Abstract
Spinal cord injury (SCI) induced changes in neurological function have significant impact on the metabolism and subsequent metabolic-related disease risk in injured individuals. This metabolic-related disease risk relationship is differential depending on the anatomic level and severity of the injury, with high level anatomic injuries contributing a greater risk of glucose and lipid dysregulation resulting in type 2 diabetes and cardiovascular disease risk elevation. Although alterations in body composition, particularly excess adiposity and its anatomical distribution in the visceral depot or ectopic location in non-adipose organs, is known to significantly contribute to metabolic disease risk, changes in fat mass and fat-free mass do not fully account for this elevated disease risk in subjects with SCI. There are other negative adaptations in body composition including reductions in skeletal muscle mass and alterations in muscle fiber type, in addition to significant reduction in physical activity, that contribute to a decline in metabolic rate and increased metabolic disease risk following SCI. Recent studies in adult humans suggest cold- and diet-induced thermogenesis through brown adipose tissue metabolism may be important for energy balance and substrate metabolism, and particularly sensitive to sympathetic nervous signaling. Considering the alterations that occur in the autonomic nervous system (SNS) (sympathetic and parasympathetic) following a SCI, significant dysfunction of brown adipose function is expected. This review will highlight metabolic alterations following SCI and integrate findings from brown adipose tissue studies as potential new areas of research to pursue.
Keywords: brown adipose tissue, white adipose tissue, insulin, glucose, thermogenesis, sympathetic nervous system
Introduction
Spinal Cord Injury and Metabolic Disease Risk
Sixty percent of individuals with spinal cord injury (SCI) are younger than 45 years, meaning that more than half of individuals with newly acquired SCI have the potential to live long and healthy lives [1]. While life expectancy is increasing among individuals with SCI who survive the first year after injury, it is still lower than that observed for the able-bodied (AB) population [2]. Lower life expectancy in SCI is thought to result from secondary health conditions associated with neurological impairment as individuals with (SCI) are at increased risk of developing obesity, type 2 diabetes (T2D) and cardiometabolic disease relative to the general population [3–8]. This association has been observed in multiple population cohorts across gender and age groups. A large body of evidence supports an accelerated trajectory of metabolic disorders in the SCI population, such that these health conditions occur at an earlier age vs. AB individuals [3;7–9]. Across studies, a relatively consistent metabolic profile of elevated circulating glucose, insulin resistance and hyperlipidemia is present in subjects with SCI [4;6;10–13]. Explanatory variables including a loss of lean mass (particularly skeletal muscle), reduced physical activity, excess energy intake relative to need, and systemic compensatory responses related to impaired autonomic function, mediated through the above mentioned variables, have been previously studied [14;15]. Although data show reduced energy expenditure in SCI subjects relative to uninjured controls [14;16;17], controlling for the difference in lean body mass and decreased physical activity does not fully account for the decline in energy expenditure and increased disease risk [17], particularly for those individuals with injuries to higher anatomic levels of the spine [5;15;17–20]. While multiple factors may contribute to this differential metabolic-related disease risk in subjects with SCI, this review highlights observations related to the anatomic level of injury, alterations in body composition, skeletal muscle biology, energy expenditure, metabolic substrate metabolism, sympathetic nervous system function, and a potential role for brown adipose tissue in these metabolic-disease risk relationships.
Level of Injury Effects
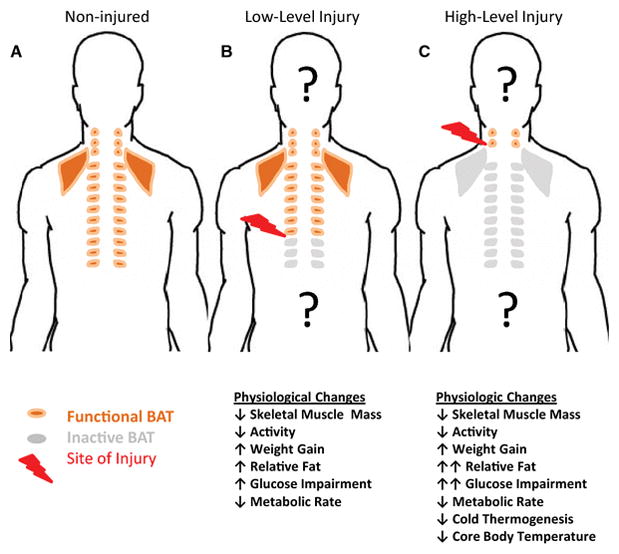
The site of anatomic injury to the spinal column is variable between individuals with injuries occurring in the upper regions of the spine (high-level injuries - cervical) resulting in greater overall neurologic impairment compared with low level injuries (thoracic and lumbar level). Metabolic impairment and disease risk are greater in individuals with high-level injuries [15;18;21]. Individuals with high-level injuries who have greater neurologic impairment present with significantly lower glucose tolerance, greater insulin resistance and impaired lipid profiles when compared with both uninjured controls and low-level injury cases [4;6;11;16]. Furthermore, individuals with high-level injuries have greater metabolic rate deficits, with thermoregulatory imbalance resulting in periods of hypothermia [22–24]. In addition to the spinal column, the ascending and descending sympathetic chain of ganglia coordinate a sensory/effector response in maintaining general physiologic homeostasis mediated in part through the catecholamine signaling, in particular norepinephrine [25]. Considering its anatomic arrangement, it is not surprising that impairment of SNS relative to the level of injury is observed, with greater impairment and resulting physiologic disruption accompanying a high-level injury [16–18;21;22;26–37]. These physiologic and metabolic alterations with high-level injuries (particularly the deficits in SNS function, metabolic rate and thermogenesis, and glucose-lipid metabolism resulting in glucose-intolerance and ultimately insulin resistance) suggest the presence of an unidentified tissue/organ integrating these phenotypes and mediating the metabolic-related disease risk – which we hypothesize as brown adipose tissue and discuss in the subsequent sections.
