Abstract
Rationale: Areas of increased lung attenuation visualized by computed tomography are associated with all-cause mortality in the general population. It is uncertain whether this association is attributable to interstitial lung disease (ILD).
Objectives: To determine whether high-attenuation areas are associated with the risk of ILD hospitalization and mortality in the general population.
Methods: We performed a cohort study of 6,808 adults aged 45–84 years sampled from six communities in the United States. High-attenuation areas were defined as the percentage of imaged lung volume with attenuation values between −600 and −250 Hounsfield units. An adjudication panel determined ILD hospitalization and death.
Measurements and Main Results: After adjudication, 52 participants had a diagnosis of ILD during 75,232 person-years (median, 12.2 yr) of follow-up. There were 48 hospitalizations attributable to ILD (crude rate, 6.4 per 10,000 person-years). Twenty participants died as a result of ILD (crude rate, 2.7 per 10,000 person-years). High-attenuation areas were associated with an increased rate of ILD hospitalization (adjusted hazard ratio, 2.6 per 1-SD increment in high-attenuation areas; 95% confidence interval, 1.9–3.5; P < 0.001), a finding that was stronger among men, African Americans, and Hispanics. High-attenuation areas were also associated with an increased rate of ILD-specific death (adjusted hazard ratio, 2.3; 95% confidence interval, 1.7–3.0; P < 0.001). Our findings were consistent among both smokers and nonsmokers.
Conclusions: Areas of increased lung attenuation are a novel risk factor for ILD hospitalization and mortality. Measurement of high-attenuation areas by screening and diagnostic computed tomography may be warranted in at-risk adults.
Keywords: subclinical interstitial lung disease, pulmonary fibrosis, mortality, hospitalizations, epidemiology
At a Glance Commentary
Scientific Knowledge on the Subject
Areas of increased lung attenuation (high-attenuation areas) visualized by computed tomography (CT) are associated with biological and physiological changes expected in subclinical interstitial lung disease (ILD) and are associated with all-cause mortality in the general population, suggesting that they may be a marker of early ILD.
What This Study Adds to the Field
Among a large cohort of community-dwelling adults sampled without regard to smoking or respiratory symptoms, a greater percentage of high-attenuation areas seen by CT was associated with higher rates of hospitalization due to ILD, death due to ILD, and respiratory symptoms at 10-year follow-up. Areas of increased lung attenuation detected by CT are a novel risk factor for ILD hospitalization and mortality among both smokers and nonsmokers.
Interstitial lung disease (ILD) affects 0.5% of the U.S. Medicare population and is responsible for 17,000 deaths annually in the United States, a number thought to vastly underestimate the true ILD mortality rate in the population (1, 2). ILD is the leading indication for lung transplant in the United States (3). Two medications slow the progression of idiopathic pulmonary fibrosis, a severe fibrotic form of ILD, but there are no curative therapies currently available or on the horizon. ILD prevention could reduce the global health burden of ILD, but limited understanding of the antecedent causes of idiopathic pulmonary fibrosis and other ILDs has hindered preventive efforts.
The investigation of subclinical ILD is a promising approach that may identify novel causes of ILD (4). Interstitial lung abnormalities (ILA), a qualitative subclinical ILD phenotype, can be visually identified by chest computed tomography (CT) in 7 to 10% of both smokers and nonsmokers and are associated with all-cause mortality (5–12). Quantitative computed tomographic densitometry can detect even more subtle areas of increased lung attenuation, termed “high-attenuation areas” (HAA). Greater HAA visualized by CT is associated with cigarette smoking, reduced lung function, biomarkers of inflammation and extracellular matrix remodeling, visually identifiable ILA at 10-year follow-up, and all-cause mortality in community-dwelling adults, supporting its construct validity as a quantitative subclinical ILD phenotype (12, 13). It remains uncertain whether HAA is associated with clinical symptoms and ILD-specific mortality.
In the present study, we hypothesized that greater HAA on chest CT is associated with increased risks of death and hospitalizations due to ILD, as well as with respiratory symptoms in community-dwelling adults. Some of the results of this study were previously reported in the form of an abstract (14).
Methods
Study Design and Participants
MESA (Multi-Ethnic Study of Atherosclerosis) is a prospective cohort study that enrolled 6,814 men and women aged 45–84 years without clinical cardiovascular disease from six communities in the United States in 2000–2002, as previously described (15). Five participants were subsequently noted to have preexisting cardiovascular events and were excluded from follow-up (see Figure E1 in the online supplement). We also excluded one participant with invalid baseline HAA measurement. All MESA participants provided informed consent, and the MESA study was approved by the institutional review boards at all centers.
HAA
Quantitative image attenuation was measured on MESA baseline cardiac computed tomographic scans using a modified version of the Pulmonary Analysis Software Suite at a single reading center by trained readers from the University of Iowa Imaging Lab (Iowa City, IA). Cardiac computed tomographic scans were obtained in 2000–2002 using multidetector CT scanners (three sites) or electron beam tomography scanners (three sites) according to a standardized protocol (Table E1), as previously described (16, 17). HAA was defined as the percentage of imaged lungs having CT attenuation values between −600 and −250 Hounsfield units, as previously described (12, 13). Percent emphysema was defined as the percentage of lung voxels less than −950 Hounsfield units (17–19).
Causes of Death and Hospitalization
MESA participants or surrogates were contacted at 9- to 12-month intervals to obtain data on deaths and hospitalizations. This information was supplemented by review of the National Death Index. Mortality data were complete as of March 2015, and hospitalization data were complete as of December 2013.
