Abstract
Background
Lower respiratory tract infections (LRTI) are a major cause of morbidity and mortality worldwide, particularly in young children and older adults. Influenza is known to cause severe disease but the risk of developing LRTI following influenza virus infection in various populations has not been systematically reviewed. Such data are important for estimating the impact specific influenza vaccine programs would have on LRTI outcomes in a community. We sought to review the published literature to determine the risk of developing LRTI following an influenza virus infection in individuals of any age.
Methods and findings
We conducted a systematic review to identify prospective studies that estimated the incidence of LRTI following laboratory-confirmed influenza virus infection. We searched PubMed, Medline, and Embase databases for relevant literature. We supplemented this search with a narrative review of influenza and LRTI. The systematic review identified two prospective studies that both followed children less than 5 years. We also identified one additional pediatric study from our narrative review meeting the study inclusion criteria. Finally, we summarized recent case-control studies on the etiology of pneumonia in both adults and children.
Conclusions
There is a dearth of prospective studies evaluating the risk of developing LRTI following influenza virus infection. Determining the burden of severe LRTI that is attributable to influenza is necessary to estimate the benefits of influenza vaccine on this important public health outcome. Vaccine probe studies are an efficient way to evaluate these questions and should be encouraged going forward.
Keywords: Influenza, Pneumonia, Lower respiratory tract infection, Systematic review
1. Introduction
Pneumonia, bronchiolitis and other lower respiratory tract infections (LRTI) continue to be a major cause of morbidity and mortality worldwide disproportionately affecting adults ≥ 70 years and children < 5 years old. The Global Burden of Disease (GBD) project estimates that nearly 2 million of the 2.74 million LRTI-associated deaths were in these vulnerable age groups and that 10% were attributable to influenza [1]. There has been a recent increase in studies of the etiology of LRTI mostly attributable to the high sensitivity and relatively low cost of polymerase chain reaction (PCR)-based laboratory assays.
The link between severe influenza disease in adults and LRTI has been recognized for at least a century. The impact of seasonal influenza in the United States has been measured by a composite measure related to LRTI, pneumonia and influenza (P&I) mortality. P&I mortality is estimated indirectly from vital records databases, and can be used to approximate the impact of influenza without laboratory confirmation due to the sharply seasonal nature of influenza virus infections in temperate countries. Similar methods are inconsistently applied to populations with a known number of at risk individuals, however, limiting the ability to calculate incidence of LRTI in the general population.
In children, recognition of severe outcomes following influenza virus infection has been comparatively recent. At first, this recognition relied on non-specific outcomes such as wintertime increases in acute respiratory disease hospitalizations [2], [3], [4], [5]. Due to the non-specific presentation of LRTI in children, laboratory confirmation is essential for proper quantification of risk when other viruses, such as respiratory syncytial virus (RSV) or human metapneumovirus (HMPV), circulate at the same time as influenza.
The reliance of previous research on non-specific outcomes has resulted in uncertainty about the relative contribution of specific viruses to the overall burden. This issue can manifest as the distinction between primary viral and secondary bacterial infections or by infection with multiple viruses, among other concerns. Thus, to validly determine the risk of LRTI following influenza virus infection, ideally large, prospective studies with active surveillance, laboratory confirmation of infection, and long-term follow up are required. In addition, these studies must be longitudinal to capture illnesses in a series of years since incidence of influenza often varies markedly, especially in the tropics [6]. With these requirements as background, we conducted a landscape review incorporating a systematic literature search meeting the above criteria to estimate of risk of LRTI following influenza virus infection; recognizing that this estimate will represent a lower bound of the true burden. Since the systematic review identified very few articles which met these rigorous criteria we also present a narrative review with recommendations on alternative study designs to determine the potential impacts of influenza vaccine to reduce the global burden of LRTI.
