Abstract
Not until the turn of this century has immunotherapy become a fundamental component of cancer treatment. While monotherapy with immune modulators such as immune checkpoint inhibitors provides a subset of patients with durable clinical benefit and possible cure, combination therapy offers the potential for anti-tumor activity in a greater number of patients. The field of immunology has provided us with a plethora of potential molecules and pathways to target. This abundance makes it impractical to empirically test all possible combinations efficiently. We recommend that potential immunotherapy combinations be chosen based on sound rationale and available data to address the mechanisms of primary and acquired immune resistance. Novel trial designs may increase the proportion of patients receiving potentially efficacious treatments and, at the same time, better define the balance of clinical activity and safety. We believe that implementing a strategic approach in the early development of immunotherapy combinations will expedite the delivery of more effective therapies with improved safety and durable outcomes.
INTRODUCTION
The hypothesis that the immune system can be manipulated to fight cancer was made over a century ago. Despite significant advances in the scientific insights of antitumor immunity, repeated prior therapeutic attempts - largely aimed at immune stimulation via cancer vaccines - have met limited success. Recently, anti-cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and anti-programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) monoclonal antibodies targeting immune inhibitory pathways referred to as checkpoints, have demonstrated durable responses in multiple tumor types including melanoma (1, 2), renal cell carcinoma (RCC) (3), non-small cell lung cancer (NSCLC) (4), bladder cancer (5), Hodgkin’s lymphoma (6), gastric cancer (7), head and neck squamous cell carcinoma (8), and microsatellite unstable colon cancer (9); these results have led to a growing number of regulatory indications.
Single agent activity is limited to a minority of patients and emerging long-term follow-up data in melanoma indicate that a substantial proportion of patients previously responding to immune checkpoint inhibitor therapy develop resistance (10–12). Evidence-based combinations may lead to therapeutic synergies to overcome resistance. The enhanced efficacy of dual CTLA-4 and PD-1 blockade in melanoma (13–15) is an example. Multiple new agents targeting various immune processes are entering clinical development. Examples include other immune checkpoint inhibitors, co-stimulatory agonists, oncolytic viruses, vaccines and adoptive cell therapy (Table 1), the broad potential of immunotherapies is being explored in novel combinations and in combination with conventional therapies.
Table 1.
Immunotherapeutic agents in current development
| Co-inhibitory molecules (targets of immune checkpoint inhibitors) | Co-stimulatory molecules (targets of immune-stimulatory agonists) |
|---|---|
|
|
| Vaccines | Adoptive T cell therapy |
|
|
| Immunosuppressive soluble factors | Cytokines |
|
|
| Oncolytic virus | T regulatory cell depletion therapy |
|
|
| Bispecific T cell engaging antibody-based technologies | Endogenous adjuvants |
|
|
CTLA-4, cytotoxic T-lymphocyte-associated antigen-4; PD-1, programmed death 1; PD-L1, programmed death ligand 1; LAG3, lymphocyte activation gene 3; TIM3, T cell immunoglobulin (Ig)-3; BTLA, B and T lymphocyte attenuator; TIGIT, T cell immunoglobulin and ITIM domain; VISTA, V-domain Ig-containing suppressor of T cell activation; KIR, killer IgG-like receptor; GITR, Glucocorticoid-induced tumour necrosis factor receptor; ICOS, Inducible T cell COStimulator; IDO-1, idoleamine-2,3-dioxygenase 1; IL, interleukin; T-VEC, Talimogene laherparepvec; TCR, T cell receptor.
A fundamental challenge for the immuno-oncology field is the rational selection of agents from a vast number of possible combinations while contending with escalating financial costs and limited resources. The current clinical trial framework will need to be modernized to support the successful development and implementation of immunotherapy combinations into standard clinical care. Key components to consider include new approaches to optimize dose determination and operational efficiency, the incorporation of clinically fitting endpoints, and the integration of biomarker assessment to guide patient selection. This paper builds upon previous recommendations by the Clinical Trial Design Task Force (CTD) of the National Cancer Institute (NCI) Investigational Drug Steering Committee (IDSC) on combination phase I trials (16) and complements other papers in the CCR Focus series, outlining guidance on the design and conduct of immunotherapy clinical trials. The complex challenges of and recommendations for combination immunotherapy development are discussed here, with an emphasis on early phase trials (Table 2).
Table 2.
Summary of recommendations
| Nonclinical studies |
|---|
|
| Early phase trials: combination selection and overall goals |
|
| Early phase clinical trial design |
|
PD-1, programmed death 1; PK, pharmacokinetic; PD, pharmacodynamic.
MECHANISMS OF RESISTANCE TO IMMUNOTHERAPY AND THE RATIONALE FOR COMBINATIONS
Characterization of the human tumor micro-environment (TME), in particular its molecular features and the presence of lymphocytic infiltration, has led to the identification of distinct immunophenotypes (Figures 1 and 2) (17). These include a T cell-infiltrated phenotype with a broad chemokine profile and type I interferon signature and a non-T cell-infiltrated phenotype that lacks inflammatory signals for recruitment of T cells (18). The predominant mechanism of immunosuppression in the T cell-infiltrated or so-called ‘inflammed’ TME is postulated to be upregulation of inhibitory pathways rendering T cells dysfunctional, providing a rationale for targeting co-inhibitory molecules (see quadrant A in Figures 1 and 2). In fact, correlative data indicate that an underlying immune-active TME characterized by the presence of CD8+ T cells may be a pre-condition for response to immune checkpoint inhibitors (19, 20). Disabling a singular pathway may be insufficient, however, and may trigger compensatory mechanisms leading to resistance (21). These may be circumvented by inhibition of additional immune checkpoints or modulation of T cell co-stimulatory molecules, which upon engagement function to promote T cell activity. This hypothesis is validated by the demonstration of synergistic activity of multi-checkpoint blockade in non-clinical studies (22, 23). In some cases, interference with antigen presentation (B in Figures 1 and 2) is the primary barrier to T cell response and may be therapeutically targeted by strategies to enhance antigen-presenting cell function. Conversely, patients with non-T cell infiltrated or ‘non-inflammed’ phenotypes are unlikely to respond to immunomodulatory agents alone (C and D in Figure 1 and 2). Such patients will likely require more intensified combination therapies to induce and promote tumor T cell infiltration or recognition of tumor antigens, through modalities such as adoptive cell therapy, inflammatory cytokines, immune stimulatory agents and vaccines. Conventional therapies, such as chemotherapy, radiotherapy, and molecularly-targeted agents also have a role in priming the immune response by causing tumor death-related antigen presentation in addition to other immunomodulatory roles, with synergism found in combination with immunotherapies in multiple nonclinical studies (24–29). Another emerging combinatorial approach involves epigenetic therapies with in vivo demonstration of synergy; growing evidence indicates that epigenetic reprogramming may suppress immune-related genes and/or tumor-specific antigens (30). An alternative pragmatic classification model stratifies the TME into four types based on the presence or absence of tumor-infiltrating lymphocytes and PD-L1 expression (31, 32). However, caveats include the lack of standardized methodology and sampling challenges in light of intratumoral heterogeneity and the adaptive and dynamic nature of immune resistance. Furthermore, relevant variables such as tumoral stromal and molecular factors, and other immune cell populations are not characterized.