Metabolism-Related Alterations Following Spinal Cord Injury
Body Composition
Due to significant neurological impairment, body composition drastically deteriorates, as early as 6 months after SCI with an excessive loss of lean mass below the level of injury and an increased total fat mass [38–40]. Lower metabolic rates as a result of reduced lean mass further accelerate the development of excess adipose tissue (obesity) in individuals with SCI. Obesity is associated with a broad range of metabolic abnormalities that affect several different organ systems in the body via atherogenic, neuro-humoral, and hemodynamic mechanisms in the able-bodied (AB) as well as SCI populations [41–43]. Methods for determining in vivo body composition, lean mass and fat mass, have expanded significantly over the last 30 years. This includes the use of whole body estimates of fat mass and fat free mass or lean mass from anthropometry (e.g. body mass index, waist circumference) and biophysical means (e.g. under water weighing or air displacement plesthymography) to more detailed tissue and regional assessments of fast mass and distribution using in vivo imaging technologies like dual x-ray absorptiometry, computed tomography and magnetic resonance imaging. Despite these advances and the numerous studies in able body populations, detailed and accurate descriptions of the amount and distribution of fat mass and adipose tissue following spinal cord injury remains limited, particularly across population variables like sex, anatomical level and severity of injury, time since injury. Whole body relative fat mass is underestimated by body mass index (BMI) with SCI populations [44–46], indicating BMI categories and disease risk relationships should be carefully considered and possibly revised to lower category values or include additional indices (e.g. waist circumference) in subjects with SCI [46–48]. More recent advances in in vivo imaging modalities open new possibilities for determining organ specific masses, fat infiltration in non-adipose depots and regional distributions of body fat (subcutaneous versus visceral) which have been shown to differentially influence disease risk and will be important for understanding the changes that occur in subjects following SCI [49–51].
Skeletal Muscle Adaptations
In individuals with American Spinal Cord Injury Association Impairment Scale (AIS) category A SCI, the activation and loading levels of the skeletal muscles below the level of the lesion are markedly reduced or absent. Without preventive measures, these muscles rapidly atrophy and show a decline in functional capacity. As soon as 6 weeks after injury, lower limb muscles are 25–45% smaller than in non-injured controls [52]. Muscle protein changes may occur more slowly than fiber atrophy; nevertheless, several months post-SCI, fatigue-resistant and oxidative muscle fibers transform into highly fatigable and glycolytic muscle fibers with impaired oxidative capacity and mitochondrial function and poor fatigue resistance [53–55]. The maintenance of normal glucose homeostasis depends on a finely balanced interaction between muscle sensitivity to insulin and insulin secretion, as up to 70% of glucose utilization occurs at the muscle level [56;57]. Changes in skeletal muscle insulin sensitivity and glucose metabolism are considered to be the initiating defect in type 2 diabetes, evident decades before beta-cell failure. The transformation of muscles from a slow oxidative to a fast glycolytic phenotype following SCI yields a muscle tissue that is insulin resistant and metabolically inflexible. Recent research [58] confirmed that human SCI muscle has histochemical and biochemical properties that are very similar to that of human diabetic muscle, including fewer Type I fibers and a predominance of Type IIax+IIx fibers. These fibers have significantly reduced glucose handling capacity under insulin-stimulated conditions due to lower levels of the insulin receptor, glucose transporter 4 (GLUT 4), hexokinase II, glycogen synthase, and pyruvate dehydrogenase-E1α.