2. Methods
2.1. Data sources and searches
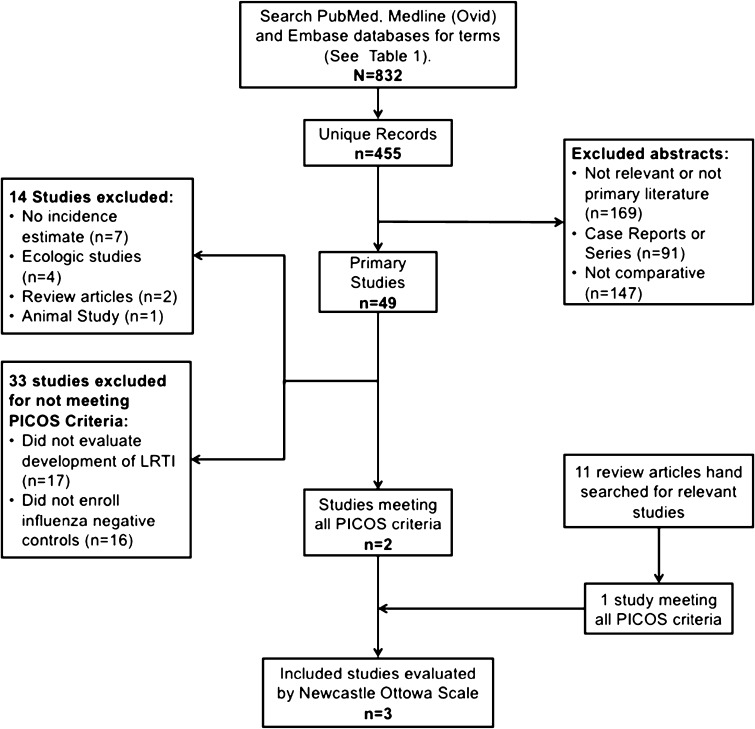
We conducted a systematic review to determine the availability and quality of literature addressing the following question: What is the risk of lower respiratory tract infections or pneumonia following seasonal or pandemic influenza virus infection compared to those without influenza virus infection? (see Fig. 1). In September 2016 we searched PubMed, Medline (Ovid), and EMBASE databases using the search terms indicated in Table 1. We attempted to capture a range of clinical presentations considered LRTI including pneumonia, acute lower respiratory illness and bronchiolitis. Search results were restricted to English abstracts only and primary literature; all study designs were included in the original search. Both articles and articles in press were included in EMBASE search parameters. Records were compiled using Endnote software and duplicates records were identified and removed. Abstracts were reviewed by a member of the study team (EM) and excluded if meeting the following exclusion criteria: (1) Not relevant to study question or not primary literature; (2) Not population-based (case report or case series); (3) Not comparative (i.e. not encompassing both influenza negative and positive; and LRTI and non-LRTI). Identified studies that met these criteria were further evaluated to determine if they met the inclusion criteria.
Fig. 1.
Results of the systematic review.
Table 1.
Search terms by database.
| Database | Influenza terms | Laboratory confirmation | LRTI termsa | ||
|---|---|---|---|---|---|
| Pubmed | Influenza, humanb | AND | “Laboratory” OR “polymerase chain reaction” | AND | |
| Medline (OVID) | Influenza, human.sh* | Laboratory OR polymerase chain reaction | |||
| EMBASE | ‘Influenza’/exp* | ‘Polymerase chain reaction’/exp* | ‘Pneumonia’/exp* |
Each term in this category was included in a separate search iteration.
These terms were mapped to database-defined terms (i.e. MESH or EMTREE terms).
indicates use of truncation in search term.
2.2. Narrative review
To supplement the systematic review, we also identified relevant review articles and meta-analyses and searched the lists of included studies for relevant primary literature references. Studies identified by the narrative review are summarized below but are excluded from the quality assessment and are reported separately from the systematic review results as they are were not identified using the pre-defined search criteria.
2.3. Study selection
We included studies that prospectively evaluated the incidence of laboratory confirmed influenza virus infection and that followed participants with evidence of infection for development of LRTI. Studies were excluded if they tested for influenza after LRTI had already developed and been identified or if the study only included persons with laboratory-evidence of influenza. Additionally we excluded studies if they collected appropriate data but did not report an incidence estimate for development of LRTI following influenza virus infection. Finally ecologic, review, and animal studies were excluded.
2.4. Data extraction and quality assessment
A data collection form was created to record the study design and incidence estimates. Full text of studies that passed the initial screening were read and information on the population, intervention, comparison, outcome and study design were recorded. Additionally details of the study population were recorded such as the size, age groups, other defining characteristics, and whether the study involved pandemic or seasonal influenza. Papers meeting the criteria for inclusion were evaluated for quality using the Newcastle-Ottawa score [7].
3. Results
3.1. Systematic and narrative review results
Our search terms identified 832 records. After excluding duplicates, 455 unique titles and abstracts were screened (Fig. 1). Screening identified 49 primary studies to be evaluated further and 11 relevant reviews (Table 2) that were hand searched for additional references. Among these 49 studies, four were ecologic in nature, two were excluded because they were either review articles or meta-analyses, and one study was excluded because it was an animal study (Fig. 1). In addition, according to the reported methods, seven of the identified studies collected data that could have been used to answer the PICOS question, but did not provide an appropriate estimate. After these exclusions, 39 studies remained to be evaluated on the basis of the PICOS criteria. Sixteen of the remaining studies enrolled patients already receiving medical care for laboratory confirmed influenza virus infection, and thus had no comparison group of uninfected individuals. Seven of these 16 studies excluded for not including a comparison group focused only on children (<18 years old) [8], [9], [10], [11], [12], [13], [14] and ten of the 16 were limited to examinations of influenza A(H1N1)pdm09 conducted during the 2009 pandemic [8], [10], [11], [12], [13], [15], [16]. Finally, 17 studies were excluded because they enrolled only participants with LRTI, and were therefore unable to estimate the incidence of developing the outcome. After these exclusions, two studies remained that met all of the PICOS criteria.
