Abstract
Background:
The search for new natural or synthetic products with antioxidant activity is commonly based on methods that involve reduction of either 2,2-diphenyl-1-picrylhydrazyl (DPPH) or 2-2-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS). However, the reported values of the effective concentrations are highly variable, even in controls. Herein, we optimize and validate both meth-ods of determining antiradical activity.
Methods:
Optimization was carried out using both a fractionated factorial design and a basic sequential simplex method, by monitoring the reduction percentage. Quercetin or Trolox were used as positive con-trol. Furthermore, for each method, linearity, precision, accuracy, robustness, plate uniformity, signal variability, and Z factor, were established.
Results:
The optimized conditions for the DPPH method were: DPPH 280 μM in ethanol and 15 min of reaction time in the dark. The linear range was between 7 and 140 μM with an R2 value of 0.9987. The optimized conditions for the ABTS method were: ABTS adjusted to 0.7 absorbance units, 70% concen-tration in ethanol, and a reaction time of 6 min in the dark. The linear range was found to be between 1 and 70% with an R2 = 0.9991. For both methods, the accuracy and precision were within limits and the Z factor value was higher than 0.89. The applicability of each method was assessed by analyzing eight plant extracts.
Conclusion:
The DPPH and ABTS reduction methods were optimized and validated on a microscale and could be expected to be implemented in any laboratory.
Keywords: ABTS, antiradical activity, DPPH, high-throughput screening methods, natural products, reduction
1. INTRODUCTION
High-throughput screening (HTS) methods are widely used for the discovery of new drugs because they provide in vitro results that can be obtained on a microscale, and also they are rapid, simple, and relatively inexpensive. In addition, the use of such methods can reduce both the amount of waste produced and the need to use laboratory animals [1].
The search for products with antioxidant activity, either natural or synthetic, has intensified in the recent years because such compounds play an important role in chronic degenerative diseases as well as in aging and lifestyle changes. The increasing number of products that require testing has required the use of both in vivo and in vitro assays. Some of these assays can be used to evaluate general mechanisms for the removal of oxidized species, such as 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2-2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), and others are based on specific mechanisms related to a particular illness (e.g., xanthine oxidase). Alam et al. (2013) published a review in which they included 10 in vivo and 19 in vitro methods [2]. The most commonly used are the in vitro tests involving scavenging of free radicals, which are simple and rapid procedures that are properly known as antiradical activity methods. Among these, the DPPH and ABTS assays are the simplest to implement and tend to achieve the most reproducible results [3].
There are numerous published methods that can be used to measure total antioxidant capacity in vitro; these can be classified into two types:
Assays based on hydrogen atom transfer (HAT) and assays based on electron transfer (ET). ET-based assays are used to measure the capacity of an antioxidant to reduce an oxidant, which changes color when reduced. The degree of color change is correlated to the concentration of the antioxidant in the sample. ET-based assays include DPPH and ABTS radical scavenging capacity assays. However, no single method is sufficient; more than one type of antioxidant capacity measurement needs to be performed to take into account the various modes of action of antioxidants [3]. For example, the ABTS assay is applicable to both hydrophilic and lipophilic antioxidant systems, whereas the DPPH assay is more suited to hydrophobic systems [4].
DPPH is a free radical that is stabilized through the delocalization of its free electron over the entire molecule in such a manner that it does not dimerize, as can occur with other free radicals. When the molecule is dissolved in ethanol, this delocalization produces an intense purple coloration that absorbs light at 517 nm. When the DPPH solution is mixed with a hydrogen atom donor, the reduced form is produced with concomitant loss of the purple coloration [2].
The ABTS method for free radical reduction is based on electron transfer between the bluish-green radical and the antioxidant agent. This reaction can be monitored at 750 nm as a decrease in the intensity of the absorbance [2].
Both assays are convenient in their application and very commonly used; nevertheless, their application is limited because they use nonphysiological radicals [4]. Antiradical activity potency is quantified through the percentage reduction of the amount of light-absorbing species (e.g., DPPH or ABTS) using a defined concentration of the antioxidant agent. Alternatively, it can be quantified by measuring the antioxidant concentration required to reduce 50% of the light-absorbing species (EC50). Nevertheless, the reported EC50 values for the same compound (used as a positive control) have large variations. For instance, using the DPPH method, Iacopini et al. (2008) determined an EC50 value of 1.66 μg/mL for quercetin [5], whereas Sadek et al. (2009) reported a value of 36 μg/mL for the same compound using the same method [6]. Presumably, the discrepancy between these results arises because each laboratory or research group “standardizes” its method without evaluating or optimizing the slight modifications they make to the original reference method.
The optimization of any analytical method is essential to achieve acceptable performance. High-throughput methods carried out in artificial environments (in vitro) may be unstable or exhibit activities below their potential. Considering this, optimization can significantly improve stability and is therefore a crucial step for the development of screening methods [7].
