Abstract
Background
Hops (Humulus lupulus (L.)) dietary supplements are of interest as herbal remedies to alleviate menopausal symptoms, such as hot flushes, depression and anxiety. So far, the evidence regarding estrogenic and related properties of hops preparations has been considered insufficient for a market authorization for menopausal indications.
Purpose
The study aims to investigate a chemically standardized hops extract regarding its safety in the uterus, as wells as its efficacy to prevent bone loss in the ovariectomized rat model.
Study Design/Methods
Female Wistar rats were ovariectomized and divided into a control group receiving phytoestrogen-free diet, a group treated with E2benzoate (0.93 mg/kg body weight/d) and a group treated with the standardized hops extract (60 mg/kg body weight/d) for 8 weeks. Micro-computed tomography of the tibiae and vertebrae, as wells as histological changes in the uterus and tibia were analyzed.
Results
Neither uterotrophic nor proliferative effects were observed in the endometrium in response to the oral 8-week administration of the hops extract. However, site-dependent skeletal effects were observed. The hops extract significantly decreased the number of osteoclasts in the tibial metaphysis and prevented reduction of the trabecular thickness that resulted from estradiol depletion. In contrast, the hops extract did not prevent the ovariectomy-induced micro-architectural changes in the lumbar vertebra. Certain parameters (e.g. thickness and number of trabeculae) were even found to be below the values determined in the ovariectomized control group.
Conclusion
Taken together, the results provide evidence for the safety of the standardized hops extract and point to a weak bone type-specific, protective effect on bone loss following estradiol depletion.
Keywords: Hops extract, 8-prenylnaringenin, Botanical dietary supplements, Ovariectomized rat, Osteoporosis, Uterus
Introduction
In addition to the usage of its female inflorescences in beer brewing as a flavoring and preserving agent, herbal remedies containing Humulus lupulus (L., Cannabaceae) (hops) are used to alleviate menopausal symptoms, such as hot flushes, depression and anxiety (Milligan et al., 2000). While hops preparations have been accepted as traditional medicinal products with sedative effects by the European Medicines Agency (EMA), the evidence regarding estrogenic and related properties of hops preparations have been considered in-sufficient for a market authorization for menopausal indications (Vlietinck, 2008). The increased interest in herbal remedies among women resulted in part from the Women’s Health Initiative study published in 2002, which associated long-term hormone therapy (HT) with an increased risk of hormone-dependent cancers (e.g. breast cancer, endometrium carcinoma) (Rossouw et al., 2002).
Since 1999, 8-prenylnaringenin (8-PN) was shown in several in vitro and in vivo studies to be the active estrogenic compound in hops (Zierau et al., 2002; Overk et al., 2005, 2008) (Fig. 1). While the abundance of 8-PN in hops is low, other more abundant prenylated phenols like xanthohumol (XN) and isoxanthohumol (IX) can be metabolically converted to 8-PN (Dietz et al., 2013; Legette et al., 2012; van Breemen et al., 2014). The conversion of IX into 8-PN can be accomplished enzymatically by hepatic CYP1A2 or by gut microflora (Bolca et al., 2007; Guo et al., 2006).
Fig. 1.
Chemical structures of prenylated hops phenols.
Upon cessation of ovarian function, low serum E2 levels perturb the bone homeostasis with an excessive bone resorption that may result in osteoporosis. Osteoporosis as a systemic skeletal disorder is characterized by low bone mass, micro-architectural deterioration, and elevated bone fragility (WHO, 1994). In 1997, XN was shown to inhibit osteoclast-induced pit formation in vitro (Tobe et al., 1997). Li et al. (2015) described an inhibitory effect of XN on osteoclastogenesis and bone resorption in vitro as well as in ovariectomized (ovx) mice. Further studies showed that subcutaneously administered 8-PN prevented the ovx-induced bone loss in rats (Hümpel et al., 2005; Miyamoto et al., 1998). However, the impact of an orally administered hops extract on bone loss has not been investigated up to now in this animal model. Regarding the estrogenic effects in the uterus, several publications described uterotrophic effects of 8-PN applied orally or subcutaneously to ovx rats (Keiler et al., 2015; Overk et al., 2008). In contrast, no uterotrophic response was found in ovx rats after dietary treatment with a hops extract (Overk et al., 2008).
The aim of the present study was to investigate parameters of efficacy and safety of an H. lupulus extract that has been biologically standardized for estrogenicity as well as chemically for XN, IX, 8-PN and 6-PN (Fig. 1) (Krause et al., 2014) for potential use in the management of menopausal complaints. Ovariectomized rats are an established preclinical model of osteoporosis caused by estrogen defficiency that has been previously used to assess bone protective effects of dietary compounds (Lelovas et al., 2008). For this purpose, female Wistar rats were ovariectomized and put on a phytoestrogen-free diet that contained either estradiol-3-benzoate (E2benzoate) or the hops extract. After 8 weeks, the bone micro-architectures of the tibiae and the lumbar vertebrae were analyzed using micro-computed tomography and the number of osteoclasts was determined by staining of tibial sections. Regarding safety, we analyzed the uterine morphology and the number of proliferating cells in the uterine luminal epithelium. Serum levels of the four hops marker constituents were quantified by LC-MS.
Materials and methods
Substances and extract
E2benzoate was purchased from Sigma-Aldrich (Hamburg, Germany). The preparation and standardization (chemical and biological) of the hops extract that was used in the present and other parallel studies has been described in detail previously (Krause et al., 2014; van Breemen et al., 2014). In brief, hops with an optimum content of prenylated flavonoids were extracted with ethanol under GLP conditions, the bitter acids removed by supercritical CO2 extraction of the dried crude extract, and the resulting spent hops extract processed to yield a dry, yellow powder (Hopsteiner, New York, NY, USA). Details of the LC-MS chemical standardization of this bulk spent hops extract for four marker compounds (XN, IX, 8PN, 6PN) have been reported using LC-MS (Krause et al., 2014) as well as by UHPLC and qHNMR (Ramos Alvarenga et al., 2014) as part of the development of hops extracts from this bulk extract. The material was stored at −25 °C until use and has shown no detectable signs of degradation over time.