Table 2.
Relevant reviews.
| Number | Reference |
|---|---|
| 1. | Anonymous, COMMISSION ON RESPIRATORY DISEASES. The transmission of primary atypical pneumonia to human volunteers; laboratory studies. Bulletin of the Johns Hopkins Hospital, 1946. 79: p. 153–67 |
| 2. | Campigotto, A. and S. Mubareka, Influenza-associated bacterial pneumonia; managing and controlling infection on two fronts. Expert Review of Antiinfective Therapy, 2015. 13(1): p. 55–68 |
| 3. | Chertow, D.S. and M.J. Memoli, Bacterial coinfection in influenza: A grand rounds review. JAMA - Journal of the American Medical Association, 2013. 309(3): p. 275–282 |
| 4. | Crotty, M.P., et al., Impact of antibacterials on subsequent resistance and clinical outcomes in adult patients with viral pneumonia: An opportunity for stewardship. Critical Care, 2015. 19(1) |
| 5. | Dawood, F.S., et al., Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis, 2012. 12(9): p. 687–95 |
| 6. | Hendrickson, C.M. and M.A. Matthay, Viral pathogens and acute lung injury: Investigations inspired by the SARS epidemic and the 2009 H1N1 influenza pandemic. Seminars in Respiratory and Critical Care Medicine, 2013. 34(4): p. 475–486 |
| 7. | Lafond, K.E., et al., Global Role and Burden of Influenza in Pediatric Respiratory Hospitalizations, 1982–2012: A Systematic Analysis. PLoS Medicine/Public Library of Science, 2016. 13(3): p. e1001977 |
| 8. | Marcos, M.A. and A. Torrres, Viral community-acquired pneumonia. Clinical Pulmonary Medicine, 2011. 18(2): p. 60–64 |
| 9. | Nair, H., et al., Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet, 2011. 378(9807): p. 1917–30 |
| 10. | Ortiz, J.R., et al., Influenza pneumonia surveillance among hospitalized adults may underestimate the burden of severe influenza disease. PLoS One, 2014. 9(11): p. e113903 |
| 11. | Ott, S.R., et al., [The impact of viruses in lower respiratory tract infections of the adult. Part III: therapy and prevention]. Pneumologie, 2010. 64(2): p. 115–23 |
| 12. | Pavia, A.T., What is the role of respiratory viruses in community-acquired pneumonia?: What is the best therapy for influenza and other viral causes of community-acquired pneumonia? Infectious Disease Clinics of North America, 2013. 27(1): p. 157–75 |
We further conducted a narrative review, examined the reference lists from 11 published reviews (Fig. 1) and identified four papers that prospectively enrolled individuals with laboratory confirmed influenza or at risk of influenza, conducted active surveillance for acute respiratory illness, performed laboratory confirmation of influenza virus infection status, and followed patients for development of LRTI following influenza virus infection. Of these 4 studies, one large prospective study did not provide LRTI incidence estimates for influenza negative cases [17] and another only performed influenza testing for the LRTI cases [18]. Importantly, the study by Brooks and colleagues relied on virus isolation and two-thirds of the influenza-associated pneumonia cases were enrolled with pneumonia and thus were not followed from the start of their illness [17]. Additionally, Budge and colleagues reported on the RESPIRA-PERU study [19] which included prospective enrollment of households with children in the Andean highlands. Influenza virus infection was determined by RT-PCR and illnesses classified as either ARI or LRTI, but did not report virus specific incidence of LRTI [19]. After excluding these studies one additional study was added to the two identified from the systematic review for final set of three studies meeting all the PICOS criteria (Fig. 1). We present the major themes identified in the course of this narrative review and end with a summary of the three studies that were able to produce estimates of the risk of developing LRTI following an influenza virus infection.
3.2. Clinical outcomes of influenza virus infection
The first major theme that emerged from our landscape review was that there has been substantial work to understand the clinical outcomes of influenza virus infection. We identified several studies that enrolled patients after they were hospitalized or sought medical care for an influenza associated respiratory illness. Many of these studies were conducted during the pandemic of 2009 and examined the outcomes of influenza A/H1N1pdm09 infection. Five were in special or sub populations (such as those with a specific chronic condition and most were small (<100 individuals). Only one study attempted to estimate the rates of hospitalization with PCR confirmed influenza A/H1N1pdm09 infection overall and stratified by pre-existing conditions. In England during the second wave of the pandemic (September 2009) infants had the highest hospitalizations rate, 28 per 100,000 population. Studies including only patients who have sought medical care, or who have been hospitalized, for laboratory confirmed influenza virus infection are efficient for determining the progression of infection particularly in subgroups of the population. For population level estimates of the burden of LRTI attributable to influenza virus infection, however, a different study design is required.