Sympathetic Nervous System & Energy Expenditure
Energy expenditure in the homeothermic organism (e.g. mammals) is related to overall body composition, with energy balance requiring a complex integration of central and peripheral affectors and effectors to balance the processes of metabolism against energy acquisition, dissipation and storage[60]. Each of these metabolic components is variable depending on environmental inputs and is mediated through the CNS and autonomic nervous system, including the parasympathetic and sympathetic nervous systems [25] (see [61–64]). The three main components of daily energy expenditure in the absence of thermal stress include resting metabolic rate (including the basal metabolic rate), activity-related energy expenditure, and the thermic effect of food (including diet-induced thermogenesis). Basal metabolic rate (BMR), referring to the minimal amount of energy expenditure in an unfed, non-active, awake state for a non-reproductive organism within the thermoneutral zone (absence of cold or heat stress), has been measured multiple ways, with most currently technologies relying on the calculation of energy expenditure based on respiratory gas analysis referred to as indirect calorimetry [65;66]. Above the range of ambient temperatures, where core body temperature can be defended by involuntary changes in vascular control and sweating for heat dissipation, energy expenditure rises [64]. Below the lower critical temperature where vascular and behavioral controls are no longer sufficient to preserve core temperature, thermogenesis is required [25]. This includes both shivering (involuntary muscle contractions) and non-shivering thermogenesis. Although not as commonly discussed in the context of daily energy expenditure, in the context of modern society where thermal exposures are modified by controlling ambient temperatures, clothing, environmental surroundings and other factors, there is increasing awareness of the “cold-induced thermogenesis” component including both shivering and non-shivering thermogenesis, that may at times significantly contribute to energy balance and body temperature maintenance [67–71]. This adaptive, facultative cold-induced thermogenesis has been clearly demonstrated in small mammals to be sympathetically controlled and mediated through brown adipose tissue (BAT) [72;73], discussed below in more detail. Basal and resting metabolic rate are generally lower following SCI when compared with non-injured controls, with high-level injuries having a larger metabolic rate deficit, although adjustment for changes in body composition and appropriate covariates may obviate these metabolic deficits at times [65;74–78]. Concomitant with these potential energy expenditure reductions, metabolic substrate utilization based on the respiratory exchange ratio (or respiratory quotient) suggest reduced carbohydrate utilization, particularly after a meal [78;79], in agreement with elevated blood glucose during this same period[80] and the observed glucose intolerance following SCI [5;6;9]. Although making up a smaller overall percentage of daily energy expenditure, diet-induced thermogenesis (or the thermic effect of feeding) and cold-induced thermogenesis have been inconsistently reported as lower in subjects with SCI, with higher level injuries having greater deficits [79;81;82]. Differences between study methodologies including the duration and type of metabolic rate measure, time since injury, standardized conditions (time of day if not whole day, composition of meals used for feeding tests, temperature monitoring of room and clothing utilized, medication usage, SNS function and anatomic level of SCI, etc.) may contribute to some of these discrepancies and should be carefully considered in performing comprehensive energy expenditure measurements [82].
Body Temperature
Given the metabolic rate deficits often observed with SCI, as well as the SNS alterations that contribute to thermal regulation, defense of core body temperature under normal ambient temperature exposures in addition to mild cold challenge might be expected to deviate from normative values of non-injured subjects. Subjects with high-level injury and greater neurologic impairment generally have significantly lower core body temperatures than low-level injuries or high-level SCI with less neurologic impairment under basal conditions [23;24;79;83–85] as well as cold thermal challenges [86–88]. This data agrees with previous observations of a “poikilothermy” phenotype in subjects following SCI [22;24;89], further suggesting alterations in SNS function in thermal regulation which may reflect increased heat loss to the environment as well as possible thermogenesis defects. Whether varying core body temperature and altered thermal balance contributes to differences in metabolic efficiency and propensity to excess weight and adipose gains following SCI remains to be fully elucidated.
Brown Adipose Tissue and Metabolism
Brown Adipose Tissue Anatomical Distribution and Function
As described above, core body temperature is defended by a balance of energy intake, expenditure and dissipation. Adipose tissue plays an important role in energy balance as an energy storage tissue, paracrine/endocrine organ and for physical thermal regulation. While the majority of research in humans over the past half century has focused on the importance of excess white adipose tissue (WAT) in obesity and related metabolic disease, the rediscovery of the presence of metabolically active brown adipose tissue (BAT) in adult humans has reopened questions related to energy balance and thermogenesis [90]. BAT is a type of adipose tissue with high metabolic activity named for its unique color derived from increased mitochondrial content, vascular/blood flow and reduced lipid content relative to the more abundant, storage form of adipose - white adipose tissue [72;91;92]. BAT contains a unique mitochondrial protein called uncoupling protein 1 (UCP1) [72]. As the name implies, this protein dissipates the intermembrane H+ gradient thereby “uncoupling” the process of substrate utilization through the electron transport chain from ATP synthesis, resulting in heat generation – a.k.a. thermogenesis [72]. SNS stimulation via release of norepinephrine is essential for BAT activation, with UCP1 levels increasing under chronic or sustained SNS signaling [73;93–96].
Over the last 7 years the presence of metabolically active BAT in humans has been reconsidered thanks in part to advances in medical imaging [90;97;98]. Positron emission tomography (PET) scans are used to identify ‘hypermetabolic’ tissues based on the uptake of a radio-labelled glucose analog. In adult human subjects, cold exposure prior to and/or during the PET scans was found to induce a symmetrical pattern of hypermetabolic tissues anatomically localized to the cervical and upper thoracic regions, particularly in lean subjects and during cold weather months [99;100]. Further investigation revealed the composition of these symmetrical depots to be similar to that of adipose tissue based on computed tomography (CT) densities and containing UCP1 and sympathetic innervation, verifying the presence of metabolically active BAT in at least a portion of healthy, adult humans [101–107]. These sites of metabolically active BAT (particularly the paravertebral depots), as well as other BAT depots in the upper thoracic/cervical regions, anatomically coincide with the SNS anatomic ganglia distribution.