A two-member adjudication panel blinded to HAA values reviewed inpatient medical records (n = 280) and death certificates (n = 39) from 66 of the 70 participants who were hospitalized with an International Classification of Diseases, Ninth Revision (ICD-9), ILD diagnosis code (ICD-9 codes 495.XX, 515.XX, or 516.XX) or died with any ICD-10 ILD diagnosis codes (ICD-10 codes J60.X–J64.X, J67.X, or J84.X). There were no available records for four participants (Figure E1). Their ICD codes are shown in Table E2. On the basis of review of these diagnosis codes, they were classified as having ILD and included in all primary analyses.
Each panel member reviewed half of the available records to determine (1) whether the study participant had ILD, (2) whether each hospitalization was respiratory related, (3) whether each hospitalization was due to ILD, (4) whether each death was respiratory related, and (5) whether each death was due to ILD. In cases of uncertainty, panel members convened to classify diagnoses and events by consensus. A random sample of 48 charts was independently reviewed by both panel members (agreement, 100%; kappa = 1.0 for ILD diagnosis, ILD death, and ILD hospitalization).
Panel members adjudicated an ILD diagnosis if any of the following were found in the inpatient medical record: physician-diagnosed or self-reported ILD, chest x-ray or computed tomographic scan with bilateral fibrosis or interstitial infiltrates not attributable to pulmonary edema, or an autopsy or death certificate diagnosis of ILD. Panel members adjudicated a respiratory hospitalization among those with an adjudicated ILD diagnosis (“respiratory hospitalization with ILD”) when a participant had an adjudicated ILD diagnosis and the primary reason for hospitalization was one of the following: acute exacerbation of ILD, pulmonary embolism, lower respiratory tract infection, pneumothorax, aspiration event, exacerbation of chronic obstructive pulmonary disease (COPD), lung transplant, respiratory failure requiring mechanical ventilation, or other acute respiratory worsening (including increased dyspnea, hypoxemia, and respiratory distress) (20). An “ILD hospitalization” was adjudicated when a participant with an adjudicated ILD diagnosis experienced a respiratory hospitalization that was determined by the reviewer to be due to the presence of ILD.
For inpatient deaths, an “ILD death” was adjudicated if all of the following criteria were met: (1) the cause of death was respiratory related on the basis of the criteria listed in Table E3, (2) the decedent had an adjudicated diagnosis of ILD, and (3) the reviewer determined that death was due to ILD. For out-of-hospital deaths, an “ILD death” was adjudicated if ILD was listed as the primary cause of death on the death certificate.
For comparison purposes, we also examined death due to all respiratory causes, as defined by the presence of any “J” ICD-10 code as the underlying cause of death, and chronic lower respiratory disease (CLRD), which includes COPD, emphysema, chronic bronchitis, and asthma (defined by ICD-10 codes J40–J47 as the underlying cause of death). MESA CLRD deaths that occurred between study entry and December 2013 were adjudicated as previously described (19).
Respiratory Symptoms
Questionnaires on cough and dyspnea were completed at the fifth MESA follow-up examination in 2010–2012. Of the 4,716 participants who completed the follow-up examination, 4,562 and 4,506 completed the following questions, respectively: (1) “When walking on level ground, do you get more breathless than people your own age?” and (2) “When walking up hills or stairs, do you get more breathless than people your own age?” Cough was ascertained in 3,442 participants enrolled in the MESA Lung ancillary study and was defined by answering yes to the following question: “Do you usually have a cough on most days for 3 or more months during the year?”
Statistical Analysis
We computed crude cause-specific mortality and hospitalization rates and used Cox regression models to examine associations between baseline HAA and time to ILD death. For hospitalizations, we used the proportional means model, which is an extension of the proportional hazards model to account for recurrent events (21). Survival time was calculated as time from study entry to ILD hospitalization or death. For recurrent events, the time interval for each subsequent event started at the end of the previous event. Follow-up time was censored upon death due to other causes. For hospitalization analyses, participants without an ILD hospitalization were censored at the time of death or last follow-up. We used logistic regression to examine associations of HAA with an adjudicated ILD diagnosis and respiratory symptoms. Hazard ratios (HRs) and odds ratios (ORs) are reported per 1-SD increment of natural log-transformed HAA.
Because HAA can be heavily influenced by study site/scanner, radiation dose, and the total volume of imaged lung, we first constructed parsimonious models adjusting for these variables (“precision variable-adjusted models”). Because of low event rates, we chose to control for additional potential confounders and precision variables using a generalized propensity score (GPS) using the CBPS package in R (22–26). The GPS included age, sex, race/ethnicity, smoking status, cigarette pack-years, body mass index (BMI), waist circumference, height, educational attainment, study site/scanner, glomerular filtration rate, and radiation dose. Total volume of imaged lung and percent emphysema were excluded from the GPS because of poor covariate balance (Table E4). Instead, they were included as individual independent variables in each regression model. The Pearson correlations between HAA and the other covariates were close to zero after GPS calculation, indicating good balance (Table E4). We also calculated a second GPS that included adjustment for additional clinical variables: alcohol use, total intentional exercise (metabolic equivalent min/wk), coronary artery calcium score, diabetes medication use, insulin use, fasting glucose, hypertension, antihypertensive medication use, systolic and diastolic blood pressures, cholesterol medication use, total and high-density lipoprotein cholesterol levels, C-reactive protein, d-dimer, and cancer history. Effect modification by smoking status, age, sex, and race/ethnicity was tested in fully adjusted models using multiplicative interaction terms. We performed sensitivity analyses that included adjustment for FVC and FEV1 and that excluded participants in the top-fifth percentile of HAA. For consistency with prior work, we also report estimates per doubling of log base 2 (binary)-transformed HAA. We used additive Cox models with penalized splines to account for nonlinearity and to generate plots (27). We tested for nonlinearity using the likelihood ratio test. We employed multiple imputation using chained equations to account for missing covariate values, which were infrequent (<1%) (28). All statistical tests were performed using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria) software.