3.3. Studies of the etiology of pneumonia
A second major theme identified by our landscape review was the recent efforts to determine the viral etiology of patients with pneumonia. We identified a number of large case-control studies that have been conducted in recent years, both in the US and in LMIC, among both adults and children to determine the viral pathogens associated with clinical pneumonia. While these studies do not seek to answer our specific PICOS question, they represent an important step forward in determining the causes of severe lower respiratory illnesses. Here we will discuss three of the largest studies to date.
The Pneumonia Etiology Research for Child Health (PERCH) and GABRIEL [20] studies are both case-control studies in children less than 5 years old seeking to determine the etiology of and risk factors for severe and very severe pneumonia in LMICs [21]. Complete results from the PERCH study have not yet been published, but preliminary findings and methodological considerations are available [22]. Cases were children 1–59 months of age who were hospitalized with severe or very severe pneumonia. Community based controls were children aged 1–59 months living in the catchment area and were frequency matched by age group [21]. In reports of preliminary findings, influenza was detected in 9–10% of tested specimens [23] and the odds of influenza detection differed by case or control status. In the GABRIEL study influenza A was also significantly more likely to be detected in the cases than the controls. The cross-sectional nature of these studies means that influenza virus infections could have occurred prior to participant enrollment in the study. If these individuals were infected with influenza virus but were no longer shedding virus at the time of presentation with LRTI, they would be misclassified. Additionally, the PERCH study enrolled subjects with respiratory illness as controls (∼33% of controls). These individuals may be misclassified based on the severity of their illness or may yet develop LRTI. The investigators conclude that excluding children with respiratory symptoms from the control group would have negatively impacted generalizability. Both of these forms of misclassification would lead to underestimates of the true risk of LRTI following influenza virus infection, thus the estimates from the PERCH and GABRIEL studies likely represent the lower bound of the true causal effect of a given pathogen.
The Etiology of Pneumonia in the Community (EPIC) enrolled patients hospitalized with radiographically confirmed community acquired pneumonia (CAP) in three U.S. children’s hospitals and five U.S. adult medical centers to estimate the burden of CAP among children and adults in the United States. 2222 children and 2063 adults provided specimens for pathogen identification [24], [25]. A convenience sample of asymptomatic controls was also enrolled starting in the second year, but a comparison by case or control status was not the primary objective of these studies. This study used hospital discharge codes, market share, and US census data adjustments to estimate the incidence rate of pathogen specific hospitalization for CAP. Overall the highest incidence of influenza-associated pneumonia hospitalizations in children was found in those < 2 years old (3.7 per 10000 child-years). Children with asthma were more likely to have a pathogen detected than children without asthma. Among adults the highest incidence rate of influenza associated pneumonia hospitalization was found among those ≥ 80 years old. Importantly, over 60% of pneumonia hospitalizations in the adult study were not associated with a pathogen, compared to only 20% in the study including children. The median time from onset to specimen collection was shorter in the study in children than it was in the adult study.
3.4. Incidence of LRTI following influenza virus infection
Finally, our landscape review identified three prospective studies that estimated the risk of LRTI following an influenza virus infection in children; no prospective, longitudinal studies matched the search criteria in older children or adults. Study design and incidence rate estimates are presented in Table 3. The earliest study, by Neuzil et al. followed 1665 children under 5 years of age for an average of 1.8 years per child (total person-time under observation of 3041 person-years) enrolled in the Vanderbilt Vaccine Clinic between 1974 and 1999 [26]. Influenza was detected during 289 (4%) medical visits associated with fever or respiratory tract illness. There was substantial variation in influenza attack rates; from 1 per 100 medical visits/year in 1978–1979 to 19 per 100 visits/year in 1994–1995. Overall, the rate of influenza associated LRTI was 8 per 1000 child-years (95% CI 5-12) (Table 3). This study included a large population of children followed over multiple years; however, the study only included medically attended ARI and more importantly, did not report incidence of LRTI in influenza negative patients to allow calculation of the attributable burden of influenza.
Table 3.