Prior to the “re-discovery” of metabolically active BAT in humans by medical imaging methods (previously described by anatomical dissection in adult humans in the 1970s [108]), animals models had suggested a potential role of defects in BAT function associated with both obesity and glucose/insulin disorders [90;97;109–111]. Since 2009, multiple studies using PET-CT and cold challenges, where the subjects are exposed to a cold room to stimulate non-shivering thermogenesis, have been published showing an inverse association between metabolically active BAT and body fat/BMI in humans [103;112–130]. Furthermore, in line with animal studies which show responsiveness to insulin, leptin, thyroid hormone, beta-3 adrenergic stimulation (norepinephrine), fatty acid uptake and protection from T2D in BAT activated states [90;131;132], human BAT activity is positively associated with lower circulating glucose and improved insulin sensitivity, and inversely associated with T2D [101;112;120;133–137].
Obesity and Sympathetic Alterations
Dysregulation of autonomic nervous function in obese animals is frequently observed in metabolism and obesity research. The primary mediator of this autonomic dysregulation has generally been considered a defect in SNS function, so much so that the acronym MONA LISA (Most Obesities kNown Are Low In Sympathetic Activity) is used to describe it [138–143]. Two common deficits of SNS-mediated metabolism reported in the individuals who are obese is a deficit of diet-induced thermogenesis and non-shivering thermogenesis [144–150]. However, a cause-effect relationship has been difficult to determine given the deficits have most often been measured in the obese state or post-weight loss subsequent to overweight/obese status. Alternatively, patients with adrenal tumors (pheochromocytoma) overproduce epinephrine and norepinephrine with accompanying hypermetabolic status including increased energy expenditure, body leanness and brown adipose tissue hyperplasia [119;151–157]. Considering the necessity of SNS function for BAT stimulation of thermogenesis [93], important and unanswered questions of human, clinical relevance remain to be answered in understanding the relationship between SNS function, BAT function and metabolic-related disease risk.
Intersection of SCI, SNS and BAT
Observations from individuals with SCI demonstrate a decreased SNS activity and obesity development post-injury [13;76;158;159]. Although a HLI model of SCI carries additional neurologic insults outside a singular SNS disruption (reduced physical activity, sensory perception, etc.), the clinical parallel and incomplete understanding of this increased disposition to develop obesity and metabolic dysregulation/disease support a need for additional clarity in this area. Thus, comparisons with LLI and non-injured, able-bodied individuals will aid in assessing the contribution of SNS control of BAT in human metabolism and physiology. Human SCI research involving impaired SNS function (high level injury) has focused on multiple metabolic tissues to the relative exclusion of BAT. This may be in part because of the previously disputed presence of metabolically active brown adipose tissue in adult humans [160], as well as controversy regarding the amount of daily energy expenditure contributed by BAT through diet induced thermogenesis (DIT) and non-shivering thermogenesis (NST) with estimated ranges from 5–15% [161;162] in adult humans. However, in animal models it is well established that sympathetic nervous control is essential for BAT thermogenesis, and that molecular, cellular and tissue level changes in BAT accompany chronic SNS activation as we demonstrated in mice with environmental temperature housing conditions [163]. While pre-clinical research is suggestive of a SNS-mediated BAT deficit [164], significant physiological differences between the rodent research model and humans stress the importance of additional research focused on the contribution (or lack thereof) of BAT to aspects of thermal regulation, metabolic rate, substrate utilization and ultimately metabolic-related disease risk in the SCI population. Given the anatomical distribution of BAT and the SNS, we hypothesize that individuals with high-level SCI (tetraplegia) will have decreased BAT amount and function, relative to the impairment of SNS input.
Conclusions
Metabolic alterations following SCI indicate an increased disease risk for metabolic-related diseases like type 2 diabetes and cardiovascular disease. While reduced energy expenditure reflecting changes in activity and body composition contribute to metabolic and physiologic deficits relative to non-injured subjects, these do not fully account for metabolic dysregulation between subjects with SCI, particularly at higher versus lower anatomic levels of injury. The SNS plays a critical role in metabolic rate and energy balance, and with impaired SNS coincident with the level and severity of SCI. While SNS alterations have previously been observed in multiple models of obesity, the cause-effect relationship has been difficult to ascertain. With the rediscovery of BAT in adult humans, a renewed interest in BAT as a mediator of SNS metabolic activity and metabolic-related disease risk has occurred. Considering the physiologic and metabolic phenotypes observed with SCI, particularly across varying anatomic levels, understanding BAT function and impairment with SCI may help improve clinical practice for subjects with SCI while bringing clarity to a broader understanding of the metabolic significance of BAT in adult humans.
Figure.
Acknowledgments
We thank UAB Center for Clinical and Translational Science (1KL2TR001419, Yarar-Fisher) and the University of Alabama at Birmingham. The opinions expressed herein are those of the authors and do not necessarily reflect the official position of any of organization with which they are affiliated.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Daniel L. Smith, Jr. and Ceren Yarar-Fisher declare they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This review article does not contain any new, primary data of studies with human or animal subjects performed by the authors.