Results
A total of 6,808 participants had complete event follow-up and HAA data. At study enrollment, the mean age (SD) was 62 (10) years, 47% were male, 38% were white, 28% were African American, 22% were Hispanic, and 12% were Chinese (Table 1). Among the sample, 41% were former smokers, and 14% were current smokers, with a median of 14.5 cigarette pack-years. Participants with higher HAA were more likely to be female, nonwhite, and never-smokers, and they tended to have a higher BMI and less emphysema. Median follow-up time was 12.2 years (75,232 person-years). Mean (SD) HAA was 5.1% (3.1%) of imaged lung volume. In unadjusted analyses, higher HAA quartiles had higher proportions of participants with a diagnosis of ILD, ILD hospitalization, and ILD death (Table E5).
Table 1.
Baseline Characteristics of 6,808 MESA Participants Overall and Stratified by Quartiles of High-Attenuation Areas
| Overall | HAA |
||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| HAA %, range | 2–75% | 2.0–3.5% | 3.5–4.2% | 4.2–5.4% | 5.4–75.2% |
| Participants, n | 6,808 | 1,702 | 1,702 | 1,702 | 1,702 |
| Age, yr | 62 ± 10 | 62 ± 10 | 62 ± 10 | 62 ± 10 | 63 ± 10 |
| Male sex | 3,213 (47) | 952 (56) | 839 (49) | 750 (44) | 672 (39) |
| Race/ethnicity | |||||
| White | 2,622 (38) | 942 (55) | 666 (39) | 586 (34) | 428 (25) |
| African American | 1,892 (28) | 385 (23) | 481 (28) | 524 (31) | 502 (29) |
| Hispanic | 1,495 (22) | 238 (14) | 349 (21) | 394 (23) | 514 (30) |
| Chinese | 804 (12) | 138 (8) | 207 (12) | 200 (12) | 259 (15) |
| BMI, kg/m2 | 28 ± 5 | 27 ± 5 | 28 ± 5 | 29 ± 6 | 30 ± 6 |
| Height, cm | 166 ± 10 | 169 ± 10 | 167 ± 10 | 166 ± 10 | 164 ± 10 |
| Weight, kg | 79 ± 17 | 75 ± 16 | 78 ± 17 | 81 ± 18 | 80 ± 18 |
| Waist circumference, cm | 98 ± 14 | 94 ± 13 | 97 ± 14 | 100 ± 14 | 101 ± 15 |
| Hip circumference, cm | 106 ± 11 | 103 ± 10 | 105 ± 11 | 107 ± 12 | 108 ± 12 |
| Smoking history | |||||
| Never-smokers | 3,085 (45) | 707 (42) | 744 (43) | 786 (46) | 848 (50) |
| Former smokers | 2,761 (41) | 785 (46) | 724 (43) | 645 (38) | 607 (36) |
| Current smokers | 967 (14) | 211 (12) | 235 (14) | 273 (16) | 248 (15) |
| Cigarette pack-years* | 14.5 (3–33) | 13 (3–32) | 15 (4–34) | 16 (4–34) | 14 (3–31) |
| Spirometry† | |||||
| FVC, L | 3.19 ± 0.95 | 3.63 ± 0.94 | 3.26 ± 0.90 | 3.04 ± 0.89 | 2.85 ± 0.92 |
| FVC, % predicted | 95 ± 16 | 102 ± 16 | 96 ± 15 | 93 ± 18 | 91 ± 17 |
| FEV1, L | 2.38 ± 0.73 | 2.61 ± 0.74 | 2.43 ± 0.71 | 2.30 ± 0.70 | 2.20 ± 0.72 |
| FEV1, % predicted | 94 ± 18 | 98 ± 19 | 94 ± 18 | 92 ± 18 | 92 ± 18 |
| FEV1/FVC ratio | 0.75 ± 0.09 | 0.72 ± 0.09 | 0.75 ± 0.09 | 0.75 ± 0.08 | 0.77 ± 0.07 |
| Computed tomography | |||||
| Total imaged lung volume, ml | 2,691 (2,208–3,253) | 3,312 (2,857–3,861) | 2,866 (2,449–3,362) | 2,569 (2,201–3,006) | 2,045 (1,711–2,466) |
| Emphysema, % | 4.2 (3.5–5.4) | 6.7 (3.8–10.1) | 3.6 (2.1–6.0) | 2.3 (1.3–4.0) | 0.9 (0.4–1.9) |
Definition of abbreviations: BMI = body mass index; HAA = high-attenuation areas; MESA = Multi-Ethnic Study of Atherosclerosis.
Data are presented as mean ± SD, n (%), or median (interquartile range), unless otherwise stated. All parameters collected at MESA baseline visit in 2000–2002, unless otherwise stated.
Among ever-smokers.
Completed at MESA exam 3–4 (years 2004–2006) in 3,832 MESA participants.
HAA and ILD Diagnosis upon Hospitalization or Death
Between 2000 and 2013, there were 7,696 event investigations among 3,339 MESA participants. There were 84 hospitalizations and 18 deaths with an ILD diagnosis code among 70 participants. After adjudication, 52 of these 70 participants were determined to have an ILD diagnosis (Table 2). The most common criterion for ILD diagnosis was a physician diagnosis of ILD (Table E6). Twenty-eight participants had at least one imaging report (chest x-ray or CT) or autopsy report noting bilateral pulmonary fibrosis or interstitial changes not due to pulmonary edema. In a fully adjusted model, each 1-SD increment in HAA was associated with a 2.4-fold greater odds of adjudicated ILD diagnosis (95% confidence interval [CI], 1.9–3.1) (Table 2).