Summary of large, prospective studies with sufficient follow up and laboratory confirmation of influenza outcomes.
| Study | Population | Study design | Surveillance method | Dates of surveillance | Influenza detection | Outcome | Incidence rate of LRTI per 1000 person years | NOS |
|---|---|---|---|---|---|---|---|---|
| Neuzil et al. [26] | Children less than 5 years; Nashville TN, USA (n = 1665) | Prospective cohort study | Passive, children < 5 years old seeking care at clinic for acute respiratory illness | August 1, 1974- July 31 1999 | Virus culture, HAI | Clinical diagnosis of lower respiratory tract disease | 8 (5–12) | 7 |
| McMorrow et al. [27] | Children less than 5 years; Kenya (n = 8493) | Prospective household and clinic-based surveillance network | Active, bi-weekly or weekly home visits | 2009–2012 | RT-PCR | Acute respiratory infection with a subjective or measured temperature of ≥ 38 °C and cough with onset in the last 10 days requiring hospitalization plus physician diagnosed lower respiratory tract infection | 17 (14–20) | 8 |
| Broor et al. [30] | Infants followed until 3 yrs old; Nepal (n = 281) | Prospective cohort study | Active, weekly home visits | October 1, 2001 – December 31 2004 | DFA | WHO ICMI classification for acute lower respiratory disease | 13 (0–69) | 7 |
A second study, by McMorrow et al., observed 9652 person years of follow up among children less than 5 years old as part of a population based surveillance network from 2008 to 2012 [27]. The source population for this study was individuals living in Kibera or Lwak, Kenya; two areas that were part of an influenza surveillance network. Active surveillance was conducted for influenza-like illness (ILI) or acute lower respiratory infections (ALRI) on a biweekly basis initially, increasing to weekly surveillance following the emergence of influenza A/H1N1pdm09 in 2009. Influenza testing by RT-PCR was performed on 3128 ILI and 2516 ALRI; the rate of influenza-associated LRTI was 17 per 1000 child-years (95% CI 14-20 per 1000 child-years). The case definition [28] for ALRI for children 0–4 years old was based on a modified World Health Organization (WHO) severe pneumonia definition [29], but often the youngest children presented with less specific syndromes; thus influenza-associated LRTI in this study may be underestimated for the youngest children. Additionally severely ill children were excluded, therefore if influenza is associated with more severe disease in this setting the incidence of influenza-associated LRTI would be underestimated.
One community based study identified by the narrative review did produce an incidence estimate. Broor et al. conducted active weekly surveillance for ARI and LRTI in children in India from October 2001 through March 2005 [30]. They enrolled 281 infants and followed them for, on average, 20 months. Influenza virus infections were identified by direct fluorescent antibody (DFA). The overall incidence of LRTI reported in this study was 13 per 1000 child-years (Table 3), but given the relatively small sample size this estimate was imprecise (95% CI 0-69). In addition, the laboratory methods used in this study are not as sensitive as molecular methods and may, therefore, also result in an underestimate of the incidence of influenza associated LRTI.
4. Discussion
Pneumonia and other LRTI remain a major source of morbidity and mortality on a global scale and influenza virus infection is recognized as the potential trigger of a sequence of events, infectious and otherwise, that can lead to development of these potentially severe illnesses. While the systematic review identified only three studies meeting the search criteria, the landscape review identified several research topics that have received substantial focus recently regarding influenza and LRTI. Nevertheless, a substantial gap remains in our knowledge of incidence of LRTI following influenza virus infection. Specifically, we identified a number of studies that have described the clinical outcomes associated with influenza virus infection, and others that have described the etiology of pneumonia. Nevertheless, prospective studies with long term follow up capable of estimating the incidence of LRTI following influenza virus infection are extremely rare. Thus, burden estimates for severe influenza-associated illnesses remain uncertain. In many countries the primary goal of the vaccination program is prevention of mortality, additional data are needed to effectively make the case for influenza vaccine programs in these countries [31], [32].
The large studies of the viral etiology of pneumonia conducted in recent years [24], [25], [33], [34] represent a major leap forward in our knowledge of what causes lower respiratory tract disease. In these studies influenza has been consistently detected in a substantial proportion of pneumonia cases, but estimates of incidence based on these data are often lower than expected. While these and other, similar studies are able to quantify the proportion of hospitalized pneumonia cases that influenza virus infections detectable by RT-PCR at the time of admission, because of their cross-sectional nature, they seek to answer a fundamentally different question than that which we ask here. Given the duration that influenza virus is detectable by PCR the relative contribution of influenza to pneumonia may be underestimated using an approach that enrolls patients after their illness has become serious enough to require hospitalization. Another persistent question for any study of pneumonia, is the manner in which pneumonia is diagnosed clinically. Using chest radiograph as opposed to clinical impressions can result in drastically different estimates depending on the study population and setting. In addition, the knowledge we have gained from these studies has led to additional questions that can be answered only with prospective studies with long term follow up. The increased sensitivity of molecular diagnostics, for example, raises questions about the interaction of various etiologic agents in producing severe disease, including both bacterial and viral pathogens. Determining the dynamics of multiple pathogen infections will require intensive follow up, including repeated specimen collection and complex systems analytical approaches. Without intensive follow-up, quantifying the burden of severe disease attributable to each specific pathogen will continue to be problematic, and will be especially difficult in tropical areas where there may be less pathogen specific seasonality.