Reference List
- 1.Spinal Cord Injury (SCI) Facts and Figures at a Glance. J Spinal Cord Med. 2016;39:243–244. doi: 10.1080/10790268.2016.1160676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen MP, Molton IR, Groah SL, Campbell ML, Charlifue S, Chiodo A, Forchheimer M, Krause JS, Tate D. Secondary health conditions in individuals aging with SCI: terminology, concepts and analytic approaches. Spinal Cord. 2012;50:373–378. doi: 10.1038/sc.2011.150. [DOI] [PubMed] [Google Scholar]
- 3.Duckworth WC, Solomon SS, Jallepalli P, Heckemeyer C, Finnern J, Powers A. Glucose intolerance due to insulin resistance in patients with spinal cord injuries. Diabetes. 1980;29:906–910. doi: 10.2337/diab.29.11.906. [DOI] [PubMed] [Google Scholar]
- 4.Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism. 1994;43:749–756. doi: 10.1016/0026-0495(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 5.Bauman WA, Adkins RH, Spungen AM, Waters RL. The effect of residual neurological deficit on oral glucose tolerance in persons with chronic spinal cord injury. Spinal Cord. 1999;37:765–771. doi: 10.1038/sj.sc.3100893. [DOI] [PubMed] [Google Scholar]
- 6.Bauman WA, Spungen AM. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med. 2001;24:266–277. doi: 10.1080/10790268.2001.11753584. [DOI] [PubMed] [Google Scholar]
- 7.Bauman WA, Spungen AM. Coronary heart disease in individuals with spinal cord injury: assessment of risk factors. Spinal Cord. 2008;46:466–476. doi: 10.1038/sj.sc.3102161. [DOI] [PubMed] [Google Scholar]
- 8.Kressler J, Cowan RE, Bigford GE, Nash MS. Reducing cardiometabolic disease in spinal cord injury. Phys Med Rehabil Clin N Am. 2014;25:573–604. viii. doi: 10.1016/j.pmr.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism. 1994;43:749–756. doi: 10.1016/0026-0495(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 10.Banerjea R, Sambamoorthi U, Weaver F, Maney M, Pogach LM, Findley T. Risk of stroke, heart attack, and diabetes complications among veterans with spinal cord injury. Arch Phys Med Rehabil. 2008;89:1448–1453. doi: 10.1016/j.apmr.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 11.Bauman WA, Adkins RH, Spungen AM, Waters RL. The effect of residual neurological deficit on oral glucose tolerance in persons with chronic spinal cord injury. Spinal Cord. 1999;37:765–771. doi: 10.1038/sj.sc.3100893. [DOI] [PubMed] [Google Scholar]
- 12.Bluvshtein V, Korczyn AD, Pinhas I, Vered Y, Gelernter I, Catz A. Insulin resistance in tetraplegia but not in mid-thoracic paraplegia: is the mid-thoracic spinal cord involved in glucose regulation? Spinal Cord. 2011;49:648–652. doi: 10.1038/sc.2010.152. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson AK. Insulin resistance and sympathetic function in high spinal cord injury. Spinal Cord. 1999;37:494–500. doi: 10.1038/sj.sc.3100844. [DOI] [PubMed] [Google Scholar]
- 14.Buchholz AC, Pencharz PB. Energy expenditure in chronic spinal cord injury. Curr Opin Clin Nutr Metab Care. 2004;7:635–639. doi: 10.1097/00075197-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Raymond J, Harmer AR, Temesi J, van KC. Glucose tolerance and physical activity level in people with spinal cord injury. Spinal Cord. 2010;48:591–596. doi: 10.1038/sc.2009.180. [DOI] [PubMed] [Google Scholar]
- 16.Schmid A, Halle M, Stutzle C, Konig D, Baumstark MW, Storch MJ, Schmidt-Trucksass A, Lehmann M, Berg A, Keul J. Lipoproteins and free plasma catecholamines in spinal cord injured men with different injury levels. Clin Physiol. 2000;20:304–310. doi: 10.1046/j.1365-2281.2000.00263.x. [DOI] [PubMed] [Google Scholar]
- 17.Monroe MB, Tataranni PA, Pratley R, Manore MM, Skinner JS, Ravussin E. Lower daily energy expenditure as measured by a respiratory chamber in subjects with spinal cord injury compared with control subjects. Am J Clin Nutr. 1998;68:1223–1227. doi: 10.1093/ajcn/68.6.1223. [DOI] [PubMed] [Google Scholar]
- 18.Storch MJ, Konig D, Bultermann D, Blum A, Vogt S, Baumstark M, Berg A, Schmid A. Lipid profile in spinal cord-injured women with different injury levels. Prev Med. 2005;40:321–325. doi: 10.1016/j.ypmed.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86:142–152. doi: 10.1097/PHM.0b013e31802f0247. [DOI] [PubMed] [Google Scholar]
- 20.Karlsson AK. Insulin resistance and sympathetic function in high spinal cord injury. Spinal Cord. 1999;37:494–500. doi: 10.1038/sj.sc.3100844. [DOI] [PubMed] [Google Scholar]
- 21.Schmid A, Huonker M, Stahl F, Barturen JM, Konig D, Heim M, Lehmann M, Keul J. Free plasma catecholamines in spinal cord injured persons with different injury levels at rest and during exercise. J Auton Nerv Syst. 1998;68:96–100. doi: 10.1016/s0165-1838(97)00127-6. [DOI] [PubMed] [Google Scholar]
