Abstract
Rationale: Mepolizumab, an IL-5–blocking antibody, reduces exacerbations in patients with severe eosinophilic asthma. Mepolizumab arrests eosinophil maturation; however, the functional phenotype of eosinophils that persist in the blood and airway after administration of IL-5 neutralizing antibodies has not been reported.
Objectives: To determine the effect of anti–IL-5 antibody on the numbers and phenotypes of allergen-induced circulating and airway eosinophils.
Methods: Airway inflammation was elicited in participants with mild allergic asthma by segmental allergen challenge before and 1 month after a single intravenous 750-mg dose of mepolizumab. Eosinophils were examined in blood, bronchoalveolar lavage, and endobronchial biopsies 48 hours after challenge.
Measurements and Main Results: Segmental challenge without mepolizumab induced a rise in circulating eosinophils, bronchoalveolar lavage eosinophilia, and eosinophil peroxidase deposition in bronchial mucosa. IL-5 neutralization before allergen challenge abolished the allergen-induced rise in circulating eosinophils and expression of IL-3 receptors, whereas airway eosinophilia and eosinophil peroxidase deposition were blunted but not eliminated. Before mepolizumab treatment, bronchoalveolar lavage eosinophils had more surface IL-3 and granulocyte–monocyte colony–stimulating factor receptors, CD69, CD44, and CD23 and decreased IL-5 and eotaxin receptors than blood eosinophils. This activation phenotype indicated by bronchoalveolar lavage eosinophil surface markers, as well as the release of eosinophil peroxidase by eosinophils in the bronchial mucosa, was maintained after mepolizumab.
Conclusions: Mepolizumab reduced airway eosinophil numbers but had a limited effect on airway eosinophil activation markers, suggesting that these cells retain functionality. This observation may explain why IL-5 neutralization reduces but does not completely eradicate asthma exacerbations.
Clinical trial registered with www.clinicaltrials.gov (NCT00802438).
Keywords: allergen challenge, IL-5, IL-3 receptor
At a Glance Commentary
Scientific Knowledge on the Subject
IL-5–blocking antibodies, such as mepolizumab, nearly eliminate circulating eosinophils and reduce the frequency and severity of exacerbations in patients with severe eosinophilic asthma. However, the effect of in vivo IL-5 neutralizing antibodies on the functional phenotype of eosinophils in the circulation and airway remains largely unknown.
What This Study Adds to the Field
This study of subjects with mild allergic asthma shows that mepolizumab prevents the slight rise in circulating eosinophils and decreases the dramatic influx of eosinophils into the airway 48 hours after segmental bronchoprovocation with allergen. Eosinophils circulating after mepolizumab are shown to have less mRNA encoding eosinophil-derived neurotoxin and reduced surface expression of the IL-3 receptor. In contrast, airway eosinophil expression of the IL-5–responsive activation markers CD23, CD44, and CD69, and receptors for the IL-5 family cytokines IL-5, IL-3, and granulocyte–macrophage colony–stimulating factor, is not altered by mepolizumab, indicating that airway eosinophils can be readily activated after administration of anti–IL-5 antibody. These observations may explain why some patients continue to have asthma exacerbations after anti–IL-5 treatment despite diminished numbers and activity of circulating eosinophils.
Airway eosinophils are a prominent component of allergic asthma. Patients with asthma with persistent eosinophilic airway inflammation tend to have more severe disease and frequent exacerbations (1). In some patients, titrating inhaled corticosteroid dose to reduce sputum eosinophils is an effective approach to lessen exacerbations (2). Nonetheless, in a subset of patients, elevated eosinophils persist despite high-dose corticosteroid treatment. Mepolizumab, a humanized monoclonal antibody with specificity for the proeosinophilic cytokine IL-5, was developed to target eosinophils (3), and received U.S. Food and Drug Administration approval in 2015 under the trade name Nucala (GlaxoSmithKline, Brentford, UK) (4). In patients with severe eosinophilic asthma, mepolizumab treatment reduced corticosteroid requirement (5, 6) and exacerbation frequency/severity (7–10). Collectively, these studies point to airway eosinophilia as a key component of some asthma exacerbations.
How eosinophils contribute to exacerbations is not completely understood. Eosinophil degranulation/cytolysis releases the cationic granule proteins major basic protein-1, eosinophil cationic protein, eosinophil-derived neurotoxin (EDN), and eosinophil peroxidase (EPX), which may contribute to epithelial damage and excessive repair (11, 12). Ex vivo studies demonstrating eosinophil generation of matrix metalloproteinase-9 (13), transforming growth factor-β1 (14), activin A (15), and semaphorin 7A (CD108) (16) support their role in tissue injury, repair, and remodeling. Furthermore, human eosinophils can influence airway inflammation by releasing cytokines (17), chemoattractants (18), and IL-1β (19), which is essential for T-helper cell type 17 development (20).
In addition to being required for eosinophil maturation and egress from the bone marrow (21), IL-5 promotes eosinophil survival and migration; release of granule proteins, cytokines, and growth factors (21); and surface expression of activation markers including CD69, CD44, and CD23; and the integrins αM (CD11b) and β2 (CD18) (22). The IL-5 receptor is composed of an IL-5–specific α chain and a signal-transducing β chain that is common to IL-3 and granulocyte–macrophage colony–stimulating factor (GM-CSF) receptors (21). Thus, the common β-chain/IL-5 family cytokines have similar and overlapping effects on mature eosinophils (21). Compared with their circulating counterparts, bronchoalveolar lavage (BAL) eosinophils have heightened levels of IL-3Rα and GM-CSFRα, whereas IL-5Rα appears to be down-regulated (23). Ex vivo exposure of blood eosinophils to IL-5 up-regulates GM-CSFRα and IL-3Rα while reducing IL-5Rα (24, 25). Consequently, expression of IL-5 family cytokine receptors on airway eosinophils may be controlled, in part, by in vivo IL-5 exposure. We hypothesized that in addition to reducing eosinophil numbers, mepolizumab would affect the activation phenotype of circulating and airway eosinophils and impact responsiveness to IL-3 and/or GM-CSF by altering receptor expression.
To test this hypothesis, we used segmental bronchoprovocation with allergen (SBP-Ag) before and after anti–IL-5 (mepolizumab) administration. Compared with the more commonly used whole-lung inhalation challenge, SBP-Ag induces an intense, but localized, airway eosinophilia in subjects with allergy (26). This approach permitted evaluation of allergen-induced activation and recruitment of eosinophils in subjects who have received anti–IL-5.
Some of the results of these studies have been previously reported in the form of an abstract (27).
Methods
Participants
The University of Wisconsin-Madison Health Sciences Institutional Review Board (Madison, WI) approved the study, and each participant provided written informed consent. Subjects had mild allergic asthma (aeroallergen skin prick test positive, FEV1 albuterol reversibility ≥ 12% or methacholine provocative concentration causing a 20% fall in FEV1 ≤ 8 mg/ml, prealbuterol FEV1 ≥ 70%, and postalbuterol FEV1 ≥ 80%) and were not receiving asthma controller medications. Additional inclusion/exclusion criteria and participant disposition are provided elsewhere (see Figure E1 in the online supplement).