Validly estimating the incidence of LRTI following influenza virus infection will also require studies with a very specific methodology. For example, due the relatively short duration of viral shedding following influenza virus infection, particularly in adult populations, active surveillance will be necessary to avoid misclassification of infection status. The hypothetical study must also be large; as approximately 10% of the general adult population and a larger percent of children will experience an influenza virus infection in any given season [refs.]. A subset of those who are infected will go on to develop LRTI and a further subset will seek medical care for that LRTI. It is this final subset that serves as the study population for the recent studies of viral etiology described in this review. Finally, the ideal study must be long enough to capture illnesses over multiple influenza seasons, we would recommend no less than three, since incidence of influenza will vary markedly. In tropical settings, there will also be an additional consideration of different seasonality of influenza, with varying periods of influenza circulation, in many cases lasting much longer than in temperate areas. Unfortunately, financial and logistical challenges make a study meeting all of these criteria likely unfeasible.
An alternative to the ideal study described above may be a vaccine probe study, which has been used to understand the public health value of a vaccination program beyond prevention of medically attended illness by estimating the vaccine preventable disease incidence (VPDI) and the attributable fraction [35]. Prevention of pneumonia by a pathogen specific vaccination program provides strong evidence for the etiologic role of that pathogen in pneumonia. Vaccine probe studies have been designed based on this principle to quantify to the potential reduction in severe outcomes, including those that are not detected at the point of care [36]. The VPDI of influenza or the attributable fraction of influenza in clinical pneumonia could be estimated with a probe study [37]. Overall vaccine effectiveness (VE) is an important consideration for studies of this type [37], and the effect of influenza vaccines against severe influenza disease is not well understood [38]. Newly developed vaccines with higher effectiveness in young children also make this approach feasible. Vaccine probe studies can also be used to examine the interaction of pathogens, for example the reduction in pneumococcal pneumonia due to influenza vaccination [39]. The etiologic role of multiple viral pathogen infections can also be examined with a vaccine probe approach, but it will be important to specify which interactions are of interest a priori and select appropriate study populations and analytic methods to examine these effects. Additional considerations for studies of attributable fraction will include the variability of influenza vaccine by season and setting, meaning that study of VE will need to be conducted simultaneously to provide valid VE estimates [39]. Importantly, ethical considerations due to, for example, the universal recommendation of influenza vaccine for age-eligible persons in the United States means that randomized probe studies will not be possible in certain settings. Despite these considerations that are necessary in the design of a vaccine probe study, they remain a particularly attractive option for future research to reduce the uncertainty around estimates of the burden of influenza-associated LRTI, especially given their direct relevance to implementation of vaccine use in prevention programs.
Disclaimer
Justin R. Ortiz was an employees of the World Health Organization at the time this study was commissioned and conducted. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions or policies of the World Health Organization.
Funding
The authors acknowledge the contributions of the Centers for Disease Control and Prevention (CK14-1402), which provides financial support to the World Health Organization Initiative for Vaccine Research.
References
- 1.Troeger C., Forouzanfar M., Rao P.C., Khalil I., Brown A., Swartz S. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the global burden of disease study 2015. Lancet Infect Dis. 2017 doi: 10.1016/S1473-3099(17)30396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullooly J.P., Barker W.H. Impact of type A influenza on children: a retrospective study. Am J Public Health. Am Public Health Assoc. 1982;72:1008–1016. doi: 10.2105/ajph.72.9.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glezen W.P., Decker M., Perrotta D.M. Survey of underlying conditions of persons hospitalized with acute respiratory disease during influenza epidemics in Houston, 1978–1981. Am Rev Respir Dis. 1987;136:550–555. doi: 10.1164/ajrccm/136.3.550. [DOI] [PubMed] [Google Scholar]