Animals
Eighteen young-adult female Wistar rats (150–200 g) were housed under controlled conditions (20 ± 1 °C, 50–80% of relative humidity; 12:12-h light-dark-cycles) in groups of six animals per cage with free access to water and food. All animal handling and experimental protocols followed the ethical standards compiled in the 1964 Declaration of Helsinki and the experimental arrangements were chosen and conducted to adhere as closely as possible to the 3R principles of animal welfare. All procedures were approved twice, once by the institutional Animal Care and Use Committee of the Technische Universität Dresden and secondly by the state Animal Care and Use Committee and licensed by the intermediate authority, Landesdirektion Sachsen (Permission number 24-9168.11-8/2012-2). At the time of bilateral ovariectomy, animals were divided into feeding groups. The unsubstituted control group (n = 6) received a phytoestrogen-free diet (Ssniff, SMR/H, 10 mm, PE-free; Ssniff GmbH, Soest, Germany) (noPE animals). The two other groups received phytoestrogen-free diets containing either 10.5 mg/kg diet E2benzoate (E2benzoate animals) or 700 mg/kg diet hops extract respectively for an intervention period of 8 weeks. The dose of the hops extract in our long-term feeding study was chosen based on the extended dose-response study of the hops extracts in the classical uterotrophic assay performed by Overk and colleagues (Overk et al., 2008). From the three exposure doses investigated by Overk and colleagues, we chose the middle dose in our study. The average exposure of the animals calculated by the daily dietary intake of the animals was 60 mg hops extract per kg body weight and 0.93 mg E2benzoate per kg body weight respectively. Eight weeks after the study onset, all animals were sacrificed by CO2-inhalation subsequently to a light O2/CO2-inhalation anesthesia.
Uteri were fixed in 4% buffered paraformaldehyde for 24 h and embedded in paraffin. Right tibiae were dissected and fixed in 4% buffered paraformaldehyde (PFA) for 48 h, subsequently decalcified using 20% EDTA (pH 8.4) for 21 d and embedded in paraffin. Left tibiae and lumbar vertebrae were fixed in 70% ethanol for micro-computed tomography analysis (μCT).
Serum metabolite analyses
Quantitative analyses of marker compounds in serum were carried out as reported previously (van Breemen et al., 2014; Yuan et al., 2012). Briefly, 0.2 ml of serum was diluted with 0.2 ml of 0.1 M sodium acetate buffer pH 5.0 containing 8-isopentyl naringenin as internal standard and then incubated with 500 units of β-glucuronidase for 3 h at 37 °C to release free aglycones from glucuronic acid conjugates. Free aglycones were then extracted with 2 × 2 ml of MTBE. Organic phases were combined, evaporated to dryness and reconstituted in 80% methanol/water (v/v) prior to injection onto LC-MS. LC-MS/MS analyses were carried out on a Shimadzu (Kyoto, Japan) Nexera UHPLC system and LCMS-8050 triple quadrupole mass spectrometer. The analytes were separated on a Waters XBridge 2.0 × 50 mm 2.5 μm C18 column using a 3-min gradient from 40–70% acetonitrile/0.1% formic acid. The flow rate was 0.4 ml/min and the column temperature was 40 °C. Analytes were quantified using negative ion electrospray and selected reaction monitoring (SRM). Two SRM transitions per analyte were used as described previously. Calibration curves were prepared by spiking standards into blank rat serum and samples processed as described above.
Histology and histomorphometry
For histological analyses, 4 μm thick longitudinal tibia sections were made. For histomorphometry, bone sections were stained with hematoxylin-eosin (H&E). Assessment of osteoclast number per area (N.Oc/T.Ar) was done by tartrate-resistant acid phosphatase (TRAP) staining. The OsteoMeasure™ Software (OsteoMetrics, Inc, Decatur, USA) was used to quantify all measures.
Embedded uterine samples were cut in 4 μm thick cross sections. Morphological analyses of uteri were performed using H&E-stained sections. Immunohistochemical analysis was performed by rehydration and protein epitope retrieval using 10 mM Tris-EDTA (pH 9) and the IHC Select® HRP/DAB kit (Merck Millipore, Darmstadt, Germany) according to the manufacturer’s protocol. Primary antibodies raised against PCNA (1:250, source: rabbit, Thermo Scientific, Rockford, USA) were used. A Keyence BZ-8100E microscope (Keyence, Neu-Isenburg, Germany) was used to visualize tissue sections. Epithelial and myometrial thickness were measured at 100 positions for each parameter and animal. The percentage of PCNA-positive cells in the uterine luminal epithelium was determined by counting all positive and negative nuclei in the epithelium in six animals per group.