Study Design
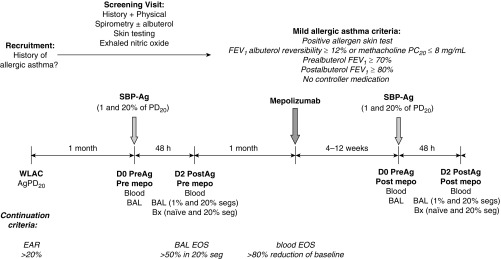
The design of this experimental, nonclinical study is shown in Figure 1. Participants with mild allergic asthma underwent whole-lung allergen inhalation challenge (WLAC) to determine AgPD20, the allergen provocation dose resulting in a 20% reduction in FEV1. An early allergic reaction, defined as a drop in FEV1 of at least 20% within 1 hour of challenge, was required for continuation. One month later, a baseline bronchoscopy with BAL was performed in two subsegments followed by SBP-Ag at a dose of 1% (low dose) or 20% (high dose) of each subject’s AgPD20. Forty-eight hours later, bronchoscopy with BAL was performed in each subsegment, and biopsies were taken from an antigen-naive site and from the high-dose segment. BAL eosinophilia greater than 50% in the high-dose segment was required for subject continuation in the study. One month after SBP-Ag, spirometry was performed to confirm continued asthma stability, and then a single 750-mg intravenous dose of mepolizumab was administered. One month after dosing, circulating eosinophils were required to be reduced by at least 80% of the baseline value, or less than 100 eosinophils/μl. If the threshold was not met, mepolizumab dosing was repeated. If the threshold was met, participants underwent bronchoscopy with BAL performed in two subsegments followed by SBP-Ag at the same 1 and 20% AgPD20 doses administered for the baseline challenge before mepolizumab. Forty-eight hours after SBP-Ag, bronchoscopy with BAL was performed in each subsegment and biopsies were taken from an antigen-naive site and from the high-dose segment.
Figure 1.
Study design. This was an experimental, nonclinical study rather than a traditional pharmaceutical industry–sponsored “clinical trial.” Participants with mild allergic asthma underwent whole-lung allergen inhalation challenge (WLAC) to determine the allergen provocation dose resulting in a 20% reduction in FEV1 (AgPD20). An early allergic reaction (EAR), defined as a drop in FEV1 ≥ 20% within 1 hour of challenge, was required for continuation. One month later, bronchoscopy with bronchoalveolar lavage (BAL) was performed in two subsegments followed by segmental bronchoprovocation with allergen (SBP-Ag) at a dose of 1% (low dose) or 20% (high dose) of the subject’s AgPD20. Forty-eight hours later, bronchoscopy with BAL was performed in each subsegment and biopsies (Bx) were taken from an antigen-naive site and from the high-dose segment. BAL eosinophilia greater than 50% in the high-dose segment was required for continuation. One month after SBP-Ag, spirometry was performed to confirm continued asthma stability, and then a single 750-mg intravenous dose of mepolizumab was administered. One month after dosing, circulating eosinophils were required to be decreased by at least 80% of the baseline value, or fewer than 100 eosinophils/μl. If the threshold was not met, mepolizumab dosing was repeated. If the threshold was met, participants underwent bronchoscopy with BAL in two subsegments followed by SBP-Ag at the same 1 and 20% AgPD20 doses administered for the baseline challenge before mepolizumab. The postmepolizumab challenge was performed in the contralateral lung. Forty-eight hours after SBP-Ag, bronchoscopy with BAL was performed in each subsegment and Bx were taken from an antigen-naive site and from the high-dose segment. D0, D2 = Day 0, Day 2; EOS = eosinophils; mepo = mepolizumab; PC20 = provocative concentration of methacholine causing a 20% fall in FEV1; PD20 = provocative dose resulting in a 20% reduction in FEV1; PreAg = before antigen administration; PostAg = after antigen administration; seg/s = segment/s.
WLAC
WLAC was performed to determine the AgPD20 for SBP-Ag and to screen for subjects with an airway response to Fel d1, GS ragweed mix, or Dermatophagoides farinae (all from Greer Labs, Lenoir, NC). To determine the AgPD20, consecutively greater concentrations of nebulized allergen were inhaled at 15-minute intervals until the FEV1 fell at least 20% compared with baseline diluent challenge.
Segmental Bronchoprovocation, BAL, and Biopsy, and Anti–IL-5 (Mepolizumab) Dosing
Baseline (Day 0) bronchoscopy with BAL (4 × 40 ml of 0.9% NaCl) was performed in two different bronchopulmonary subsegments that were in close proximity. Because of concern that the anti-eosinophil effect of mepolizumab might be overcome by an intense SBP-Ag–induced airway eosinophilia, two doses of allergen were administered in two separate segments. Allergen was instilled through a wedged bronchoscope into each of the two subsegments at either 1 or 20% of the participant’s AgPD20. Forty-eight hours later (Day 2), bronchoscopy with BAL (4 × 40 ml of 0.9% NaCl) was performed in each subsegment, and endobronchial biopsies were obtained at the site of the 20% SBP-Ag challenge and from an allergen-naive (control) site. One month after SBP-Ag, a single 750-mg dose (or in one participant, two doses) of mepolizumab (generously donated by GlaxoSmithKline, study No. MEA110170) was administered intravenously. Circulating eosinophils were monitored weekly for 6 months after mepolizumab administration. One month after administration of mepolizumab, bronchoscopy, BAL, SBP-Ag, and biopsy procedures were performed by the same methods as before mepolizumab administration, except in the contralateral lung. SBP-Ag was administered at identical concentrations in segments/subsegments in the contralateral lung.
Immunocytochemistry
EPX was detected as described by Protheroe and colleagues (28) and in the online supplement, using monoclonal anti-EPX (clone MM25-82.2.1) antibody (28) provided by James J. Lee and Nancy A. Lee (Mayo Clinic, Scottsdale, AZ).
Immunohistochemical Scoring
A numerical value (0–10) was assigned, based on modification of the semiquantitative scoring system established by Protheroe and colleagues (28) and described in the online supplement. The staining score was calculated as the sum of each of these criteria: (1) the gross EPX score (0, 0.5, or 1) based on the overall staining profile; (2) the percentage area (0–4) of mucosal EPX staining; and (3) the degree (0–5) of EPX staining.
Flow Cytometric Analysis
See the online supplement for methods and gating strategy (Figure E2).
Eosinophil Purification
Blood and BAL eosinophils were purified by negative selection and density gradient centrifugation, respectively (see the online supplement). Only cells with more than 96% purity were used for analysis by quantitative polymerase chain reaction (Table E1).
Reverse Transcription Followed by Quantitative Polymerase Chain Reaction
Reverse transcription followed by quantitative polymerase chain reaction (RT-qPCR) was performed as previously reported (15), using specific primers described in the online supplement. Data were normalized to β-glucuronidase and presented as fold change in 2–∆∆Ct compared with each individual’s premepolizumab circulating eosinophils, which was fixed at 100%.
ELISAs
See the online supplement for the highly sensitive “in-house” sandwich ELISAs.
Statistical Analysis
Baseline subject characteristics were compared by study completion status, using the two-sample t test for continuous measures and Fisher’s exact test for categorical measures. Outcome data were compared using longitudinal mixed effects models with fixed effect terms for pre-/postmepolizumab, pre-/postantigen challenge, and antigen dose subsegment and a random-effect subject term (29). Further details are described in the online supplement. A two-sided P value less than 0.05 was considered significant. Analyses were conducted with SAS version 9.4 (SAS Institute, Cary, NC).
Results
Subject Characteristics
As shown in Figure E1, 38 participants were enrolled for screening. Twenty-six participants met the inclusion criteria and underwent WLAC, 17 qualified to undergo bronchoscopy, and 10 had a sufficient eosinophil response after SBP-Ag to continue the study and receive mepolizumab. Baseline characteristics of participants who completed the study were not significantly different from those who were discontinued (Table 1). The subjects who completed the study had mild asthma, which was stable at the time of mepolizumab infusion (Table E1). Only one participant required a second dose of mepolizumab to achieve an 80% reduction in circulating eosinophils 1 month after dosing. Forty-eight hours after SBP-Ag without mepolizumab, there was a small but statistically significant decrease in FEV1 percent predicted and an increase in fractional exhaled nitric oxide (Table 2). After mepolizumab, allergen challenge still induced a significant rise in fractional exhaled nitric oxide, and levels did not correlate with BAL eosinophil numbers or percentage after segmental allergen challenge (data not shown). In these subjects with mild allergic asthma, mepolizumab had no significant effect on pulmonary function before or after challenge (Table 2).
Table 1.