The quality of a bioassay is defined by the robustness and reproducibility of the signal. This allows the biological process to be quantified both in the absence of a test compound and in the presence of inactive compounds [8]. The purpose of high-throughput methods is to produce reliable data that is relevant for its application in humans. Therefore, the measurements must exhibit low variability and a high signal-to-noise ratio so that both false positives and false negatives can be minimized [9].
Reported studies that include an evaluation of a particular biological activity based on assays or methods rarely provide comprehensive optimization and/or analytical validation data. Generally, it is simply stated that a previously used method has been adopted and that slight modifications have been made to it. However, several of these modifications are made to critical conditions (variables or factors) of the respective method. Thus, the method should be optimized and, most importantly, it should be ensured that these “slight changes” do not affect either the response or the potency of the antiradical agent.
In this study, the optimization and validation of two antiradical activity methods are reported; both of the methods were carried out with microplates. Their use in high-throughput methods to trace antioxidant activity is proposed.
2. MATERIAL AND METHODS
2.1. Chemicals and Reagents
The following reagents were purchased directly from Sigma-Aldrich: 2,2-diphenyl-1-picrylhydrazyl (DPPH); 2-2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS); 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox); quercetin; potassium phosphate dibasic; potassium phosphate monobasic; and potassium persulfate. Reagent grade methanol and ethanol were purchased from J.T. Baker. Polystyrene 96-well flat-bottom plates were purchased from Corning, USA. All experiments were carried out with a Multiskan FC plate reader (Thermo Scientific, US).
2.2. Samples
Eight plant extracts from North of México were used to evaluate the application of the optimized and validated methods: The aerial parts of Quercus canbyira, Turnera diffusa, Leucophyllum frutescens, Teucrium bicolor, Salvia texana, and Salvia beateflora, as well the fruit peel of Vitis vinifera and the root of Jatropha dioica, were used to obtain hydroalcoholic extracts (90:10). All extracts were kindly provided by researchers from the Analytical Chemistry Department (Medicine Faculty, UANL,).
2.3. Optimization
Experimental design and data handling were carried out using STATGRAPHICS Centurion XVI software (Statpoint Technologies, Inc., USA). Optimization of the DPPH reduction method was performed with a half-fraction factorial design 2^5-1, with no blocks, and with a random central point and one replicate. The ABTS method optimization was carried out using a full factorial design; this was randomized and included one replicate, as the fractionated factorial was not sufficient. Thirty-two experiments were conducted with DPPH and 128 with ABTS. For both methods, the reduction percentage of the light-absorbing species was calculated in the absence of an antiradical agent (negative control) and in the presence of an antiradical agent. This reduction percentage was considered the response of the method. In all cases, the percentage reduction was calculated using Equation (1):
| (1) |
where A is the absorbance of the negative control and B is the absorbance in the presence of an antiradical agent.
For DPPH, the positive control was quercetin (4 μg/mL), and for ABTS, the positive control was Trolox (3 μg/mL). All experiments were performed in polystyrene 96-well plates with a final volume of 200 μL. Absorbance readings were carried out with a microplate reader (Multiskan FC, Thermo Scientific) employing the recommended wavelengths for each method.
Factors or variables included in the optimization process for the DPPH reduction method were: DPPH concentration (μM), incubation time (min), light/darkness, solvent, and wavelength. Factors or variables included in the optimization process for the ABTS method were: ABTS concentration (%), ABTS absorbance, incubation time (min), light/darkness, solvent, and incubation temperature (°C). In all cases, the highest or lowest previously reported values were used for subsequent experiments.
Pareto diagrams were employed to simplify the evaluation of the effect caused by the variables included in this study on the response of the system.
The factors with the highest influence on the response were optimized using the basic sequential simplex method. The number of initial experiments was calculated according to N + 1, where N is the number of factors to be optimized. Initial experiments were set with the highest or lowest levels, as previously reported for each method. After each group of experiments, one of the conditions was discarded, and new conditions were calculated for each variable (VN) according to the basic sequential simplex method and Equation (2):
| (2) |
where VN is the new value for each factor, xoptimal is the average value of the factors that produced the optimal results, and Vlow is the value that produced the lowest response.
2.4. Validation
2.4.1. Linearity
An evaluation of the linearity of the method was carried out using three experimental approximations:
A calibration curve was constructed by using only the absorbance of the analyte (DPPH or ABTS) at different concentrations to evaluate the response of the equipment.
A calibration curve was constructed using the percentage reduction of five concentration levels of the positive control: quercetin or Trolox.
A standard curve was constructed using the percentage reduction values of the five concentration levels of the same positive controls used above, but added in extract from Jatropha dioica that does not possess antiradical activity.