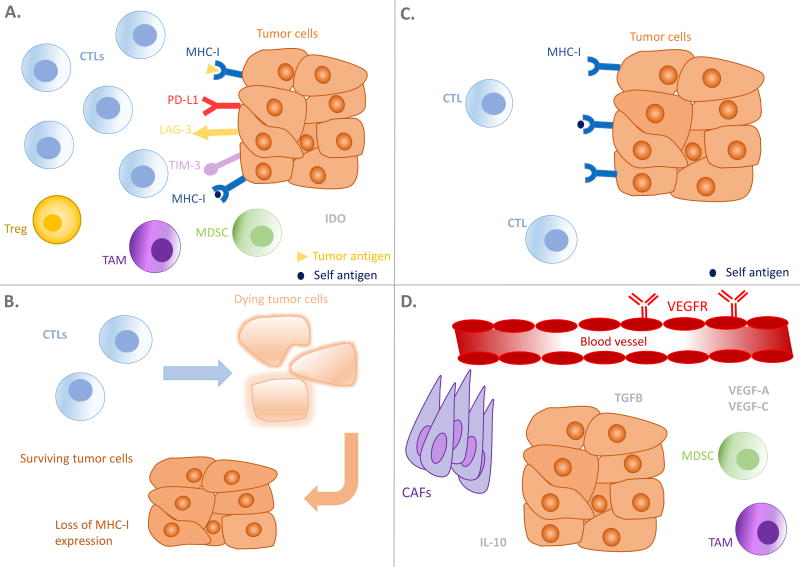
Figure 1. Schematic representation of examples of mechanisms of resistance.
CTL, cytotoxic T lymphocyte; Treg, T-regulatory cell; TAM, tumor-associated macrophage; MDSC, myeloid-derived suppressor cell; MHC-I, major histocompatibility complex-I; PD-L1, programmed death ligand 1; LAG-3, lymphocyte-activation gene 3; TGFβ, transforming growth factor-β; vascular endothelial growth factor receptor VEGFR; VEGF, vascular endothelial growth factor; CCL2, chemokine ligand 2; CAFs, cancer-associated fibroblasts; IL-10, interleukin 10.
The tumor microenvironment (TME) in A is T cell-rich, however T cells have been rendered dysfunctional by upregulated co-inhibitory pathways and/or immunosuppressive cells and metabolites. In B, another frequent mechanism of immune evasion is demonstrated, that is, the loss or downregulation of MHC-I expression, thereby affecting antigen presentation and recognition by T cells. The TME in C is characterized by poor immunogenicity and expression of tumor antigens, leading to minimal chemokine expression and T cell infiltration. Lack of co-stimulation may also leave the T cells present to be anergic or unresponsive. D shows a number of processes tumor exploit to prevent T cell recruitment, including adverse stromal factors, secretion of suppressive soluble factors (e.g. TGF-β and II-10) and dysfunctional tumor vasculature, which is turn is maintained by proangiogenic growth factors such as VEGF and fibroblast growth factor, and immunosuppressive myeloid cells such as MDSCs and TAMs.
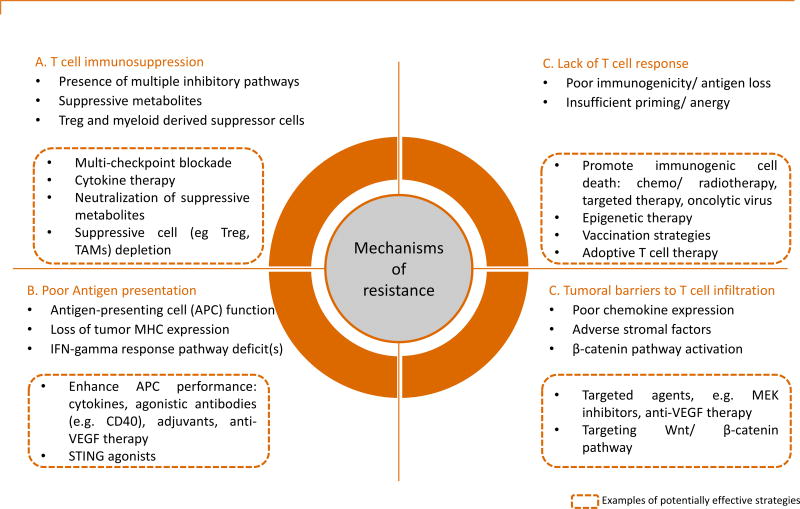
Figure 2. Potential mechanisms of resistance to immunotherapy and examples of therapeutic strategies.
APC, antigen-presenting cell; IFNγ, interferon gamma; Treg, regulatory T cells; TAMs, tumor-associated macrophages; MEK, mitogen-activated protein kinase kinase; VEGF, vascular endothelial growth factor; MHC, major histocompatibility complex; STING, stimulator of interferon genes.
Arguably the greatest challenge in the immuno-oncology field is primary and acquired resistance to therapy. While stratification of the TME provides a context for understanding anti-tumor immunity and guidance for treatment selection, more translational studies are essential for dissecting the molecular complexities of immune resistance. Interestingly, two recent genomic profiling studies linked acquired resistance to anti-PD-1 therapy and primary resistance to CTLA-4 blockade to defects in the pathways regulating interferon receptor signalling (33, 34). Additionally, tumor-intrinsic active β-catenin signalling has been identified as one potential mechanism of T cell exclusion (35). Another study in melanoma patients found innately anti-PD-1 therapy-resistant tumors display upregulation of genes involved in mesenchymal transition, cell adhesion, and angiogenesis, suggesting that these biological processes and their effects on the TME may impede anti-tumor immunity (36). Lastly, pharmacological factors including drug exposure and clearance, receptor occupancy and tumor penetrance; patient-intrinsic factors such as age, gender and body weight; and other cellular processes may also affect treatment and should be considered. Figures 1 and 2 illustrate examples of tumor immune escape mechanisms and a number of suggested combinatorial strategies to target these.
LESSONS LEARNED FROM PRIOR IMMUNOTHERAPY COMBINATIONS
Immunotherapy-immunotherapy combinations
The combination of the anti-CTLA-4 antibody, ipilimumab, and the anti-PD-1 antibody, nivolumab, is the most clinically studied immunotherapy doublet thus far (13–15, 37, 38), and the key lessons learnt are detailed in Table 3. In summary, the combination achieved enhanced activity characterized by earlier and deeper antitumor responses for a greater proportion of patients with melanoma, compared with monotherapy. Early survival data in the randomized phase III study in melanoma found that the combination significantly improved overall survival compared with ipilimumab (hazard ratio, 0.55; P<0.0001). At the present time with a minimum follow-up of 28 months, median overall survival has not been reached for the combination and nivolumab alone arms. In any case, the study is not powered for a statistical comparison between these two arms (39). Despite considerable activity, substantial treatment-related toxicities may challenge the clinical application of combination CTLA-4 and PD-1 blockade. Interestingly, the candidate dose regimen selected has differed among trials in melanoma, NSCLC, and RCC, primarily due to a determination in early studies that non-melanoma populations did not tolerate the regimen containing a higher ipilimumab dose (13, 37, 38). Differential dosing highlights potential tumor-specific differences in immune checkpoint inhibitor tolerability and efficacy, and the importance of thorough dose exploration studies. Pharmacological analyses have shown that treatment efficacy and toxicity is likely to be dose-dependent for ipilimumab while anti-PD-1 agents demonstrated relatively flat exposure-efficacy relationships (40–42). Moreover, despite regulatory approvals in Europe and North America, the optimal combination dose regimen in melanoma is still not clear and is the subject of an ongoing trial (NCT02714218). The challenges of varying dosing strategies of immune checkpoint inhibitors are further discussed by Baik and colleagues (43).