Micro-computed tomography
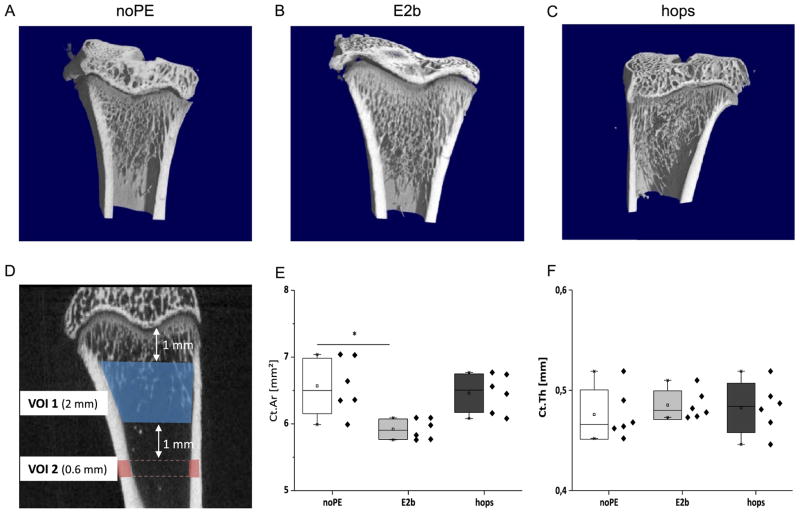
Micro-computed tomography (vivaCT 75, Scanco Medical, Brüttisellen, Switzerland) on the excised tibiae and lumbar vertebrae was performed using an isotropic voxel size of 20 μm (55 keV, 110 μA, 220 ms, 1000 projections). Calibration of the scanner took place weekly using hydroxyapatite phantoms. For tibia, morphological analysis of a region of 2 mm thickness was performed 1 mm distal to the tibial growth plate (Fig. 4A). Contours were drawn manually adjacent to the endocortical boundary. For the analysis, the Image Programming Language software (IPL) from Scanco Medical was used to assess bone volume ratio (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), connectivity density (Conn.D), cortical area (Ct.AR) and cortical thickness (Ct.Th) (Bouxsein et al., 2010). The threshold was set to 330 mg HA/cm3 for cancellous bone and to 580 mg HA/cm3 for cortical bone. Morphological analyses of two lumbar vertebrae per animal (LV 3 and LV4) 1 mm proximal to the growth plate of the caudal half were done using semi-automated contouring (Fig. 5A).
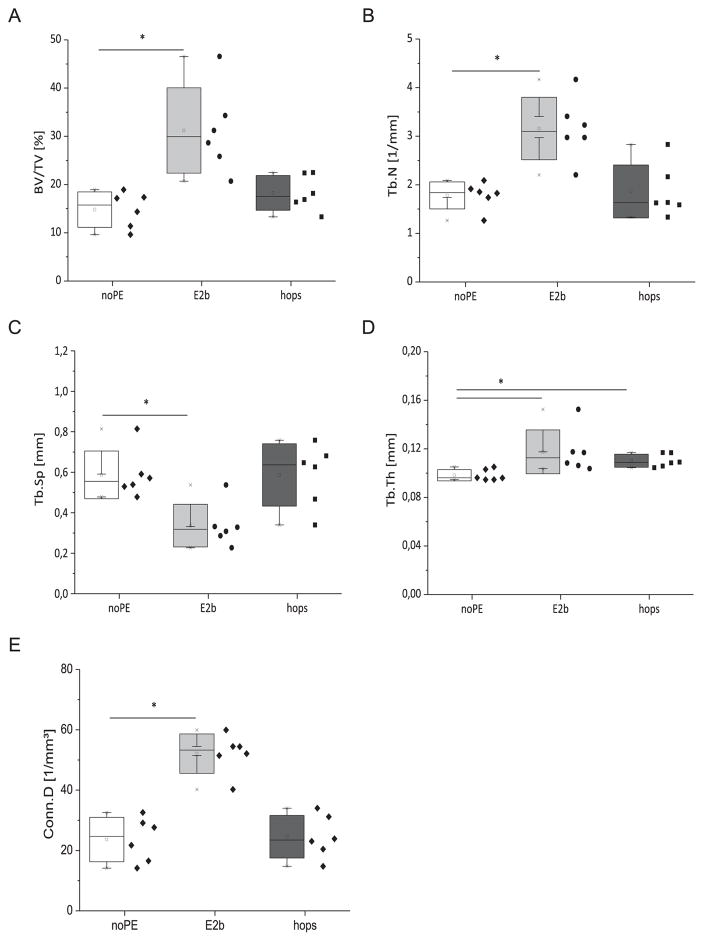
Fig. 4.
Micro-architectural parameters of the tibial metaphysis. Bar graphs and scatter plots show Bone Volume Density (BV/TV, A), Trabecular Number (Tb.N, B), Trabecular Separation (Tb.Sp, C), Trabecular Thickness (Tb.Th, D) and Connectivity Density (Conn.D, E) for each treatment group (n = 6) quantified by μCT. The daily exposure dose of the animals was 0.93 mg/kg body weight for E2benzoate (E2b) and 60 mg/kg body weight for the hops extract (hops) respectively. * denotes statistically significant differences compared to the noPE control group (p < 0.05).