All experiments were carried out in triplicate. The obtained data were treated with the least-squares method to obtain the plot’s equation as well as the determination coefficient. For strategy A, the employed response was absorbance, whereas for strategies B and C, the percentage reduction (%) was calculated, which was represented as a function of the concentration.
2.4.2. Precision
Precision was assessed using the same plant extract from Jatropha dioica, either with or without adding the positive control at three concentration levels in triplicate. Quercetin at 8, 4, and 0.5 μg/mL concentrations was used for DPPH, whereas Trolox at 5, 3, and 1 μg/mL concentrations was used for ABTS. The relative standard deviation (%RSD) was calculated for the respective percentage of free radical reduction, according to Equation (3):
| (3) |
where s is the standard deviation of each measurement and x is the average of such measurements.
The procedure was evaluated on the same day to obtain the inter-day precision, and on different days to evaluate intra-day precision.
2.4.3. Accuracy
Accuracy was evaluated in two ways:
The error percentage was evaluated with a plant extract from Jatropha dioica, either with or without positive control at three concentration levels in triplicate. Quercetin (8, 4, and 0.5 μg/mL) was used for DPPH, whereas Trolox (5, 3, and 1 μg/mL) was used for ABTS. The error percentage was calculated according to Equation (4):
| (4) |
where C°x is the added concentration and cx is the experimentally determined concentration.
A calibration curve was constructed and regression analysis was used to recalculate the standard concentrations. Five concentration levels were used. Quercetin (8, 6, 4, 2, and 0.5 μg/mL) was used for DPPH, whereas Trolox (5, 4, 3, 2, and 1 μg/mL) was used for ABTS. Using the equation of a straight line, the concentration of each standard was recalculated. Subsequently, the correlation between both concentrations was studied using least-squares regression analysis. Accuracy was evaluated from the determination coefficient value as well as from the slopes from the recalculation curves.
2.4.4. Robustness
Robustness evaluation was performed by slightly modifying those conditions that exhibited a significant effect during the optimization process. In the case of DPPH, the experiments were conducted by varying DPPH concentration (275 and 285 μM). For the ABTS method, the effects of using 65 and 75% ABTS concentrations and varying the absorbance adjustment of the solution to 0.65 and 0.75 values were evaluated. Additionally, in both methods, other variables, including solvent variations using DMSO (0.15 and 0.25%) as well as water addition to 25% of the final volume, were examined.
All experiments were carried out in triplicate. The robustness of each method was evaluated using Tukey’s test (α=0.05), which indicates whether there is a significant percentage reduction and hence whether the method is robust with respect to the evaluated conditions.
2.4.5. Plate Uniformity, Signal Variability, and Z Factor
Plate uniformity and signal variability were assessed through absorbance measurements of the respective solutions possessing different percentage reductions [6]:
Maximum absorbance with 80% of the analyte (i.e., 20% reduction of the light-absorbing species).
Mean absorbance with 50% of the light-absorbing species.
Minimum absorbance with 20% of the analyte (i.e., 80% reduction of the light-absorbing species).
To obtain three different percentage reductions of the light-absorbing species, quercetin was used at 1.26, 4.33, and 7.41 μg/mL concentrations, whereas Trolox was used at 1.22, 2.95, and 4.7 μg/mL concentrations, for the maximum, mean, and minimum signals, respectively.
Each solution was measured in 32 wells of the same 96-well plate and the procedure was performed with three different plates. The position of each solution was changed in each plate (rows or columns of wells). All assays were carried out with reagents prepared independently and evaluated on different days. %RSD was calculated for each solution.
The Z factor was also quantified in each plate according to Equation (5):
| (5) |
where n is the number of tests.
2.5. Applicability of the Antiradical Activity Reduction Methods
Once the methods were optimized and validated, their application was evaluated with eight methanol plant extracts.
The DPPH free radical reduction method was carried out by mixing 100 μL of the plant extract sample at different concentrations (250–0.25 μg/mL ethanol) with 100 μL of DPPH (280 μM in ethanol). A negative control was included (corresponding to 100% DPPH) in which ethanol was added instead of the sample. The mixture was incubated in the dark for 15 min at room temperature, and then the absorbance was measured at 540 nm with a Multiskan FC microplate reader.
For the ABTS free radical reduction method, a 7 mM solution of ABTS in 2.45 mM aqueous potassium persulfate was prepared. The solution was incubated for 12–16 h in the dark to produce the ABTS+ free radical. This solution was diluted with ethanol to adjust the absorbance value to 0.7. To evaluate antiradical activity, 60 μL of a plant extract sample solution (150–0.25 μg/mL ethanol) was mixed with 140 μL of the ABTS+ solution (A=0.7). A negative control was included (corresponding to 100% ABTS) in which ethanol was added instead of the sample. The mixture was incubated in the dark for 6 min at room temperature and the absorbance was measured at 750 nm with a Multiskan FC microplate reader.