Baseline Characteristics of Participants*
| Total Enrolled | Completed | Screen Failed | P Value | |
|---|---|---|---|---|
| Number | 38 | 10 | 28 | — |
| Age, yr | 26 ± 7 | 25 ± 5 | 26 ± 8 | 0.90 |
| Female/male, n | 18/20 | 3/7 | 15/13 | 0.28 |
| FEV1, % predicted | 90 ± 16 | 96 ± 19 | 88 ± 15 | 0.21 |
| FEV1/FVC | 0.77 ± 0.07 | 0.78 ± 0.08 | 0.77 ± 0.07 | 0.75 |
| Methacholine PC20, mg/ml† | 1.7 ± 2.8 | 1.5 ± 2.7 | 1.8 ± 2.8 | 0.90 |
Definition of abbreviation: PC20 = provocative concentration of methacholine causing a 20% fall in FEV1.
Data are expressed as means ± SD unless indicated otherwise.
Geometric mean ± median absolute deviation; n = 13 participants for screen failed.
Table 2.
Effect of Mepolizumab on SBP-Ag–Induced Changes in Pulmonary Function
| Premepolizumab |
Postmepolizumab |
P Value Pre- vs. Postmepolizumab |
||||||
|---|---|---|---|---|---|---|---|---|
| Pre–SBP-Ag | 48 h Post–SBP-Ag | P Value | Pre–SBP-Ag | 48 h Post–SBP-Ag | P Value | Pre–SBP-Ag | Post–SBP-Ag | |
| FEV1, L* | 4.1 (3.4–4.7) | 4.0 (3.3–4.6) | 0.08 | 4.1 (3.4–4.7) | 4.0 (3.4–4.6) | 0.10 | 0.80 | 0.90 |
| FEV1, % predicted* | 96.3 (85.0–107.6) | 92.7 (82.1–103.3) | 0.04 | 95.1 (84.2–106.0) | 92.7 (84.0–101.4) | 0.08 | 0.38 | 1.00 |
| FVC, L* | 5.5 (4.4–6.5) | 5.4 (4.3–6.5) | 0.34 | 5.4 (4.4–6.4) | 5.4 (4.4–6.4) | 0.38 | 0.77 | 0.85 |
| FEV1/FVC* | 0.76 (0.70–0.82) | 0.76 (0.68–0.84) | 0.81 | 0.76 (0.70–0.83) | 0.75 (0.69–0.80) | 0.14 | 0.74 | 0.48 |
| PEFR, L/min* | 3.4 (2.7–4.0) | 3.3 (2.6–4.0) | 0.12 | 3.4 (2.7–4.1) | 3.2 (2.6–3.7) | 0.07 | 0.74 | 0.50 |
| Bronchodilator reversibility,† % | 6.8 ± 4.8 | 9.3 ± 5.0 | 0.05 | 6.7 ± 4.7 | 8.2 ± 5.1 | 0.23 | 0.94 | 0.41 |
| FeNO, ppb‡ | 40 (29–54) | 62 (49–77) | 0.003 | 32 (22–47) | 58 (39–85) | 0.0002 | 0.11 | 0.56 |
Definition of abbreviations: FeNO = fractional exhaled nitric oxide; PEFR = peak expiratory flow rate; ppb = parts per billion; SBP-Ag = segmental bronchoprovocation with allergen.
Least-squares means with 95% confidence intervals.
Albuterol (180 μg); data are shown as mean ± SD.
Geometric means with 95% confidence intervals.
Effect of Anti–IL-5 on Circulating and Airway Eosinophils
One week after mepolizumab administration, the median value for circulating eosinophils decreased by approximately 75% (Figure E3). The median percent decrease in eosinophil levels remained greater than 75% for 3 months (n = 10) and returned toward the predosing level by 6 months (n = 8). Table E2 shows there were no significant changes in absolute numbers of neutrophils, monocytes, or lymphocytes; however, basophils, which are known to express IL-5Rα (3), were reduced by approximately 33% at 1 week, and this reduction persisted for 2 months after dosing.
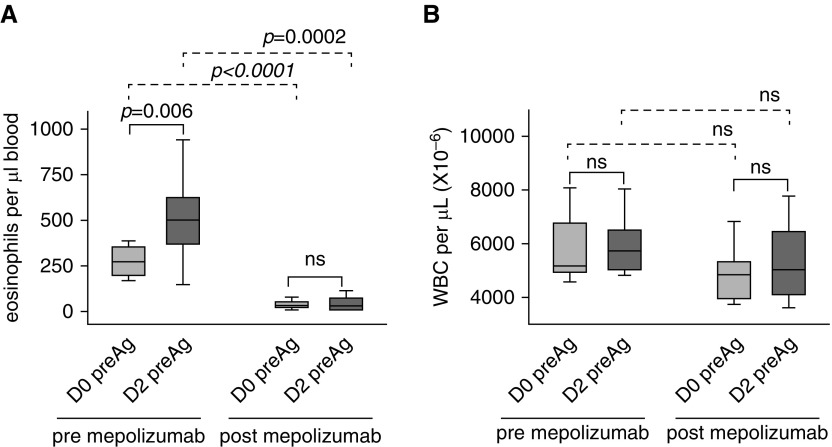
Although there was no change in total circulating white blood cells (Figure 2 and Table E2), localized airway allergen challenge induced an increase in circulating eosinophils (P = 0.006) that did not occur when allergen challenge was performed after mepolizumab (Figure 2).
Figure 2.
Mepolizumab prevents an allergen-induced rise in circulating eosinophils. (A) Before administration of mepolizumab, there was a significant rise in circulating eosinophil counts 48 hours (D2) after airway allergen challenge. The allergen-induced rise did not occur when challenge was given 1 month postmepolizumab. (B) WBC counts were not changed by allergen challenge or mepolizumab. Data represent medians with first and third quartiles; whisker lines represent the 10th and 90th percentiles (n = 10). Pre- versus postallergen P values are indicated above the solid lines; pre- versus postmepolizumab P values are indicated above the dashed lines. D0, D2 = Day 0, Day 2; ns = not significant; preAg = before segmental allergen challenge; WBC = white blood cells.
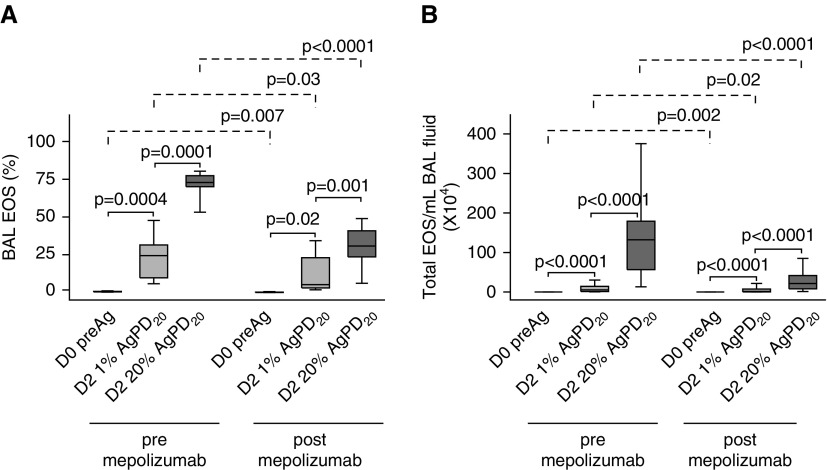
Forty-eight hours after SBP-Ag without mepolizumab, there was an allergen dose–dependent influx of inflammatory cells, with eosinophils accounting for a median (range) of 24% (5–49%) and 73% (52–81%) of the total BAL cells in the low- and high-dose segments, respectively (Figure 3A). Despite the prolonged abrogation of blood eosinophils by mepolizumab (Figure E3), SBP-Ag still induced a dose-dependent increase in BAL eosinophils, which rose from a baseline median (range) of 0.1% (0–0.1%) to 5% (2–31%) and 31% (4–49%) of the total BAL cells after low- and high-dose challenge, respectively. Nonetheless, allergen-induced differences (pre/post SBP-Ag deltas) in eosinophil total numbers and percentage in both the low- and high-dose segments were significantly less (P ≤ 0.001) after mepolizumab. Compared with the response before mepolizumab, there was a median decrease in eosinophils per milliliter of BAL fluid of 62 and 84% in the low- and high-dose segments, respectively (Figure 3B). Mepolizumab had no effect on other BAL cell populations in the naive and low-dose segment, but slightly increased the percentages of lymphocytes in the high-dose segment (Table E3).