- 4.Izurieta H.S., Thompson W.W., Kramarz P., Shay D.K., Davis R.L., DeStefano F. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med Massachusetts Med Soc. 2000;342:232–239. doi: 10.1056/NEJM200001273420402. [DOI] [PubMed] [Google Scholar]
- 5.Neuzil K.M., Mellen B.G., Wright P.F., Mitchel E.F., Griffin M.R. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med Massachusetts Med Soc. 2000;342:225–231. doi: 10.1056/NEJM200001273420401. [DOI] [PubMed] [Google Scholar]
- 6.Ng S., Gordon A. Influenza burden and transmission in the tropics. Curr Epidemiol Rep. 2015;2:89–100. doi: 10.1007/s40471-015-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. [cited 13 Oct 2017]. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 8.Caselli D., Carraro F., Castagnola E., Ziino O., Frenos S., Milano G.M. Morbidity of pandemic H1N1 influenza in children with cancer. Pediatr Blood Cancer. 2010;55:226–228. doi: 10.1002/pbc.22619. [DOI] [PubMed] [Google Scholar]
- 9.Chi C.-Y., Wang S.-M., Lin C.-C., Wang H.-C., Wang J.-R., Su I.-J. Clinical features of children infected with different strains of influenza B in southern Taiwan. Pediatr Infect Dis J. 2008;27:640–645. doi: 10.1097/INF.0b013e31816be008. [DOI] [PubMed] [Google Scholar]
- 10.Cost C., Brock E., Adams-Huet B., Siegel J.D., Ardura M.I. 2009 pandemic influenza A (H1N1) virus infection in pediatric oncology and hematopoietic stem cell transplantation patients. Pediatr Blood Cancer. 2011;56:127–133. doi: 10.1002/pbc.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das R.R., Sami A., Lodha R., Jain R., Broor S., Kaushik S. Clinical profile and outcome of swine flu in Indian children. Indian Pediatr. 2011;48:373–378. doi: 10.1007/s13312-011-0085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esposito S., Daleno C., Tagliabue C., Scala A., Picciolli I., Taroni F. Antibody response of healthy children to pandemic A/H1N1/2009 influenza virus. Virol J. 2011;8:563. doi: 10.1186/1743-422X-8-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavaldà J., Cabral E., Alonso E., Perez-Romero P., Pérez A., Quintero J. Influenza A H1N1/2009 infection in pediatric solid organ transplant recipients. Transpl Infect Dis. 2012;14:584–588. doi: 10.1111/tid.12005. [DOI] [PubMed] [Google Scholar]
- 14.Liu C.Y., Der Wang J, Yu J.T., Wang L.C., Lin M.C., Lee H.F. Influenza B virus-associated pneumonia in pediatric patients: clinical features, laboratory data, and chest X-ray findings. Pediatr Neonatol. 2014;55:58–64. doi: 10.1016/j.pedneo.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Campbell C.N.J., Mytton O.T., McLean E.M., Rutter P.D., Pebody R.G., Sachedina N. Hospitalization in two waves of pandemic influenza A(H1N1) in England. Epidemiol Infect. 2011;139:1560–1569. doi: 10.1017/S0950268810002657. [DOI] [PubMed] [Google Scholar]
- 16.Michaan N, Amzallag S, Laskov I, Cohen Y, Fried M, Lessing JB, et al. Maternal and neonatal outcome of pregnant women infected with H1N1 influenza virus (Swine Flu). J Matern Neonatal Med. Taylor & Francis; 2012; 25: 130–2. doi: 10.3109/14767058.2011.562569. [DOI] [PubMed]
- 17.Brooks W.A., Goswami D., Rahman M., Nahar K., Fry A.M., Balish A. Influenza is a major contributor to childhood pneumonia in a tropical developing country. Pediatr Infect Dis J. 2010;29:216–221. doi: 10.1097/INF.0b013e3181bc23fd. [DOI] [PubMed] [Google Scholar]
- 18.Hasan K., Jolly P., Marquis G., Roy E., Podder G., Alam K. Viral etiology of pneumonia in a cohort of newborns till 24 months of age in Rural Mirzapur, Bangladesh. Scand J Infect Dis. 2006;38:690–695. doi: 10.1080/00365540600606473. [DOI] [PubMed] [Google Scholar]
- 19.Budge P.J., Griffin M.R., Edwards K.M., Williams J.V., Verastegui H., Hartinger S.M. A household-based study of acute viral respiratory illnesses in Andean children. Pediatr Infect Dis J. 2014;33:443–447. doi: 10.1097/INF.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bénet T., Sánchez Picot V., Messaoudi M., Chou M., Eap T., Wang J. Microorganisms associated with pneumonia in children <5 years of age in developing and emerging countries: the GABRIEL pneumonia multicenter, prospective, case-control study. Clin Infect Dis. Oxford University Press. 2017;65:604–612. doi: 10.1093/cid/cix378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deloria-Knoll M., Feikin D.R., Scott J.A.G., O’Brien K.L., DeLuca A.N., Driscoll A.J. Identification and selection of cases and controls in the pneumonia etiology research for child health project. Clin Infect Dis. Oxford University Press. 2012;54:S117–S123. doi: 10.1093/cid/cir1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Brien K.L., Baggett H.C., Brooks W.A., Feikin D.R., Hammitt L.L., Howie S.R.C. Introduction to the epidemiologic considerations, analytic methods, and foundational results from the pneumonia etiology research for child health study. Clin Infect Dis. 2017;64:S179–S184. doi: 10.1093/cid/cix142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driscoll A.J., Karron R.A., Bhat N., Thumar B., Kodani M., Fields B.S. Evaluation of fast-track diagnostics and TaqMan array card real-time PCR assays for the detection of respiratory pathogens. J Microbiol Methods. 