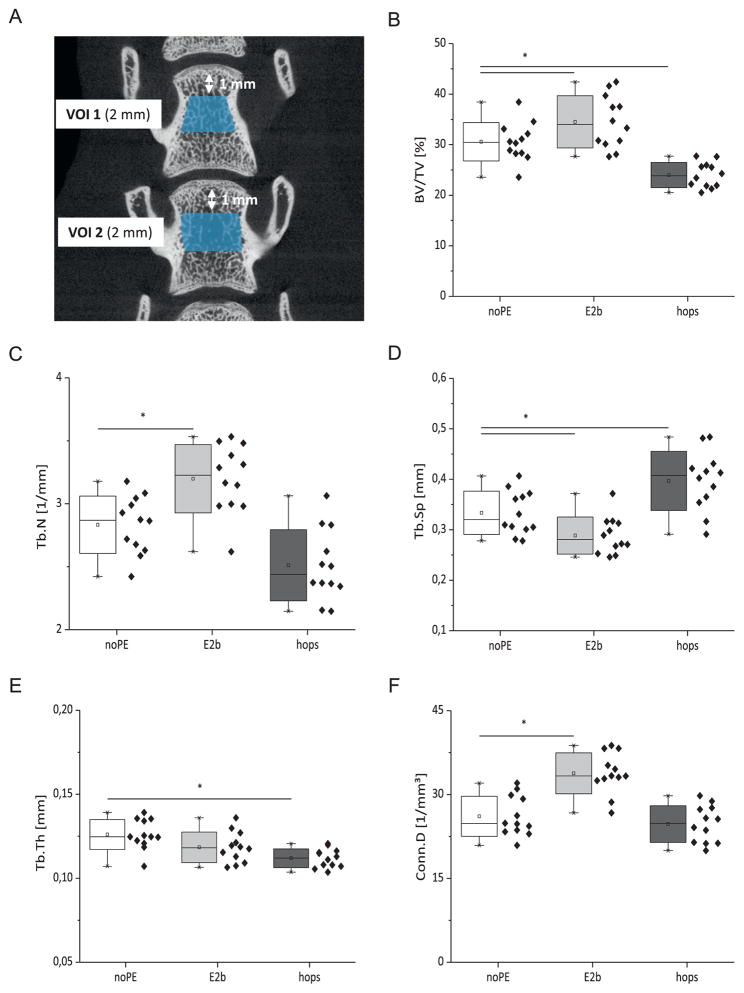
Fig. 5.
Micro-architectural parameters of the lumbar vertebrae (LV 3 and LV 4). μCT image of the spine showing VOI 1 (LV 3) and VOI 2 (LV 4, A). Bar graphs and scatter plots show Bone Volume Density (BV/TV, B), Trabecular Number (Tb.N, C), Trabecular Separation (Tb.Sp, D), Trabecular Thickness (Tb.Th, E) and Connectivity Density (Conn.D, F) for each treatment group (n = 6) quantified by μCT. The daily exposure dose of the animals was 0.93 mg/kg body weight for E2benzoate (E2b) and 60 mg/kg body weight for the hops extract (hops) respectively. * denotes statistically significant differences compared to the noPE control group (p < 0.05).
Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by the Bonferroni post-hoc test to determine significant differences compared to the noPE control group. Results were considered as statistically significant at p ≤0.05.
Results
Serum concentration of prenylated hops phenols
Composition of the hops extract and the serum levels of 8-PN, 6-PN, IX and XN at the end of the intervention are shown in Table 1. As reported previously (Krause et al., 2014), using a LC-MS/MS method, XN is the major constituent of the extract (35.78 %), followed by 6-PN (2.18 %), IX (1.35 %) and 8-PN with (0.42 %). Accordingly, there is an approximate 100:1 ratio between the abundance of XN and 8-PN. The measurement of these markers in serum after the 8-week intervention indicated a different proportion of these markers compared to that present in the extract. The average serum level of the minor constituent, 8-PN was found to be higher than that of the major component, XN, at 26.7 nM vs. 22.6 nM, respectively. The average serum levels of 6-PN and IX were 2.9 nM and 5.8 nM, respectively. It should be noted that no free aglycones could be detected in serum, which is in agreement with previous studies showing that glucuronic acid conjugates are the dominant circulating form of prenylated phenols (Legette et al., 2012; van Breemen et al., 2014).
Table 1.
Concentrations of 8-prenylnaringenin (8-PN), 6-prenylnaringenin (6-PN), isoxanthohumol (IX) and xanthohumol (XN) in the hop extract and serum levels of the free aglycones after the 8-week intervention period in the group receiving the diet enriched with the hop extract with a daily exposure of 60 mg hop extract per kg body weight (n = 6, mean ± SD).
| Compound | % in the extract (g/100 g dry weight, according to Krause et al. (2014)) | Serum levels after 8 week Intervention (nM) |
|---|---|---|
| 8-PN | 0.42 | 26.7 ± 16.9 |
| 6-PN | 2.18 | 2.9 ± 1.4 |
| IX | 1.35 | 5.8 ± 5.6 |
| XN | 35.78 | 22.6 ± 4.6 |
Uterine morphology
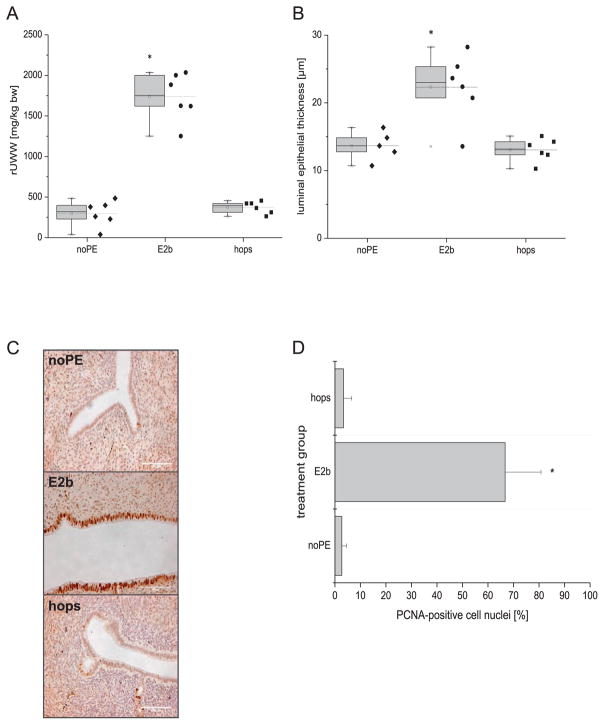
Oral administration of E2benzoate leads to a 6-fold higher relative uterine wet weight compared to the noPE control group after the 8-week intervention period (Fig. 2A). In contrast, the hops-enriched diet did not increase the uterine wet weight. No estrogenic effects in response to treatment with the hops extract were observed regarding histomorphometric parameters of the uterus. While E2benzoate increased the luminal epithelial thickness (22.3 μm), the values measured in the hops treated group were nearly equal to those from the control animals (13.1 μm vs. 13.7 μm, p < 0.05, Fig. 2B). Immunohistochemical PCNA staining showed that 67% of the luminal epithelial cell nuclei were PCNA-positive and therefore undergoing proliferation in the E2benzoate-treated group (Fig. 2C and D). The percentage of PCNA-positive luminal epithelial cells in the hops-treated and the noPE control animals was 3% (Fig. 2C, and D).