Figure 3.
Mepolizumab decreases, but does not eliminate, allergen-induced bronchoalveolar lavage (BAL) eosinophils. Both before and after mepolizumab, there was an antigen dose–dependent increase in the percentage of BAL eosinophils (A) and total numbers of BAL cells per milliliter of BAL fluid (B) 48 hours (D2) after segmental bronchoprovocation with allergen. The magnitude of the antigen-induced eosinophilia was significantly lower after mepolizumab. Data represent medians with first and third quartiles; whisker lines represent the 10th and 90th percentiles (n = 10). P values for pre- versus post-1% and post-1% versus 20% segmental bronchoprovocation with allergen are indicated above the solid lines; pre- versus postmepolizumab P values are indicated above the dashed lines. AgPD20 = allergen provocation dose resulting in a 20% reduction in FEV1; D0, D2 = Day 0, Day 2; EOS = eosinophils; preAg = before antigen administration.
Effect of Mepolizumab on Eosinophil Expression of IL-5–Inducible Cell Surface Receptors and Release of Granule Proteins
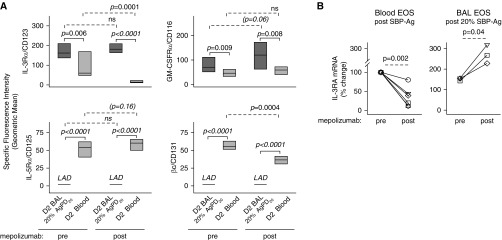
Flow cytometric comparison of BAL versus circulating eosinophils obtained 48 hours (Day 2) after SBP-Ag showed elevated levels of IL-3Rα and GM-CSFRα, whereas IL-5Rα and βc were diminished (Figure 4A), which is similar to our prior work (23). Mepolizumab did not significantly alter the patterns of cytokine receptor expression on the cell surface of airway eosinophils (Figure 4A). Circulating eosinophils obtained after administration of mepolizumab had significantly lower levels of surface IL-3Rα (P = 0.0001; Figure 4A) and mRNA (P = 0.002; Figure 4B), along with decreased surface βc (P = 0.0004; Figure 4A), and a trend toward increased surface IL-5Rα (P = 0.16). The decreased surface density of IL-3Rα was limited to eosinophils (Figure E2).
Figure 4.
Mepolizumab decreases expression of the IL-3 receptor on circulating eosinophils but has no effect on IL-5 family cytokine receptor expression on BAL eosinophils. Forty-eight hours after segmental bronchoprovocation with allergen (SBP-Ag), the cytokine receptor expression pattern was assessed on blood eosinophils and on airway eosinophils obtained from the segment challenged with 20% of the participant’s AgPD20 (allergen provocation dose resulting in a 20% reduction in FEV1) (P values indicated above the solid lines). After mepolizumab administration, IL-3 receptor α chain (IL-3Rα/CD123/IL3RA) and β chain (βc/CD131/CSF2RB) were significantly diminished on blood eosinophils. Overall, however, mepolizumab did not alter the pattern of receptor expression on blood versus BAL eosinophils (P values indicated above the dashed lines). (A) Cell surface protein. Data represent medians with first and third quartiles (n = 9). (B) mRNA fold change normalized to each individual’s premepolizumab circulating eosinophils. Symbols represent values for individual subjects (n = 6 for blood eosinophils; n = 3 for BAL eosinophils). BAL = bronchoalveolar lavage; D2 = Day 2; EOS = eosinophils; GM-CSFRα = granulocyte–macrophage colony–stimulating factor receptor α chain; IL-5Rα = IL-5 receptor α chain; LAD = below the level of accurate detection; ns = not significant.
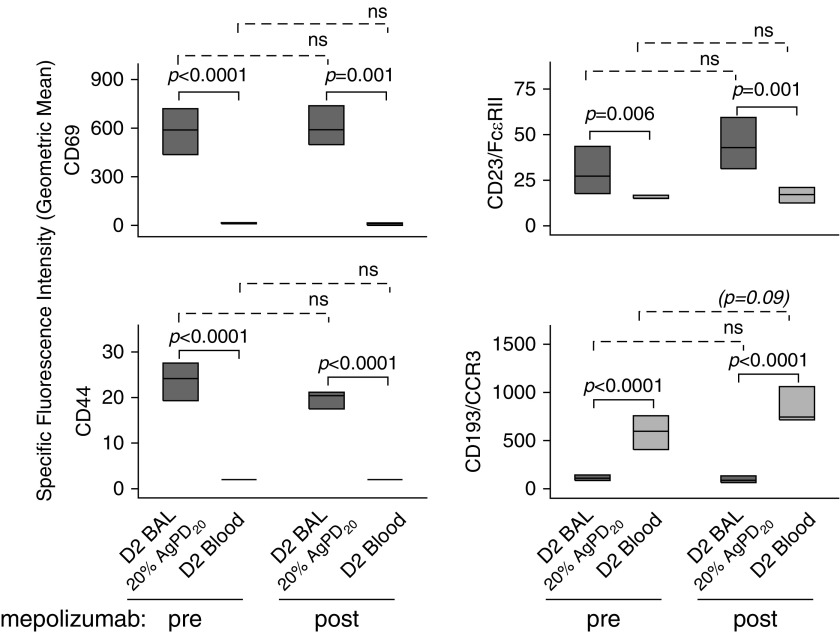
Although mepolizumab attenuated IL-3R expression on blood eosinophils, further analysis of the phenotype of blood and BAL eosinophils demonstrated no significant changes in the level of the IL-5–inducible (22, 25, 30, 31) activation markers CD69, CD44, CD23 (Figure 5), CD66e, and CD108 (data not shown). Flow cytometric comparison of BAL versus circulating eosinophils showed that levels of CD69, CD44, and CD23 were significantly elevated, whereas the level of CD193 (CCR3), which is down-regulated by IL-5 (25), was decreased on BAL eosinophils compared with blood eosinophils. This BAL versus blood eosinophil pattern did not change after mepolizumab administration (Figure 5).
Figure 5.
Mepolizumab has no significant effect on IL-5–responsive cell surface activation markers on blood or BAL eosinophils. Forty-eight hours after segmental bronchoprovocation with allergen, the expression pattern of activation markers was assessed on blood eosinophils and on airway eosinophils obtained from the segment challenged with 20% of the participant’s AgPD20 (allergen provocation dose resulting in a 20% reduction in FEV1) (P values indicated above the solid lines). The differences in activation marker expression did not change after mepolizumab administration (P value indicated above the dashed lines). Data represent medians with first and third quartiles (CD44 and CD23, n = 8; CCR3, n = 7; CD69, n = 6). BAL = bronchoalveolar lavage; D2 = Day 2; FcεRII = low-affinity receptor for IgE; ns = not significant.