- 22.Menard MR, Hahn G. Acute and chronic hypothermia in a man with spinal cord injury: environmental and pharmacologic causes. Arch Phys Med Rehabil. 1991;72:421–424. [PubMed] [Google Scholar]
- 23.Colachis SC., III Hypothermia associated with autonomic dysreflexia after traumatic spinal cord injury. Am J Phys Med Rehabil. 2002;81:232–235. doi: 10.1097/00002060-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Khan S, Plummer M, Martinez-Arizala A, Banovac K. Hypothermia in patients with chronic spinal cord injury. J Spinal Cord Med. 2007;30:27–30. doi: 10.1080/10790268.2007.11753910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landsberg L, Saville ME, Young JB. Sympathoadrenal system and regulation of thermogenesis. Am J Physiol. 1984;247:E181–E189. doi: 10.1152/ajpendo.1984.247.2.E181. [DOI] [PubMed] [Google Scholar]
- 26.Duale H, Hou S, Derbenev AV, Smith BN, Rabchevsky AG. Spinal cord injury reduces the efficacy of pseudorabies virus labeling of sympathetic preganglionic neurons. J Neuropathol Exp Neurol. 2009;68:168–178. doi: 10.1097/NEN.0b013e3181967df7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucin KM, Sanders VM, Popovich PG. Stress hormones collaborate to induce lymphocyte apoptosis after high level spinal cord injury. J Neurochem. 2009;110:1409–1421. doi: 10.1111/j.1471-4159.2009.06232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucin KM, Sanders VM, Jones TB, Malarkey WB, Popovich PG. Impaired antibody synthesis after spinal cord injury is level dependent and is due to sympathetic nervous system dysregulation. Exp Neurol. 2007;207:75–84. doi: 10.1016/j.expneurol.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attia M, Engel P. Thermoregulatory set point in patients with spinal cord injuries (spinal man) Paraplegia. 1983;21:233–248. doi: 10.1038/sc.1983.37. [DOI] [PubMed] [Google Scholar]
- 30.Chu A, Burnham RS. Reliability and validity of tympanic temperature measurement in persons with high spinal cord injuries. Paraplegia. 1995;33:476–479. doi: 10.1038/sc.1995.104. [DOI] [PubMed] [Google Scholar]
- 31.Garstang SV, Miller-Smith SA. Autonomic nervous system dysfunction after spinal cord injury. Phys Med Rehabil Clin N Am. 2007;18:275-vii. doi: 10.1016/j.pmr.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Hagen EM, Rekand T, Gronning M, Faerestrand S. Cardiovascular complications of spinal cord injury. Tidsskr Nor Laegeforen. 2012;132:1115–1120. doi: 10.4045/tidsskr.11.0551. [DOI] [PubMed] [Google Scholar]
- 33.Khan S, Plummer M, Martinez-Arizala A, Banovac K. Hypothermia in patients with chronic spinal cord injury. J Spinal Cord Med. 2007;30:27–30. doi: 10.1080/10790268.2007.11753910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koeppen AH. Subnormal body temperatures in spinal cord injury. Neurology. 1985;35:1259–1260. doi: 10.1212/wnl.35.8.1259-c. [DOI] [PubMed] [Google Scholar]
- 35.Taylor RG. Spinal cord injury: its many complications. Am Fam Physician. 1973;8:138–146. [PubMed] [Google Scholar]
- 36.Thijssen DH, Eijsvogels TM, Hesse M, Ballak DB, Atkinson G, Hopman MT. The effects of thoracic and cervical spinal cord lesions on the circadian rhythm of core body temperature. Chronobiol Int. 2011;28:146–154. doi: 10.3109/07420528.2010.540364. [DOI] [PubMed] [Google Scholar]
- 37.Weaver LC, Fleming JC, Mathias CJ, Krassioukov AV. Disordered cardiovascular control after spinal cord injury. Handb Clin Neurol. 2012;109:213–233. doi: 10.1016/B978-0-444-52137-8.00013-9. [DOI] [PubMed] [Google Scholar]
- 38.Castro MJ, Apple DF, Jr, Staron RS, Campos GE, Dudley GA. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol (1985) 1999;86:350–358. doi: 10.1152/jappl.1999.86.1.350. [DOI] [PubMed] [Google Scholar]
- 39.Kocina P. Body composition of spinal cord injured adults. Sports Med. 1997;23:48–60. doi: 10.2165/00007256-199723010-00005. [DOI] [PubMed] [Google Scholar]
- 40.Spungen AM, Bauman WA, Wang J, Pierson RN., Jr Measurement of body fat in individuals with tetraplegia: a comparison of eight clinical methods. Paraplegia. 1995;33:402–408. doi: 10.1038/sc.1995.90. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, Cao Y, Allen V, Richards JS. Weight matters: physical and psychosocial well being of persons with spinal cord injury in relation to body mass index. Arch Phys Med Rehabil. 2011;92:391–398. doi: 10.1016/j.apmr.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 42.Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, Allison TG, Batsis JA, Sert-Kuniyoshi FH, Lopez-Jimenez F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 2008;32:959–966. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gater DR., Jr Obesity after spinal cord injury. Phys Med Rehabil Clin N Am. 2007;18:333–51. vii. doi: 10.1016/j.pmr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Gupta N, White KT, Sandford PR. Body mass index in spinal cord injury -- a retrospective study. Spinal Cord. 2006;44:92–94. doi: 10.1038/sj.sc.3101790. [DOI] [PubMed] [Google Scholar]