Fig. 2.
Relative uterine wet weight (A), morphological (B) and immunohistochemical analyses (C–D) of the uterus. Representative stains of paraffin-embedded uterine sections (scale bar = 100 μm) (C) and percentage of PCNA-positive cell nuclei in the luminal epithelium (D). The daily exposure dose of the animals was 0.93 mg/kg body weight for E2benzoate (E2b) and 60 mg/kg body weight for the hops extract (hops) respectively. * denotes statistical significant differences compared to the noPE control group (p < 0.05).
Bone morphology
The results for the bone morphology analysis vary between tibia and lumbar vertebrae. The outcome of the μCT analysis of the tibia is shown in Figs. 3 and 4, representative μCT images are shown in Fig. 3A–C. The cortical area was significantly lower in the E2benzoate-substituted animals compared to the noPE animals (5.92 mm3 vs. 6.57 mm3, p < 0.05, Fig. 3E). No significant differences were observed for Ct.Ar between the noPE and the hops group. The cortical thickness was comparable between all three groups (0.48 mm in the noPE group, 0.49 mm in the E2b group and 0.48 mm in the hops group, Fig. 3F). Feeding with an E2benzoate containing diet led to much higher BV/TV, Tb.N, Tb.Th and Conn.D values (Fig. 4A, B, D, E) and to a lower trabecular separation compared to the noPE animals (Fig. 4C). Except for trabecular thickness that was moderately increased compared to the noPE animals (0.110 ± 0.005 vs. 0098 ± 0.005, Fig. 4E), micro-architectural parameters in the tibia were not altered in the hops group (Fig. 4).
Fig. 3.
Representative μCT images (A–C) and cortical bone parameters of proximal tibiae (E–F). μCT image of the proximal tibia showing the VOI 1 (trabecular bone) and VOI 2 (cortical bone, D). Bar graphs and scatter plots show Cortical Area (Ct.Ar, E) and Cortical Thickness (Ct.Th, F) for each treatment group (n = 6). The daily exposure dose of the animals was 0.93 mg/kg body weight for E2benzoate (E2b) and 60 mg/kg body weight for the hops extract (hops) respectively. * denotes statistical significant differences compared to the noPE control group (p < 0.05).
Histomorphometric parameters analyzed in longitudinal sections of paraffin-embedded tibiae are shown in Table 2. Bone volume ratio (34.8 vs. 21.1, p < 0.05) and bone perimeter (9.79 vs. 6.59, p < 0.05) were significantly higher and the number of osteoclasts lower (3.31 vs. 5.62, p < 0.05) in the E2benzoate-treated animals compared to the noPE control group. While no impact of the hops extract on BV/TV and B.Pm was observed, the number of osteoclasts relative to the bone perimeter and the tissue area analyzed was significantly lower in the hops animals compared to the control group (3.68 vs. 5.62 and 23.7 vs. 40.2 respectively, p < 0.05, Table 2).
Table 2.
Histomorphometric parameters of the proximal tibia quantified by analysis of HE- and TRAP-stained paraffin sections. Data are shown as group mean ± SD (n = 6). * Statistical significance compared to the noPE group (p < 0.05). BV/TV: bone volume ratio, B.Pm: bone perimeter, N.Oc: osteoclast number, T.Ar: tissue area.
| Parameter | noPE | E2benzoate | Hops |
|---|---|---|---|
| BV/TV (%) | 21.1 ± 5.3 | 34.8 ± 5.7* | 21.3 ± 7.8 |
| B.Pm (mm) | 6.59 ± 0.82 | 9.79 ± 1.81* | 5.91 ± 1.41 |
| N.Oc/B.Pm (1/mm) | 5.62 ± 1.77 | 3.31 ± 1.23* | 3.68 ± 0.99* |
| N.Oc/T.Ar (1/mm2) | 40.2 ± 8.4 | 35.4 ± 12.6 | 23.7 ± 7.1* |
p < 0.05.
The ovx-induced deterioration of the micro-architecture of the lumbar vertebrae was completely prevented by the E2benzoate substitution (Fig. 5B–F). In the hops animals all parameters determined by μCT, except for Conn.D (Fig. 5F), were significantly lower compared to the unsubstituted control group (p < 0.05).
Discussion
In 2014, Krause et al. described the chemical standardization of a hops botanical supplement which has also been standardized for estrogenic properties (Krause et al., 2014). Subsequently, the pharmacokinetic of this hops extract was investigated in five post-menopausal women (van Breemen et al., 2014). The present paper describes the evaluation of the standardized hops extract with regard to safety (uterotrophy) and efficacy (prevention of bone loss) in the preclinical ovx rat model.