For both DPPH and ABTS, the calculations used to obtain the percentage reduction of the light-absorbing species were those used for the optimization and validation of the method (Eq. (1)). A linear regression plot was constructed using the respective percentage reduction from each sample. The EC50 was assessed by curve interpolation for each of the plant extract samples. The results were also expressed as Quercetin equivalents for DPPH (μmol QE/g of Fresh Weight) and Trolox equivalents for ABTS (μmol TE/g of Fresh Weight) using standard curves. Quercetin standard solutions were prepared at concentrations from 1.65 to 26.47 μM and the t concentration of Trolox standard solutions ranged from 1 to 23.97 μM.
3. RESULTS AND DISCUSSION
A literature survey of assays for in vitro antiradical activity measurements that include full procedures, the positive controls employed in the study, and their respective mean effective concentrations, showed that the positive control most used for the DPPH reduction method is quercetin; Trolox is the most commonly used positive control with the ABTS reduction method.
The bibliographic review revealed important differences with respect to the mean effective concentrations of the positive control, i.e., quercetin and Trolox, although in many cases, these were described as “slight variations”. Herein, to optimize the method, these modifications were taken into account using two different levels of positive control for each of the working conditions. It should be noted that, although the maximum absorption wavelength is known for each species, our aim is to optimize high-throughput assays, i.e., microscale assays; therefore, the wavelength used for analysis was included in the optimization process. Furthermore, microplate reader filters that include wavelengths close to the maximum absorption wavelength of the positive control were used.
The design of the experimental approach involves varying all factors in a simultaneous and systematic manner, enabling the measurement of either the effect of a specific factor on the response or that of several interacting factors on the response. Experimental design, such as screening, factorial (full or fractionated), response surface, and experimental schedules generated computationally, can be used during optimization [10]. Generally, whereas the full factorial design is the easiest to implement and interpret, the fractionated factorial design typically involves fewer experiments. The choice of design is often based on the number of factors involved in the experimental method [11].
The reported range for the factor values are generally used to set the extreme operation values [12]. In this study, optimization was also carried out using the extreme values of each of the conditions identified in the initial search. Optimization of both DPPH and ABTS methods was undertaken using a 2^5-1 fractionated factorial design with no blocks and with a random central point and one replicate.
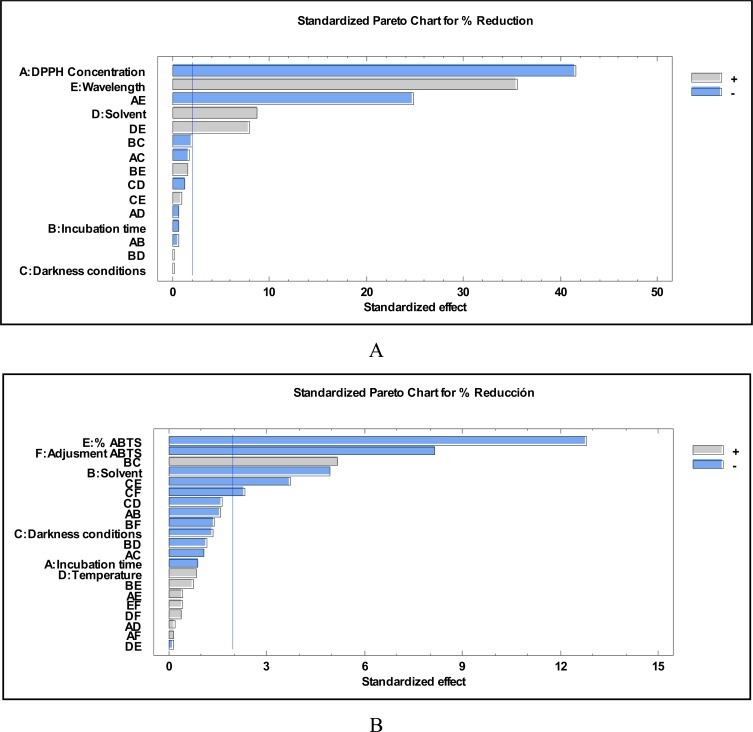
Percentage reduction of absorbance was monitored or evaluated as the response. The influence of each factor on the percentage reduction was plotted in a Pareto chart (Fig. 1). Variables that showed a significant influence on the DPPH reduction response were: DPPH concentration, wavelength, solvent, and the interaction between the three. Those for ABTS reduction were: ABTS concentration, ABTS absorbance adjustment, solvent, and light/dark conditions.
Fig. (1).
Pareto diagrams obtained from the optimization of reduction assays: DPPH (A) and ABTS (B).