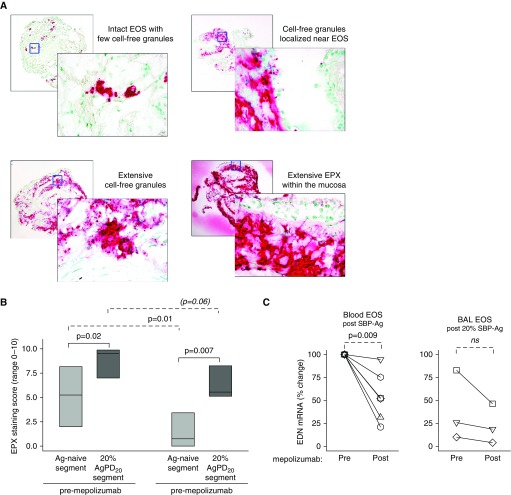
The flow cytometric studies suggested that mepolizumab does not affect the surface activation phenotype of airway eosinophils. Therefore, endobronchial biopsies obtained 48 hours after the high-dose challenge were interrogated for the presence of EPX, which is the only granule protein with absolute specificity for eosinophils (11). Release of EPX and cell-free EPX-containing granules by tissue eosinophils was evident when SBP-Ag was performed after mepolizumab administration. Similar to reports in eosinophil esophagitis (28), EPX-stained sections of endobronchial biopsies displayed four staining patterns (Figure 6A) including intact EPX-positive eosinophils, cell-free granules localized near eosinophils, extensive deposition of cell-free EPX-containing granules, and/or extensive EPX deposition within the mucosa. In these subjects with allergic asthma, samples obtained from the allergen-naive segment displayed clusters of eosinophils and, in some cases, widespread distribution of cell-free granules (Figure E4). In segments that had received allergen challenge, there was pronounced EPX staining demonstrating granule release and extensive EPX deposition within the mucosa. One month after mepolizumab administration, the EPX staining score was significantly less in the antigen-naive site (P = 0.01; n = 8) (Figure 6B), and there was a decrease in the pre/post SBP-Ag delta after mepolizumab (P = 0.02; n = 8). Although the staining score was decreased, granule release was evident in most subjects, and there was impressive EPX staining of mucosal tissue from 25% of the subjects (Figure E4).
Figure 6.
Eosinophil release of eosinophil peroxidase (EPX) and EPX-containing granules is reduced, but still present, in bronchial mucosa after mepolizumab administration. (A) Representative photomicrographs, taken using a ×10 objective (rear photographs) and a ×40 objective (forward photographs), demonstrating EPX staining patterns. (B) After mepolizumab administration, the EPX staining score (medians with first and third quartiles) was significantly less for the antigen-naive site and tended to decrease for the site that had been challenged with 20% of the participant’s AgPD20 (allergen provocation dose resulting in a 20% reduction in FEV1) (n = 8). (C) EDN mRNA fold change normalized to each individual’s premepolizumab circulating eosinophils. Symbols represent values for individual subjects (n = 6 for blood eosinophils; n = 3 for BAL eosinophils). Pre– versus post–SBP-Ag P values are indicated above the solid lines; pre- versus postmepolizumab P values are indicated above the dashed lines. Ag = antigen; BAL = bronchoalveolar lavage; EDN = eosinophil-derived neurotoxin; EOS = eosinophils; ns = not significant; SBP-Ag = segmental bronchoprovocation with allergen.
Detailed functional assays were limited by the low number of circulating eosinophils available for purification after mepolizumab administration (Table E1). However, sufficient eosinophils of very high purity were obtained from BAL (n = 3 participants) and blood (n = 6 participants) for RT-qPCR analysis. Although eosinophils synthesize and store granule proteins (major basic protein-1 and EPX) during eosinophilopoiesis, we previously established that only ribonuclease A family member 2 (RNASE 2)/EDN mRNA is detectable in unstimulated BAL eosinophils (32) and blood eosinophils (33). After mepolizumab administration, EDN mRNA (n = 6) was significantly decreased in blood eosinophils (Figure 6C). Because of the small sample size for highly pure BAL eosinophils, meaningful changes could not be assessed.
Effect of Mepolizumab on BAL Fluid Concentrations of IL-5 Family Cytokines
Before administration of mepolizumab, there was an allergen dose–dependent increase in BAL fluid concentrations of IL-5, IL-3, and GM-CSF (Table E4). After mepolizumab administration, IL-5 levels in the low-dose subsegment were modestly, but significantly increased. To assess how IL-5/mepolizumab complexes might affect the IL-5 values obtained by ELISA, BAL fluids were acid treated before performing the ELISA. Dissociation of IL-5/mepolizumab complexes by pH shock resulted in a small, but statistically significant increase in IL-5 detection from a median (first and third quartiles) of 409 (49–741) pg/ml to 447 (66–1,107) pg/ml (P = 0.01) in BAL fluid from the segment challenged with a high dose of allergen after administration of mepolizumab. Acid treatment had no effect on IL-5 detection in BAL fluid from the low-dose or control segments after mepolizumab administration or in BAL fluid obtained when SBP-Ag was performed before mepolizumab administration, indicating that IL-5/mepolizumab complexes only slightly reduce the values of the IL-5 ELISA.
Discussion
Capitalizing on the unique approach of segmental allergen challenge to induce robust localized airway eosinophilia, we observed that, in addition to preventing the allergen-induced rise in circulating eosinophils, IL-5 blockade with mepolizumab decreased allergen-induced BAL eosinophilia. Remarkably, the eosinophil-reducing effect of a single 750-mg dose of mepolizumab was maintained for 4 weeks (at the time of allergen challenge) and was not completely overpowered by the potent signal provided by SBP-Ag. Our estimate of the magnitude of the mepolizumab-mediated decrease in airway eosinophil numbers was in line with clinical efficacy studies showing greater than 75% reduction in BAL eosinophils in patients with asthma who received three monthly infusions of mepolizumab (7, 34).
Although the eosinophil-reducing effect of mepolizumab was remarkable, sufficient numbers remained in the circulation and airway to allow investigation of mepolizumab-associated changes in the activation phenotype of eosinophils before and after airway allergen challenge. The most striking effect of mepolizumab was attenuation of IL-3Rα levels on circulating eosinophils, which suggests that IL-5 neutralization affects IL-3Rα expression during hematopoiesis rather than simply preventing the allergen-induced rise in levels of surface IL-3Rα that we previously reported (23). On the basis of ex vivo studies of bone marrow– or cord blood–derived progenitors, it is thought that IL-3 and GM-CSF induce proliferation of eosinophil lineage–committed IL-5Rα–positive progenitors, whereas IL-5 induces terminal differentiation to mature eosinophils (35, 36). It is tempting to speculate that in addition to preventing IL-5–mediated terminal differentiation, mepolizumab also reduces IL-3 receptor expression, thereby restricting expansion of eosinophil-committed progenitors. Menzies-Gow and colleagues have shown that mepolizumab arrests eosinophil maturation (37). Studies characterizing the cell surface phenotype of eosinophil-committed progenitors would be warranted to more fully understand the role of IL-5 (and IL-3) in eosinophil hematopoiesis and the effect of mepolizumab on this process.
In contrast to circulating eosinophils, and contrary to our hypothesis, administration of mepolizumab did not reduce expression of the GM-CSF or IL-3 receptor, or the increase in IL-5–inducible membrane proteins on airway eosinophils. On the basis of previous studies showing attenuated IL-5Rα expression (protein and mRNA) on BAL eosinophils (23) and persistent down-regulation of IL-5Rα and enhanced IL-3Rα and GM-CSFRα levels after ex vivo exposure of eosinophils to IL-5 (24, 25), we were surprised that neutralization of IL-5 did not affect levels of any of the IL-5 cytokine family receptors on BAL eosinophils. These observations are consistent with studies showing no mepolizumab-mediated change in circulating eosinophil expression of IL-5Rα in asthma (38) or eosinophilic esophagitis (39), but are in opposition to a report that IL-5Rα is increased (40) after mepolizumab treatment of patients with various hypereosinophil-related diseases. In addition to a lack of an effect on IL-5 family cytokine receptors, mepolizumab had little effect on BAL eosinophil surface expression of IL-5–inducible activation markers such as CD69, CD44, and CD23. These observations are in accord with our finding that mepolizumab does not blunt increased expression and activation of β2-integrins on BAL eosinophils (41).