The total serum levels of the aglycones of the four prenylated phenols (XN, IX, 8-PN, 6-PN) after an 8-week dietary supplementation with the hops extract showed that on average 8-PN had the highest concentration (26.7 nM), followed by XN (22.6 nM) (Table 1). In contrast, the abundance of the estrogen, 8-PN, in the hops extract is about 10-fold lower than that of XN. Considering this large dynamic range of closely related constituents, our results support earlier conclusions that analogues such as IX and XN can act as precursors that are converted metabolically to 8-PN via cyclization of XN to IX and/or O-demethylation of IX (Legette et al., 2012; van Breemen et al., 2014). Interestingly, the 8-PN serum levels quantified in our study following a dietary exposure to the hops extract (60 mg/kg bw/d and accordingly 0.26 mg/kg bw/d 8-PN) are 2.5-fold higher than those observed after intraperitoneal treatment of ovx rats with 40 mg/kg bw/d pure 8-PN as described by Overk et al. (2008). An explanation might be that the additional 8-PN was formed by gut microbiota during oral administration of the hops extract. Another possible explication might be a time-dependent accumulation of 8-PN as the administration period in the study from Overk and colleagues was three weeks compared to 8 weeks in our study. Although three groups received a diet enriched with increasing doses of a hops extract in the study by Overk et al., they had not determined 8-PN serum levels in those hops-treated groups (Overk et al., 2008). While we only investigated the serum levels of the free aglycones, Overk et al. determined the plasma levels of 8-PN metabolites being in accordance to the metabolites found after the incubation of 8-PN with liver microsomes in a previous publication (Nikolić et al., 2004).
The E2benzoate treatment prevented the ovx-induced uterine atrophy representing the positive control. In contrast to the oral E2benzoate administration, no uterotrophic effect was induced by the dietary treatment with the hops extract (Fig. 2A). Also, no estrogenic effects in response to the hops extract were observed with regard to luminal epithelial thickness and the number of proliferating cells in the luminal epithelia (Fig. 2B–D). This is in line with the study by Overk et al., who did not observe any uterotrophic response to a dietary administration of hops extract (4, 40 and 400 mg/kg diet) (Overk et al., 2008). Although 8-PN was shown to be a potent ERα agonist and to promote proliferation in MCF-7 cells (Helle et al., 2014; Milligan et al., 2000), it is striking that no proliferation was observed in our study. This might be explained by the recently shown AhR agonistic activity of 6-PN (Wang et al., 2016) as several publications described a AhR-ER cross-talk in the uterus comprising a modulation of ER signaling by AhR activation (Buchanan et al., 2000; Shanle and Xu, 2011). The histological analysis of the mammary gland after this 8-week administration of the hops extract did not show any unwanted side effects providing further evidence for the safety of the hops extract (data not shown).
Rather than assessing hops extract, as in our study, previous reports have only evaluated the impact of single substances in preclinical models. For example, pure 8-PN has been investigated to observe bone protective effects after s.c. treatment of ovx rats in rather high dosages (30 and 18 mg/kg/d) (Hümpel et al., 2005; Miyamoto et al., 1998). However, a recent study using a lower dosage of 1.77 mg/kg 8-PN did not observe any impact on ovx-induced bone loss and no uterotrophic effects (Hoffmann et al., 2016). Our study revealed a mild protective effect on the trabecular thickness in the tibial metaphysis in response to the 8-week dietary hops treatment, with an effect comparable to that induced by the E2benzoate treatment (Fig. 4D). All other micro-architectural parameters (cortical and trabecular bone) determined by μCT analysis in the hops group were comparable to those of the noPE control group (Figs. 3 and 4). The histological analyses of tibial sections yielded significant lower numbers of osteoclasts in the hops group, thus comparable to that reduction induced by E2benzoate (Table 2). Our results are similar to two in vitro studies that described 8-PN-induced reduction of the osteoclast number by decreasing osteoclast formation and increasing apoptosis as well as by diminishing osteoclast differentiation (Luo et al., 2014; Ming et al., 2013). To our knowledge, this is the first time that the effect of a standardized hops extract on osteoclast number has been shown in vivo. This might be promising for the use of hops extracts in postmenopausal women if a long-term application to treat menopausal complaints might retard bone loss by a sustained reduction of the osteoclast number.
However, the outcome of the μCT analysis of the lumbar vertebrae contradicts the observations made for the tibiae (Fig. 5). The microarchitectural changes among the animals on the hops extract-enriched diet were more severe than those of the control group. This indicates a bone type-specific effect. Bone site-specific effects in the ovx rat model have already been described elsewhere. Bonnet et al. reported a fundamentally different response of spine bone cells compared to bone cells of the appendicular bone after propranolol treatment of ovx rats (Bonnet et al., 2006). Recently, Noorafshan et al. (2015) showed site-dependent differences (lumbar vertebra vs. tibia) in ovx rats treated with a black olive extract. Finding an explanation for the different effects on lumbar vertebrae and tibiae will require further investigation aimed at underlying mechanisms and the clarification which is the more relevant endpoint for estimation of bone effects.
Conclusion
In conclusion, our results showed that the standardized hops extract did not induce unwanted proliferative effects in the endometrium of ovariectomized rats after a dietary exposure of 8 weeks. The outcome of the bone histomorphometric analyses point to weak bone protective properties of the hops extract on E2 depletion-induced bone loss in the preclinical model, with differential effects depending on the bone type analyzed. One notable observation was the unexpected high serum levels of 8-PN relative to the levels of the other major marker compounds. Considering the large dynamic range present in the extract (ca. 100:1 for the XH/8-PN pair), the observed high 8-PN levels might indicate the existence of unknown in vivo metabolic pathways causing inter-conversion of closely related prenylated hops phenols.
Acknowledgments
Support for this work was through the Deutsche Forschungsgemeinschaft DFG KR 3768/2-1, DFG BE 5466/2-1, grant P50 AT000155 from the Office of Dietary Supplements, and the National Center for Complementary and Alternative Medicine. We thank Harald Schwarz and Martin Bindel of Hopsteiner for their consultation and for providing the spent hops extract.