Table 3.
Lessons learned from the ipilimumab and nivolumab combination
|
|
|
|
|
|
|
|
|
|
Ipi, ipilimumab; nivo, nivolumab; m, months; HR, hazard ratio; AE, adverse event; PD-L1, programmed death ligand 1; vs, versus; PD-1, programmed death 1; NSCLC, non-small cell lung cancer; RCC, renal cell carcinoma; QXW, every X weeks; CTLA-4, cytotoxic T-lymphocyte-associated antigen-4; irAEs, immune-related adverse events.
Another critical challenge in immuno-oncology is patient selection. Although antitumor activity was enriched in the PD-L1 positive population in the landmark phase III study in melanoma, incremental progression-free survival (PFS) gains compared with single-agent nivolumab was greater in the PD-L1 negative population in subgroup analysis, suggesting PD-L1 may have a role in selecting patients who require doublet therapy (15). Nevertheless, the therapeutic success of pairing ipilimumab and nivolumab has spurred the ongoing investigation of similar combinations in various tumor types (44), including the combination of anti-PD-L1 antibody, durvalumab and anti-CTLA-4 antibody, tremelimumab which has demonstrated encouraging results in NSCLC in a phase Ib study (45). Additionally, numerous novel immunotherapy combinations are in various stages of development, often with CTLA-4 or PD-1/PD-L1 inhibitors as backbones, and many are exhibiting promising early activity and safety profiles. Examples include idoleamine-2,3-dioxygenase 1 (IDO-1) inhibitor with ipilimumab (46) in melanoma and the anti-PD-1 antibody, pembrolizumab (47) in selected tumor types, and intratumoral injections of oncolytic virus, Talimogene laherparepvec (T-VEC) in combination with pembrolizumab in melanoma (48). In fact, currently there are over 800 clinical trials testing approximately 20 anti-PD-1/PD-L1/PD-L2 agents alone and in combinations for numerous indications (49).
Immunotherapy in combination with chemotherapy, radiotherapy and molecularly-targeted agents
A phase II study in advanced NSCLC showed that the addition of ipilimumab to platinum-based chemotherapy in a ‘phased’ fashion (cycle 3 to cycle 6) modestly improved median immune-related PFS (50). In another phase II study in non-squamous NSCLC, pembrolizumab combined with chemotherapy demonstrated improved response rate and PFS, compared with chemotherapy alone, with acceptable tolerability (51).
Pre-clinical and clinical data suggest that radiotherapy is a promising modality for combinatorial immunotherapy strategies. In addition to debulking tumor and releasing tumor antigens, radiotherapy has well-established immunomodulatory effects which may counteract mechanisms of resistance such as poor-immunogenicity and T-cell exclusion, and elicit systemic abscopal effects (52). This was aptly demonstrated in a proof-of-principle trial in advanced solid tumor patients, where the combination of radiotherapy to a single metastatic lesion with granulocyte-macrophage colony stimulating factor produced objective abscopal responses in 27% of patients (53). To date, clinical trials employing radiotherapy and CTLA-4 blockade have not conclusively shown clear benefit, including a negative phase III trial of ipilimumab versus placebo after radiotherapy in prostate cancer, but do confirm the safety of the combination (26, 54, 55). Trials combining radiotherapy with PD-1/PD-L1 checkpoint blockade are underway. A recent secondary analysis of the KEYNOTE-001 trial suggests that patients treated with PD-1 blockade who had received prior radiotherapy experienced an improved median progression-free survival (4.4 versus 2.1 months, p=0.019) and median overall survival (10.7 versus 5.3 months, p=0.026) (56), but these findings should be interpreted cautiously and require confirmation in prospective randomized trials. Immune-stimulatory agents such as interleukin-2 (57) in melanoma and RCC and a Toll-like receptor agonist (58) in low grade B cell lymphoma have demonstrated promising early results in combination with radiotherapy.
Early phase data of anti-PD-1/PD-L1 agents combined with standard-of-care molecularly-targeted agents in multiple tumor types appear to be well tolerated, although efficacy outcomes are largely pending (59–61). Recently, novel combinations of anti-PD-1/PD-L1 therapy in early phase trials with the MEK inhibitor cobimetinib in colorectal cancer (62); immunomodulatory agent lenolidamide in relapsed/refractory multiple myeloma (63); and antiandrogen enzalutamide in enzalutamide-resistant prostate cancer (64) have resulted in compelling efficacy despite expectedly limited monotherapy activity. The finding in the last example complements correlative data demonstrating PD-L1 upregulation in enzalutamide-resistant prostate cancer cells (65), suggesting that the molecular features within the TME may evolve in response to treatment pressures, and hence by inference, sensitivity to immunotherapy may fluctuate at different stages of the disease process. Genomic factors such as possible underlying mismatch repair defects, defects in DNA proofreading due to loss of function of DNA polymerase epsilon (66) or BRCA2 mutations may have also influenced the antitumor activity seen in this subgroup and remain to be explored.
Despite these early sources of enthusiasm, important caveats remain when combining immunotherapy with conventional therapy. Firstly, toxicities may be potentiated and unanticipated, as evidenced by the first clinical experiences combining anti-CTLA-4 antibodies with the BRAF inhibitor vemurafenib and the vascular endothelial growth factor receptor (VEGFR) inhibitor sunitinib where hepatoxicity and rapid-onset acute renal failure, respectively, led to trial closures (29, 67, 68). Interestingly, a number of reports of patients receiving vemurafenib following treatment with anti-CTLA-4 and anti-PD-1 antibodies suggest sequential therapies may also lead to severe cutaneous and systemic adverse events (69–71). Although there is currently limited understanding of the pharmacological and immune-mediated mechanisms underlying these toxicities, these findings emphasize the need to demonstrate safety of new combinations in the clinical trial setting, even for agents with regulatory approval and non-overlapping toxicity profiles. The adequate washout periods in the case of sequential treatments are also not known and clinicians need to monitor vigilantly for potential augmented toxicities. Secondly, timing and sequencing of treatments are likely to have an impact on efficacy. Emerging evidence suggests that the immune responses induced by molecularly-targeted agents may be early and transient, and low CD8+ T cell density is seen at treatment progression (72). In the case of chemotherapy and radiation, the immunomodulatory effects are complex and some of these effects may be suppressive (73). Additionally, there is considerable variability in both modalities in terms of treatment type and quality, dose and fractionation, and schedule and timing. Limited mechanistic data are available to guide how to best combine these treatments and immunotherapy in light of these variables. Thus, caution must be exercised as the consequence of compromising the efficacy of established treatments is substantial, particularly in curative settings, and every effort should be made to elucidate individual and combined immunomodulatory and pharmacological effects.
