receptors alters the stress/anxiety response and plays a role in alcohol drinking and addiction (Gilpin et al., 2008, Heilig, 2004, Huang et al., 2010, Lowery et al., 2010, Zhang et al.,). Systemic or central administration of CRF and CRF1 receptor agonists enhance alcohol consumption, while CRF1 receptor antagonists blunt high levels of drinking in DID and other voluntary consumption paradigms without altering low, non-binge levels of alcohol consumption (Chu et al., 2007, Lowery et al., 2010, Olive et al., 2003, Sparta et al., 2008, Valdez et al., 2002). Expression of CRF and its receptors are altered in limbic and cortical brain regions during binge alcohol drinking, chronic alcohol exposure, and withdrawal (Huang et al., 2010, Pastor et al., 2008, Zorrilla et al., 2001), suggesting that plasticity upregulating central extra-hypothalamic CRF systems may contribute to increased alcohol consumption during the development of alcohol dependence (Le et al., 2000). In contrast, the anti-stress neuropeptide Y (NPY) system blunts excessive alcohol consumption and produces anxiolysis, predominantly via activation of NPY receptor 1 (Y1R) (Deo et al., 2010, Sparta et al., 2004). It is believed that NPY activation during alcohol or stress exposure serves to maintain or re-achieve homeostasis via direct or indirect functional interactions with the CRF system (Kash and Winder, 2006, Sajdyk et al., 2004), however the site and nature of the interaction between these systems is poorly understood. And, repeated binge drinking may disrupt the typical balance between the signaling or function of these peptide systems, leading to an increasingly dysregulated response over alcohol/stress exposures that perpetuate a trajectory toward neuropsychiatric disease.
4.3. The bed nuclei of the stria terminalis (BNST) as a site for peptide-peptide interactions
The BNST is a limbic structure enriched with neuropeptides including CRF and NPY that receives dense synaptic input from several cortical, limbic, thalamic, and hindbrain structures and projects to many brain areas directly involved in the regulation of anxiety and alcohol/drug-related behaviors (Dong et al., 2001, Dong and Swanson, 2003, Dong and Swanson, 2004, Erb et al., 2001, Georges and Aston-Jones, 2001, McDonald et al., 1999). As a limbic “hub” within the extended amygdala, it is a site of integration of stress and reward information (Carboni et al., 2000, Choi et al., 2007, McElligott et al., 2013, Meloni et al., 2006, Walker et al., 2003) and anatomically situated to mediate the negative affective state associated with chronic alcohol use (Koob, 2003, Koob, 2008). Previous research has shown that the BNST mediates stress responsivity and stress-induced relapse to drug seeking, potentially via CRF-dependent mechanisms (Erb et al., 2001, Sahuque et al., 2006, Wang et al., 2006). Pharmacological manipulations of CRF or NPY in the BNST can alter alcohol-drinking behaviors and chronic alcohol exposure and withdrawal alter the function and synaptic plasticity of BNST neurons, including CRF neurons (Francesconi et al., 2009, Silberman et al., 2013).
We recently provided the first evidence that the central NPY and CRF systems interact directly to modulate binge alcohol drinking behavior, and that long-lasting plasticity in this interaction occurs with as few as three cycles of DID binge drinking (Pleil et al., 2015b). We showed that CRF neurons in the BNST drive binge alcohol drinking behavior and anxiety in mice. NPY signaling in the BNST blunts binge drinking by activating postsynaptic Gi-coupled Y1Rs located specifically on CRF neurons, increasing the trafficking/surface expression of gamma-aminobutyric acid type A (GABAA) receptors to the postsynaptic membrane through a protein kinase A-dependent mechanism and leading to greater synaptic inhibition of CRF neurons. We hypothesized that this mechanism of NPY would be down/dysregulated after three cycles of DID, providing a functional mechanism for the escalation of alcohol consumption and preference that has been shown to emerge at this time point. However, we surprisingly found that this Y1R-mediated synaptic effect and Y1R protein expression were upregulated, providing increased homeostatic inhibition of CRF neurons, for at least ten days following the last binge drinking episode. We also found this same synaptic upregulation of Y1R in alcohol-dependent rhesus monkeys using a chronic voluntary alcohol drinking paradigm, suggesting that this adaptation occurs across mammalian species and states of alcohol dependence. Therefore, this appears to be a critical node of circuitry that undergoes plasticity that is initiated across binge drinking episodes and sustained into the development of dependence. This finding has led us to question whether there is an upregulation of excitatory synaptic transmission at BNST CRF neurons that necessitates increased Y1R-mediated inhibition as a protective mechanism.
4.4. Thalamo-limbic drive of binge drinking behavior
The excitatory neurotransmitter glutamate promotes alcohol drinking and plays an important role in alcohol dependence. For example, systemically-administered glutamate receptor antagonists reduce binge alcohol consumption during DID (Gupta et al., 2008) and are used to block relapsed drinking behavior in alcoholics (Bouza et al., 2004). There is evidence that the site of some of these glutamate-dependent effects may be via modulation of BNST CRF neurons, as chronic alcohol exposure alters synaptic modulation of CRF neurons and produces CRF-dependent alterations in the intrinsic excitability of some BNST neurons (Francesconi et al., 2009, Silberman et al., 2013). Therefore, we have recently begun identifying and characterizing what excitatory inputs to BNST CRF neurons may provide feed-forward modulation of their activity to promote binge drinking behavior.
Preliminary anatomical mapping studies using retrograde and anterograde tracers have identified many previously identified and several novel glutamatergic (VGLUT2-positive) inputs to the BNST, including the paraventricular nucleus of the thalamus (PVT) (Myers et al., 2014). Like the BNST, the PVT is implicated in compulsive drug/alcohol seeking, anxiety and negative affect, and reinstatement of drug and alcohol seeking (James et al., 2011, Zhu et al., 2011). Given its glutamatergic connectivity with the BNST and involvement in reward-seeking and anxiety, we hypothesize that it may be involved in the regulation of binge drinking via excitatory drive onto CRF neurons in the BNST. Preliminary evidence using chemogenetic manipulation of PVT VGLUT2 neurons suggests that these neurons drive binge-drinking behavior, and ongoing studies are evaluating whether they do so via their projection to the BNST. In addition, slice electrophysiology experiments show that PVT glutamate neurons provide direct, monosynaptic excitatory input to CRF neurons in the BNST. We are currently examining the plasticity in PVT glutamate neurons, BNST CRF neurons, and the synaptic connections between them across binge drinking episodes.
4.5. Conclusions and new research directions
While we are making progress toward understanding broader circuit context and signaling mechanisms of BNST CRF neuron drive of binge drinking behavior, the PVT is just one of many glutamatergic inputs and NPY just one of many neuropeptides that may highly regulate the function of BNST CRF neurons. There are also many other known brain regions, neural pathways, and molecules involved in the regulation of binge alcohol drinking and stress responsivity that we and others are actively characterizing. We are currently examining how gonadal and stress hormones modulate these known and newly identified circuits and peptidergic mechanisms in a sex-dependent fashion. The rate of binge drinking is increasing across the U.S., and at a particularly alarming rate in women, who are more susceptible to developing comorbid expression of alcohol dependence and mood disorders. However, females have been underrepresented or excluded from much of the pre-clinical and clinical research for the development of pharmacological treatments. We believe that acute hormone signaling dynamically regulates peptide and circuit function to modulate these behaviors and confers increased susceptibility to neuropsychiatric disease in females, and that understanding these modulatory processes will lead to better treatment strategies.
5. Disruption of mTORC1 signaling contributes to synaptic deficits caused by chronic stress: reversal by rapid-acting antidepressants - Eric Wohleb and Ronald Duman, Yale University
The precise molecular and cellular mechanisms underlying symptoms of major depressive disorder (MDD) remain unclear, but considerable headway has been made in understanding the neurobiology of depressive-like behaviors in rodent models. Clinical and preclinical studies indicate that behavioral symptoms of MDD stem from neurobiological alterations that include dendritic atrophy and synaptic loss on pyramidal neurons in the PFC as well as the hippocampus. In preclinical models of MDD these neuroplasticity deficits can be provoked by psychosocial and environmental stressors, which include repeated exposure to social defeat, restraint, or chronic unpredictable stress (Christoffel et al., 2011b, Duman and Aghajanian, 2012, Duman et al., 2016). Indeed seminal work shows that repeated exposure to stressors reduced dendritic complexity of pyramidal neurons in the medial PFC, which leads to impaired regulation of emotion and cognition (Lupien et al., 2009, Popoli et al., 2012, Radley et al., 2008). Of note, these findings in rodent models recapitulate clinical findings as long-term psychosocial stress in humans is shown to similarly disrupt cortical connectivity and promote attentional control impairments (Liston et al., 2006, Liston et al., 2009).