The observation that mepolizumab did not affect surface expression of IL-5–inducible activation markers on BAL eosinophils raises the possibility that, although their numbers are substantially reduced, the remaining airway eosinophils retain their functionality. This notion is supported by evidence of eosinophil degranulation in the bronchial mucosa when allergen challenge occurred after mepolizumab administration, along with the clinical efficacy studies showing persistence of mucosal eosinophil granules after mepolizumab treatment of patients with mild (34) or severe eosinophilic asthma (7). Whether granule release occurred via exocytosis, piecemeal degranulation, or cytolysis (12, 42–44) is not known. Regardless, cell-free granules express surface receptors and can release toxic granule proteins as well as cytokines in response to a variety of agonists including IFN-γ, eotaxin, and cysteinyl leukotrienes (45, 46). Thus, the residual cell-free granules present after mepolizumab could contribute to persistent airway inflammation and damage to mucosal tissue. The preservation of the active eosinophil phenotype in the airway after mepolizumab administration may explain why some patients continue to have asthma exacerbations after treatment with mepolizumab, despite the significant reduction in eosinophil number.
The postmepolizumab observations that SBP-Ag–induced airway eosinophilia occurs despite the near ablation of circulating eosinophils, and the striking difference between surface levels of IL-3Rα on circulating versus BAL eosinophils, raise questions concerning the origin and activation mechanism(s) of eosinophils that are in the airway lumen after mepolizumab treatment. It is possible that a limited number of circulating eosinophils do express a high level of IL-3Rα and are selectively recruited/retained in the airway. However, we believe it is more likely that at least a portion of BAL eosinophils originates from allergen-induced release of circulating eosinophil progenitors (47) and/or expansion of resident eosinophil progenitors in the bronchial mucosa (48). In nonallergic individuals, local airway eosinophil differentiation can occur in response to inhaled IL-5 (49) and in individuals with allergic asthma, recruitment/differentiation of eosinophil progenitors in the airway is reduced after mepolizumab treatment (37), suggesting that in situ maturation of eosinophils is, to some extent, governed by IL-5. Nonetheless, patients with severe eosinophilic asthma have exaggerated eosinophilopoiesis in the bronchial mucosa that is not affected by treatment with mepolizumab (50). This may be due to inadequate neutralization of IL-5 that is produced locally by stromal cells (51), innate lymphoid cells (52), or eosinophil progenitors (53) themselves. In addition, there may be an IL-5–independent component of bronchial tissue eosinophilopoiesis. Candidate factors include the combination of GM-CSF and leukotriene D4 (54) or IL-3 plus thymic stromal lymphopoietin or IL-33 (53).
A major strength of this study was our ability to examine both circulating and airway eosinophils after administration of mepolizumab, and, unlike previously published studies of patients with severe asthma, the effects of mepolizumab on eosinophils were not confounded by corticosteroid effects. Another strength is the use of a well-established experimental model of allergen-induced eosinophilic airway inflammation in individuals with allergic asthma, which allowed interrogation of mepolizumab on airway cell numbers and functional phenotype. However, we recognize that our study design has limitations. First, the induction of profound type 2 inflammation may have limited the IL-5–neutralizing capacity of mepolizumab. Levels of IL-5 in BAL fluid were increased to the same extent by SBP-Ag before and after mepolizumab treatment. Others have demonstrated that IL-5/mepolizumab complexes are present in serum (40), and we noted a small but significant increase in detection of IL-5 after acid treatment of BAL fluid. Although we believe that the majority of IL-5 in the BAL fluid is neutralized by mepolizumab, we cannot be completely certain that there is not a small amount of free and functional IL-5 in the airway. Second, SBP-Ag induces intense local airway eosinophilia that is much greater than what is typically seen in asthma. When planning the study design, we believed the unique opportunity to study airway eosinophils outweighed the physiological limitations. This is the first report evaluating the effect of mepolizumab on the functional phenotype of airway eosinophils. Third, induction of airway eosinophilia by SBP-Ag does not model the induction of nonallergic eosinophilic asthma (55). Although the sources of IL-5 may differ in nonallergic versus allergic asthma (type 2 innate lymphoid cells [ILC2] vs. ILC2 plus adaptive helper T type 2 cells) (56), there are no reported differences in eosinophils, and mepolizumab significantly reduces asthma exacerbations in eosinophil-associated asthma regardless of allergen sensitization (55). Notwithstanding its shortcomings, using the combined approach of SBP-Ag before and after mepolizumab administration permitted purification of circulating and BAL eosinophils from a limited number of subjects. Although current technologies did not permit us to study the ex vivo potential of eosinophils to respond to IL-5 family cytokines, in vivo activation could be inferred from RT-qPCR analysis.
We realize that our small sample size limits any meaningful consideration of the mechanisms of mepolizumab’s clinical action. The prolonged time commitment and inclusion of four bronchoscopies affected subject retention and limited the sample size. Nonetheless, statistically significant differences in numbers of BAL eosinophils pre- versus postmepolizumab were of sufficient power to halt recruitment when 10 participants had completed the study. Finally, given the experimental nature of our intervention, we cannot directly extrapolate our findings to the bedside. However, it is tempting to speculate that our study may explain why some patients continue to have asthma exacerbations despite the near elimination of circulating eosinophil after anti–IL-5 therapy. We also recognize that exacerbations could be due to neutrophilic airway inflammation that occurs in response to infection (57).
In summary, using an experimental model to induce intense eosinophilic airway inflammation in individuals with allergic asthma, we demonstrated that mepolizumab has potent anti-eosinophil effects leading to dramatic reduction in circulating and airway eosinophilia. Although it does not completely eliminate circulating eosinophils, mepolizumab affects their activation status as assessed by EDN mRNA expression, and may attenuate their response to IL-3 by preventing their expression of IL-3 receptors. Despite the low level of circulating eosinophils after mepolizumab treatment, SBP-Ag still induced recruitment, and/or maturation of eosinophils in the airway. The observation that BAL eosinophil expression of cytokine receptors and IL-5–responsive activation markers is not altered by mepolizumab may indicate that airway eosinophils retain their specific functions (release of granule proteins, cytokines, chemokines, and growth factors) and may explain why some patients continue to have asthma exacerbations after anti–IL-5 treatment despite diminished numbers and activity of circulating eosinophils.
Acknowledgments
Acknowledgment
The authors thank the participants in this study for their time and commitment to this research. The authors thank GlaxoSmithKline for kindly providing mepolizumab as a gift. The authors thank Larissa De Lain, B.S., and Lei Shi, Ph.D., for processing BAL samples; Elizabeth Schwantes, M.S., Paul Fichtinger, B.S., Ellen Cook, Ph.D., and Jim Stahl, Ph.D., for purification of blood and BAL eosinophils through the Laboratory Core (S.M., Principal Investigator [PI]); Larissa De Lain, B.S., for assistance with RT-qPCR work; Jami Hauer, B.S., for assistance with ELISAs; Drew Roenneburg, B.S., for EPX staining of biopsy tissue; Mary Jo Jackson, B.S.N., Michele Wolff, B.S.N., Holly Eversoll, B.S.N., and Evelyn Falibene, B.S., for participant recruitment and screening; Richard Cornwell, M.D., and Keith Meyer, M.D., for assistance with bronchoscopies through the Clinical Core (L.C.D., PI); Gina Crisafi, B.S., for assistance through the Administrative Core (N.N.J., PI), and Kathleen Schell, B.S., and Dagna Sheerar, B.S., for advice on flow cytometry.
Footnotes
Supported by National Institutes of Health Program Project grant P01 HL88594, General Clinical Research Center grant M01 RR03186, and Clinical and Translational Science Award grant UL1 RR25011. Mepolizumab was a gift from GlaxoSmithKline. GSK had no role in the conduct of the study, data analysis or interpretation, preparation of the manuscript, or the decision to submit the manuscript for publication.