GOALS AND CHALLENGES OF IMMUNOTHERAPY COMBINATIONS
Scientific challenges: prioritizing evidence-based combinations
Current combination selection is largely empiric, based on availability and a supposition of complementary and non-redundant mechanisms of action. As more targets and therapies are discovered, prioritizing the most promising combinations and rational sequencing of therapies will be crucial. Combinations must be designed to address clinical and biological challenges and should provide a significant advantage over monotherapy by deactivating mechanisms of immune escape, or substantially augmenting responses, while maintaining acceptable tolerability. Given these objectives, industry collaborations should be strongly encouraged to avoid duplication of efforts and investigational pipelines, in order to minimize cost, redundant resource utilization and regulatory pressures. Goals and recommendations for early phase combination immunotherapy trials are summarized in Table 2, beginning with a strong scientific hypothesis supported by nonclinical or clinical data.
Currently, there are substantial limitations to nonclinical studies, including suboptimal reproducibility (74, 75), publication bias and insufficiently characterized combination index (defined as a quantitative measure of combination drug effects) (76). In immunotherapy research, an additional barrier arises from inherent differences in immune systems across species and tumor antigen repertoire, leading to poor recapitulation of host immune effects, as highlighted by unforeseen severe immune-mediated toxicities in the first-in-human (FIH) study of a CD28 agonist (77). Active efforts are being made to improve the reliability of nonclinical models to better simulate clinical complexities (78), including the development of alternative translational models such as various immunocompetent allograft mouse models and ‘humanized’ mouse models in which murine immune-related genes or proteins are replaced with human equivalents (79). Another example is the use of companion canines that develop spontaneous tumors in the setting of an intact immune system (27). The advantages of canine models include large population size and tumor and immune system characteristics that are more akin to that of humans compared with rodent models (80).
Limitations notwithstanding, nonclinical studies are an excellent platform for mechanistic and exploratory studies and have helped to guide the selection of current immunotherapy combinations. Recommended aims of nonclinical studies to consider when designing experimental conditions are detailed in Table 2. Additionally, considering the limited predictive capacity of nonclinical studies, initial small proof-of-principle clinical studies with high efficacy bars may also be appropriate to select combinations to take forward.
Patient selection considerations and biomarkers
An imperative for immunotherapy combinations is to focus on populations with unmet needs, particularly those who are unlikely to derive benefit from monotherapy. However, currently there is no precise method of biomarker-driven patient selection. PD-L1 expression is the most mature biomarker for anti-PD-1/PD-L1 therapy and several companion diagnostic PD-L1 assays have been approved by the US Food and Drug Administration (FDA). It enriches for responders in some but not consistently in all disease indications, and a negative result cannot reliably predict non-response (3, 15, 81). Additional drawbacks include variability in assay techniques and quantitative cut-offs (81). The initial results of a collaborative project evaluating the analytical comparability of the four PD-L1 companion assays used in NSCLC, found that while three assays demonstrated similar tumor cell PD-L1 expression, inter-observer discrepancy was high for immune cell PD-L1 expression. Notably, the use of alternative assays would lead to discordance in PD-L1 positivity and the treatment-determining threshold in 37% of cases (82).
To refine personalized treatment selection, intensive efforts have been invested in biomarker discovery for immunotherapies and these approaches are likely to be complementary to PD-L1 expression. For example, the aforementioned stratification of the TME based on PD-L1 status and lymphocytic infiltration has been described to guide treatment options (32, 83). Although several groups found tumor mutational burden and neoantigen load were positively associated with immune checkpoint inhibitor response, there was overlap in the range of mutations and neoantigens between the responders and nonresponders (84, 85). A phase II study demonstrated the utility of DNA mismatch repair status as a predictor of response to PD-1 blockade (9). Mismatch repair-deficiency results in microsatellites and far greater numbers of mutation-associated neoantigens which is thought to be the basis of increased immune infiltrates and improved immunotherapy response in these tumors, compared with mismatch repair-proficient tumors (9). Other promising emerging biomarkers include gene expression patterns and signatures. Elevated baseline expression of immune-related genes, including T helper type-1 and interferon-gamma pathway-related genes is associated with favorable response to immunotherapies in multiple tumor types (8, 20, 86, 87). Conversely, analyses from The Cancer Genome Atlas in 13 tumor histologies showed that increased Wnt/β-catenin pathway signalling correlates with absence of T cell gene expression and may mediate both primary and acquired resistance to immunotherapy (35, 88).
It is foreseeable that in the near future, novel techniques such as immune monitoring, tumor antigen profiling, T cell receptor sequencing and gene expression signatures at multiple treatment time points can provide integrated multidimensional and dynamic data on a patient’s immune milieu, offering hope for individualized treatment selection (89). The challenges and future directions of immune biomarkers are discussed by Mehnert and colleagues in this CCR Focus series (90). At present, in the absence of validated biomarkers, one approach for combination trials may be to restrict eligibility to salvage settings for patients who are or likely to be monotherapy-refractory. One example is a randomized phase II study assessing the efficacy of ipilimumab versus ipilimumab and nivolumab in anti-PD-1 therapy-refractory patients (NCT02731729).
Dose selection and the need for innovative trial designs
Traditional rule-based designs, such as the classic ‘3+3’ design, that use toxicity-driven dose escalation to define the maximum tolerated dose (MTD) and assume a linear dose-efficacy-toxicity relationship, are unlikely to be adequate for immunotherapies. Immune-driven effects are difficult to predict and depend on a myriad of poorly understood factors beyond drug dose and exposure. Well-tolerated agents may also achieve the desired target effect without producing significant detectable toxicity. In fact, in many immune checkpoint inhibitor phase I trials, MTDs were not reached with few dose-limiting toxicity (DLT) events (91). Immune-related adverse events (IrAEs) may also be delayed, and will not be sufficiently captured by the DLT observational period (generally the first cycle of treatment). Moreover, combination agents introduce further challenges including pharmacodynamic (PD) and pharmacokinetic (PK) interactions, potentially overlapping or additive toxicity profiles, and multiple possible combinations of MTDs and schedules. These complexities necessitate novel integrated approaches in trial design.
Model-based designs and Bayesian methods were developed to provide more precise estimates of the recommended phase II dose (RP2D), by building on a pre-study a priori dose-toxicity curve, then using accumulating data during the trial to update the curve and inform prospective dose escalation decisions. Features of contemporary designs can be extended to include other clinically relevant endpoints such as efficacy, pharmacology parameters and long term tolerability (92). Parallel PK/PD assessments help to define pharmacologic properties for each agent and in combination, and may inform dose determination. For example, pharmacological data (in this case, the dose sufficient to maintain target drug levels) assisted in determining the dose of the anti-PD-L1 antibody, atezolizumab in a phase I study when MTD was not reached (93).