2014;107:222–226. doi: 10.1016/j.mimet.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain S., Self W.H., Wunderink R.G., Fakhran S., Balk R., Bramley A.M. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med Massachusetts Med Soc. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain S., Williams D.J., Arnold S.R., Ampofo K., Bramley A.M., Reed C. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med Massachusetts Med Soc. 2015;372:835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neuzil K.M., Zhu Y., Griffin M.R., Edwards K.M., Thompson J.M., Tollefson S.J. Burden of interpandemic influenza in children younger than 5 years: A 25-year prospective study. J Infect Dis. 2002;185:147–152. doi: 10.1086/338363. [DOI] [PubMed] [Google Scholar]
- 27.McMorrow ML, Emukule GO, Njuguna HN, Bigogo G, Montgomery JM, Nyawanda B, et al. The unrecognized burden of influenza in young kenyan children, 2008–2012. In Viboud C, editor. PLoS One. Public Library of Science; 2015;10:e0138272, https://doi.org/10.1371/journal.pone.0138272 [DOI] [PMC free article] [PubMed]
- 28.Scott JAG, Wonodi C, Moïsi JC, Deloria-Knoll M, DeLuca AN, Karron RA, et al. The definition of pneumonia, the assessment of severity, and clinical standardization in the pneumonia etiology research for child health study. Clin Infect Dis. Oxford University Press; 2012; 54 Suppl 2: S109-16. doi:10.1093/cid/cir1065. [DOI] [PMC free article] [PubMed]
- 29.WHO | Integrated Management of Childhood Illness (IMCI). WHO. World Health Organization; 2017. Available: http://www.who.int/maternal_child_adolescent/topics/child/imci/en/.
- 30.Broor S., Parveen S., Bharaj P., Prasad V.S., Srinivasulu K.N., Sumanth K.M. A prospective three-year cohort study of the epidemiology and virology of acute respiratory infections of children in rural India. PLoS One. Public Library of Science. 2007;2 doi: 10.1371/journal.pone.0000491. e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Preferred product characteristics for next-generation influenza vaccines [Internet]. Geneva; 2017. Available: http://apps.who.int/iris/bitstream/10665/258767/1/9789241512466-eng.pdf.
- 32.Neuzil K.M., Bresee J.S., de la Hoz F., Johansen K., Karron R.A., Krishnan A. Data and product needs for influenza immunization programs in low- and middle-income countries: Rationale and main conclusions of the WHO preferred product characteristics for next-generation influenza vaccines. Vaccine. 2017;35:5734–5737. doi: 10.1016/j.vaccine.2017.08.088. [DOI] [PubMed] [Google Scholar]
- 33.Klugman K.P., Rodgers G.L. PERCH in perspective: what can it teach us about pneumonia etiology in children? Clin Infect Dis. 2017;64:S185–S187. doi: 10.1093/cid/cix075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falsey A.R., McElhaney J.E., Beran J., Van Essen G.A., Duval X., Esen M. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. J Infect Dis. 2014;209:1873–1881. doi: 10.1093/infdis/jit839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gessner B.D., Sutanto A., Linehan M., Djelantik I.G.G., Fletcher T., Gerudug I.K. Incidences of vaccine-preventable Haemophilus influenzae type b pneumonia and meningitis in Indonesian children: Hamlet-randomised vaccine-probe trial. Lancet (London, England) 2005;365:43–52. doi: 10.1016/s0140-6736(04)17664-2. Available: http://www.ncbi.nlm.nih.gov/pubmed/15643700. [DOI] [PubMed] [Google Scholar]
- 36.Nunes M.C., Cutland C.L., Jones S., Downs S., Weinberg A., Ortiz J.R. Efficacy of maternal influenza vaccination against all-cause lower respiratory tract infection hospitalizations in young infants: results from a randomized controlled trial. Clin Infect Dis. 2017;65:1066–1071. doi: 10.1093/cid/cix497. Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gessner B.D., Brooks W.A., Neuzil K.M., Vernet G., Bright R.A., Tam J.S. Vaccines as a tool to estimate the burden of severe influenza in children of low-resourced areas (November 30-December 1, 2012, Les Pensieres, Veyrier-du-Lac, France) Vaccine. 2013:3222–3228. doi: 10.1016/j.vaccine.2013.05.017. Elsevier. [DOI] [PubMed] [Google Scholar]
- 38.Petrie J.G., Ohmit S.E., Cheng C.K., Martin E.T., Malosh R.E., Lauring A.S. Influenza vaccine effectiveness against antigenically drifted influenza higher than expected in hospitalized adults: 2014–2015. Clin Infect Dis. 2016;63 doi: 10.1093/cid/ciw432. [DOI] [PubMed] [Google Scholar]
- 39.Feikin D.R., Scott J.A.G., Gessner B.D. The Lancet. Elsevier; 2014. Use of Vaccines as Probes to Define Disease Burden [Internet] pp. 1762–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]