Subsequently, factors that influenced the response in each method were optimized using the basic sequential simplex method. A group of experiments was conducted for each method, following the basic sequential simplex method rules, and those with the worst responses were discarded. New conditions were then calculated, and again, the worst responses were discarded. The simplex procedure was stopped when a negative value for some condition was found.
In the case of DPPH reduction, the highest percentage reduction values and the highest absorbance were obtained with the conditions established in the fourth experiment. These conditions were considered optimal.
With the ABTS method, higher percentage reduction values were obtained using the conditions established in the first experiment (with 60.1% reduction); under these conditions, the ABTS concentration was 50% with an absorbance value of 0.288. As previously noted, given that this method is used to evaluate the ability of a compound to reduce ABTS, any agent capable of reducing ABTS will also lead to a reduction in the level of the absorbance. Therefore, the concentration of ABTS should be optimized to obtain higher absorbance values to increase the percentage reduction. Three assays were conducted under the conditions established in the first experiment, modifying ABTS concentration between 70 and 90% (classical optimization). The highest potency (ABTS percentage reduction) was found using an ABTS concentration of 70%. Furthermore, under these conditions, an acceptable %RSD and an optimal absorbance value were obtained. Therefore, the conditions established from this experiment, using an ABTS concentration of 70%, were considered optimal for the ABTS reduction method.
The optimized methods were validated by following the recommendations and criteria set forth in international guidelines [8, 13, 14]. The figures of merit included in the validation process were linearity, precision, accuracy, robustness, plate uniformity, signal variability, and Z factor.
According to the ICH guidelines [14], the linearity of an analytical procedure is its ability (within a defined range) to obtain results directly proportional to the concentration (or amount) of the analyte in the sample. However, in biological activity assays, in which the potency of a compound is evaluated, linearity must be considered the ability of the procedure to obtain a potency interval (in this case, of reduction percentages) directly proportional to the concentration (or amount) of the test compound. Considering both definitions, three approximations were included in this study to establish the linearity of each method. In each case, the plots were obtained using the least-squares method (Fig. 1).
The direct analysis of solutions containing different concentrations of the analyte. The determination coefficients (R2) for DPPH and ABTS were 0.9987 and 0.9991, respectively. The linear concentration interval of DPPH was between 7 and 140 μM in ethanol (equivalent to DPPH reductions of 86.6 and 0%, respectively), and that of ABTS was between 1 and 70% in ethanol (equivalent to ABTS reductions of 99.0 and 0%, respectively).
The analysis of positive control solutions at five concentration levels. The determination coefficients (R2) for the DPPH and ABTS reduction methods were 0.9941 and 0.9947, and the slope values were 9.758 and 16.539, respectively. The linear range for quercetin concentration was between 8 and 0.5 μg/mL (with DPPH reductions of 86.59 and 10.49%, equivalent to 7.2 and 121.7 μM DPPH, respectively), whereas for Trolox, it was between 6 and 0.25 μg/mL (with ABTS reductions of 96.43 and 2.10%, equivalent to 3.45 and 68.52% ABTS, respectively).
The analysis of positive control solutions at five concentration levels added to a extract from Jatropha dioica. Determination coefficients (R2) of the DPPH and ABTS reduction percentages were 0.9914 and 0.995, and the slope values were 9.8482 and 14.734, respectively. The linear range of quercetin concentration was between 8 and 0.5 μg/mL (with DPPH reductions of 86.69 and 7.80%, equivalent to 7.04 and 125 μM DPPH, respectively), whereas for Trolox, the linear range was between 6 and 0.25 μg/mL (with ABTS reductions of 92.5 and 10.34%, equivalent to 5.25 and 62.75% ABTS, respectively).
Based on the linear intervals, the maximum and minimum potencies can be assessed for each method. In the case of DPPH, its minimum potency is a 10.5% reduction (equivalent to 121.7 µM DPPH) and its maximum potency is an 86.6% reduction (equivalent to 7.2 µM DPPH). For the ABTS method, its minimum potency was a 10.3% reduction (equivalent to 62.75% ABTS) and its maximum potency was a 92.5% reduction (equivalent to 5.25% ABTS). It is important to note that these values relate specifically to the concentrations of the free radical (DPPH or ABTS).
Precision was expressed as percentage of the relative standard deviation (%RSD). Results obtained are presented in Table 1. In all cases, the precision values were within the acceptable ranges recommended by the Biological Assay Validation Guidelines [8, 13]. The %RSD was less than 6.8 and 9.75% for DPPH and ABTS, respectively. Therefore, both methods showed good intra- and inter-day precision.
Table 1.