While it is evident that stress-induced neuronal atrophy in corticolimbic brain regions contributes to the neurobiology of depressive-like behavior, the molecular mechanisms underlying these processes remain poorly understood. The mechanistic target of rapamycin complex 1 (mTORC1) pathway is a critical regulator of protein synthesis-dependent synaptic plasticity and formation of new synapses. Thus, inhibition of this pathway could contribute to the atrophy of neurons seen in corticolimbic brain regions in depressed patients and rodent stress models. In support of this possibility, a postmortem study showed that protein levels of mTOR as well as a downstream mediator p70 ribosomal protein S6-kinase (p70S6K) were reduced in the PFC of depressed subjects (Jernigan et al., 2011). Consistent with these findings, initial studies in our laboratory revealed that chronic unpredictable stress exposure (CUS) for 3 weeks decreased mTORC1-p70S6K signaling in the PFC (Fig. 1).
5.1. Stress-induced up-regulation of REDD1 drives synaptic deficits and depressive-like behaviors via decreased mTORC1-p70S6K signaling
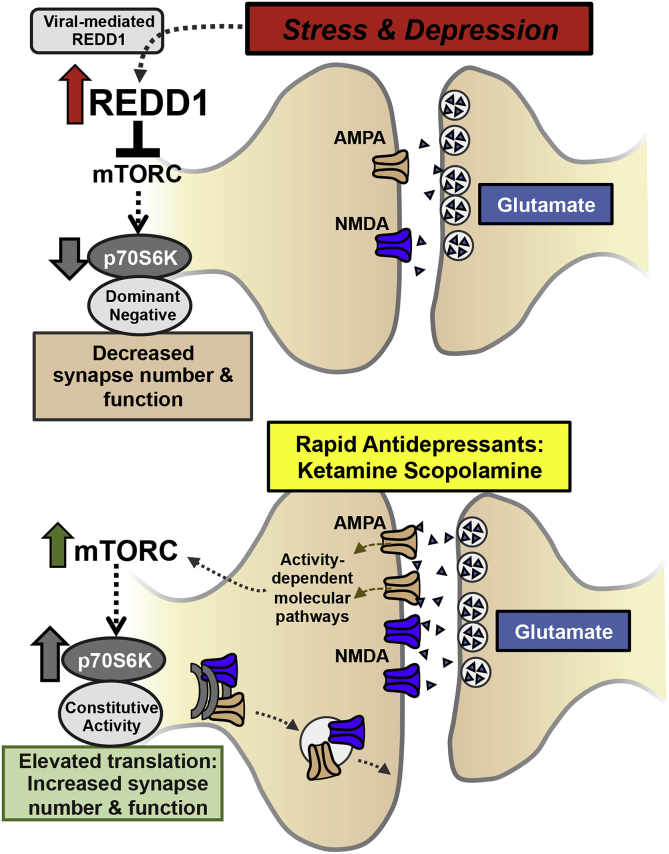
To further examine the role of mTORC1-p70S6K signaling, studies were conducted to test the hypothesis that stress caused neuronal atrophy and synaptic deficits by modulating this signaling pathway. The up-stream negative regulator of mTORC1, referred to as REDD1 (regulated in development and DNA damage responses 1), was identified as a candidate because REDD1 is induced by glucocorticoids and activation of the hypothalamic-pituitary-adrenal axis, a hallmark neuroendocrine target of stress. Indeed, our studies demonstrated that CUS exposure increased levels of REDD1 mRNA and protein in the PFC (Ota et al., 2014). In addition, postmortem gene expression analyses showed that REDD1 was elevated in the dorsolateral PFC of depressed individuals. Further viral-mediated over-expression of REDD1 in the rat medial PFC was sufficient to decrease the phosphorylated and activated forms of mTORC and p70S6K and caused depressive-like behaviors. Moreover, DiOlistic labeling of pyramidal neurons in the medial PFC showed that REDD1 over-expression significantly decreased synaptic spine density on apical dendrites (Fig. 2). In contrast, mice lacking REDD1 expression were resilient to CUS-induced depressive-like behavior, as well as decreased mTORC1-p70S6K signaling and the synaptic deficits in the medial PFC caused by chronic stress exposure. These findings indicate that stress-induced REDD1 expression leads to decreased mTORC1-dependent signaling, synaptic deficits in the medial PFC, and development of depressive-like behaviors (Ota et al., 2014). This is the first direct evidence that mTORC1 signaling plays an important role in mediating the synaptic and behavioral deficits associated with chronic stress and MDD (Fig. 2).
Fig. 2.
mTORC1-p70S6 kinase signaling in synaptic plasticity effects of stress and rapid antidepressant actions. In rodents exposed to stress and patients with MDD, REDD1 expression is increased leading to reduced mTORC1-p70S6 kinase signaling and decreased synapse number and function. Viral-mediated overexpression of REDD1 or dominant negative forms of p70S6 kinase cause similar neuronal and behavioral effects. In contrast, treatment with ketamine or scopolamine causes glutamate influx in the PFC promoting AMPA receptor activation, which increased mTORC1-p70S6K signaling, contributing to increased synaptic plasticity underlying antidepressant responses. Similar antidepressant responses can be attained with viral-mediated expression of constitutively active forms of p70S6 kinase.
To further examine the role of mTORC1 signaling in the neurobiology of MDD and antidepressant treatment, studies were conducted to determine the influence of increasing or decreasing the functional activity of p70S6K (Dwyer et al., 2015). These studies were directed by previous work that showed rapid-acting antidepressants promote synaptic plasticity through activation of mTORC1 signaling (Li et al., 2010, Voleti et al., 2013). In order to examine the role of downstream p70S6K in antidepressant behavioral effects, viral constructs were designed with either constitutively active or dominant negative forms of p70S6K, which allowed for broad control of translational pathways. As expected cell culture experiments showed that the constitutively active p70S6K increased overall protein translation, while the dominant negative p70S6K limited protein production (Dwyer et al., 2015) (Fig. 2). Infusion of the constitutively active p70S6K in the medial PFC of rats produced antidepressant-like effects and prevented stress-induced anhedonia. In contrast, rats that received the dominant negative form of p70S6K showed depressive-like behaviors and ketamine failed to produce an antidepressant response under these conditions of p70S6K blockade (Dwyer et al., 2015). These findings demonstrate that manipulations in p70S6K activity modulate depressive-like behavioral states and further reinforce the role of mTORC1-p70S6K signaling in the pathophysiology and treatment of MDD. In the end, our results support development of molecules that inhibit REDD1 or activate mTORC1-p70S6K signaling as novel therapeutic targets for the treatment of depression (Fig. 2).
5.2. GABA interneurons in the prefrontal cortex are critical mediators of rapid-acting antidepressants
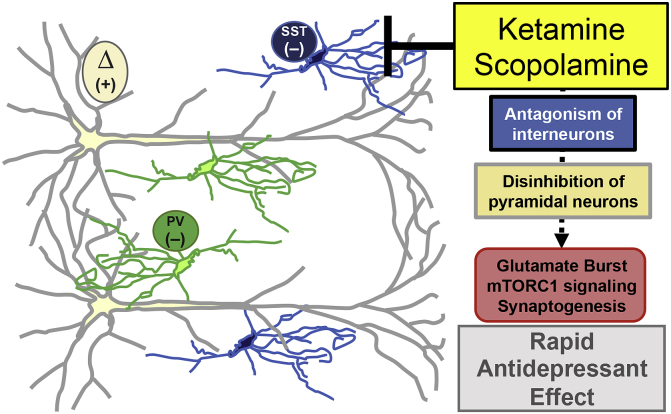
The development of more effective pharmacological therapies for MDD is important as the therapeutic efficacy of clinical treatments are limited, and some patients develop treatment-resistance, failing to respond to two or more different antidepressant agents (Souery et al., 2006). The discovery of rapid-acting antidepressant drugs, including scopolamine, a nonselective muscarinic acetylcholine receptor (mAChR) antagonist, and ketamine, an NMDA receptor antagonist have had a dramatic effect on the antidepressant treatment field (Drevets et al., 2013, Duman et al., 2016). Preclinical research indicates that scopolamine and ketamine induce rapid antidepressant responses through glutamate release, neuronal activity-dependent mTORC1 signaling and increased synaptic plasticity in the medial PFC (Li et al., 2010, Navarria et al., 2015, Voleti et al., 2013). The converging physiological and molecular pathways utilized by ketamine and scopolamine suggest that microcircuitry in the medial PFC is engaged to initiate glutamate release and subsequent downstream mTORC1 signaling (Monteggia and Kavalali, 2013, Wohleb et al., 2016b). In particular, ketamine and scopolamine may promote glutamate release by antagonism of inhibitory interneurons, thereby disinhibiting pyramidal neurons in the medial PFC (Homayoun and Moghaddam, 2007, Moghaddam et al., 1997, Wohleb et al., 2016b) (Fig. 3). Further recent reports indicate that specific neurotransmitter receptor subtypes mediate these neuronal and behavioral effects; in particular the M1-type AChR mediates, at least in part, the effects of scopolamine (Navarria et al., 2015, Voleti et al., 2013, Witkin et al., 2014). Through Cre-dependent conditional knockdown of M1-AChR we showed that scopolamine engaged GABA interneurons in the medial PFC to initiate antidepressant responses. Further studies revealed that M1-AChR expression on somatostatin interneurons in the medial PFC are required for the antidepressant-like effects of scopolamine (Wohleb et al., 2016b). In the end, these studies provided evidence that scopolamine antagonism of PFC interneurons provides the initial cellular trigger leading to glutamate release and subsequent activity-dependent mTORC1 signaling underlying rapid antidepressant responses (Fig. 3). Similar studies are currently being conducted to examine the cellular trigger for ketamine, and if this also includes disinhibition of glutamate transmission via blockade of NMDA receptors on GABAergic neurons in the medial PFC.