Table 2.
Associations between Baseline High-Attenuation Areas and Interstitial Lung Disease Diagnosis in 6,808 MESA Participants over 12.2 Years of Follow-up
| Overall | P Value | |
|---|---|---|
| Total number of participants | 6,808 | |
| Number with adjudicated ILD diagnosis | 52 | |
| OR per SD of HAA (95% CI), adjusted for | ||
| Site/scanner, radiation dose (mA), total volume of imaged lung | 2.4 (1.9–3.1) | <0.001 |
| Plus demographics, smoking, emphysema* | 2.4 (1.8–3.2) | <0.001 |
| Plus clinical variables† | 2.4 (1.9–3.1) | <0.001 |
Definition of abbreviations: CI = confidence interval; HAA = high-attenuation areas; ILD = interstitial lung disease; MESA = Multi-Ethnic Study of Atherosclerosis; OR = odds ratio.
Adjusted for total volume of imaged lung; percent emphysema; and generalized propensity score 1, which included age, sex, race/ethnicity, smoking status, cigarette pack-years, body mass index, waist circumference, height, educational attainment, study site/scanner, glomerular filtration rate, and radiation dose.
Adjusted for total volume of imaged lung; percent emphysema; and generalized propensity score 2, which included age, sex, race/ethnicity, smoking status, cigarette pack-years, body mass index, waist circumference, height, educational attainment, study site/scanner, glomerular filtration rate, radiation dose, alcohol use, total intentional exercise (metabolic equivalent min/wk), coronary artery calcium, diabetes medication use, insulin use, fasting glucose, hypertension, antihypertensive medication use, systolic and diastolic blood pressures, cholesterol medication use, total and high-density lipoprotein cholesterol levels, C-reactive protein, d-dimer, and cancer history.
HAA and Hospitalization
Among 52 participants with ILD (adjudicated upon hospitalization or death) over 75,232 person-years, there were 80 respiratory hospitalizations (crude rate, 10.6 per 10,000 person-years; 95% CI, 8.5–13.2) (Table 3), of which 48 were due to ILD (crude rate, 6.4 per 10,000 person-years; 95% CI, 4.8–8.5). The most common reason for respiratory hospitalization was increased dyspnea, followed by respiratory tract infection or pneumonia (Table E3). There were seven elective admissions for surgical lung biopsy, which we did not consider respiratory hospitalizations.
Table 3.
Associations between Baseline High-Attenuation Areas and Respiratory Hospitalizations in 6,808 MESA Participants over 12.2 Years of Follow-up
| Respiratory Hospitalization with ILD | P Value | ILD Hospitalization | P Value | |
|---|---|---|---|---|
| Events | 80 | 48 | ||
| Person-years | 75,232 | 75,232 | ||
| Event rate per 10,000 person-years (95% CI) | 10.6 (8.5–13.2) | 6.4 (4.8–8.5) | ||
| HR per SD of HAA (95% CI), adjusted for | ||||
| Site/scanner, radiation dose (mA), total volume of imaged lung | 2.3 (1.6–3.3) | <0.001 | 2.5 (1.8–3.4) | <0.001 |
| Plus demographics, smoking, emphysema* | 2.4 (1.7–3.5) | <0.001 | 2.6 (1.9–3.6) | <0.001 |
| Plus clinical variables† | 2.4 (1.7–3.3) | <0.001 | 2.6 (1.9–3.5) | <0.001 |
Definition of abbreviations: CI = confidence interval; HAA = high-attenuation areas; HR = hazard ratio; ILD = interstitial lung disease; MESA = Multi-Ethnic Study of Atherosclerosis.
Adjusted for total volume of imaged lung; percent emphysema; and generalized propensity score 1, which included age, sex, race/ethnicity, smoking status, cigarette pack-years, body mass index, waist circumference, height, educational attainment, study site/scanner, glomerular filtration rate, and radiation dose.
Adjusted for total volume imaged lung; percent emphysema; and generalized propensity score 2, which included age, sex, race/ethnicity, smoking status, cigarette pack-years, body mass index, waist circumference, height, educational attainment, study site/scanner, glomerular filtration rate, radiation dose, alcohol use, total intentional exercise (metabolic equivalent min/wk), coronary artery calcium, diabetes medication use, insulin use, fasting glucose, hypertension, antihypertensive medication use, systolic and diastolic blood pressures, cholesterol medication use, total and high-density lipoprotein cholesterol levels, C-reactive protein, d-dimer, and cancer history.
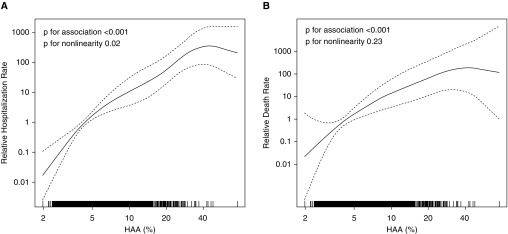
In fully adjusted models, HAA was associated with increased rates of respiratory hospitalization among those with ILD (HR, 2.4; 95% CI, 1.7–3.3) and ILD hospitalization (HR, 2.6; 95% CI, 1.9–3.5) (Table 3). There was statistical evidence that this association was nonlinear (P = 0.02 for nonlinearity) (Figure 1A), but this was due to one participant with HAA of 75%. After exclusion of this participant, there was a linear association between HAA and ILD hospitalization (P = 0.17 for nonlinearity) (Figure E2). There was strong evidence that sex and race/ethnicity modified the association between HAA and ILD hospitalization (P ≤ 0.01 for interaction) (Table E7). The association between HAA and ILD was stronger among men (HR, 3.1; 95% CI, 2.3–4.1) than among women (HR, 2.0; 95% CI, 1.4–2.7). African Americans and Hispanics tended to have a stronger association than did white individuals (Table E7). There was moderate evidence of effect modification by smoking status (P = 0.08 for interaction), with a trend toward a stronger association among ever-smokers (HR, 2.9; 95% CI, 2.2–3.8) than among never-smokers (HR, 2.0; 95% CI, 1.3–3.0).
