Summary
In this issue of Structure Lynch et al (2017) reveal that the interaction between two key proteins in the bacterial flagellar motor results in a shared structural domain. This unusual arrangement keeps the corresponding genes together through the course of evolution.
Bacterial flagellum, an organelle that propels a bacterial cell in liquid environments, is one the most complex supramolecular structures in nature. It can be subdivided in three principal parts: the basal body (motor), the hook (joint), and the helical filament (propeller). In well-studied enteric bacteria Escherichia coli and Salmonella enterica, as well as in distantly related Bacillus subtilis, more than 50 genes, distributed in more than 10 operons contribute to the assembly, function, and regulation of the flagella (Aizawa et al., 2002; Macnab, 2003) and many flagellar components vary between different bacterial species leading to a substantial structural diversity of bacterial flagellar motors (Minamino and Imada, 2015). Consequently, the evolutionary history of flagellum has proven difficult to reconstruct until a substantial number of sequenced bacterial genomes became available. Comparative genomic analysis using a few dozens of genomes of flagellated species representing diverse bacterial phyla revealed an ancient core of 24 structural genes that were likely present in the last common ancestor of Bacteria (Liu and Ochman, 2007). This core included two key elements of the flagellar motor, FliF and FliG. FliF is a single transmembrane protein, whose multiple copies form the MS (‘membrane/supramembrane’) ring, the first circular structure to form during the assembly of flagella (Macnab, 2003). FliG is a cytoplasmic protein, which is a part of the C (‘cytoplasmic’) ring on the cytoplasmic face of the MS ring, also called a switch complex, because it is responsible for switching the direction of motor rotation. Interaction between the C-terminal region of FliF (FliFC) and the N-terminal domain of FliG (FliGN) is a required for a correct position of the switch complex relative to the MS ring, which in turn is crucial for further assembly and function of the flagellar motor (Macnab, 2003). In this issue of Structure, Lynch et al. (2017) reported an insight into this key interaction, which not only explained how a key unit of the flagellar motor is built via protein-protein interaction, but also led to an interesting hypothesis about the origins of this interaction.
Lynch et al (2017) solved a crystal structure of a complex between FliFC and FliGN and used solution structure NMR, small-angle X-ray scattering and biochemical data to refine the interaction. The FliFC:FliGN structure was used to model the MS/C-ring interface in the context of cryo-EM density for isolated rotors (Thomas et al., 2006), which in turn was refined by targeted cross-linking and motility assays of mutants carrying structure-guided substitutions. As a result, the authors were able to update the architecture of the upper and lower switch. They clarified that the stoichiometry of FliF to FliG is 1:1 and showed that twenty-five copies of the FliF:FliG complex fit well around the upper switch, which is in agreement with the results of recent homology modeling of the periplasmic region of FliF (Bergeron, 2016).
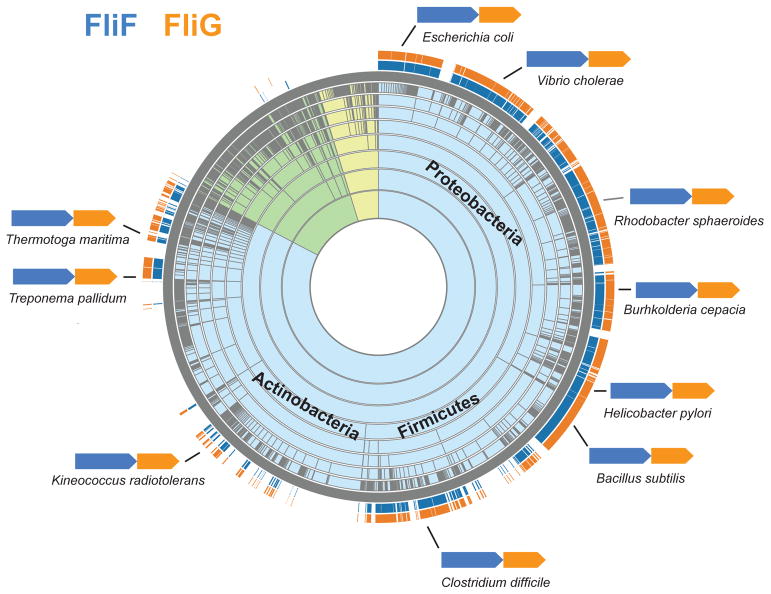
One of the key findings by Lynch et al. (2017) is that interacting elements of FliFC and FliGN form a structural domain that aligns well with the FliG middle (FliGM) and C-terminal (FliGC) domains. This finding led the authors to propose that FliFC: FliGN and FliGM originated from a common ancestral domain by duplication. Several lines of genomic evidence seem to support the authors’ idea that two proteins FliF and FliG, are tightly linked together to ensure the MS ring/switch complex interaction, while preventing FliGN interaction with FliM, which is reserved exclusively for FliGM. First, relatedness of FliGN and FliGM is confirmed by the leading domain database Pfam, which places both domains along with FliGC in the same superfamily (Pfam clan FliG, accession number CL0436) solely on the basis of sequence similarity. Second, analysis of hundreds of bacterial genomes shows that fliF and fliG genes are not only always encoded together, but also the exact orientation of these genes, fliF>fliG, is preserved in bacteria from different phyla (Fig. 1). The end of fliF is usually separated from the start of fliG by only a few base pairs and in some instances they overlap. This tight coupling favors the Lynch et al. (2017) hypothesis about the origin of FliFC-FliGN interaction. The uniqueness of fliG/fliG genomic conservation is contrasted by the fact that that gene order for the majority of other flagellar genes is not conserved: the order and organization of many flagellar genes have undergone extensive shuffling and rearrangements among bacterial lineages (Liu and Ochman, 2017). Third, the region where FliFC terminates and FliGN begins coincides with the FliM-binding motif of FliGM (Paul et al., 2011). Separating the ancestral FliGN domain derived from FliGM duplication into two (FliFC and the modern FliGN) at what would be the FliM interaction site prevented incorrect binding of FliGN to FliM during MS/C-ring assembly. Finally, domain duplication and shuffling leading to the current FliF:FliG organization proposed by Lynch et al (2017) is in agreement with the evolutionary scenario proposed by Liu and Ochman (2007), where the core components of the bacterial flagellum originated through the successive duplication of a few genes.