- 45.Yarar-Fisher C, Chen Y, Jackson AB, Hunter GR. Body mass index underestimates adiposity in women with spinal cord injury. Obesity (Silver Spring) 2013;21:1223–1225. doi: 10.1002/oby.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buchholz AC, Bugaresti JM. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord. 2005;43:513–518. doi: 10.1038/sj.sc.3101744. [DOI] [PubMed] [Google Scholar]
- 47.Laughton GE, Buchholz AC, Martin Ginis KA, Goy RE. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord. 2009;47:757–762. doi: 10.1038/sc.2009.33. [DOI] [PubMed] [Google Scholar]
- 48.Edwards LA, Bugaresti JM, Buchholz AC. Visceral adipose tissue and the ratio of visceral to subcutaneous adipose tissue are greater in adults with than in those without spinal cord injury, despite matching waist circumferences. Am J Clin Nutr. 2008;87:600–607. doi: 10.1093/ajcn/87.3.600. [DOI] [PubMed] [Google Scholar]
- 49.Gorgey AS, Wells KM, Austin TL. Adiposity and spinal cord injury. World J Orthop. 2015;6:567–576. doi: 10.5312/wjo.v6.i8.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu HH, Chen J, Shen W. Segmentation and quantification of adipose tissue by magnetic resonance imaging. MAGMA. 2015 doi: 10.1007/s10334-015-0498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Machann J, Horstmann A, Born M, Hesse S, Hirsch FW. Diagnostic imaging in obesity. Best Pract Res Clin Endocrinol Metab. 2013;27:261–277. doi: 10.1016/j.beem.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Castro MJ, Apple DF, Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol. 1999;80:373–378. doi: 10.1007/s004210050606. [DOI] [PubMed] [Google Scholar]
- 53.McCully KK, Mulcahy TK, Ryan TE, Zhao Q. Skeletal muscle metabolism in individuals with spinal cord injury. J Appl Physiol (1985) 2011;111:143–148. doi: 10.1152/japplphysiol.00094.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bickel CS, Yarar-Fisher C, Mahoney ET, McCully KK. Neuromuscular Electrical Stimulation-Induced Resistance Training After SCI: A Review of the Dudley Protocol. Top Spinal Cord Inj Rehabil. 2015;21:294–302. doi: 10.1310/sci2104-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes. 2001;50:150–158. doi: 10.2337/diabetes.50.1.150. [DOI] [PubMed] [Google Scholar]
- 56.Bickel CS, Slade JM, VanHiel LR, Warren GL, Dudley GA. Variable-frequency-train stimulation of skeletal muscle after spinal cord injury. J Rehabil Res Dev. 2004;41:33–40. doi: 10.1682/jrrd.2004.01.0033. [DOI] [PubMed] [Google Scholar]
- 57.Zierath JR, Krook A, Wallberg-Henriksson H. Insulin action in skeletal muscle from patients with NIDDM. Mol Cell Biochem. 1998;182:153–160. [PubMed] [Google Scholar]
- 58.Albers PH, Pedersen AJ, Birk JB, Kristensen DE, Vind BF, Baba O, Nohr J, Hojlund K, Wojtaszewski JF. Human muscle fiber type-specific insulin signaling: impact of obesity and type 2 diabetes. Diabetes. 2015;64:485–497. doi: 10.2337/db14-0590. [DOI] [PubMed] [Google Scholar]
- 59.Yarar-Fisher C, Bickel CS, Windham ST, McLain AB, Bamman MM. Skeletal muscle signaling associated with impaired glucose tolerance in spinal cord-injured men and the effects of contractile activity. J Appl Physiol (1985) 2013;115:756–764. doi: 10.1152/japplphysiol.00122.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinez JA, Fruhbeck G. Regulation of energy balance and adiposity: a model with new approaches. Rev Esp Fisiol. 1996;52:255–258. [PubMed] [Google Scholar]
- 61.Krassioukov AV, Karlsson AK, Wecht JM, Wuermser LA, Mathias CJ, Marino RJ. Assessment of autonomic dysfunction following spinal cord injury: rationale for additions to International Standards for Neurological Assessment. J Rehabil Res Dev. 2007;44:103–112. doi: 10.1682/jrrd.2005.10.0159. [DOI] [PubMed] [Google Scholar]
- 62.Alexander MS, Biering-Sorensen F, Bodner D, Brackett NL, Cardenas D, Charlifue S, Creasey G, Dietz V, Ditunno J, Donovan W, Elliott SL, Estores I, Graves DE, Green B, Gousse A, Jackson AB, Kennelly M, Karlsson AK, Krassioukov A, Krogh K, Linsenmeyer T, Marino R, Mathias CJ, Perkash I, Sheel AW, Schilero G, Schurch B, Sonksen J, Stiens S, Wecht J, Wuermser LA, Wyndaele JJ. International standards to document remaining autonomic function after spinal cord injury. Spinal Cord. 2009;47:36–43. doi: 10.1038/sc.2008.121. [DOI] [PubMed] [Google Scholar]