Abbreviations
- 6-PN
6-prenylnaringenin
- 8-PN
8-prenylnaringenin
- BV/TV
bone volume ratio
- Conn.D
connectivity density
- E2benzoate
estradiol-3-benzoat
- IX
isoxanthohumol
- μCT
micro-computed tomography
- PE
phytoestrogen
- TB.N
trabecular number
- Tb.Th
trabecular thickness
- Tb.Sp
trabecular separation
- XN
xanthohumol
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Bolca S, Possemiers S, Maervoet V, Huybrechts I, Heyerick A, Vervarcke S, Depypere H, De Keukeleire D, Bracke M, De Henauw S, Verstraete W, Van de Wiele T. Microbial and dietary factors associated with the 8-prenylnaringenin producer phenotype: a dietary intervention trial with fifty healthy post-menopausal Caucasian women. Br J Nutr. 2007;98:950–959. doi: 10.1017/S0007114507749243. [DOI] [PubMed] [Google Scholar]
- Bonnet N, Laroche N, Vico L, Dolleans E, Benhamou CL, Courteix D. Dose effects of propranolol on cancellous and cortical bone in ovariectomized adult rats. J Pharmacol Exp Ther. 2006;318:1118–1127. doi: 10.1124/jpet.106.105437. [DOI] [PubMed] [Google Scholar]
- Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using microcomputed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- Buchanan DL, Sato T, Peterson RE, Cooke PS. Antiestrogenic effects of 2,3,7,8-Tetrachlorodibenzo-p-dioxin in mouse uterus: critical role of the aryl hydrocarbon receptor in stromal tissue. Toxicol Sci. 2000;57:302–311. doi: 10.1093/toxsci/57.2.302. [DOI] [PubMed] [Google Scholar]
- Dietz BM, Hagos GK, Eskra JN, Wijewickrama GT, Anderson JR, Nikolić D, Guo J, Wright B, Chen SN, Pauli GF, van Breemen RB, Bolton JL. Differential regulation of detoxification enzymes in hepatic and mammary tissue by hops (Humulus lupulus) in vitro and in vivo. Mol Nutr Food Res. 2013;57:1055–1066. doi: 10.1002/mnfr.201200534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Nikolić D, Chadwick LR, Pauli GF, van Breemen RB. Identification of human hepatic cytochrome P450 enzymes involved in the metabolism of 8-prenylnaringenin and isoxanthohumol from hops (Humulus lupulus L.) Drug Metab Dispos. 2006;34:1152–1159. doi: 10.1124/dmd.105.008250. [DOI] [PubMed] [Google Scholar]
- Helle J, Kräker K, Bader MI, Keiler AM, Zierau O, Vollmer G, Welsh J, Kretzschmar G. Assessment of the proliferative capacity of the flavanones 8-prenylnaringenin, 6-(1.1-dimethylallyl)naringenin and naringenin in MCF-7 cells and the rat mammary gland. Mol Cell Endocrinol. 2014;392:125–135. doi: 10.1016/j.mce.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Hoffmann DB, Griesel MH, Brockhusen B, Tezval M, Komrakova M, Menger B, Wassmann M, Stuermer KM, Sehmisch S. Effects of 8-prenylnaringenin and whole-body vibration therapy on a rat model of osteopenia. J Nutr Metab. 2016;2016:6893137. doi: 10.1155/2016/6893137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hümpel M, Isaksson P, Schaefer O, Kaufmann U, Ciana P, Maggi A, Schleuning WD. Tissue specificity of 8-prenylnaringenin: protection from ovariectomy induced bone loss with minimal trophic effects on the uterus. J Steroid Biochem Mol Biol. 2005;97:299–305. doi: 10.1016/j.jsbmb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Keiler AM, Dörfelt P, Chatterjee N, Helle J, Bader MI, Vollmer G, Kretzschmar G, Kuhlee F, Thieme D, Zierau O. Assessment of the effects of naringenin-type flavanones in uterus and vagina. J Steroid Biochem Mol Biol. 2015;145:49–57. doi: 10.1016/j.jsbmb.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Krause E, Yuan Y, Hajirahimkhan A, Dong H, Dietz BM, Nikolić D, Pauli GF, Bolton JL, van Breemen RB. Biological and chemical standardization of a hop (Humulus lupulus) botanical dietary supplement. Biomed Chromatogr BMC. 2014;28:729–734. doi: 10.1002/bmc.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legette L, Ma L, Reed RL, Miranda CL, Christensen JM, Rodriguez-Proteau R, Stevens JF. Pharmacokinetics of xanthohumol and metabolites in rats after oral and intravenous administration. Mol Nutr Food Res. 2012;56:466–474. doi: 10.1002/mnfr.201100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA. The laboratory rat as an animal model for osteoporosis research. Comp Med. 2008;58:424–430. [PMC free article] [PubMed] [Google Scholar]
- Li J, Zeng L, Xie J, Yue Z, Deng H, Ma X, Zheng C, Wu X, Luo J, Liu M. Inhibition of osteoclastogenesis and bone resorption in vitro and in vivo by a prenylflavonoid xanthohumol from hops. Sci Rep. 2015;5:17605. doi: 10.1038/srep17605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Kang L, Ma Y, Chen H, Kuang H, Huang Q, He M, Peng W. Effects and mechanisms of 8-prenylnaringenin on osteoblast MC3T3-E1 and osteoclast-like cells RAW264.7. Food Sci Nutr. 2014;2:341–350. doi: 10.1002/fsn3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan SR, Kalita JC, Pocock V, Kauter VVD, Stevens JF, Deinzer ML, Rong H, Keukeleire DD. The endocrine activities of 8-prenylnaringenin and related hop (Humulus lupulus L.) flavonoids. J Clin Endocrinol Metab. 2000;85:4912–4915. doi: 10.1210/jcem.85.12.7168. [DOI] [PubMed] [Google Scholar]