Fig. 3.
GABA interneurons in the medial PFC mediate antidepressant effects of ketamine and scopolamine. GABA interneurons in the medial PFC tonically inhibit excitatory pyramidal neurons (Δ). Rapid antidepressant drugs antagonize GABA interneurons (somatostatin (SST); parvalbumin (PV)), leading to disinhibition of pyramidal neurons and subsequent stimulation of neuronal activity-dependent molecular pathways in pyramidal neurons that underlie behavioral responses to ketamine and scopolamine.
5.3. Potential sex differences in stress-induced neuronal atrophy in the medial prefrontal cortex and responses to rapid-acting antidepressants
While the majority of studies performed in the lab have used males, it is important to consider sex-dependent differences in responses to stress and antidepressant treatment. Indeed varied neurobiology and neuroendocrine responses in males and females likely contribute to disproportionate prevalence of affective disorders in females (Kessler, 2003, Kessler and Bromet, 2013). Interestingly, rodent models indicate that estrogen signaling prevents repeated restraint-induced synaptic deficits in the medial PFC, preventing cognitive deficits (Wei et al., 2014, Yuen et al., 2012). This is consistent with studies showing that repeated stress exposure does not cause pyramidal neuron atrophy in the medial PFC of females; in fact pyramidal neurons projecting to basolateral amygdala appear to increase dendritic complexity (Garrett and Wellman, 2009, Shansky et al., 2010). Further research into the specific neurocircuitry influenced by stress exposure will lend insight into the pathophysiology leading to differences in susceptibility to affective disorders.
Sex-dependent differences in neurobiology are also implicated in the response to rapid-acting antidepressants, particularly scopolamine (Wohleb et al., 2016a). Clinical studies comparing males and females treated with scopolamine revealed that females have larger antidepressant and anxiolytic responses (Furey et al., 2010). It is important to point out there is no indication that antidepressant responses to ketamine differ between males and females. In either case these findings warrant further research into potential sex-dependent differences in the neurocircuitry and molecular mechanisms mediating rapid antidepressant responses.
5.4. Conclusions and future research directions
Altogether studies reviewed here support the notion that disruption of mTORC1-p70S6K signaling leads to stress-induced synaptic deficits and contributes to development of depressive-like behaviors. Indeed viral-mediated techniques to promote mTORC1-p70S6K signaling lead to enhanced synaptic plasticity and prevent depressive-like behaviors. These studies are consistent with preclinical studies showing rapid-acting antidepressants engage mTORC1-p70S6K signaling to reverse stress-induced synaptic deficits on pyramidal neurons. Recent studies aimed to uncover the PFC microcircuitry that triggers activity-dependent mTORC1-p70S6K signaling revealed a critical role of GABA interneurons. Further research into the microcircuitry of the medial PFC and specific receptor expression in interneuron subtypes targeted by rapid-acting antidepressants will undoubtedly uncover innovative pharmacological pathways for the treatment of affective disorders.
6. General summary
The work presented in this symposium highlights several themes for understanding the neurobiological changes associated with repeated exposure to salient environmental stimuli. An important idea from each perspective is that protracted challenges have the capacity to alter subsequent behavioral response strategies. On the one hand, responses to chronic stress are considered to be adaptive from an ecological standpoint, regardless of individual variations and/or their implications for altering the developmental trajectory. Nevertheless, many of the adaptations to repeated stress highlighted in each presentation increase vulnerability to stress-related psychiatric disorders. Thus, adaptation appears to come with associated costs, and/or depends on the context under which adaptation is defined (i.e., for survival vis-à-vis increased disease risk). Moreover, each presentation highlights aspects of core circuitry that provides a substrate for stress-induced synaptic and cellular alterations. Dr. Pleil's presentation provides mechanistic insight regarding the synaptic changes in “stress” pathways involving BNST CRF neurons that drive binge drinking behavior. Dr. Russo's work directs attention to differential circuit and synaptic perturbations within and involving the NAc as a function of stress endophenotype that lay some of the groundwork for mapping the circuits and mechanisms of altered hedonic response as part of depression-like behaviors. Drs. Wohleb and Duman have significantly advanced understanding of the cellular mechanisms underlying chronic stress-induced regressive structural and functional plasticity in the prefrontal cortex, and have made inroads into understanding how novel therapeutic approaches may intercede on signaling events to reverse these alterations. Dr. Bath's work raises the novel hypothesis that early-life stress may accelerate regional brain maturation and precocious expression of corresponding behaviors. From an adaptive perspective, stress effects on the timing of neurodevelopmental events may support a bias toward earlier maturation of structures supporting emotional responding, self-preservation, and reproductive development. If accelerated maturation is restricted to select regions of the brain, the mismatch in timing of regional development could contribute to increased susceptibility for pathological behavior.
While these limbic and related structures have long since been appreciated to be involved in stress-related pathologies, these sets of studies highlight novel and integrative perspectives that have advanced understanding how these loci interact to produce stress-related alterations at the circuit and behavioral level, and also provide a clear mechanistic basis for how these circuits function in resilient and susceptible endophenotypes, whether naturally occurring or provoked by early-life experiences. Finally, their implementation with studies directed toward describing how these circuits and mechanisms are altered by antidepressant treatments have provided additional insight into innovative pathways for the treatment of stress-related mental health disorders.
Acknowledgements
Research reported in this review was supported by an NIH IDeA Networks of Biomedical Research Excellence grant P20GM103430 (KGB); R01 MH090264, R01 MH104559, and P50AT008661-01 (SJR); NIH grants K99AA023559 and F32AA021043 (KEP); NIH grants R37MH045481, R01MH093897, and the state of Connecticut (EW and RD); NIH grant R01MH095972 (JJR).
Contributor Information
Kevin G. Bath, Email: Kevin_Bath@Brown.edu.
Jason J. Radley, Email: jason-radley@uiowa.edu.