Although 3+3 designs have been used in the majority of past phase I trials owing to their operational simplicity, model-based, Bayesian and hybrid approaches are increasingly adopted as the therapeutic landscape and statistical capabilities evolve (92, 94). The modified toxicity probability interval design, which couples a rule-based dose-finding scheme with guidance by Bayesian posterior estimates (95), is employed in numerous ongoing immunotherapy combination trials. To delineate the degree of additive toxicity by a combination regimen, a randomized Bayesian phase I design has been proposed in which dose determination is based on the difference of probability of DLTs between the control (single agent) arm and the combination arm (96). Furthermore, multiple statistical designs have been described using both toxicity and efficacy endpoints, and may be well-suited to immunotherapies combination trials to assess for early activity (92, 97). One such example is the parallel phase I/II zone design which utilizes rule-based dose escalation and subsequent Bayesian adaptive randomization to maximize the number of patients treated with the most effective dose combinations (97).
The choice of dose escalation design should be individualized, taking into consideration non-clinical and single agent pharmacology data, desired trial outcomes and aims, target patient population characteristics and the intended drug development plan. In combination trials, where a wide range of dose pairings are possible, a pragmatic approach may be to identify an effective dose range or a number of admissible schedules for further evaluation in subsequent expansion or phase II studies (98). Although regimen selection is preferred prior to the registration study, post-approval dose optimization may be necessary. The aforementioned trial of ipilimumab and nivolumab is an example (NCT02714218). Additionally, the duration of anti-PD-1 therapy sufficient to trigger durable immune responses is currently undefined and is the theme of ongoing investigation (NCT02821013).
Aside from dose escalation trials, population pharmacological modelling correlating exposure and other PK data with toxicity, efficacy and other multifactorial endpoints may have a complementary role in supporting dose selection and may further characterize the target therapeutic window (40–42). Indeed, flat-dosing of nivolumab and pembrolizumab were found to be comparable to weight-based dosing in population PK/PD analyses, leading to FDA approval of flat dosing for a number of indications (99). Moreover, in the case of pembrolizumab, findings from translational PK/PD murine modelling and human simulations were applied to select a minimum effective dose to guide ongoing clinical evaluation (100).
Measures of success: assessing outcomes
As discussed by Anagnostou and colleagues in this series, the determination of clinically meaningful efficacy endpoints in immunotherapy trials is contentious, owing to atypical immune response patterns (101–103). Delayed anti-tumor effect can lead to late separation of survival curves in randomized trials, affecting study duration and statistical power in detecting differences in the overall treatment effect (104). Sustained stable disease in the absence of tumor shrinkage can also be seen in a subset of patients and is associated with improved survival (105). Conversely, concurrent PD-1 and CTLA-4 inhibition is associated with deep and early tumor responses, with complete response rates approaching 20% in the first line treatment of melanoma (14, 15). Thus, endpoint selection needs to appropriately capture the expected biology of the agent(s) under investigation based on the mechanisms of action and disease setting; in therapeutic combinations, this is likely to be driven by the most active agent. For agents with delayed or cytostatic activity, disease control rate, PFS or overall survival may be preferred, although the latter may be confounded by subsequent treatments. For combinations anticipated to have substantial activity, response-based endpoints such as complete response rate, durable response rate, or composite measures encapsulating both depth and duration of response are likely reliable measures of early efficacy and surrogates for long-term survival. As previously recommended by the IDSC, randomized combination phase II trials are preferred to single-arm studies to firmly establish efficacy (106). Furthermore, early phase trials should incorporate comprehensive PD assessments and biomarkers to correlate clinical outcomes with mechanistic biological effects.
Importantly, an acceptable balance between toxicity and efficacy is fundamental to the success and clinical utility of drug therapies, and is of particular concern when agents are combined. Immune-mediated tissue injuries are wide-ranging and variable in presentation and time of onset (107). A systematic review found substantial heterogeneity in the completeness and quality of irAE reporting across immune checkpoint inhibitor trials (108). A further consideration in clinical trials of novel combinations is that the causality attribution of adverse events may be problematic, particularly in the absence of monotherapy comparator treatment arms. Additionally, health-related quality of life (HRQoL) is a key consideration and a goal of anticancer care alongside lengthening life. In particular, chronic low grade toxicities can significantly affect patient wellbeing, but are generally under-reported in clinical trials and not captured as DLTs. Encouragingly, HRQoL is increasingly assessed in late phase immunotherapy trials using existing instruments, with improvements shown compared with standard-of-care therapies (109, 110). To more precisely evaluate the kinetics and clinical impact of immune-driven toxicities, standardized reporting of irAEs and patient-reported HRQoL - ideally utilizing tools developed for immunotherapies - should be routinely incorporated into combination clinical trials, in both palliative and adjuvant settings.
Improving drug development efficiency: the tension between speed and safety
The unprecedented success of immune checkpoint inhibitors has generated tremendous enthusiasm to expedite the development of new immunotherapeutics and combinations. In response, clinical trial designs are evolving from the conventional sequential phase I–II–III model, characterized by lengthy timelines and high failure rates (111), to approaches such as seamless phase I/II and phase I/III trials, and the use of large cohort expansions in FIH trials (112–114). Early phase trials using novel designs can be geared toward answering more complex objectives and emerging hypotheses beyond dose-finding, such as preliminary efficacy and biomarker evaluation. For example, multi-cohort FIH trials of novel agents using a common anti-PD-1/PD-L1 backbone under a master protocol can rapidly screen for the most effective combinations for further investigation. Additionally, expansion cohorts are increasingly utilized as an early enrichment strategy, often to estimate efficacy in disease-specific or biomarker-specific groups and in exceptional cases, have supported accelerated approval (113, 114). However, to mitigate the risks of these streamlined approaches, maintain quality control and safeguard patient interests, clearly defined objectives, flexible statistical designs, pre-determined futility rules and scheduled independent oversight by external data and safety monitors are required. To avoid the immense financial and human costs of negative large late-phase trials, it must be stressed that abbreviated development pathways should be restricted to agents showing substantial activity and foreseeable advantages over standard therapies.
Recognizing the need for therapeutic combinations and in an endeavour to promote industry collaboration, the FDA has provided guidance on the co-development of unmarketed drugs, with an emphasis on frequent interactions with the FDA during the investigational and marketing process. Furthermore, FDA-directed expedited programs, such as breakthrough designation, provide intensive regulatory support that can work in concert with accelerated clinical development strategies outlined above (113). Lastly, cumbersome processes can hinder trial conduct and accrual (115), and efforts should be directed to reform the existing clinical trials system to improve operational efficiency, as recommended by reports from the NCI and the Institute of Medicine (116, 117).
CONCLUSIONS
CTLA-4 and PD-1/PD-L1-based immunotherapies have produced durable responses and even long-term survival in patients with advanced cancer, pioneering the concept of the ‘clinical cure’. Owing to their success and accumulating scientific knowledge in tumor immunology, novel therapies and combinations are rapidly entering development, with unprecedented therapeutic potential to transform cancer care. However, the conventional nonclinical and clinical framework predominantly designed for cytotoxic drug development may not be adequate for immunotherapies and these shortcomings are amplified in the combination setting. A coordinated effort from industry, regulatory authorities, and the scientific and medical communities is required to meet these challenges and much progress has already been made. An immediate goal in the field is the rational selection of combinations based on mechanistic evidence or robust biological rationale to overcome intrinsic and acquired immune resistance, particularly to anti-PD-1/PD-L1 agents. Novel trial designs and statistical methodologies tailored to immunotherapy characteristics should be applied to investigate these combinations effectively and in an efficient manner. As biomarker-based techniques mature, they will help to lend longitudinal insight into tumor-immune interactions, identify predictors of response, and refine patient selection with the eventual goal of personalized medicine.