- Ming LG, Lv X, Ma XN, Ge BF, Zhen P, Song P, Zhou J, Ma HP, Xian CJ, Chen KM. The prenyl group contributes to activities of phytoestrogen 8-prenynaringenin in enhancing bone formation and inhibiting bone resorption in vitro. Endocrinology. 2013;154:1202–1214. doi: 10.1210/en.2012-2086. [DOI] [PubMed] [Google Scholar]
- Miyamoto M, Matsushita Y, Kiyokawa A, Fukuda C, Iijima Y, Sugano M, Akiyama T. Prenylflavonoids: a new class of non-steroidal phytoestrogen (Part 2). Estrogenic effects of 8-isopentenylnaringenin on bone metabolism. Planta Med. 1998;64:516–519. doi: 10.1055/s-2006-957505. [DOI] [PubMed] [Google Scholar]
- Nikolić D, Li Y, Chadwick LR, Grubjesic S, Schwab P, Metz P, van Breemen RB. Metabolism of 8-prenylnaringenin, a potent phytoestrogen from hops (Humulus lupulus), by human liver microsomes. Drug Metab Dispos. 2004;32:272–279. doi: 10.1124/dmd.32.2.272. [DOI] [PubMed] [Google Scholar]
- Noorafshan A, Dabbaghmanesh MH, Tanideh N, Koohpeyma F, Rasooli R, Hajihoseini M, Bakhshayeshkaram M, Hosseinabadi OK. Stereological study of the effect of black olive hydroalcoholic extract on osteoporosis in vertebra and tibia in ovariectomized rats. Osteoporos Int. 2015;26:2299–2307. doi: 10.1007/s00198-015-3126-x. [DOI] [PubMed] [Google Scholar]
- Overk CR, Guo J, Chadwick LR, Lantvit DD, Minassi A, Appendino G, Chen SN, Lankin DC, Farnsworth NR, Pauli GF, van Breemen RB, Bolton JL. In vivo estrogenic comparisons of Trifolium pratense (red clover), Humulus lupulus (hops), and the pure compounds isoxanthohumol and 8-prenylnaringenin. Chem Biol Interact. 2008;176:30–39. doi: 10.1016/j.cbi.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overk CR, Yao P, Chadwick LR, Nikolić D, Sun Y, Cuendet MA, Deng Y, Hedayat AS, Pauli GF, Farnsworth NR, van Breemen RB, Bolton JL. Comparison of the in vitro estrogenic activities of compounds from hops (Humulus lupulus) and red clover (Trifolium pratense) J Agric Food Chem. 2005;53:6246–6253. doi: 10.1021/jf050448p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos Alvarenga RF, Friesen JB, Nikolić D, Simmler C, Napolitano JG, van Breemen R, Lankin DC, McAlpine JB, Pauli GF, Chen SN. K-Targeted metabolomic analysis extends chemical subtraction to DESIGNER extracts: selective depletion of extracts of hops (Humulus lupulus) J Nat Prod. 2014;77:2595–2604. doi: 10.1021/np500376g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SAA, Howard BV, Johnson KC, Kotchen JM, Ockene J Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Shanle EK, Xu W. Endocrine disrupting chemicals targeting estrogen receptor signaling: identification and mechanisms of action. Chem Res Toxicol. 2011;24:6–19. doi: 10.1021/tx100231n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe H, Muraki Y, Kitamura K, Komiyama O, Sato Y, Sugioka T, Maruyama HB, Matsuda E, Nagai M. Bone resorption inhibitors from hop extract. Biosci Biotechnol Biochem. 1997;61:158–159. doi: 10.1271/bbb.61.158. [DOI] [PubMed] [Google Scholar]
- van Breemen RB, Yuan Y, Banuvar S, Shulman LP, Qiu X, Alvarenga RFR, Chen SN, Dietz BM, Bolton JL, Pauli GF, Krause E, Viana M, Nikolić D. Pharmacokinetics of prenylated hop phenols in women following oral administration of a standardized extract of hops. Mol Nutr Food Res. 2014;58:1962–1969. doi: 10.1002/mnfr.201400245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlietinck AJ. Assessment report for herbal substance(s), herbal preparation(s) or combinations thereof with traditional use—Humulus lupulus L., flos. EMEA. 2008;2006:513618. [Google Scholar]
- Wang S, Dunlap TL, Howell CE, Mbachu OC, Rue EA, Phansalkar R, Chen SN, Pauli GF, Dietz BM, Bolton JL. Hop (Humulus lupulus L.) extract and 6-prenylnaringenin induce P450 1A1 catalyzed estrogen 2-hydroxylation. Chem Res Toxicol. 2016;29:1142–1150. doi: 10.1021/acs.chemrestox.6b00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Assessment of fracture risk and its application to screening for post-menopausal osteoporosis. Report of a WHO Study Group World Health Organ. Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- Yuan Y, Qiu X, Nikolić D, Dahl JH, van Breemen RB. Method development and validation for ultra-high-pressure LC/MS/MS determination of hop prenylflavonoids in human serum. J AOAC Int. 2012;95:1744–1749. doi: 10.5740/jaoacint.11-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierau O, Gester S, Schwab P, Metz P, Kolba S, Wulf M, Vollmer G. Estrogenic activity of the phytoestrogens naringenin, 6-(1,1-dimethylallyl)naringenin and 8-prenylnaringenin. Planta Med. 2002;68:449–451. doi: 10.1055/s-2002-32089. [DOI] [PubMed] [Google Scholar]