References
- Allsworth J.E., Weitzen S., Boardman L.A. Early age at menarche and allostatic load: data from the third national health and nutrition examination survey. Ann. Epidemiol. 2005;15:438–444. doi: 10.1016/j.annepidem.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Anda R.F., Chapman D.P., Felitti V.J., Edwards V., Williamson D.F., Croft J.B., Giles W.H. Adverse childhood experiences and risk of paternity in teen pregnancy. Obstet. Gynecol. 2002;100:37–45. doi: 10.1016/s0029-7844(02)02063-x. [DOI] [PubMed] [Google Scholar]
- Anda R.F., Croft J.B., Felitti V.J., Nordenberg D., Giles W.H., Williamson D.F., Giovino G.A. Adverse childhood experiences and smoking during adolescence and adulthood. JAMA. 1999;282:1652–1658. doi: 10.1001/jama.282.17.1652. [DOI] [PubMed] [Google Scholar]
- Anda R.F., Felitti V.J., Bremner J.D., Walker J.D., Whitfield C., Perry B.D., Dube S.R., Giles W.H. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur. Arch. Psychiatry Clin. Neurosci. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S.L., Teicher M.H. Delayed effects of early stress on hippocampal development. Neuropsychopharmacol. official Publ. Am. Coll. Neuropsychopharmacol. 2004;29:1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Bagot R.C., Parise E.M., Pena C.J., Zhang H.X., Maze I., Chaudhury D., Persaud B., Cachope R., Bolanos-Guzman C.A., Cheer J.F., Deisseroth K., Han M.H., Nestler E.J. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat. Commun. 2015;6:7062. doi: 10.1038/ncomms8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale T.L., Vale W.W. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Balmer T.S., Carels V.M., Frisch J.L., Nick T.A. Modulation of perineuronal nets and parvalbumin with developmental song learning. J. Neurosci. official J. Soc. Neurosci. 2009;29:12878–12885. doi: 10.1523/JNEUROSCI.2974-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath K.G., Manzano-Nieves G., Goodwill H. Early life stress accelerates neural and behavioral maturation of the hippocampus in male mice. Horm. Behav. 2016;82:64–71. doi: 10.1016/j.yhbeh.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouza C., Angeles M., Munoz A., Amate J.M. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99:811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- Breese G.R., Chu K., Dayas C.V., Funk D., Knapp D.J., Koob G.F., Le D.A., O'Dell L.E., Overstreet D.H., Roberts A.J., Sinha R., Valdez G.R., Weiss F. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin. Exp. Res. 2005;29:185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Randall P., Scott T.M., Bronen R.A., Seibyl J.P., Southwick S.M., Delaney R.C., McCarthy G., Charney D.S., Innis R.B. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am. J. psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse. 2009;10:198–210. doi: 10.1177/1524838009334448. [DOI] [PubMed] [Google Scholar]
- Breslau N., Davis G.C., Andreski P., Peterson E.L., Schultz L.R. Sex differences in posttraumatic stress disorder. Arch. Gen. Psychiatry. 1997;54:1044–1048. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- Breslau N., Davis G.C., Peterson E.L., Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Arch. Gen. Psychiatry. 1997;54:81–87. doi: 10.1001/archpsyc.1997.01830130087016. [DOI] [PubMed] [Google Scholar]
- Britt J.P., Benaliouad F., McDevitt R.A., Stuber G.D., Wise R.A., Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.W., Young K.E., Anda R.F., Felitti V.J., Giles W.H. Re: asthma and the risk of lung cancer. findings from the Adverse Childhood Experiences (ACE) Cancer Causes Control. 2006;17:349–350. doi: 10.1007/s10552-005-0420-5. [DOI] [PubMed] [Google Scholar]
- Brunson K.L., Avishai-Eliner S., Hatalski C.G., Baram T.Z. Neurobiology of the stress response early in life: evolution of a concept and the role of corticotropin releasing hormone. Mol. Psychiatry. 2001;6:647–656. doi: 10.1038/sj.mp.4000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubser M., Deutch A.Y. Stress induces Fos expression in neurons of the thalamic paraventricular nucleus that innervate limbic forebrain sites. Synapse. 1999;32:13–22. doi: 10.1002/(SICI)1098-2396(199904)32:1<13::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Burt V.K., Stein K. Epidemiology of depression throughout the female life cycle. J. Clin. psychiatry. 2002;63(Suppl. 7):9–15. [PubMed] [Google Scholar]
- Callaghan B.L., Richardson R. Early emergence of adult-like fear renewal in the developing rat after chronic corticosterone treatment of the dam or the pups. Behav. Neurosci. 2014;128:594–602. doi: 10.1037/bne0000009. [DOI] [PubMed] [Google Scholar]
- Cameron H.A., Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Cameron N.M. Maternal programming of reproductive function and behavior in the female rat. Front. Evol. Neurosci. 2011;3:10. doi: 10.3389/fnevo.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron N.M., Fish E.W., Meaney M.J. Maternal influences on the sexual behavior and reproductive success of the female rat. Horm. Behav. 2008;54:178–184. doi: 10.1016/j.yhbeh.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Cancedda L., Putignano E., Sale A., Viegi A., Berardi N., Maffei L. Acceleration of visual system development by environmental enrichment. J. Neurosci. official J. Soc. Neurosci. 2004;24:4840–4848. doi: 10.1523/JNEUROSCI.0845-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E., Silvagni A., Rolando M.T., Di Chiara G. Stimulation of in vivo dopamine transmission in the bed nucleus of stria terminalis by reinforcing drugs. J. Neurosci. 2000;20:RC102. doi: 10.1523/JNEUROSCI.20-20-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattarji S., Tomar A., Suvrathan A., Ghosh S., Rahman M.M. Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat. Neurosci. 2015;18:1364–1375. doi: 10.1038/nn.4115. [DOI] [PubMed] [Google Scholar]
- Chen Y., Dube C.M., Rice C.J., Baram T.Z. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J. Neurosci. official J. Soc. Neurosci. 2008;28:2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D.C., Furay A.R., Evanson N.K., Ostrander M.M., Ulrich-Lai Y.M., Herman J.P. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J. Neurosci. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel D.J., Golden S.A., Dumitriu D., Robison A.J., Janssen W.G., Ahn H.F., Krishnan V., Reyes C.M., Han M.H., Ables J.L., Eisch A.J., Dietz D.M., Ferguson D., Neve R.L., Greengard P., Kim Y., Morrison J.H., Russo S.J. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J. Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel D.J., Golden S.A., Russo S.J. Structural and synaptic plasticity in stress-related disorders. Rev. Neurosci. 2011;22:535–549. doi: 10.1515/RNS.2011.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K., Koob G.F., Cole M., Zorrilla E.P., Roberts A.J. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol. Biochem. Behav. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland W.E., Wolke D., Angold A., Costello E.J. Adult psychiatric outcomes of bullying and being bullied by peers in childhood and adolescence. JAMA psychiatry. 2013;70:419–426. doi: 10.1001/jamapsychiatry.2013.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello E.J., Sung M., Worthman C., Angold A. Pubertal maturation and the development of alcohol use and abuse. Drug Alcohol Depend. 2007;88(Suppl. 1):S50–S59. doi: 10.1016/j.drugalcdep.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Covington H.E., 3rd, Lobo M.K., Maze I., Vialou V., Hyman J.M., Zaman S., LaPlant Q., Mouzon E., Ghose S., Tamminga C.A., Neve R.L., Deisseroth K., Nestler E.J. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J. Neurosci. official J. Soc. Neurosci. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B.R., Olney J.J., Lowery-Gionta E.G., Sprow G.M., Rinker J.A., Navarro M., Kash T.L., Thiele T.E. Repeated cycles of binge-like ethanol (EtOH)-drinking in male C57BL/6J mice augments subsequent voluntary EtOH intake but not other dependence-like phenotypes. Alcohol Clin. Exp. Res. 2013;37:1688–1695. doi: 10.1111/acer.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culpepper L. Why do you need to move beyond first-line therapy for major depression? J. Clin. Psychiatry. 2010;71(Suppl. 1):4–9. doi: 10.4088/JCP.9104su1c.01. [DOI] [PubMed] [Google Scholar]
- De Munck S., Portzky G., Van Heeringen K. Epidemiological trends in attempted suicide in adolescents and young adults between 1996 and 2004. Crisis. 2009;30:115–119. doi: 10.1027/0227-5910.30.3.115. [DOI] [PubMed] [Google Scholar]
- Deo G.S., Dandekar M.P., Upadhya M.A., Kokare D.M., Subhedar N.K. Neuropeptide Y Y1 receptors in the central nucleus of amygdala mediate the anxiolytic-like effect of allopregnanolone in mice: behavioral and immunocytochemical evidences. Brain Res. 2010;1318:77–86. doi: 10.1016/j.brainres.2009.12.088. [DOI] [PubMed] [Google Scholar]
- Dietz P.M., Spitz A.M., Anda R.F., Williamson D.F., McMahon P.M., Santelli J.S., Nordenberg D.F., Felitti V.J., Kendrick J.S. Unintended pregnancy among adult women exposed to abuse or household dysfunction during their childhood. JAMA. 1999;282:1359–1364. doi: 10.1001/jama.282.14.1359. [DOI] [PubMed] [Google Scholar]