Author Contributions: N.N.J., E.A.K., and L.C.D. were responsible for study design; E.A.K., S.E., L.Y.L., and M.W.J. conceived and designed experiments; E.A.K., L.C.D., M.D.E., and S.M. analyzed participants’ data and maintained participant-associated database; M.D.E. was responsible for statistical analyses; E.A.K., L.Y.L., M.W.J., and D.F.M. contributed to flow cytometry experiments and interpretation; S.E. and L.Y.L. performed reverse transcription–quantitative polymerase chain reaction experiments; E.A.K. quantified immunohistochemical staining; and all authors contributed intellectually to the project through data interpretation and manuscript preparation. All authors approved the final version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201611-2234OC on September 1, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Volbeda F, Broekema M, Lodewijk ME, Hylkema MN, Reddel HK, Timens W, Postma DS, ten Hacken NH. Clinical control of asthma associates with measures of airway inflammation. Thorax. 2013;68:19–24. doi: 10.1136/thoraxjnl-2012-201861. [DOI] [PubMed] [Google Scholar]
- 2.Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, Wardlaw AJ, Pavord ID. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360:1715–1721. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 3.Smith DA, Minthorn EA, Beerahee M. Pharmacokinetics and pharmacodynamics of mepolizumab, an anti–interleukin-5 monoclonal antibody. Clin Pharmacokinet. 2011;50:215–227. doi: 10.2165/11584340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Keating GM. Mepolizumab: first global approval. Drugs. 2015;75:2163–2169. doi: 10.1007/s40265-015-0513-8. [DOI] [PubMed] [Google Scholar]
- 5.Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, Ortega HG, Pavord ID SIRIUS Investigators. Oral glucocorticoid–sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 6.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, Humbert M, Katz LE, Keene ON, Yancey SW, et al. MENSA Investigators. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 7.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O’Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 9.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, Ortega H, Chanez P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 10.Yancey SW, Ortega HG, Keene ON, Mayer B, Gunsoy NB, Brightling CE, Bleecker ER, Haldar P, Pavord ID. Meta-analysis of asthma-related hospitalization in mepolizumab studies of severe eosinophilic asthma. J Allergy Clin Immunol. 2017;139:1167–1175.e2. doi: 10.1016/j.jaci.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014;289:17406–17415. doi: 10.1074/jbc.R113.546218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persson C, Uller L. Theirs but to die and do: primary lysis of eosinophils and free eosinophil granules in asthma. Am J Respir Crit Care Med. 2014;189:628–633. doi: 10.1164/rccm.201311-2069OE. [DOI] [PubMed] [Google Scholar]
- 13.Kelly EA, Liu LY, Esnault S, Quinchia Johnson BH, Jarjour NN. Potent synergistic effect of IL-3 and TNF on matrix metalloproteinase 9 generation by human eosinophils. Cytokine. 2012;58:199–206. doi: 10.1016/j.cyto.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minshall EM, Leung DY, Martin RJ, Song YL, Cameron L, Ernst P, Hamid Q. Eosinophil-associated TGF-β1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1997;17:326–333. doi: 10.1165/ajrcmb.17.3.2733. [DOI] [PubMed] [Google Scholar]
- 15.Kelly EA, Esnault S, Johnson SH, Liu LY, Malter JS, Burnham ME, Jarjour NN. Human eosinophil activin A synthesis and mRNA stabilization are induced by the combination of IL-3 plus TNF. Immunol Cell Biol. 2016;94:701–708. doi: 10.1038/icb.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esnault S, Kelly EA, Johansson MW, Liu LY, Han ST, Akhtar M, Sandbo N, Mosher DF, Denlinger LC, Mathur SK, et al. Semaphorin 7A is expressed on airway eosinophils and upregulated by IL-5 family cytokines. Clin Immunol. 2014;150:90–100. doi: 10.1016/j.clim.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spencer LA, Bonjour K, Melo RC, Weller PF. Eosinophil secretion of granule-derived cytokines. Front Immunol. 2014;5:496. doi: 10.3389/fimmu.2014.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu LY, Mathur SK, Sedgwick JB, Jarjour NN, Busse WW, Kelly EA. Human airway and peripheral blood eosinophils enhance Th1 and Th2 cytokine secretion. Allergy. 2006;61:589–597. doi: 10.1111/j.1398-9995.2006.01060.x. [DOI] [PubMed] [Google Scholar]
- 19.Esnault S, Kelly EA, Nettenstrom LM, Cook EB, Seroogy CM, Jarjour NN. Human eosinophils release IL-1β and increase expression of IL-17A in activated CD4+ T lymphocytes. Clin Exp Allergy. 2012;42:1756–1764. doi: 10.1111/j.1365-2222.2012.04060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17–producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 21.Takatsu K. Interleukin-5 and its receptor molecules. In: Lee JJ, Rosenberg HF, editors. Eosinophils in health and disease. Oxford: Elsevier; 2013. pp. 97–103. [Google Scholar]
- 22.Johansson MW. Activation states of blood eosinophils in asthma. Clin Exp Allergy. 2014;44:482–498. doi: 10.1111/cea.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, Jarjour NN, Busse WW, Kelly EA. Decreased expression of membrane IL-5 receptor α on human eosinophils. I. Loss of membrane IL-5 receptor α on airway eosinophils and increased soluble IL-5 receptor α in the airway after allergen challenge. J Immunol. 2002;169:6452–6458. doi: 10.4049/jimmunol.169.11.6452. [DOI] [PubMed] [Google Scholar]
- 24.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, Jarjour NN, Busse WW, Kelly EAB. Decreased expression of membrane IL-5 receptor α on human eosinophils. II. IL-5 down-modulates its receptor via a proteinase-mediated process. J Immunol. 2002;169:6459–6466. doi: 10.4049/jimmunol.169.11.6459. [DOI] [PubMed] [Google Scholar]
- 25.Gregory B, Kirchem A, Phipps S, Gevaert P, Pridgeon C, Rankin SM, Robinson DS. Differential regulation of human eosinophil IL-3, IL-5, and GM-CSF receptor α-chain expression by cytokines: IL-3, IL-5, and GM-CSF down-regulate IL-5 receptor α expression with loss of IL-5 responsiveness, but up-regulate IL-3 receptor α expression. J Immunol. 2003;170:5359–5366. doi: 10.4049/jimmunol.170.11.5359. [DOI] [PubMed] [Google Scholar]
- 26.Calhoun WJ, Jarjour NN, Gleich GJ, Stevens CA, Busse WW. Increased airway inflammation with segmental versus aerosol antigen challenge. Am Rev Respir Dis. 1993;147:1465–1471. doi: 10.1164/ajrccm/147.6_Pt_1.1465. [DOI] [PubMed] [Google Scholar]
- 27.Kelly EA, Denlinger LC, Liu L, Mathur SK, Esnault S, DeLain LP, Schwantes EA, Cook EB, Stahl JL, Jarjour NN.Utilization of segmental bronchoprovocation to study the effect of anti–IL-5 (mepolizumab) on eosinophil phenotype and recruitment to the airway in subjects with atopic asthma [abstract]. Presented at the 7th International Eosinophil Society Symposium. June 21–25, 2011, Québec City, Canada. [Google Scholar]
- 28.Protheroe C, Woodruff SA, de Petris G, Mukkada V, Ochkur SI, Janarthanan S, Lewis JC, Pasha S, Lunsford T, Harris L, et al. A novel histologic scoring system to evaluate mucosal biopsies from patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2009;7:749–755.e11. doi: 10.1016/j.cgh.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 30.Hartnell A, Robinson DS, Kay AB, Wardlaw AJ. CD69 is expressed by human eosinophils activated in vivo in asthma and in vitro by cytokines. Immunology. 1993;80:281–286. [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto K, Appiah-Pippim J, Schleimer RP, Bickel CA, Beck LA, Bochner BS. CD44 and CD69 represent different types of cell-surface activation markers for human eosinophils. Am J Respir Cell Mol Biol. 1998;18:860–866. doi: 10.1165/ajrcmb.18.6.3159. [DOI] [PubMed] [Google Scholar]