Figure 1.
Continuous associations between high-attenuation areas (HAA) and (A) interstitial lung disease (ILD) hospitalization and (B) ILD mortality. Models are adjusted for total volume of imaged lung; percent emphysema; and generalized propensity score 2, which included age, sex, race/ethnicity, smoking status, cigarette pack-years, body mass index, waist circumference, height, educational attainment, study site/scanner, glomerular filtration rate, radiation dose, alcohol use, total intentional exercise (metabolic equivalent min/wk), coronary artery calcium, diabetes medication use, insulin use, fasting glucose, hypertension, antihypertensive medication use, systolic and diastolic blood pressures, cholesterol medication use, total and high-density lipoprotein cholesterol levels, C-reactive protein, d-dimer, and cancer history. Solid line is the overall effect estimate, and dashed lines are the 95% confidence bands. Each vertical hash mark in the rug plot along the x-axis represents one study participant.
HAA and ILD-Specific Mortality
There were 1,225 deaths in MESA over 75,232 person-years. Of these, 84 were due to respiratory causes (crude rate, 11.2 per 10,000 person-years; 95% CI, 9.0–13.8) (Table 4), 20 were adjudicated to be due to ILD (crude rate, 2.7 per 10,000 person-years; 95% CI, 1.7–4.1), and 42 were due to CLRD (crude rate, 5.6 per 10,000 person-years; 95% CI, 4.1–7.6). In fully adjusted models, HAA was associated with an increased rate of ILD death (HR, 2.3; 95% CI, 1.7–3.0; P = 0.23 for nonlinearity) (Figure 1B). In precision-adjusted analyses, HAA was inversely associated with death due to CLRD (HR, 0.5; 95% CI, 0.3–0.9). Further adjustment for demographics and smoking did not significantly change this association (Table E8). In models further adjusted for emphysema, HAA was not associated with CLRD death (HR, 0.9; 95% CI, 0.6–1.5) (Tables 4 and E8). There was no evidence of effect modification by sex, age, race/ethnicity, or smoking status on the risk of ILD death, with similar findings among all subgroups, including smokers and nonsmokers (Table E7).
Table 4.
Associations between Baseline High-Attenuation Areas and Cause of Death in 6,808 MESA Participants over 12.2 Years of Follow-up
| Underlying Cause of Death | Overall | P Value |
|---|---|---|
| Respiratory | ||
| Deaths | 84 | |
| Person-years | 75,232 | |
| Mortality rate per 10,000 person-years | 11.2 (9.0–13.8) | |
| HR per SD of HAA (95% CI), adjusted for | ||
| Site/scanner, radiation dose (mA), total volume of imaged lung | 1.4 (1.1–1.8) | 0.01 |
| Plus demographics, smoking, emphysema* | 1.5 (1.2–1.9) | <0.001 |
| Plus clinical variables† | 1.5 (1.2–1.9) | <0.001 |
| ILD | ||
| Deaths | 20 | |
| Person-years | 75,232 | |
| Mortality rate per 10,000 person-years | 2.7 (1.7–4.1) | |
| HR per SD of HAA (95% CI), adjusted for | ||
| Site/scanner, radiation dose (mA), total volume of imaged lung | 2.5 (1.9–3.3) | <0.001 |
| Plus demographics, smoking, emphysema* | 2.2 (1.6–3.0) | <0.001 |
| Plus clinical variables† | 2.3 (1.7–3.0) | <0.001 |
| CLRD | ||
| Deaths | 42 | |
| Person-years | 75,232 | |
| Mortality rate per 10,000 person-years | 5.6 (4.1–7.6) | |
| HR per SD of HAA (95% CI), adjusted for | ||
| Site/scanner, radiation dose (mA), total volume of imaged lung | 0.5 (0.3–0.8) | 0.01 |
| Plus demographics, smoking, emphysema* | 0.9 (0.6–1.5) | 0.74 |
| Plus clinical variables† | 0.9 (0.6–1.5) | 0.72 |
Definition of abbreviations: CI = confidence interval; CLRD = chronic lower respiratory disease; HAA = high-attenuation areas; HR = hazard ratio; ILD = interstitial lung disease; MESA = Multi-Ethnic Study of Atherosclerosis.
Adjusted for total volume of imaged lung; percent emphysema; and generalized propensity score 1, which included age, sex, race/ethnicity, smoking status, cigarette pack-years, body mass index, waist circumference, height, educational attainment, study site/scanner, glomerular filtration rate, and radiation dose.
Adjusted for total volume of imaged lung; percent emphysema; and generalized propensity score 2, which included age, sex, race/ethnicity, smoking status, cigarette pack-years, body mass index, waist circumference, height, educational attainment, study site/scanner, glomerular filtration rate, radiation dose, alcohol use, total intentional exercise (metabolic equivalent min/wk), coronary artery calcium, diabetes medication use, insulin use, fasting glucose, hypertension, antihypertensive medication use, systolic and diastolic blood pressures, cholesterol medication use, total and high-density lipoprotein cholesterol levels, C-reactive protein, d-dimer, and cancer history.