- Ding J.B., Guzman J.N., Peterson J.D., Goldberg J.A., Surmeier D.J. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron. 2010;67:294–307. doi: 10.1016/j.neuron.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H.W., Petrovich G.D., Watts A.G., Swanson L.W. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J. Comp. Neurol. 2001;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dong H.W., Swanson L.W. Projections from the rhomboid nucleus of the bed nuclei of the stria terminalis: implications for cerebral hemisphere regulation of ingestive behaviors. J. Comp. Neurol. 2003;463:434–472. doi: 10.1002/cne.10758. [DOI] [PubMed] [Google Scholar]
- Dong H.W., Swanson L.W. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J. Comp. Neurol. 2004;468:277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Dong M., Dube S.R., Felitti V.J., Giles W.H., Anda R.F. Adverse childhood experiences and self-reported liver disease: new insights into the causal pathway. Arch. Intern Med. 2003;163:1949–1956. doi: 10.1001/archinte.163.16.1949. [DOI] [PubMed] [Google Scholar]
- Drevets W.C., Zarate C.A., Jr., Furey M.L. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol. psychiatry. 2013;73:1156–1163. doi: 10.1016/j.biopsych.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube S.R., Anda R.F., Felitti V.J., Chapman D.P., Williamson D.F., Giles W.H. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the Adverse Childhood Experiences Study. JAMA. 2001;286:3089–3096. doi: 10.1001/jama.286.24.3089. [DOI] [PubMed] [Google Scholar]
- Dube S.R., Anda R.F., Felitti V.J., Edwards V.J., Williamson D.F. Exposure to abuse, neglect, and household dysfunction among adults who witnessed intimate partner violence as children: implications for health and social services. Violence Vict. 2002;17:3–17. doi: 10.1891/vivi.17.1.3.33635. [DOI] [PubMed] [Google Scholar]
- Duman R.S., Aghajanian G.K. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R.S., Aghajanian G.K., Sanacora G., Krystal J.H. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med. 2016;22:238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer J.M., Maldonado-Aviles J.G., Lepack A.E., DiLeone R.J., Duman R.S. Ribosomal protein S6 kinase 1 signaling in prefrontal cortex controls depressive behavior. Proc. Natl. Acad. Sci. U. S. A. 2015;112:6188–6193. doi: 10.1073/pnas.1505289112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S., Salmaso N., Rodaros D., Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacol. Berl. 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Fan A.Z., Russell M., Stranges S., Dorn J., Trevisan M. Association of lifetime alcohol drinking trajectories with cardiometabolic risk. J. Clin. Endocrinol. Metab. 2008;93:154–161. doi: 10.1210/jc.2007-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti V.J., Anda R.F., Nordenberg D., Williamson D.F., Spitz A.M., Edwards V., Koss M.P., Marks J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Foster H., Hagan J., Brooks-Gunn J. Growing up fast: stress exposure and subjective “weathering” in emerging adulthood. J. health Soc. Behav. 2008;49:162–177. doi: 10.1177/002214650804900204. [DOI] [PubMed] [Google Scholar]
- Francesconi W., Berton F., Repunte-Canonigo V., Hagihara K., Thurbon D., Lekic D., Specio S.E., Greenwell T.N., Chen S.A., Rice K.C., Richardson H.N., O'Dell L.E., Zorrilla E.P., Morales M., Koob G.F., Sanna P.P. Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. J. Neurosci. 2009;29:5389–5401. doi: 10.1523/JNEUROSCI.5129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis T.C., Chandra R., Friend D.M., Finkel E., Dayrit G., Miranda J., Brooks J.M., Iniguez S.D., O'Donnell P., Kravitz A., Lobo M.K. Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol. psychiatry. 2014;77:212–222. doi: 10.1016/j.biopsych.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T., Reinhold E., Koutsouleris N., Reiser M., Meisenzahl E.M. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J. Psychiatr. Res. 2010;44:799–807. doi: 10.1016/j.jpsychires.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Funk C.K., O'Dell L.E., Crawford E.F., Koob G.F. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J. Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey M.L., Khanna A., Hoffman E.M., Drevets W.C. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacol. official Publ. Am. Coll. Neuropsychopharmacol. 2010;35:2479–2488. doi: 10.1038/npp.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett J.E., Wellman C.L. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 2009;162:195–207. doi: 10.1016/j.neuroscience.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gater R., Tansella M., Korten A., Tiemens B.G., Mavreas V.G., Olatawura M.O. Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization Collaborative Study on Psychological Problems in General Health Care. Arch. Gen. Psychiatry. 1998;55:405–413. doi: 10.1001/archpsyc.55.5.405. [DOI] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L., Telzer E.H., Humphreys K.L., Goff B., Shapiro M., Flannery J., Lumian D.S., Fareri D.S., Caldera C., Tottenham N. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol. Sci. 2014;25:2067–2078. doi: 10.1177/0956797614550878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H., Hare T.A., Bookheimer S.Y., Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U. S. A. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George D.T., Nutt D.J., Dwyer B.A., Linnoila M. Alcoholism and panic disorder: is the comorbidity more than coincidence? Acta Psychiatr. Scand. 1990;81:97–107. doi: 10.1111/j.1600-0447.1990.tb06460.x. [DOI] [PubMed] [Google Scholar]
- Georges F., Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J. Neurosci. 2001;21:RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin N.W., Richardson H.N., Koob G.F. Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin. Exp. Res. 2008;32:1535–1542. doi: 10.1111/j.1530-0277.2008.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N., Takesian A.E., Feng G., Fagiolini M., Hensch T.K. Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron. 2014;83:894–905. doi: 10.1016/j.neuron.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S.A., Christoffel D.J., Heshmati M., Hodes G.E., Magida J., Davis K., Cahill M.E., Dias C., Ribeiro E., Ables J.L., Kennedy P.J., Robison A.J., Gonzalez-Maeso J., Neve R.L., Turecki G., Ghose S., Tamminga C.A., Russo S.J. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat. Med. 2013;19:337–344. doi: 10.1038/nm.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E., Tanapat P. Stress and hippocampal neurogenesis. Biol. psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Gould E., Tanapat P., Cameron H.A. Adrenal steroids suppress granule cell death in the developing dentate gyrus through an NMDA receptor-dependent mechanism. Brain research. Dev. brain Res. 1997;103:91–93. doi: 10.1016/s0165-3806(97)00079-5. [DOI] [PubMed] [Google Scholar]
- Gould E., Woolley C.S., Cameron H.A., Daniels D.C., McEwen B.S. Adrenal steroids regulate postnatal development of the rat dentate gyrus: II. Effects of glucocorticoids and mineralocorticoids on cell birth. J. Comp. Neurol. 1991;313:486–493. doi: 10.1002/cne.903130309. [DOI] [PubMed] [Google Scholar]
- Gould E., Woolley C.S., McEwen B.S. Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. J. Comp. Neurol. 1991;313:479–485. doi: 10.1002/cne.903130308. [DOI] [PubMed] [Google Scholar]
- Gould E., Woolley C.S., McEwen B.S. The hippocampal formation: morphological changes induced by thyroid, gonadal and adrenal hormones. Psychoneuroendocrinology. 1991;16:67–84. doi: 10.1016/0306-4530(91)90071-z. [DOI] [PubMed] [Google Scholar]
- Gupta T., Syed Y.M., Revis A.A., Miller S.A., Martinez M., Cohn K.A., Demeyer M.R., Patel K.Y., Brzezinska W.J., Rhodes J.S. Acute effects of acamprosate and MPEP on ethanol Drinking-in-the-Dark in male C57BL/6J mice. Alcohol Clin. Exp. Res. 2008;32:1992–1998. doi: 10.1111/j.1530-0277.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- Hankin B.L. Development of sex differences in depressive and co-occurring anxious symptoms during adolescence: descriptive trajectories and potential explanations in a multiwave prospective study. J. Clin. Child. Adolesc. Psychol. 2009;38:460–472. doi: 10.1080/15374410902976288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes D.M., Knapp D.J., Breese G.R., Thiele T.E. Comparison of basal neuropeptide Y and corticotropin releasing factor levels between the high ethanol drinking C57BL/6J and low ethanol drinking DBA/2J inbred mouse strains. Alcohol Clin. Exp. Res. 2005;29:721–729. doi: 10.1097/01.ALC.0000164375.16838.F3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Heilig M., Koob G.F. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M., Thorsell A. Brain neuropeptide Y (NPY) in stress and alcohol dependence. Rev. Neurosci. 2002;13:85–94. doi: 10.1515/revneuro.2002.13.1.85. [DOI] [PubMed] [Google Scholar]
- Heun-Johnson H., Levitt P. Early-life stress paradigm transiently alters maternal behavior, dam-pup interactions, and offspring vocalizations in mice. Front. Behav. Neurosci. 2016;10:142. doi: 10.3389/fnbeh.2016.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis S.D., Anda R.F., Dube S.R., Felitti V.J., Marchbanks P.A., Marks J.S. The association between adverse childhood experiences and adolescent pregnancy, long-term psychosocial consequences, and fetal death. Pediatrics. 2004;113:320–327. doi: 10.1542/peds.113.2.320. [DOI] [PubMed] [Google Scholar]