Precision and accuracy evaluation (n = 3).
| DPPH Reduction | ||||
|---|---|---|---|---|
| Level | Quercetin (μg/mL) | %RSD (Intra-day) | %RSD (Inter-day) | %error |
| High | 8 | 1.75 | 2.64 | 1.55 |
| Medium | 4 | 6.80 | 6.79 | –5.19 |
| Low | 0.5 | 1.57 | 2.64 | –5.81 |
| ABTS Reduction | ||||
| Level | Trolox (μg/mL) | %RSD (Intra-day) | %RSD (Inter-day) | %error |
| High | 5 | 6.34 | 0.77 | –0.20 |
| Medium | 3 | 2.32 | 1.62 | –1.98 |
| Low | 1 | 9.75 | 3.02 | –1.82 |
Accuracy was expressed as an error percentage and was acceptable for both DPPH and ABTS methods at the three concentration levels used; the % error values lay between –5.81 and 1.55%. Additionally, accuracy was evaluated through back-calculation of the positive control concentration. A correlation coefficient (R2) value of 0.9914 and a slope of 0.996 were obtained for quercetin concentration using the DPPH method, whereas an R2 value of 0.9911 and a slope of 0.999 were obtained for Trolox. These data confirm the accuracy of both methods [15], as they exhibit a good correlation and their slopes approximate to unity.
To establish the robustness of both methods, slight changes to variables that showed a significant effect on the response during the optimization process were evaluated.
DPPH free radical concentration was the only significant factor found during the optimization studies of the DPPH reduction method. Given this, the effect on the response was evaluated when a sub-optimal concentration of DPPH was used. According to Tukey’s test, the results obtained with 5 μM variations of the DPPH final concentration did not produce significant changes in absorbance (with absorbance ranges between 0.73 and 0.78).
In the ABTS reduction method, both initial absorbance adjustment (after generation of the ABTS radical) and the final free radical concentration were the most significant factors identified during the optimization studies. Variations in absorbance adjustment between 0.65 and 0.75, as well as variations in the final ABTS concentrations between 65 and 75%, did not produce significant differences in the reduction percentages compared with the optimized value.
Although ethanol is used as solvent in both methods, compounds and extracts or fractions that are not soluble in this solvent alone are sometimes analyzed. Therefore, it was necessary to evaluate the effect of other solvents in which the samples are solubilized. The inclusion of either water or DMSO had no significant effect on the reduction percentages compared with ethanol; however, the inclusion of 0.25% DMSO in the ABTS reduction method resulted in a significant difference. Therefore, it is recommended that water be used at a final concentration lower than 25% and that DMSO be used at a final concentration lower than 0.15% in the ABTS method.
The guidelines for developing high-throughput assays presented by Eli-Lilly [8] recommend assessing plate uniformity, signal variability, and the Z factor in all methods that use microplates. Therefore, variations of the response signal (absorbance) originating from differences in the microplates and microplate readers were evaluated to verify that there was no significant difference between the microplate wells. Experimentally, signal detection was carried out using three absorbance intensities (low, medium, and high) with analyte solutions of different concentrations and placed in different wells of three different plates. Using the obtained signal of each solution, the %RSD was calculated for each of the plates; %RSD values less than 15% indicate good precision, good uniformity, and low signal variability. The Z factor is used to quantify the advantages of using a particular method as a high-throughput assay on a large scale, and is defined as a signal window that is a function of four parameters (mean and standard deviations of both positive and negative controls). It can be used to indicate the extent of separation of noise from the signal [16], with the recommended acceptance criterion being Z ≥ 0.4 [17].
The results obtained with both methods are shown in Table 2. In the case of the DPPH reduction method, the %RSD values were between 1.22 and 12.7% for each of the signals recorded on different plates. The %RSD of the reduction percentages of the average signal in each of the plates were between 5.27 and 13.88%. The Z factor for three plates was higher than 0.94. Based on the acceptance criteria with respect to the Z factor, the DPPH reduction method carried out with a microplate is therefore considered an excellent procedure with good plate uniformity and low signal variability.
Table 2.
Plate uniformity, signal variability, and Z factor.
| DPPH Reduction Method | ABTS Reduction Method | |||||
|---|---|---|---|---|---|---|
| Plate 1 | Plate 2 | Plate 3 | Plate 1 | Plate 2 | Plate 3 | |
| Max signal (%RSD) | 1.39 | 1.22 | 2.13 | 13.09 | 11.18 | 13.16 |
| Medium signal (%RSD) | 5.77 | 5.74 | 2.04 | 5.08 | 5.08 | 5.56 |
| Low signal (%RSD) | 12.7 | 6.60 | 4.56 | 3.34 | 4.64 | 5.43 |
| % Reduction of medium signal | 42.56 | 41.69 | 42.19 | 49.55 | 51.95 | 50.19 |
| %RSD of % reduction | 13.88 | 13.53 | 5.27 | 13.44 | 12.35 | 13.81 |
| Z factor | 0.94 | 0.97 | 0.97 | 0.93 | 0.91 | 0.89 |
For the ABTS reduction method, the obtained %RSD values were between 3.34 and 13.16% for signals recorded on different plates. The reduction percentages of the average signal were calculated for each plate, and their %RSD values were between 12.35 and 13.81%. The quantified Z factor for all three plates was higher than 0.89. Based on the acceptance criteria with respect to the Z factor, the ABTS reduction method carried out with a microplate is therefore considered an excellent procedure with good plate uniformity and low signal variability.