HAA and Respiratory Symptoms
Greater HAA at baseline was associated with a higher odds of dyspnea at 9.5-year follow-up. In fully adjusted models, HAA was associated with increased odds of dyspnea on level ground (OR, 1.3; 95% CI, 1.1–1.4; P < 0.001) (Table 5) and dyspnea walking up hills (OR, 1.1; 95% CI, 1.0–1.2; P = 0.04). HAA was not associated with cough (P = 0.70).
Table 5.
Associations between Baseline High-Attenuation Areas and Dyspnea and Cough at 9.5 Years of Follow-up
| n | Events | OR per SD of HAA (95% CI)* | P Value | |
|---|---|---|---|---|
| Dyspnea on level ground | 4,562 | 467 | 1.3 (1.1–1.4) | <0.001 |
| Dyspnea walking up hills | 4,506 | 1,070 | 1.1 (1.0–1.2) | 0.04 |
| Chronic cough | 3,442 | 334 | 1.0 (0.8–1.1) | 0.70 |
Definition of abbreviation: CI = confidence interval; OR = odds ratio; HAA = high-attenuation areas.
Adjusted for total volume of imaged lung; percent emphysema; and generalized propensity score 2, which included age, sex, race/ethnicity, smoking status, cigarette pack-years, body mass index, waist circumference, height, educational attainment, study site/scanner, glomerular filtration rate, radiation dose, alcohol use, total intentional exercise (metabolic equivalent min/wk), coronary artery calcium, diabetes medication use, insulin use, fasting glucose, hypertension, antihypertensive medication use, systolic and diastolic blood pressures, cholesterol medication use, total and high-density lipoprotein cholesterol levels, C-reactive protein, d-dimer, and cancer history.
Additional Analyses
In a subset of participants who completed spirometry, additional adjustment for FVC and FEV1 (measured in liters) did not meaningfully change our results (Table E9). Our findings remained consistent in sensitivity analyses that excluded participants with HAA values above the 95th percentile (HAA, >9.8%) (Table E10). Table E11 shows adjusted effect estimates per doubling of HAA.
Discussion
We have shown that HAA in the lung fields visualized by cardiac CT is associated with an adjudicated diagnosis of ILD, with increased risk of hospitalization and death due to ILD, and with exertional dyspnea during a median 12.2-year follow-up in a multiethnic cohort of middle-aged and older community-dwelling adults, independent of smoking status and other potential confounders. The lack of an association with CLRD events provides evidence that HAA is specific for ILD and is not related to COPD, asthma, or other lung diseases. These findings provide strong evidence that HAA represents structural alveolar changes that may precede clinically relevant ILD and that HAA is a novel ILD risk factor that may help identify adults at risk for the development of clinically significant ILD events who can be enrolled in future clinical trials of ILD prevention.
Although the pathological abnormalities that increase CT lung attenuation in the general population remain unknown, our data support the hypothesis that, at least in some cases, HAA is a result of subclinical alveolar epithelial injury, alveolar inflammation, remodeling of the extracellular matrix, and interstitial fibrosis. HAA may reflect early pathological changes that lead to a number of different ILDs, including autoimmune disease and sarcoidosis, which may explain the higher proportions of women, never-smokers, and African Americans/Hispanics in higher HAA quartiles. Indeed, we have previously shown that autoimmune serologies are associated with greater HAA (as well as ILA) (29). In some individuals, HAA might also represent hydrostatic pulmonary edema, pulmonary infection, or basilar atelectasis due to obesity or incomplete inspiration. However, the totality of our work establishes HAA as a quantitative measure of subclinical ILD (12, 13). For example, greater HAA on baseline MESA cardiac computed tomographic scans is associated with greater serum matrix metalloproteinase 7 and IL-6 levels, indicating that HAA detects extracellular matrix remodeling and inflammation (12). Greater HAA is also associated with reduced FVC (but not with airflow obstruction), reduced exercise capacity, and all-cause mortality (12). We have previously shown the consistency of our results after adjustment for left ventricular function and renal function, as well as across all strata of BMI (12). MESA excluded participants with clinically diagnosed heart failure and cognitive impairment that would preclude completion of study procedures, and those with an acute infectious process were rescheduled (15). The strong associations between HAA and adjudicated ILD endpoints support the clinical relevance of this measure in middle-aged and older adults undergoing CT for a nonclinical indication.
Researchers in prior studies have examined the progression of subclinical ILD and have shown strong and reproducible associations of both the presence and progression of ILA with all-cause mortality, indicating that visually identifiable interstitial changes have clinical implications (10, 11). We have previously shown that HAA is associated with the presence of ILA 10 years later (12). We now extend these findings by showing that HAA has clinical relevance and can progress to clinical ILD events. Whereas ILA remains an important subclinical ILD phenotype, HAA may have a number of advantages over ILA, particularly as a screening tool for at-risk individuals and in research settings. For example, HAA may precede ILA, and HAA is an automated, highly reproducible, easy-to-measure, and inexpensive metric, whereas measurement of ILA is a qualitative assessment performed by a trained reader. Prior studies have shown that HAAs and ILA capture different information. For example, in one study, HAA had a low positive predictive value (<20%) for ILA in cross-sectional analyses of smokers and community-dwelling adults, and HAA was not associated with the MUC5B promoter single-nucleotide polymorphism mutation (whereas ILA was) (30). In the present study, we found consistent associations between HAA and clinical ILD events even after exclusion of the top ranges of HAA. These findings support HAA as a distinct phenotype of subclinical ILD.