- Hodes G.E., Brancato A., Bregman D., Ahn F.H., Russo S.J. Sub-chronic variable stress induced sex-specific effects on glutamatergic signaling in the nucleus accumbens. Soc. Neurosci. Abstr. 2016;42 doi: 10.1016/j.neuroscience.2017.03.014. 62.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes G.E., Pfau M.L., Purushothaman I., Ahn H.F., Golden S.A., Christoffel D.J., Magida J., Brancato A., Takahashi A., Flanigan M.E., Menard C., Aleyasin H., Koo J.W., Lorsch Z.S., Feng J., Heshmati M., Wang M., Turecki G., Neve R., Zhang B., Shen L., Nestler E.J., Russo S.J. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J. Neurosci. 2015;35:16362–16376. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H., Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J. Neurosci. official J. Soc. Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar C.E., Gunnar M.R. The developmental effects of early life stress: an overview of current theoretical frameworks. Curr. Dir. Psychol. Sci. 2013;22:400–406. doi: 10.1177/0963721413488889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.M., Overstreet D.H., Knapp D.J., Angel R., Wills T.A., Navarro M., Rivier J., Vale W., Breese G.R. Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is brain site specific and mediated by CRF-1 receptors: relation to stress-induced sensitization. J. Pharmacol. Exp. Ther. 2010;332:298–307. doi: 10.1124/jpet.109.159186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z.J., Kirkwood A., Pizzorusso T., Porciatti V., Morales B., Bear M.F., Maffei L., Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Hultman R., Mague S.D., Li Q., Katz B.M., Michel N., Lin L., Wang J., David L.K., Blount C., Chandy R., Carlson D., Ulrich K., Carin L., Dunson D., Kumar S., Deisseroth K., Moore S.D., Dzirasa K. Dysregulation of prefrontal cortex-mediated slow-evolving limbic dynamics drives stress-induced emotional pathology. Neuron. 2016;91:439–452. doi: 10.1016/j.neuron.2016.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaguma Y., Kurobe N., Shinohara H., Kato K. Sensitive immunoassay for rat parvalbumin: tissue distribution and developmental changes. Biochim. Biophys. Acta. 1991;1075:68–74. doi: 10.1016/0304-4165(91)90076-s. [DOI] [PubMed] [Google Scholar]
- James M.H., Charnley J.L., Flynn J.R., Smith D.W., Dayas C.V. Propensity to 'relapse' following exposure to cocaine cues is associated with the recruitment of specific thalamic and epithalamic nuclei. Neuroscience. 2011;199:235–242. doi: 10.1016/j.neuroscience.2011.09.047. [DOI] [PubMed] [Google Scholar]
- Jernigan C.S., Goswami D.B., Austin M.C., Iyo A.H., Chandran A., Stockmeier C.A., Karolewicz B. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog. neuro-psychopharmacology Biol. psychiatry. 2011;35:1774–1779. doi: 10.1016/j.pnpbp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B., Leeton J., McLeod I., Wood C. Factors influencing the age of menarche in a lower socio-economic group in Melbourne. Med. J. Aust. 1972;2:533–535. doi: 10.5694/j.1326-5377.1972.tb47460.x. [DOI] [PubMed] [Google Scholar]
- Karatsoreos I.N., McEwen B.S. Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cogn. Sci. 2011;15:576–584. doi: 10.1016/j.tics.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Kash T.L., Winder D.G. Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropharmacology. 2006;51:1013–1022. doi: 10.1016/j.neuropharm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Keita G.P. Psychosocial and cultural contributions to depression in women: considerations for women midlife and beyond. J. Manag. Care Pharm. 2007;13:S12–S15. doi: 10.18553/jmcp.2007.13.9-a.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C. Epidemiology of women and depression. J. Affect. Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kessler R.C. The costs of depression. Psychiatr. Clin. North Am. 2012;35:1–14. doi: 10.1016/j.psc.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Archives general psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Bromet E.J. The epidemiology of depression across cultures. Annu. Rev. public health. 2013;34:119–138. doi: 10.1146/annurev-publhealth-031912-114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khibnik L.A., Beaumont M., Doyle M., Heshmati M., Slesinger P.A., Nestler E.J., Russo S.J. Stress and cocaine trigger divergent and cell type-specific regulation of synaptic transmission at single spines in nucleus accumbens. Biol. Psychiatry. 2016;79:898–905. doi: 10.1016/j.biopsych.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur. Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob G.F. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehner C. Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta Psychiatr. Scand. 2003;108:163–174. doi: 10.1034/j.1600-0447.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- Kumar S., Hultman R., Hughes D., Michel N., Katz B.M., Dzirasa K. Prefrontal cortex reactivity underlies trait vulnerability to chronic social defeat stress. Nat. Commun. 2014;5:4537. doi: 10.1038/ncomms5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlant Q., Chakravarty S., Vialou V., Mukherjee S., Koo J.W., Kalahasti G., Bradbury K.R., Taylor S.V., Maze I., Kumar A., Graham A., Birnbaum S.G., Krishnan V., Truong H.T., Neve R.L., Nestler E.J., Russo S.J. Role of nuclear factor kappaB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol. psychiatry. 2009;65:874–880. doi: 10.1016/j.biopsych.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A.D., Harding S., Juzytsch W., Watchus J., Shalev U., Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacol. Berl. 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Li N., Lee B., Liu R.J., Banasr M., Dwyer J.M., Iwata M., Li X.Y., Aghajanian G., Duman R.S. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell S.G., Schwandt M.L., Sun H., Sparenborg J.D., Bjork K., Kasckow J.W., Sommer W.H., Goldman D., Higley J.D., Suomi S.J., Heilig M., Barr C.S. Functional NPY variation as a factor in stress resilience and alcohol consumption in rhesus macaques. Arch. Gen. Psychiatry. 2008;67:423–431. doi: 10.1001/archgenpsychiatry.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C., Gan W.B. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16074–16079. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C., McEwen B.S., Casey B.J. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc. Natl. Acad. Sci. U. S. A. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C., Miller M.M., Goldwater D.S., Radley J.J., Rocher A.B., Hof P.R., Morrison J.H., McEwen B.S. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. official J. Soc. Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Wong T.P., Pozza M.F., Lingenhoehl K., Wang Y., Sheng M., Auberson Y.P., Wang Y.T. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Lowery E.G., Spanos M., Navarro M., Lyons A.M., Hodge C.W., Thiele T.E. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology. 2010;35:1241–1252. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta E.G., Navarro M., Li C., Pleil K.E., Rinker J.A., Cox B.R., Sprow G.M., Kash T.L., Thiele T.E. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J. Neurosci. 2012;32:3405–3413. doi: 10.1523/JNEUROSCI.6256-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature reviews. Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Magarinos A.M., Orchinik M., McEwen B.S. Morphological changes in the hippocampal CA3 region induced by non-invasive glucocorticoid administration: a paradox. Brain Res. 1998;809:314–318. doi: 10.1016/s0006-8993(98)00882-8. [DOI] [PubMed] [Google Scholar]
- McDonald A.J., Shammah-Lagnado S.J., Shi C., Davis M. Cortical afferents to the extended amygdala. Ann. N. Y. Acad. Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- McElligott Z.A., Fox M.E., Walsh P.L., Urban D.J., Ferrel M.S., Roth B.L., Wightman R.M. Noradrenergic synaptic function in the bed nucleus of the stria terminalis varies in animal models of anxiety and addiction. Neuropsychopharmacology. 2013;38:1665–1673. doi: 10.1038/npp.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. N. Y. Acad. Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Gould E.A., Sakai R.R. The vulnerability of the hippocampus to protective and destructive effects of glucocorticoids in relation to stress. Br. J. psychiatry. Suppl. 1992:18–23. [PubMed] [Google Scholar]
- McEwen B.S., McEwen C.A. 2016. Response to Jerome Kagan's essay on stress. Perspect. Psychol. Sci. 2016;11:451–455. doi: 10.1177/1745691616646635. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Morrison J.H. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni E.G., Gerety L.P., Knoll A.T., Cohen B.M., Carlezon W.A., Jr. Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. J. Neurosci. 2006;26:3855–3863. doi: 10.1523/JNEUROSCI.4957-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt T.E., Caspi A., Belsky J., Silva P.A. Childhood experience and the onset of menarche: a test of a sociobiological model. Child. Dev. 1992;63:47–58. doi: 10.1111/j.1467-8624.1992.tb03594.x. [DOI] [PubMed] [Google Scholar]