The analysis of complex samples, such as natural extracts, can be affected by interfering substances; therefore, it is necessary to validate the method in the presence of potentially interfering species. It is also important to note that the antioxidant capacities depend on not only extract composition, but also the conditions of the test used. To address these issues, several extracts obtained from different plants were analyzed using both the optimized and validated reduction methods. The ABTS assay is particularly relevant for analysis of plant extracts because the wavelength absorption at 734 nm essentially eliminates color interference from the sample. All extracts were kindly provided by researchers from the Analytical Chemistry Department. Some of these plant extracts have previously been shown to exhibit antioxidant activity.
All samples were evaluated at concentrations between 1 and 250 μg/mL for the DPPH assay, and between 1 and 150 μg/mL for the ABTS assay. In each case, a plot was constructed using the least-squares method, and an interpolation was performed to identify the effective concentration of sample (μg/mL) that reduces 50% of DPPH or ABTS (EC50) (Table 3). The EC50 is also expressed as Quercetin or Trolox equivalents, respectively (Table 4). It is important to note that the values determined are specifically related to the concentrations of the free radical (DPPH or ABTS).
Table 3.
Median effective concentration of antiradical activity in methanolic plant extracts.
| DPPH Reduction Method | ABTS Reduction Method | |||
|---|---|---|---|---|
| Test Sample | EC50 (μg/mL) | SD (μg/mL) | EC50 (μg/mL) | SD (μg/mL) |
| Quercus canbyira | 21.74 | 0.47 | 5.33 | 0.14 |
| Turnera diffusa | 23.97 | 0.68 | 10 | 0.3 |
|
Leucophyllum frutescens |
51.14 | 0.91 | 27.05 | 0.58 |
| Teucrium bicolor | 53.52 | 2.13 | 16.9 | 0.47 |
| Salvia texana | 56.94 | 1.9 | 28.44 | 0.96 |
| Salvia beateflora | 92.1 | 1.93 | 25.42 | 0.39 |
| Vitis vinifera | 147.47 | 1.79 | 21.31 | 0.39 |
| Jatropha dioica | > 250 | > 150 | ||
Table 4.
Median effective concentration of antiradical activity in methanolic plant extracts expressed in μM QE/g FW for DPPH and in μM TE/g FW.
| DPPH Reduction Method | ABTS Reduction Method | |||
|---|---|---|---|---|
| Test Sample | EC50 (μM QE/g FW) | SD (μM QE/g FW) | EC50 (μM TE/g FW) | SD (μM TE/g FW) |
| Quercus canbyira | 659.75 | 14.54 | 1350.63 | 36.79 |
| Turnera diffusa | 598.17 | 17.17 | 719.78 | 21.85 |
| Leucophyllum frutescens | 280.43 | 4.97 | 266.20 | 5.63 |
| Teucrium bicolor | 267.96 | 10.56 | 426.02 | 11.74 |
| Salvia texana | 251.84 | 8.35 | 253.15 | 8.62 |
| Salvia beateflora | 155.70 | 3.22 | 283.25 | 4.35 |
| Vitis vinifera | 97.24 | 1.18 | 337.92 | 6.11 |
| Jatropha dioica | < 57.36 | < 28.80 | ||
The results show that both methods can be used to distinguish between samples having antioxidant activity (Q. canbyira and T. diffusa) and those without activity (J. dioica). Q. canbyira and T. diffusa showed the lowest EC50 (μg/mL) and therefore the best antioxidant activity of the samples evaluated. Moreover, the latter two plant extracts have a higher effective concentration of antiradical activity as expressed as Quercetin and Trolox equivalents per gram of fresh weight, and therefore a higher antioxidant capacity. In contrast, J. dioica showed almost no antioxidant activity by either methods; this extract exhibited the lowest number of Quercetin and Trolox equivalents per gram of fresh weight. These findings confirm that both methods are comparable.
CONCLUSION
Two methods for measuring antiradical activity were optimized and the conditions that produced the best potency and signal (absorbance) were selected. Both methods were validated, and the linearity, range, precision, accuracy and robustness were established, as indicated by several guidelines for developing biological assays. The DPPH and ABTS reduction methods were applied to eight plant extracts and the EC50 was assessed for each sample.