- 32.Esnault S, Kelly EA, Schwantes EA, Liu LY, DeLain LP, Hauer JA, Bochkov YA, Denlinger LC, Malter JS, Mathur SK, et al. Identification of genes expressed by human airway eosinophils after an in vivo allergen challenge. PLoS One. 2013;8:e67560. doi: 10.1371/journal.pone.0067560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen ZJ, Hu J, Esnault S, Dozmorov I, Malter JS. RNA Seq profiling reveals a novel expression pattern of TGF-β target genes in human blood eosinophils. Immunol Lett. 2015;167:1–10. doi: 10.1016/j.imlet.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil’s role remains uncertain as anti–interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- 35.Gauvreau GM, Ellis AK, Denburg JA. Haemopoietic processes in allergic disease: eosinophil/basophil development. Clin Exp Allergy. 2009;39:1297–1306. doi: 10.1111/j.1365-2222.2009.03325.x. [DOI] [PubMed] [Google Scholar]
- 36.Ackerman SJ, Du J. Transcriptional regulation of eosinophil lineage commitment and differentiation. In: Lee JJ, Rosenberg HF, editors. Eosinophils in health and disease. Oxford: Elsevier; 2012. pp. 76–89. [Google Scholar]
- 37.Menzies-Gow A, Flood-Page P, Sehmi R, Burman J, Hamid Q, Robinson DS, Kay AB, Denburg J. Anti–IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol. 2003;111:714–719. doi: 10.1067/mai.2003.1382. [DOI] [PubMed] [Google Scholar]
- 38.Büttner C, Lun A, Splettstoesser T, Kunkel G, Renz H. Monoclonal anti–interleukin-5 treatment suppresses eosinophil but not T-cell functions. Eur Respir J. 2003;21:799–803. doi: 10.1183/09031936.03.00027302. [DOI] [PubMed] [Google Scholar]
- 39.Conus S, Straumann A, Simon HU. Anti–IL-5 (mepolizumab) therapy does not alter IL-5 receptor α levels in patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2009;123:269–, author reply 269–270. doi: 10.1016/j.jaci.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 40.Stein ML, Villanueva JM, Buckmeier BK, Yamada Y, Filipovich AH, Assa’ad AH, Rothenberg ME. Anti–IL-5 (mepolizumab) therapy reduces eosinophil activation ex vivo and increases IL-5 and IL-5 receptor levels. J Allergy Clin Immunol. 2008;121:1473–1483, 1483.e1–1483.e4. doi: 10.1016/j.jaci.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansson MW, Gunderson KA, Kelly EA, Denlinger LC, Jarjour NN, Mosher DF. Anti–IL-5 attenuates activation and surface density of β2-integrins on circulating eosinophils after segmental antigen challenge. Clin Exp Allergy. 2013;43:292–303. doi: 10.1111/j.1365-2222.2012.04065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melo RC, Weller PF. Piecemeal degranulation in human eosinophils: a distinct secretion mechanism underlying inflammatory responses. Histol Histopathol. 2010;25:1341–1354. doi: 10.14670/hh-25.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ueki S, Melo RC, Ghiran I, Spencer LA, Dvorak AM, Weller PF. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood. 2013;121:2074–2083. doi: 10.1182/blood-2012-05-432088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radonjic-Hoesli S, Wang X, de Graauw E, Stoeckle C, Styp-Rekowska B, Hlushchuk R, Simon D, Spaeth PJ, Yousefi S, Simon HU. Adhesion-induced eosinophil cytolysis requires the receptor-interacting protein kinase 3 (RIPK3)-mixed lineage kinase-like (MLKL) signaling pathway, which is counterregulated by autophagy. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.01.044. (In press) [DOI] [PubMed] [Google Scholar]
- 45.Neves JS, Perez SA, Spencer LA, Melo RC, Reynolds L, Ghiran I, Mahmudi-Azer S, Odemuyiwa SO, Dvorak AM, Moqbel R, et al. Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc Natl Acad Sci USA. 2008;105:18478–18483. doi: 10.1073/pnas.0804547105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neves JS, Radke AL, Weller PF. Cysteinyl leukotrienes acting via granule membrane-expressed receptors elicit secretion from within cell-free human eosinophil granules. J Allergy Clin Immunol. 2010;125:477–482. doi: 10.1016/j.jaci.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibson PG, Manning PJ, O’Byrne PM, Girgis-Gabardo A, Dolovich J, Denburg JA, Hargreave FE. Allergen-induced asthmatic responses: relationship between increases in airway responsiveness and increases in circulating eosinophils, basophils, and their progenitors. Am Rev Respir Dis. 1991;143:331–335. doi: 10.1164/ajrccm/143.2.331. [DOI] [PubMed] [Google Scholar]
- 48.Rådinger M, Bossios A, Sjöstrand M, Lu Y, Malmhäll C, Dahlborn AK, Lee JJ, Lötvall J. Local proliferation and mobilization of CCR3+ CD34+ eosinophil-lineage–committed cells in the lung. Immunology. 2011;132:144–154. doi: 10.1111/j.1365-2567.2010.03349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Menzies-Gow AN, Flood-Page PT, Robinson DS, Kay AB. Effect of inhaled interleukin-5 on eosinophil progenitors in the bronchi and bone marrow of asthmatic and non-asthmatic volunteers. Clin Exp Allergy. 2007;37:1023–1032. doi: 10.1111/j.1365-2222.2007.02735.x. [DOI] [PubMed] [Google Scholar]
- 50.Sehmi R, Smith SG, Kjarsgaard M, Radford K, Boulet LP, Lemiere C, Prazma CM, Ortega H, Martin JG, Nair P. Role of local eosinophilopoietic processes in the development of airway eosinophilia in prednisone-dependent severe asthma. Clin Exp Allergy. 2016;46:793–802. doi: 10.1111/cea.12695. [DOI] [PubMed] [Google Scholar]
- 51.Fanat AI, Thomson JV, Radford K, Nair P, Sehmi R. Human airway smooth muscle promotes eosinophil differentiation. Clin Exp Allergy. 2009;39:1009–1017. doi: 10.1111/j.1365-2222.2009.03246.x. [DOI] [PubMed] [Google Scholar]
- 52.Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria JP, O’Byrne PM, Gauvreau GM, Boulet LP, Lemiere C, Martin J, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137:75–86.e78. doi: 10.1016/j.jaci.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 53.Hui CCK, McNagny KM, Denburg JA, Siracusa MC. In situ hematopoiesis: a regulator of TH2 cytokine–mediated immunity and inflammation at mucosal surfaces. Mucosal Immunol. 2015;8:701–711. doi: 10.1038/mi.2015.17. [DOI] [PubMed] [Google Scholar]
- 54.Braccioni F, Dorman SC, O’Byrne PM, Inman MD, Denburg JA, Parameswaran K, Baatjes AJ, Foley R, Gauvreau GM. The effect of cysteinyl leukotrienes on growth of eosinophil progenitors from peripheral blood and bone marrow of atopic subjects. J Allergy Clin Immunol. 2002;110:96–101. doi: 10.1067/mai.2002.125000. [DOI] [PubMed] [Google Scholar]
- 55.Ortega H, Chupp G, Bardin P, Bourdin A, Garcia G, Hartley B, Yancey S, Humbert M. The role of mepolizumab in atopic and nonatopic severe asthma with persistent eosinophilia. Eur Respir J. 2014;44:239–241. doi: 10.1183/09031936.00220413. [DOI] [PubMed] [Google Scholar]
- 56.Brusselle GG, Maes T, Bracke KR. Eosinophils in the spotlight: eosinophilic airway inflammation in nonallergic asthma. Nat Med. 2013;19:977–979. doi: 10.1038/nm.3300. [DOI] [PubMed] [Google Scholar]
- 57.Denlinger LC, Sorkness RL, Lee WM, Evans MD, Wolff MJ, Mathur SK, Crisafi GM, Gaworski KL, Pappas TE, Vrtis RF, et al. Lower airway rhinovirus burden and the seasonal risk of asthma exacerbation. Am J Respir Crit Care Med. 2011;184:1007–1014. doi: 10.1164/rccm.201103-0585OC. [DOI] [PMC free article] [PubMed] [Google Scholar]