- Moghaddam B., Adams B., Verma A., Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. official J. Soc. Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia L.M., Kavalali E.T. Scopolamine and ketamine: evidence of convergence? Biol. psychiatry. 2013;74:712–713. doi: 10.1016/j.biopsych.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Moriceau S., Sullivan R.M. Corticosterone influences on Mammalian neonatal sensitive-period learning. Behav. Neurosci. 2004;118:274–281. doi: 10.1037/0735-7044.118.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M.B., Zimmermann S., Sillaber I., Hagemeyer T.P., Deussing J.M., Timpl P., Kormann M.S., Droste S.K., Kuhn R., Reul J.M., Holsboer F., Wurst W. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat. Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- Murray C.J., Atkinson C., Bhalla K., Birbeck G., Burstein R., Chou D., Dellavalle R., Danaei G., Ezzati M., Fahimi A., Flaxman D., Foreman Gabriel S., Gakidou E., Kassebaum N., Khatibzadeh S., Lim S., Lipshultz S.E., London S., Lopez, MacIntyre M.F., Mokdad A.H., Moran A., Moran A.E., Mozaffarian D., Murphy T., Naghavi M., Pope C., Roberts T., Salomon J., Schwebel D.C., Shahraz S., Sleet D.A., Murray, Abraham J., Ali M.K., Atkinson C., Bartels D.H., Bhalla K., Birbeck G., Burstein R., Chen H., Criqui M.H., Dahodwala, Jarlais, Ding E.L., Dorsey E.R., Ebel B.E., Ezzati M., Fahami, Flaxman S., Flaxman A.D., Gonzalez-Medina D., Grant B., Hagan H., Hoffman H., Kassebaum N., Khatibzadeh S., Leasher J.L., Lin J., Lipshultz S.E., Lozano R., Lu Y., Mallinger L., McDermott M.M., Micha R., Miller T.R., Mokdad A.A., Mokdad A.H., Mozaffarian D., Naghavi M., Narayan K.M., Omer S.B., Pelizzari P.M., Phillips D., Ranganathan D., Rivara F.P., Roberts T., Sampson U., Sanman E., Sapkota A., Schwebel D.C., Sharaz S., Shivakoti R., Singh G.M., Singh D., Tavakkoli M., Towbin J.A., Wilkinson J.D., Zabetian A., Murray, Abraham J., Ali M.K., Alvardo M., Atkinson C., Baddour L.M., Benjamin E.J., Bhalla K., Birbeck G., Bolliger I., Burstein R., Carnahan E., Chou D., Chugh S.S., Cohen A., Colson K.E., Cooper L.T., Couser W., Criqui M.H., Dabhadkar K.C., Dellavalle R.P., Jarlais, Dicker D., Dorsey E.R., Duber H., Ebel B.E., Engell R.E., Ezzati M., Felson D.T., Finucane M.M., Flaxman S., Flaxman A.D., Fleming T., Foreman, Forouzanfar M.H., Freedman G., Freeman M.K., Gakidou E., Gillum R.F., Gonzalez-Medina D., Gosselin R., Gutierrez H.R., Hagan H., Havmoeller R., Hoffman H., Jacobsen K.H., James S.L., Jasrasaria R., Jayarman S., Johns N., Kassebaum N., Khatibzadeh S., Lan Q., Leasher J.L., Lim S., Lipshultz S.E., London S., Lopez, Lozano R., Lu Y., Mallinger L., Meltzer M., Mensah G.A., Michaud C., Miller T.R., Mock C., Moffitt T.E., Mokdad A.A., Mokdad A.H., Moran A., Naghavi M., Narayan K.M., Nelson R.G., Olives C., Omer S.B., Ortblad K., Ostro B., Pelizzari P.M., Phillips D., Raju M., Razavi H., Ritz B., Roberts T., Sacco R.L., Salomon J., Sampson U., Schwebel D.C., Shahraz S., Shibuya K., Silberberg D., Singh J.A., Steenland K., Taylor J.A., Thurston G.D., Vavilala M.S., Vos T., Wagner G.R., Weinstock M.A., Weisskopf M.G., Wulf S., Murray, Collaborators, U.S.B.o.D The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B., Mark Dolgas C., Kasckow J., Cullinan W.E., Herman J.P. Central stress-integrative circuits: forebrain glutamatergic and GABAergic projections to the dorsomedial hypothalamus, medial preoptic area, and bed nucleus of the stria terminalis. Brain Struct. Funct. 2014;219:1287–1303. doi: 10.1007/s00429-013-0566-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata H., Watanabe K., Murakami Y., Gao P., Tsuiji K., Nishimura K., Plotnikoff G.A., Kurihara N., Irie Y., Ishige A. Stress on a postpartum mother inhibits the secretion of growth hormone in the offspring and causes persistent growth impairment. Methods Find. Exp. Clin. Pharmacol. 2009;31:433–441. doi: 10.1358/mf.2009.31.7.1407221. [DOI] [PubMed] [Google Scholar]
- Naninck E.F., Hoeijmakers L., Kakava-Georgiadou N., Meesters A., Lazic S.E., Lucassen P.J., Korosi A. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus. 2015;25:309–328. doi: 10.1002/hipo.22374. [DOI] [PubMed] [Google Scholar]
- Navarria A., Wohleb E.S., Voleti B., Ota K.T., Dutheil S., Lepack A.E., Dwyer J.M., Fuchikami M., Becker A., Drago F., Duman R.S. Rapid antidepressant actions of scopolamine: role of medial prefrontal cortex and M1-subtype muscarinic acetylcholine receptors. Neurobiol. Dis. 2015;82:254–261. doi: 10.1016/j.nbd.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino T.M., Klein D.N., Lewinsohn P.M., Rohde P., Seeley J.R. Latent trajectory classes of depressive and anxiety disorders from adolescence to adulthood: descriptions of classes and associations with risk factors. Compr. Psychiatry. 2010;51:224–235. doi: 10.1016/j.comppsych.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive M.F., Mehmert K.K., Koenig H.N., Camarini R., Kim J.A., Nannini M.A., Ou C.J., Hodge C.W. A role for corticotropin releasing factor (CRF) in ethanol consumption, sensitivity, and reward as revealed by CRF-deficient mice. Psychopharmacol. Berl. 2003;165:181–187. doi: 10.1007/s00213-002-1248-2. [DOI] [PubMed] [Google Scholar]
- Ota K.T., Liu R.J., Voleti B., Maldonado-Aviles J.G., Duric V., Iwata M., Dutheil S., Duman C., Boikess S., Lewis D.A., Stockmeier C.A., DiLeone R.J., Rex C., Aghajanian G.K., Duman R.S. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat. Med. 2014;20:531–535. doi: 10.1038/nm.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor R., McKinnon C.S., Scibelli A.C., Burkhart-Kasch S., Reed C., Ryabinin A.E., Coste S.C., Stenzel-Poore M.P., Phillips T.J. Corticotropin-releasing factor-1 receptor involvement in behavioral neuroadaptation to ethanol: a urocortin1-independent mechanism. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9070–9075. doi: 10.1073/pnas.0710181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell S.S., Bath K.G., Casey B.J., Ninan I., Lee F.S. Selective early-acquired fear memories undergo temporary suppression during adolescence. Proc. Natl. Acad. Sci. U. S. A. 2011;108:1182–1187. doi: 10.1073/pnas.1012975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil K.E., Lopez A., McCall N., Jijon A.M., Bravo J.P., Kash T.L. Chronic stress alters neuropeptide Y signaling in the bed nucleus of the stria terminalis in DBA/2J but not C57BL/6J mice. Neuropharmacology. 2012;62:1777–1786. doi: 10.1016/j.neuropharm.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil K.E., Lowery-Gionta E.G., Crowley N.A., Li C., Marcinkiewcz C.A., Rose J.H., McCall N.M., Maldonado-Devincci A.M., Morrow A.L., Jones S.R., Kash T.L. Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology. 2015;99:735–749. doi: 10.1016/j.neuropharm.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil K.E., Rinker J.A., Lowery-Gionta E.G., Mazzone C.M., McCall N.M., Kendra A.M., Olson D.P., Lowell B.B., Grant K.A., Thiele T.E., Kash T.L. NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat. Neurosci. 2015;18:545–552. doi: 10.1038/nn.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M., Yan Z., McEwen B.S., Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature reviews. Neuroscience. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratchett L.C., Pelcovitz M.R., Yehuda R. Trauma and violence: are women the weaker sex? Psychiatr. Clin. North Am. 2010;33:465–474. doi: 10.1016/j.psc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Radley J., Morilak D., Viau V., Campeau S. Chronic stress and brain plasticity: mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders. Neurosci. Biobehav Rev. 2015;58:79–91. doi: 10.1016/j.neubiorev.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley J.J., Rocher A.B., Rodriguez A., Ehlenberger D.B., Dammann M., McEwen B.S., Morrison J.H., Wearne S.L., Hof P.R. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J. Comp. neurology. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J.S., Best K., Belknap J.K., Finn D.A., Crabbe J.C. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol. Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rice C.J., Sandman C.A., Lenjavi M.R., Baram T.Z. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo S.J., Murrough J.W., Han M.H., Charney D.S., Nestler E.J. Neurobiology of resilience. Nat. Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo S.J., Nestler E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahuque L.L., Kullberg E.F., McGeehan A.J., Kinder J.R., Hicks M.P., Blanton M.G., Janak P.H., Olive M.F. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacol. Berl. 2006;186:122–132. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk T.J., Shekhar A., Gehlert D.R. Interactions between NPY and CRF in the amygdala to regulate emotionality. Neuropeptides. 2004;38:225–234. doi: 10.1016/j.npep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Shansky R.M., Hamo C., Hof P.R., Lou W., McEwen B.S., Morrison J.H. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb. cortex. 2010;20:2560–2567. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A., Truitt W., Rainnie D., Sajdyk T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress. 2005;8:209–219. doi: 10.1080/10253890500504557. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Sanghavi M., Mintun M.A., Gado M.H. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J. Neurosci. official J. Soc. Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Wang P.W., Gado M.H., Csernansky J.G., Vannier M.W. Hippocampal atrophy in recurrent major depression. Proc. Natl. Acad. Sci. U. S. A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., Cummings J., Roldan L.A., Jan Y.N., Jan L.Y. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Silberman Y., Matthews R.T., Winder D.G. A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis. J. Neurosci. 2013;33:950–960. doi: 10.1523/JNEUROSCI.2949-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R., Fox H.C., Hong K.A., Bergquist K., Bhagwagar Z., Siedlarz K.M. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2008;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y., Surmeier D.J., Redgrave P., Kimura M. Thalamic contributions to Basal Ganglia-related behavioral switching and reinforcement. J. Neurosci. 2011;31:102–106. doi: 10.1523/JNEUROSCI.4634-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souery D., Papakostas G.I., Trivedi M.H. Treatment-resistant depression. J. Clin. psychiatry. 2006;67(Suppl. 6):16–22. [PubMed] [Google Scholar]