Acknowledgments
DD is an Ontario Institute of Cancer Research (OICR) Research Fellow and is supported by the OICR through funding provided by the Government of Ontario.
Footnotes
Potential conflicts of interest
AMM reports receiving clinical trial funding from Genentech, Transgene, Merck, Incyte; EHR is an employee at Merck; MOB has served on advisory boards for Merck, Bristol-Myers Squibb, Novartis, EMD Serono, and Immunocore. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373(19):1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2015;33(18):2004–12. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–20. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17(6):717–26. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 8.Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956–65. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 9.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18(7):2039–47. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, et al. Three-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. J Clin Oncol. 2016;34 doi: 10.1093/annonc/mdz011. (suppl; abstr 9503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, et al. Association of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced Melanoma. Jama. 2016;315(15):1600–9. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 13.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paller CJ, Bradbury PA, Ivy SP, Seymour L, LoRusso PM, Baker L, et al. Design of phase I combination trials: recommendations of the Clinical Trial Design Task Force of the NCI Investigational Drug Steering Committee. Clin Cancer Res. 2014;20(16):4210–7. doi: 10.1158/1078-0432.CCR-14-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whiteside TL, Demaria S, Rodriguez-Ruiz ME, Zarour HM, Melero I. Emerging Opportunities and Challenges in Cancer Immunotherapy. Clin Cancer Res. 2016;22(8):1845–55. doi: 10.1158/1078-0432.CCR-16-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–22. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61(7):1019–31. doi: 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107(9):4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J Immunother Cancer. 2014;2:3. doi: 10.1186/2051-1426-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120(4):1111–24. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Correale P, Del Vecchio MT, La Placa M, Montagnani F, Di Genova G, Savellini GG, et al. Chemotherapeutic drugs may be used to enhance the killing efficacy of human tumor antigen peptide-specific CTLs. J Immunother. 2008;31(2):132–47. doi: 10.1097/CJI.0b013e31815b69c8. [DOI] [PubMed] [Google Scholar]
- 26.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–7. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monjazeb AM, Kent MS, Grossenbacher SK, Mall C, Zamora AE, Mirsoian A, et al. Blocking Indolamine-2,3-Dioxygenase Rebound Immune Suppression Boosts Antitumor Effects of Radio-Immunotherapy in Murine Models and Spontaneous Canine Malignancies. Clin Cancer Res. 2016;22(17):4328–40. doi: 10.1158/1078-0432.CCR-15-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19(5):1225–31. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu-Lieskovan S, Robert L, Homet Moreno B, Ribas A. Combining targeted therapy with immunotherapy in BRAF-mutant melanoma: promise and challenges. J Clin Oncol. 2014;32(21):2248–54. doi: 10.1200/JCO.2013.52.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghoneim HE, Zamora AE, Thomas PG, Youngblood BA. Cell-Intrinsic Barriers of T Cell-Based Immunotherapy. Trends Mol Med. 2016;22(12):1000–11. doi: 10.1016/j.molmed.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015;75(11):2139–45. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Chen L. Classification of Advanced Human Cancers Based on Tumor Immunity in the MicroEnvironment (TIME) for Cancer Immunotherapy. JAMA Oncol. 2016;2(11):1403–4. doi: 10.1001/jamaoncol.2016.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med. 2016;375(9):819–29. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, et al. Loss of IFN-gamma Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell. 2016;167(2):397–404. doi: 10.1016/j.cell.2016.08.069. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–5. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 36.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell. 2016;165(1):35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18(1):31–41. doi: 10.1016/S1470-2045(16)30624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hammers HJ, Plimack ER, Infante JR, Rini BI, McDermott DF, Ernstoff M, et al. Expanded cohort results from CheckMate 016: A phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2015;33 doi: 10.1200/JCO.2016.72.1985. (suppl; abstr 4516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larkin J, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival (OS) results from a phase III trial of nivolumab (NIVO) combined with ipilimumab (IPI) in treatment-naïve patients with advanced melanoma (CheckMate 067) AACR. 2017 [Google Scholar]
- 40.Feng Y, Masson E, Dai D, Parker SM, Berman D, Roy A. Model-based clinical pharmacology profiling of ipilimumab in patients with advanced melanoma. Br J Clin Pharmacol. 2014;78(1):106–17. doi: 10.1111/bcp.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chatterjee M, Turner DC, Felip E, Lena H, Cappuzzo F, Horn L, et al. Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol. 2016;27(7):1291–8. doi: 10.1093/annonc/mdw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agrawal S, Feng Y, Roy A, Kollia G, Lestini B. Nivolumab dose selection: challenges, opportunities, and lessons learned for cancer immunotherapy. J Immunother Cancer. 2016;4:72. doi: 10.1186/s40425-016-0177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baik CS, Rubin EH, Forde PM, Mehnert JM, Collyar D, Butler MO, et al. Limitations and Challenges in Immuno-Oncology Clinical Trials. Clin Can Res. 2017;23 doi: 10.1158/1078-0432.CCR-16-3066. xx-xx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antonia SJ, Lopez-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883–95. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 45.Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17(3):299–308. doi: 10.1016/S1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gibney GT, Hamid O, Gangadhar TC, Lutzky J, Olszanski AJ, Gajewski T, et al. Preliminary results from a phase 1/2 study of INCB024360 combined with ipilimumab (ipi) in patients (pts) with melanoma. J Clin Oncol. 2014;32 5s, (suppl; abstr 3010) [Google Scholar]
- 47.Gangadhar TC, Hamid O, Smith DC, Bauer TM, Wasser JS, Luke JJ, et al. Preliminary results from a Phase I/II study of epacadostat (incb024360) in combination with pembrolizumab in patients with selected advanced cancers. J Immunother Cancer. 2015;3(Suppl 2):O7. [Google Scholar]
- 48.Ribas A, Puzanov I, Gajewski T, Long GV, Dummer R, Kirkwood JM, et al. A multicenter, open-label trial of talimogene laherparepvec (T-VEC) plus pembrolizumab vs pembrolizumab monotherapy in previously untreated, unresected, stage IIIB-IV melanoma. J Clin Oncol. 2015;33 (suppl; abstr TPS9081) [Google Scholar]
- 49.Brawley L. With 20 Agents, 803 Trials, and 166,736 Patient Slots, Is Pharma Investing Too Heavily in PD-1 Drug Development. The Cancer Letter, Oct. 2016 < https://cancerletter.com/articles/20161007_1/>viewed May 2017.