Quantitative and qualitative imaging techniques are just beginning to be used to characterize subclinical ILD, but these approaches have been used extensively in other disease states, such as pulmonary emphysema. Quantitative measures of emphysema have yielded important insights into this disease, including new knowledge about the relationship between emphysema and both left ventricular filling and mortality even in those without airflow obstruction (19, 31). Importantly, quantitative measures of low lung attenuation and qualitative visual assessment of emphysema are distinct phenotypes (32). For example, in a genome-wide association study, qualitative and quantitative emphysema were associated with different genetic polymorphisms (33). In this study, up to 12% of participants without visually identified emphysema based on CT had high percentages of quantitatively detected emphysema. In a different study, the agreement between qualitative and quantitative emphysema measures based on CT was poor (kappa = 0.26) (34). Similarly, ILA and HAA may have important complementary roles in studies of early ILD, which may lead to novel and distinct insights into the pathogenesis of ILD.
Our study has a number of limitations. First, we used cardiac CT, which images approximately 70% of the total lung, to measure HAA. We have previously shown good correlation and agreement between cardiac CT and full-lung CT with regard to HAA (13). Second, although we controlled for a number of potential confounders, including BMI, waist circumference, total volume of imaged lung, and renal function, residual confounding by body size, decreased muscle strength, or technical variation in HAA measurement is possible. The MESA CT protocol included extensive quality control to minimize breath artifacts. These factors seem unlikely to explain the strong associations between HAA and clinical ILD events found in our study. Third, we lack information on baseline disease status. However, those who had already undergone chest CT within the past year and those with a serious, life-limiting medical condition were not eligible for participation in MESA, making it unlikely that MESA included those with preexisting clinically evident ILD at baseline. Fourth, there were relatively few ILD events in our study, a finding that is expected for a rare disease. Nevertheless, we were able to detect meaningful associations between HAA and incident ILD deaths and hospitalizations while controlling for potential confounding factors. Fifth, although review of medical records by an adjudication panel is a major strength of our study, we lacked information regarding ILD diagnosis for those participants who were not hospitalized or deceased, likely underestimating event rates in our study. In addition, although clinical CT reports from hospital admissions were available, we were not able to visually review computed tomographic imaging performed for clinical indications.
In summary, our findings establish the clinical relevance of HAA by showing that even small increments in HAA are associated with an increased risk of clinical ILD events. HAA, an easily quantifiable measure even based on clinically indicated computed tomographic images, may be a clinically useful risk stratification tool in future studies. Perhaps most important, our data support HAA as a research tool in studies of novel causes of ILD and ILD prevention.
Acknowledgments
Acknowledgment
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at www.mesa-nhlbi.org.
Footnotes
Supported by the National Institutes of Health under contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 and grants UL1-TR-000040, UL1-TR-001079, R01-HL-103676, RC1-HL100543, R01-HL-093081, R01-HL-077612, T32-HL-105323, and K24-HL-131937; by the Pulmonary Fibrosis Foundation; and by the Rocco Guinta Research Fund.
Author Contributions: A.J.P., E.C.O., D.J.L., R.G.B., and S.M.K.: conceived of and designed the study; A.J.P., E.C.O., R.G.B., K.H.S., E.A.H., E.D.M., and D.J.L.: acquired the data; A.J.P., E.C.O., R.G.B., E.A.H., E.J.B., E.A.H., I.J.E., K.H.S., A.R., E.D.M., G.R., S.M.K., and D.J.L.: analyzed the data; A.J.P.: drafted the initial manuscript. All authors contributed to interpretation of data, revised the manuscript for important intellectual content, approved the final version of the manuscript, and agree to be accountable for all aspects of the work.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201703-0555OC on June 14, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, Collard HR. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir Med. 2014;2:566–572. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 2.Hutchinson JP, McKeever TM, Fogarty AW, Navaratnam V, Hubbard RB. Increasing global mortality from idiopathic pulmonary fibrosis in the twenty-first century. Ann Am Thorac Soc. 2014;11:1176–1185. doi: 10.1513/AnnalsATS.201404-145OC. [DOI] [PubMed] [Google Scholar]
- 3.Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, Taylor DO, Kucheryavaya AY, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult lung and heart/lung transplantation report—2008. J Heart Lung Transplant. 2008;27:957–969. doi: 10.1016/j.healun.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Rosas IO, Dellaripa PF, Lederer DJ, Khanna D, Young LR, Martinez FJ. Interstitial lung disease: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Ann Am Thorac Soc. 2014;11(Suppl 3):S169–S177. doi: 10.1513/AnnalsATS.201312-429LD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, Nishino M, Araki T, Zazueta OE, Kurugol S, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med. 2013;368:2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin GY, Lynch D, Chawla A, Garg K, Tammemagi MC, Sahin H, Misumi S, Kwon KS. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology. 2013;268:563–571. doi: 10.1148/radiol.13120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyle TJ, Washko GR, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Divo MJ, Celli BR, Sciurba FC, Silverman EK, et al. COPDGene Investigators. Interstitial lung abnormalities and reduced exercise capacity. Am J Respir Crit Care Med. 2012;185:756–762. doi: 10.1164/rccm.201109-1618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estépar RS, Lynch DA, Brehm JM, et al. COPDGene Investigators. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Washko GR, Lynch DA, Matsuoka S, Ross JC, Umeoka S, Diaz A, Sciurba FC, Hunninghake GM, San José Estépar R, Silverman EK, et al. Identification of early interstitial lung disease in smokers from the COPDGene Study. Acad Radiol. 2010;17:48–53. doi: 10.1016/j.acra.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, Okajima Y, Dupuis J, Latourelle JC, Cho MH, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators; COPDGene Investigators. Association between interstitial lung abnormalities and all-cause mortality. JAMA. 2016;315:672–681. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araki T, Putman RK, Hatabu H, Gao W, Dupuis J, Latourelle JC, Nishino M, Zazueta OE, Kurugol S, Ross JC, et al. Development and progression of interstitial lung abnormalities in the Framingham Heart Study. Am J Respir Crit Care Med. 2016;194:1514–1522. doi: 10.1164/rccm.201512-2523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Podolanczuk AJ, Oelsner EC, Barr RG, Hoffman EA, Armstrong HF, Austin JH, Basner RC, Bartels MN, Christie JD, Enright PL, et al. High attenuation areas on chest computed tomography in community-dwelling adults: the MESA study. Eur Respir J. 2016;48:1442–1452. doi: 10.1183/13993003.00129-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lederer DJ, Enright PL, Kawut SM, Hoffman EA, Hunninghake G, van Beek EJ, Austin JH, Jiang R, Lovasi GS, Barr RG. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-Lung Study. Am J Respir Crit Care Med. 2009;180:407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Podolanczuk AJ, Oelsner E, Barr RG, Hoffman EA, Easthausen IJ, Enright PL, Hinckley Stukovsky KD, RoyChoudhury A, Michos ED, Raghu G, et al. High attenuation areas on chest CT and clinical respiratory outcomes in community-dwelling adults: the Multi-Ethnic Study of Atherosclerosis (MESA) [abstract] Am J Respir Crit Care Med. 2017;195:A7418. doi: 10.1164/rccm.201703-0555OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 16.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman EA, Jiang R, Baumhauer H, Brooks MA, Carr JJ, Detrano R, Reinhardt J, Rodriguez J, Stukovsky K, Wong ND, et al. Reproducibility and validity of lung density measures from cardiac CT scans—the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16:689–699. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152:653–657. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 19.Oelsner EC, Carr JJ, Enright PL, Hoffman EA, Folsom AR, Kawut SM, Kronmal RA, Lederer DJ, Lima JA, Lovasi GS, et al. Per cent emphysema is associated with respiratory and lung cancer mortality in the general population: a cohort study. Thorax. 2016;71:624–632. doi: 10.1136/thoraxjnl-2015-207822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durheim MT, Collard HR, Roberts RS, Brown KK, Flaherty KR, King TE, Jr, Palmer SM, Raghu G, Snyder LD, Anstrom KJ, et al. IPFnet investigators. Association of hospital admission and forced vital capacity endpoints with survival in patients with idiopathic pulmonary fibrosis: analysis of a pooled cohort from three clinical trials. Lancet Respir Med. 2015;3:388–396. doi: 10.1016/S2213-2600(15)00093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Stat Soc Series B Stat Methodol. 2000;62:711–730. [Google Scholar]
- 22.Fong C, Ratkovic M, Hazlett C, Yang X, Imai K. CBPS: covariate balancing propensity score. R package version 0.12. 2016.
- 23.Feng P, Zhou XH, Zou QM, Fan MY, Li XS. Generalized propensity score for estimating the average treatment effect of multiple treatments. Stat Med. 2012;31:681–697. doi: 10.1002/sim.4168. [DOI] [PubMed] [Google Scholar]
- 24.Imai K, van Dyk DA. Causal inference with general treatment regimes: generalizing the propensity score. J Am Stat Assoc. 2004;99:854–866. [Google Scholar]
- 25.Imai K, Ratkovic M. Covariate balancing propensity score. J R Stat Soc Series B Stat Methodol. 2014;76:243–263. [Google Scholar]
- 26.Imbens GW. The role of the propensity score in estimating dose–response functions. Biometrika. 2000;87:706–710. [Google Scholar]
- 27.Ramsey J, Ripley B. 2015. pspline: penalized smoothing splines. R package version 1.0-17.
- 28.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 29.Bernstein EJ, Barr RG, Austin JHM, Kawut SM, Raghu G, Sell JL, Hoffman EA, Newell JD, Jr, Watts JR, Jr, Nath PH, et al. Rheumatoid arthritis-associated autoantibodies and subclinical interstitial lung disease: the Multi-Ethnic Study of Atherosclerosis. Thorax. 2016;71:1082–1090. doi: 10.1136/thoraxjnl-2016-208932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kliment CR, Araki T, Doyle TJ, Gao W, Dupuis J, Latourelle JC, Zazueta OE, Fernandez IE, Nishino M, Okajima Y, et al. A comparison of visual and quantitative methods to identify interstitial lung abnormalities. BMC Pulm Med. 2015;15:134. doi: 10.1186/s12890-015-0124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, Jiang R, Kawut SM, Kronmal RA, Lima JA, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castaldi PJ, San José Estépar R, Mendoza CS, Hersh CP, Laird N, Crapo JD, Lynch DA, Silverman EK, Washko GR. Distinct quantitative computed tomography emphysema patterns are associated with physiology and function in smokers. Am J Respir Crit Care Med. 2013;188:1083–1090. doi: 10.1164/rccm.201305-0873OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong X, Cho MH, Anderson W, Coxson HO, Muller N, Washko G, Hoffman EA, Bakke P, Gulsvik A, Lomas DA, et al. ECLIPSE Study NETT Investigators. Genome-wide association study identifies BICD1 as a susceptibility gene for emphysema. Am J Respir Crit Care Med. 2011;183:43–49. doi: 10.1164/rccm.201004-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hersh CP, Washko GR, Jacobson FL, Gill R, Estepar RS, Reilly JJ, Silverman EK. Interobserver variability in the determination of upper lobe-predominant emphysema. Chest. 2007;131:424–431. doi: 10.1378/chest.06-1040. [DOI] [PubMed] [Google Scholar]