Figure 1.
Taxonomic distribution and gene order of fliF and fliG. The distribution of fliF and fliG shown on a taxonomic species tree was generated using the Aquerium tool (http://aquerium.utk.edu/) and the gene order was retrieved from the MiST database (http://mistdb.com/). Background color of the sunburst tree indicates species of Bacteria (light blue), Archaea (light yellow) and Eukaryota (light green).
Interestingly, in addition to FliGN, FliGM, and FliGC, the FliG superfamily has yet another member, a protein domain termed MgtE_N. Similarly to FliG domains, MgtE_N is a part of a protein complex, the MgtE transporter, and it is also found in a similar cellular location – at the interface of the inner membrane and the cytoplasm, where it interacts with two other domains of the transporter. Relatedness of MgtE_N to FliG domains is confirmed both by structure (Hattori et al., 2007) and sequence similarity (the same Pfam clan) indicating their likely common ancestry. This, in turn, is in line with the current view that bacterial flagella originated from simpler secretion systems and other types of transporters (Pallen and Matzke, 2006).
In conclusion, the study by Lynch et al. (2017) provides important new details on the assembly and function of the bacterial flagellar motor and contributes to our understanding of its evolution.
Acknowledgments
The work in my laboratory is supported by NIH grants R01GM072285 and R01DE024463.
References
- Aizawa S-I, Zhulin IB, Marquez-Magana L, Ordal GW. In: Chemotaxis and motilityBacillus subtilis and its Closest Relatives: from Genes to Cells. Sonenshein AL, et al., editors. ASM Press; Washington, D.C: 2002. pp. 437–452. [Google Scholar]
- Bergeron JR. Structural modeling of the flagellum MS ring protein FliF reveals similarities to the type III secretion system and sporulation complex. PeerJ. 2016;4:e1718. doi: 10.7717/peerj.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M, Tanaka Y, Fukai S, Ishitani R, Nureki O. Crystal structure of the MgtE Mg2+ transporter. Nature. 2007;448:1072–1076. doi: 10.1038/nature06093. [DOI] [PubMed] [Google Scholar]
- Liu R, Ochman H. Stepwise formation of the bacterial flagellar motor. Proc Natl Acad Sci USA. 2007;104:7116–7121. doi: 10.1073/pnas.0700266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MJ, Levenson R, Kim EA, Sircar R, Blair DF, Dahlquist FW, Crane BR. Co-folding of a FliF-FliG split domain forms the basis of the MS:C ring interface within the bacterial flagellar motor. Structure. 2017;XXX:xxxx–xxxx. doi: 10.1016/j.str.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab RM. How bacteria assemble flagella. Annu Rev Microbiol. 2003;57:77–100. doi: 10.1146/annurev.micro.57.030502.090832. [DOI] [PubMed] [Google Scholar]
- Minamino T, Imada K. The bacterial flagellar motor and its structural diversity. Trends Microbiol. 2015;23:267–274. doi: 10.1016/j.tim.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Pallen MJ, Matzke NJ. From The Origin of Species to the origin of bacterial flagella. Nat Rev Microbiol. 2006;4:784–790. doi: 10.1038/nrmicro1493. [DOI] [PubMed] [Google Scholar]
- Paul K, Gonzalez-Bonet G, Bilwes AM, Crane BR, Blair D. Architecture of the flagellar rotor. EMBO J. 2011;30:2962–2971. doi: 10.1038/emboj.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DR, Francis NR, Xu C, DeRosier DJ. The three-dimensional structure of the flagellar rotor from a clockwise-locked mutant of Salmonella enterica serovar Typhimurium. Journal of Bacteriology. 2006;188:7039–48. doi: 10.1128/JB.00552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]