- 50.Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30(17):2046–54. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 51.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demaria S, Golden EB, Formenti SC. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol. 2015;1(9):1325–32. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- 53.Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16(7):795–803. doi: 10.1016/S1470-2045(15)00054-6. [DOI] [PubMed] [Google Scholar]
- 54.Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24(7):1813–21. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–12. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017 doi: 10.1016/S1470-2045(17)30380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seung SK, Curti BD, Crittenden M, Walker E, Coffey T, Siebert JC, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2--tumor and immunological responses. Sci Transl Med. 2012;4(137):137ra74. doi: 10.1126/scitranslmed.3003649. [DOI] [PubMed] [Google Scholar]
- 58.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010;28(28):4324–32. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bendell JC, Powderly JD, Lieu CH, Eckhardt SG, Hurwitz H, Hochster HS, et al. Safety and efficacy of MPDL3280A (anti-PDL1) in combination with bevacizumab (bev) and/or FOLFOX in patients (pts) with metastatic colorectal cancer (mCRC) J Clin Oncol. 2015;33 (suppl 3; abstr 704) [Google Scholar]
- 60.Creelan BC, Chow LQ, Kim DW, Kim SW, Yeh T, Karakunnel JJ, et al. Safety and tolerability results from a phase I study of MEDI4736, a human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib in patients (pts) with non-small-cell lung cancer (NSCLC) J Clin Oncol. 2015;33 (suppl; abstr 3047) [Google Scholar]
- 61.Ribas A, Butler M, Lutzky J, Lawrence DP, Robert C, Miller W, et al. Phase I study combining anti-PD-L1 (MEDI4736) with BRAF (dabrafenib) and/or MEK (trametinib) inhibitors in advanced melanoma. J Clin Oncol. 2015;33 (suppl; abstr 3003) [Google Scholar]
- 62.Bendell JC, Kim TW, Goh BC, Wallin J, Oh DY, Han SW, et al. Clinical activity and safety of cobimetinib (cobi) and atezolizumab in colorectal cancer (CRC) J Clin Oncol. 2016;34 (suppl; abstr 3502) [Google Scholar]
- 63.Mateos MV, Orlowski RZ, DiCapua Siegel DS, Reece DE, Moreau P, Ocio EM, et al. Pembrolizumab in combination with lenalidomide and low-dose dexamethasone for relapsed/refractory multiple myeloma (RRMM): Final efficacy and safety analysis. J Clin Oncol. 2016;34 (suppl; abstr 8010) [Google Scholar]
- 64.Graff JN, Alumkal JJ, Drake CG, Thomas GV, Redmond WL, Farhad M, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016;7(33):52810–7. doi: 10.18632/oncotarget.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bishop JL, Sio A, Angeles A, Roberts ME, Azad AA, Chi KN, et al. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget. 2015;6(1):234–42. doi: 10.18632/oncotarget.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mehnert JM, Panda A, Zhong H, Hirshfield K, Damare S, Lane K, et al. Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J Clin Invest. 2016;126(6):2334–40. doi: 10.1172/JCI84940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368(14):1365–6. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]
- 68.Rini BI, Stein M, Shannon P, Eddy S, Tyler A, Stephenson JJ, Jr, et al. Phase 1 dose-escalation trial of tremelimumab plus sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2011;117(4):758–67. doi: 10.1002/cncr.25639. [DOI] [PubMed] [Google Scholar]
- 69.Harding JJ, Pulitzer M, Chapman PB. Vemurafenib sensitivity skin reaction after ipilimumab. N Engl J Med. 2012;366(9):866–8. doi: 10.1056/NEJMc1114329. [DOI] [PubMed] [Google Scholar]
- 70.Khoja L, Butler MO, Chappell MA, Hogg D, Joshua AM. Increased treatment-related toxicity subsequent to an anti-PD-1 agent. Curr Oncol. 2015;22(4):e320–2. doi: 10.3747/co.22.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson DB, Wallender EK, Cohen DN, Likhari SS, Zwerner JP, Powers JG, et al. Severe cutaneous and neurologic toxicity in melanoma patients during vemurafenib administration following anti-PD-1 therapy. Cancer Immunol Res. 2013;1(6):373–7. doi: 10.1158/2326-6066.CIR-13-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cooper ZA, Reuben A, Spencer CN, Prieto PA, Austin-Breneman JL, Jiang H, et al. Distinct clinical patterns and immune infiltrates are observed at time of progression on targeted therapy versus immune checkpoint blockade for melanoma. Oncoimmunology. 2016;5(3):e1136044. doi: 10.1080/2162402X.2015.1136044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wirsdorfer F, Cappuccini F, Niazman M, de Leve S, Westendorf AM, Ludemann L, et al. Thorax irradiation triggers a local and systemic accumulation of immunosuppressive CD4+ FoxP3+ regulatory T cells. Radiat Oncol. 2014;9:98. doi: 10.1186/1748-717X-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature. 2012;483(7391):531–3. doi: 10.1038/483531a. [DOI] [PubMed] [Google Scholar]
- 75.Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov. 2011;10(9):712. doi: 10.1038/nrd3439-c1. [DOI] [PubMed] [Google Scholar]
- 76.Ocana A, Amir E, Yeung C, Seruga B, Tannock IF. How valid are claims for synergy in published clinical studies? Ann Oncol. 2012;23(8):2161–6. doi: 10.1093/annonc/mdr608. [DOI] [PubMed] [Google Scholar]
- 77.Hunig T. The storm has cleared: lessons from the CD28 superagonist TGN1412 trial. Nat Rev Immunol. 2012;12(5):317–8. doi: 10.1038/nri3192. [DOI] [PubMed] [Google Scholar]
- 78.Klevorn LE, Teague RM. Adapting Cancer Immunotherapy Models for the Real World. Trends Immunol. 2016;37(6):354–63. doi: 10.1016/j.it.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zitvogel L, Pitt JM, Daillere R, Smyth MJ, Kroemer G. Mouse models in oncoimmunology. Nat Rev Cancer. 2016;16(12):759–73. doi: 10.1038/nrc.2016.91. [DOI] [PubMed] [Google Scholar]
- 80.Park JS, Withers SS, Modiano JF, Kent MS, Chen M, Luna JI, et al. Canine cancer immunotherapy studies: linking mouse and human. J Immunother Cancer. 2016;4:97. doi: 10.1186/s40425-016-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grigg C, Rizvi NA. PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction? J Immunother Cancer. 2016;4:48. doi: 10.1186/s40425-016-0153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12(2):208–22. doi: 10.1016/j.jtho.2016.11.2228. [DOI] [PubMed] [Google Scholar]
- 83.Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13(3):143–58. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 84.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ulloa-Montoya F, Louahed J, Dizier B, Gruselle O, Spiessens B, Lehmann FF, et al. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J Clin Oncol. 2013;31(19):2388–95. doi: 10.1200/JCO.2012.44.3762. [DOI] [PubMed] [Google Scholar]