- Sparta D.R., Fee J.R., Hayes D.M., Knapp D.J., MacNeil D.J., Thiele T.E. Peripheral and central administration of a selective neuropeptide Y Y1 receptor antagonist suppresses ethanol intake by C57BL/6J mice. Alcohol Clin. Exp. Res. 2004;28:1324–1330. doi: 10.1097/01.ALC.0000139829.67958.1A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta D.R., Sparrow A.M., Lowery E.G., Fee J.R., Knapp D.J., Thiele T.E. Blockade of the corticotropin releasing factor type 1 receptor attenuates elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin. Exp. Res. 2008;32:259–265. doi: 10.1111/j.1530-0277.2007.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens D.C., Byrum C.E., McQuoid D.R., Greenberg D.L., Payne M.E., Blitchington T.F., MacFall J.R., Krishnan K.R. Hippocampal volume in geriatric depression. Biol. psychiatry. 2000;48:301–309. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- Stuber G.D., Sparta D.R., Stamatakis A.M., van Leeuwen W.A., Hardjoprajitno J.E., Cho S., Tye K.M., Kempadoo K.A., Zhang F., Deisseroth K., Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R.M., Landers M., Yeaman B., Wilson D.A. Good memories of bad events in infancy. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi L.K. Glucocorticoids and the hippocampus. Developmental interactions facilitating the expression of behavioral inhibition. Mol. Neurobiol. 1996;13:213–226. doi: 10.1007/BF02740624. [DOI] [PubMed] [Google Scholar]
- Takahashi L.K., Kim H. Relative contributions of pituitary-adrenal hormones to the ontogeny of behavioral inhibition in the rat. Physiol. Behav. 1995;57:711–716. doi: 10.1016/0031-9384(94)00324-6. [DOI] [PubMed] [Google Scholar]
- Tanapat P., Galea L.A., Gould E. Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. Int. J. Dev. Neurosci. 1998;16:235–239. doi: 10.1016/s0736-5748(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Anderson C.M., Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E563–E572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A., Polcari A., Andersen S.L. Length of time between onset of childhood sexual abuse and emergence of depression in a young adult sample: a retrospective clinical report. J. Clin. psychiatry. 2009;70:684–691. doi: 10.4088/jcp.08m04235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele T.E., Crabbe J.C., Boehm S.L., 2nd “Drinking in the Dark” (DID): a simple mouse model of binge-like alcohol intake. Curr. Protoc. Neurosci. 2014;68 doi: 10.1002/0471142301.ns0949s68. 9 49 41-49 49 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele T.E., Navarro M. “Drinking in the dark” (DID) procedures: a model of binge-like ethanol drinking in non-dependent mice. Alcohol. 2014;48:235–241. doi: 10.1016/j.alcohol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuvemo T., Gustafsson J., Proos L.A. Growth hormone treatment during suppression of early puberty in adopted girls. Swedish Growth Hormone Advisory Group. Acta Paediatr. 1999;88:928–932. doi: 10.1080/08035259950168388. [DOI] [PubMed] [Google Scholar]
- Tuvemo T., Jonsson B., Gustafsson J., Albertsson-Wikland K., Aronson A.S., Hager A., Ivarson S., Kristrom B., Marcus C., Nilsson K.O., Westgren U., Westphal O., Aman J., Proos L.A. Final height after combined growth hormone and GnRH analogue treatment in adopted girls with early puberty. Acta Paediatr. 2004;93:1456–1462. doi: 10.1080/08035250410021793. [DOI] [PubMed] [Google Scholar]
- Valdez G.R., Roberts A.J., Chan K., Davis H., Brennan M., Zorrilla E.P., Koob G.F. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin. Exp. Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- van de Wiel A., de Lange D.W. Cardiovascular risk is more related to drinking pattern than to the type of alcoholic drinks. Neth J. Med. 2008;66:467–473. [PubMed] [Google Scholar]
- Voleti B., Navarria A., Liu R.J., Banasr M., Li N., Terwilliger R., Sanacora G., Eid T., Aghajanian G., Duman R.S. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol. psychiatry. 2013;74:742–749. doi: 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A., Jadhav S., Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vythilingam M., Heim C., Newport J., Miller A.H., Anderson E., Bronen R., Brummer M., Staib L., Vermetten E., Charney D.S., Nemeroff C.B., Bremner J.D. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am. J. psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker H.R. Epidemiology and comorbidity of depressive disorders. Ther. Umsch. Rev. Ther. 2000;57:53–58. doi: 10.1024/0040-5930.57.2.53. [DOI] [PubMed] [Google Scholar]
- Walker D.L., Toufexis D.J., Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur. J. Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Wall N.R., De La Parra M., Callaway E.M., Kreitzer A.C. Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron. 2013;79:347–360. doi: 10.1016/j.neuron.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Fang Q., Liu Z., Lu L. Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacol. Berl. 2006;185:19–28. doi: 10.1007/s00213-005-0262-6. [DOI] [PubMed] [Google Scholar]
- Wang P.S., Simon G., Kessler R.C. The economic burden of depression and the cost-effectiveness of treatment. Int. J. methods psychiatric Res. 2003;12:22–33. doi: 10.1002/mpr.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Gould E., Daniels D.C., Cameron H., McEwen B.S. Tianeptine attenuates stress-induced morphological changes in the hippocampus. Eur. J. Pharmacol. 1992;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- Wei J., Yuen E.Y., Liu W., Li X., Zhong P., Karatsoreos I.N., McEwen B.S., Yan Z. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol. psychiatry. 2014;19:588–598. doi: 10.1038/mp.2013.83. [DOI] [PubMed] [Google Scholar]
- Weissman M.M., Bland R.C., Canino G.J., Faravelli C., Greenwald S., Hwu H.G., Joyce P.R., Karam E.G., Lee C.K., Lellouch J., Lepine J.P., Newman S.C., Rubio-Stipec M., Wells J.E., Wickramaratne P.J., Wittchen H., Yeh E.K. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. [PubMed] [Google Scholar]
- Williamson D.F., Thompson T.J., Anda R.F., Dietz W.H., Felitti V. Body weight and obesity in adults and self-reported abuse in childhood. Int. J. Obes. Relat. Metab. Disord. 2002;26:1075–1082. doi: 10.1038/sj.ijo.0802038. [DOI] [PubMed] [Google Scholar]
- Wissman A.M., McCollum A.F., Huang G.Z., Nikrodhanond A.A., Woolley C.S. Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens. Neuropharmacology. 2011;61:217–227. doi: 10.1016/j.neuropharm.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin J.M., Overshiner C., Li X., Catlow J.T., Wishart G.N., Schober D.A., Heinz B.A., Nikolayev A., Tolstikov V.V., Anderson W.H., Higgs R.E., Kuo M.S., Felder C.C. M1 and m2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine. J. Pharmacol. Exp. Ther. 2014;351:448–456. doi: 10.1124/jpet.114.216804. [DOI] [PubMed] [Google Scholar]
- Wittchen H.U., Knauper B., Kessler R.C. Lifetime risk of depression. Br. J. psychiatry. Suppl. 1994:16–22. [PubMed] [Google Scholar]
- Wohleb E.S., Gerhard D., Thomas A., Duman R.S. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr. Neuropharmacol. 2016;126:2482–2494. doi: 10.2174/1570159X14666160309114549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb E.S., Wu M., Gerhard D.M., Taylor S.R., Picciotto M.R., Alreja M., Duman R.S. GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J. Clin. Invest. 2016;126:2482–2494. doi: 10.1172/JCI85033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R., Brand S., Yang R.K. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol. Psychiatry. 2005;59:660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Yuen E.Y., Wei J., Liu W., Zhong P., Li X., Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Sakharkar, A.J., Shi, G., Ugale, R., Prakash, A., Pandey, S.C., Neuropeptide Y signaling in the central nucleus of amygdala regulates alcohol-drinking and anxiety-like behaviors of alcohol-preferring rats. Alcohol Clin Exp Res 34, 451–461. [DOI] [PMC free article] [PubMed]
- Zhu L., Wu L., Yu B., Liu X. The participation of a neurocircuit from the paraventricular thalamus to amygdala in the depressive like behavior. Neurosci. Lett. 2011;488:81–86. doi: 10.1016/j.neulet.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Zorrilla E.P., Valdez G.R., Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacol. Berl. 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]