The DPPH and ABTS reduction methods described in this study can be implemented in any laboratory by following the procedure exactly as described since both methods depend on the concentration of the reagent used. When implementation is being performed, a validation must be carried out with positive controls in solution. If a modification of the conditions is necessary, an optimization process is recommended to obtain the best response. Method validation is also recommended under the new working conditions.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
Author contributions: GGG performed the study and analysed data; RSA designed research, wrote paper; MGT analysed data, wrote paper; RCR analysed data, wrote paper; NWT contributed important reagents, wrote paper.
This work was funded by the SEP-CONACYT Ciencia Básica 2012 (No. 180977) and 2013 (No. 220882), as well as the Red Farmoquimicos (CONACYT N° 271520) and Red Metabolómica de plantas (PRODEP No. 103-5/15/14156).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Szymański P., Markowicz M., Mikiciuk-Olasik E. Adaptation of high-throughput screening in drug discovery-toxicological screening tests. Int. J. Mol. Sci. 2012;13:427–452. doi: 10.3390/ijms13010427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam M.N., Bristi N.J., Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013;21:143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudonné S., Vitrac X., Coutière P., Woillez M., Mérillon J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD and ORAC assays. J. Agric. Food Chem. 2009;57:1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- 4.Floegel A., Kim D., Chung S., Koo S.I., Chun O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011;24:1043–1048. [Google Scholar]
- 5.Iacopini P., Baldib M., Storchic P., Sebastiani L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008;21:589–598. [Google Scholar]
- 6.Sadek E.S., Makris D.P., Kefalas P. Polyphenolic composition and antioxidant characteristics of kumquat (Fortunella margarita) peel fractions. Plant Foods Hum. Nutr. 2009;64:297–302. doi: 10.1007/s11130-009-0140-1. [DOI] [PubMed] [Google Scholar]
- 7.Macarrón R., Hertzberg R.P. Design and implementation of high throughput screening assays. Mol. Biotechnol. 2011;47:270–285. doi: 10.1007/s12033-010-9335-9. [DOI] [PubMed] [Google Scholar]
- 8.Eli Lilly and Company and the National Institutes of Health Chemical Genomics Center . Guidance for Assay Development and HTS, Version 5. Indianapolis: Eli Lilly and Company and the National Institutes of Health Chemical Genomics Center; 2007. [Google Scholar]
- 9.Fallarero A., Hanski L., Vuorela P. How to translate a bioassay into a screening assay for natural products: General considerations and implementation of antimicrobial screens. Planta Med. 2014;80:1182–1199. doi: 10.1055/s-0034-1383061. [DOI] [PubMed] [Google Scholar]
- 10.Altekar M., Homon C.A., Kashem M.A., Mason S.W., Nelson R.M., Patnaude L.A., Yingling J., Taylor P.B. Assay optimization: A statistical design of experiments approach. J. Lab. Autom. 2006;11:33–41. doi: 10.1016/j.cll.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Granados-Guzmán G., Waksman de Torres N., Castro-Ríos R., Salazar-Aranda R. Ensayos de alto rendimiento utilizados en farmacognosia: Selección, optimización y validación de métodos de inhibición enzimática por espectrofotometría UV-visible. JPPRes. 2014;2(1):1–13. [High-throughput screening assay used in pharmacognosy: Selection, optimization and validation of methods of enzymatic inhibition by UV-visible spectrophotometry]. [Google Scholar]
- 12.Lutz M.W., Menius J.A., Choi T.D., Laskody R.G., Domanico P.L., Goetz A.S., Saussy D.L. Experimental design for high-throughput screening. Drug Discov. Today. 1996;1:277–286. [Google Scholar]
- 13.United States Pharmacopeial Convention, Inc. Biological Assay Validation (Chapter 1033). U.S. Pharmacopeia & National Formulary; United States Pharmacopeial Convention, Inc.: Rockwell,, pp.; 2010. pp. 1–25. [Google Scholar]
- 14.ICH Expert Working Group Q2 (R1) Validation of Analytical Procedures: Text and Methodology. ICH Harmonised Tripartite Guideline.; International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; Chicago. 2005. [Google Scholar]
- 15.Rozet E., Marini R.D., Ziemons E., Boulanger B., Hubert P. Advances in validation, risk and uncertainty assessment of bioanalytical methods. J. Pharm. Biomed. Anal. 2011;55:848–858. doi: 10.1016/j.jpba.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Iversen P.W., Beck B., Chen Y.F., Dere W., Devanarayan V., Eastwood B.J. HTS Assay Validation. In: Sittampalam G.S., Gal-Edd N., Arkin M., Auld D., Austin C., Bejcek B., editors. Assay Guidance Manual. Bethesda: Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2012. [Google Scholar]
- 17.Zhang J.H., Chung T.D., Oldenburg K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]