- 87.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–46. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 88.Luke JJ, Bao R, Spranger, et al. Correlation of WNT/β-catenin pathway activation with immune exclusion across most human cancers. J Clin Oncol. 2016;34 doi: 10.1158/1078-0432.CCR-18-1942. (suppl; abstr 3004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yuan J, Hegde PS, Clynes R, Foukas PG, Harari A, Kleen TO, et al. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J Immunother Cancer. 2016;4:3. doi: 10.1186/s40425-016-0107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mehnert JM, Monjazeb AM, Beerthuijzen JMT, Collyar D, Rubinstein L, Harris LN. The Challenge for Development of Valuable Immuno-Oncology Biomarkers. Clin Can Res. 2017;23 doi: 10.1158/1078-0432.CCR-16-3063. xx-xx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Postel-Vinay S, Aspeslagh S, Lanoy E, Robert C, Soria JC, Marabelle A. Challenges of phase 1 clinical trials evaluating immune checkpoint-targeted antibodies. Ann Oncol. 2016;27(2):214–24. doi: 10.1093/annonc/mdv550. [DOI] [PubMed] [Google Scholar]
- 92.Harrington JA, Wheeler GM, Sweeting MJ, Mander AP, Jodrell DI. Adaptive designs for dual-agent phase I dose-escalation studies. Nat Rev Clin Oncol. 2013;10(5):277–88. doi: 10.1038/nrclinonc.2013.35. [DOI] [PubMed] [Google Scholar]
- 93.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iasonos A, O’Quigley J. Adaptive dose-finding studies: a review of model-guided phase I clinical trials. J Clin Oncol. 2014;32(23):2505–11. doi: 10.1200/JCO.2013.54.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ji Y, Liu P, Li Y, Bekele BN. A modified toxicity probability interval method for dose-finding trials. Clin Trials. 2010;7(6):653–63. doi: 10.1177/1740774510382799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dejardin D, Lesaffre E, Hamberg P, Verweij J. A randomized phase I Bayesian dose escalation design for the combination of anti-cancer drugs. Pharm Stat. 2014;13(3):196–207. doi: 10.1002/pst.1618. [DOI] [PubMed] [Google Scholar]
- 97.Huang X, Biswas S, Oki Y, Issa JP, Berry DA. A parallel phase I/II clinical trial design for combination therapies. Biometrics. 2007;63(2):429–36. doi: 10.1111/j.1541-0420.2006.00685.x. [DOI] [PubMed] [Google Scholar]
- 98.Yap TA, Omlin A, de Bono JS. Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol. 2013;31(12):1592–605. doi: 10.1200/JCO.2011.37.6418. [DOI] [PubMed] [Google Scholar]
- 99. [Accessed Feb 2017];FDA Drug Approvals and Databases. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs.
- 100.Lindauer A, Valiathan CR, Mehta K, Sriram V, de Greef R, Elassaiss-Schaap J, et al. Translational Pharmacokinetic/Pharmacodynamic Modeling of Tumor Growth Inhibition Supports Dose-Range Selection of the Anti-PD-1 Antibody Pembrolizumab. CPT Pharmacometrics Syst Pharmacol. 2017;6(1):11–20. doi: 10.1002/psp4.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anagnostou V, Yarchoan M, Hansen AR, Sharon E, Collyar D, Chow LQM, Forde PM. Immuno-oncology Trial Endpoints: Capturing Clinically Meaningful Activity. Clin Can Res. 2017;23 doi: 10.1158/1078-0432.CCR-16-3065. xx-xx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 103.Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of Immune-Related Response Criteria and RECIST v1.1 in Patients With Advanced Melanoma Treated With Pembrolizumab. J Clin Oncol. 2016;34(13):1510–7. doi: 10.1200/JCO.2015.64.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen TT. Statistical issues and challenges in immuno-oncology. J Immunother Cancer. 2013;1:18. doi: 10.1186/2051-1426-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hughes T, Klairmont M, Broucek J, Iodice G, Basu S, Kaufman HL. The prognostic significance of stable disease following high-dose interleukin-2 (IL-2) treatment in patients with metastatic melanoma and renal cell carcinoma. Cancer Immunol Immunother. 2015;64(4):459–65. doi: 10.1007/s00262-014-1652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seymour L, Ivy SP, Sargent D, Spriggs D, Baker L, Rubinstein L, et al. The design of phase II clinical trials testing cancer therapeutics: consensus recommendations from the clinical trial design task force of the national cancer institute investigational drug steering committee. Clin Cancer Res. 2010;16(6):1764–9. doi: 10.1158/1078-0432.CCR-09-3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473–86. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 108.Chen TW, Razak AR, Bedard PL, Siu LL, Hansen AR. A systematic review of immune-related adverse event reporting in clinical trials of immune checkpoint inhibitors. Ann Oncol. 2015;26(9):1824–9. doi: 10.1093/annonc/mdv182. [DOI] [PubMed] [Google Scholar]
- 109.Schadendorf D, Dummer R, Hauschild A, Robert C, Hamid O, Daud A, et al. Health-related quality of life in the randomised KEYNOTE-002 study of pembrolizumab versus chemotherapy in patients with ipilimumab-refractory melanoma. Eur J Cancer. 2016;67:46–54. doi: 10.1016/j.ejca.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 110.Cella D, Grunwald V, Nathan P, Doan J, Dastani H, Taylor F, et al. Quality of life in patients with advanced renal cell carcinoma given nivolumab versus everolimus in CheckMate 025: a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):994–1003. doi: 10.1016/S1470-2045(16)30125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kaitin KI, DiMasi JA. Pharmaceutical innovation in the 21st century: new drug approvals in the first decade, 2000–2009. Clin Pharmacol Ther. 2011;89(2):183–8. doi: 10.1038/clpt.2010.286. [DOI] [PubMed] [Google Scholar]
- 112.Prowell TM, Theoret MR, Pazdur R. Seamless Oncology-Drug Development. N Engl J Med. 2016;374(21):2001–3. doi: 10.1056/NEJMp1603747. [DOI] [PubMed] [Google Scholar]
- 113.Theoret MR, Pai-Scherf LH, Chuk MK, Prowell TM, Balasubramaniam S, Kim T, et al. Expansion Cohorts in First-in-Human Solid Tumor Oncology Trials. Clin Cancer Res. 2015;21(20):4545–51. doi: 10.1158/1078-0432.CCR-14-3244. [DOI] [PubMed] [Google Scholar]
- 114.Poole RM. Pembrolizumab: first global approval. Drugs. 2014;74(16):1973–81. doi: 10.1007/s40265-014-0314-5. [DOI] [PubMed] [Google Scholar]
- 115.Dilts DM, Cheng SK, Crites JS, Sandler AB, Doroshow JH. Phase III clinical trial development: a process of chutes and ladders. Clin Cancer Res. 2010;16(22):5381–9. doi: 10.1158/1078-0432.CCR-10-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Institute of Medicine (IOM) A National Cancer Clinical Trials System for the 21st century: reinvigorating the NCI Cooperative Group Program. Washington (DC): The National Academies Press; 2010. [PubMed] [Google Scholar]
- 117.National Cancer Institute (NCI) Report of the Operational Efficiency Working Group: compressing the timeline for cancer clinical trial activation. Bethesda (MD): National Cancer Institute; 2010. [Google Scholar]
- 118.Weber JS, Gibney G, Sullivan RJ, Sosman JA, Slingluff CL, Jr, Lawrence DP, et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol. 2016 doi: 10.1016/S1470-2045(16)30126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]