- 63.Wecht JM, La Fountaine MF, Handrakis JP, West CR, Phillips A, Ditor DS, Sharif H, Bauman WA, Krassioukov AV. Autonomic Nervous System Dysfunction Following Spinal Cord Injury: Cardiovascular, Cerebrovascular, and Thermoregulatory Effects. Curr Phys Med Rehabil Rep. 2015:197–205. [Google Scholar]
- 64.Cabanac M. Temperature regulation. Annu Rev Physiol. 1975;37:415–439. doi: 10.1146/annurev.ph.37.030175.002215. [DOI] [PubMed] [Google Scholar]
- 65.Yilmaz B, Yasar E, Goktepe S, Alaca R, Yazicioglu K, Dal U, Mohur H. Basal metabolic rate and autonomic nervous system dysfunction in men with spinal cord injury. Obesity (Silver Spring) 2007;15:2683–2687. doi: 10.1038/oby.2007.320. [DOI] [PubMed] [Google Scholar]
- 66.Jequier E, Schutz Y. Long-term measurements of energy expenditure in humans using a respiration chamber. Am J Clin Nutr. 1983;38:989–998. doi: 10.1093/ajcn/38.6.989. [DOI] [PubMed] [Google Scholar]
- 67.Raven PB, Wilkerson JE, Horvath SM, Bolduan NW. Thermal, metabolic, and cardiovascular responses to various degrees of cold stress. Can J Physiol Pharmacol. 1975;53:293–298. doi: 10.1139/y75-041. [DOI] [PubMed] [Google Scholar]
- 68.Dauncey MJ. Influence of mild cold on 24 h energy expenditure, resting metabolism and diet-induced thermogenesis. Br J Nutr. 1981;45:257–267. doi: 10.1079/bjn19810102. [DOI] [PubMed] [Google Scholar]
- 69.van Marken Lichtenbelt WD, Daanen HA. Cold-induced metabolism. Curr Opin Clin Nutr Metab Care. 2003;6:469–475. doi: 10.1097/01.mco.0000078992.96795.5f. [DOI] [PubMed] [Google Scholar]
- 70.van Ooijen AM, van Marken Lichtenbelt WD, van Steenhoven AA, Westerterp KR. Seasonal changes in metabolic and temperature responses to cold air in humans. Physiol Behav. 2004;82:545–553. doi: 10.1016/j.physbeh.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Wijers SL, Saris WH, van Marken Lichtenbelt WD. Individual thermogenic responses to mild cold and overfeeding are closely related. J Clin Endocrinol Metab. 2007;92:4299–4305. doi: 10.1210/jc.2007-1065. [DOI] [PubMed] [Google Scholar]
- 72.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 73.Steiner G, Loveland M, Schonbaum E. Effect of denervation on brown adipose tissue metabolism. Am J Physiol. 1970;218:566–570. doi: 10.1152/ajplegacy.1970.218.2.566. [DOI] [PubMed] [Google Scholar]
- 74.Mollinger LA, Spurr GB, el Ghatit AZ, Barboriak JJ, Rooney CB, Davidoff DD, Bongard RD. Daily energy expenditure and basal metabolic rates of patients with spinal cord injury. Arch Phys Med Rehabil. 1985;66:420–426. [PubMed] [Google Scholar]
- 75.Alexander LR, Spungen AM, Liu MH, Losada M, Bauman WA. Resting metabolic rate in subjects with paraplegia: the effect of pressure sores. Arch Phys Med Rehabil. 1995;76:819–822. doi: 10.1016/s0003-9993(95)80545-1. [DOI] [PubMed] [Google Scholar]
- 76.Jeon JY, Steadward RD, Wheeler GD, Bell G, McCargar L, Harber V. Intact sympathetic nervous system is required for leptin effects on resting metabolic rate in people with spinal cord injury. J Clin Endocrinol Metab. 2003;88:402–407. doi: 10.1210/jc.2002-020939. [DOI] [PubMed] [Google Scholar]
- 77.Bauman WA, Spungen AM, Wang J, Pierson RN., Jr The relationship between energy expenditure and lean tissue in monozygotic twins discordant for spinal cord injury. J Rehabil Res Dev. 2004;41:1–8. doi: 10.1682/jrrd.2004.01.0001. [DOI] [PubMed] [Google Scholar]
- 78.Buchholz AC, McGillivray CF, Pencharz PB. Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. Am J Clin Nutr. 2003;77:371–378. doi: 10.1093/ajcn/77.2.371. [DOI] [PubMed] [Google Scholar]
- 79.Monroe MB, Tataranni PA, Pratley R, Manore MM, Skinner JS, Ravussin E. Lower daily energy expenditure as measured by a respiratory chamber in subjects with spinal cord injury compared with control subjects. Am J Clin Nutr. 1998;68:1223–1227. doi: 10.1093/ajcn/68.6.1223. [DOI] [PubMed] [Google Scholar]
- 80.Aksnes AK, Brundin T, Hjeltnes N, Maehlum S, Wahren J. Meal-induced rise in resting energy expenditure in patients with complete cervical spinal cord lesions. Paraplegia. 1993;31:462–472. doi: 10.1038/sc.1993.75. [DOI] [PubMed] [Google Scholar]
- 81.Buchholz AC, Pencharz PB. Energy expenditure in chronic spinal cord injury. Curr Opin Clin Nutr Metab Care. 2004;7:635–639. doi: 10.1097/00075197-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 82.Nevin AN, Steenson J, Vivanti A, Hickman IJ. Investigation of measured and predicted resting energy needs in adults after spinal cord injury: a systematic review. Spinal Cord. 2016;54:248–253. doi: 10.1038/sc.2015.193. [DOI] [PubMed] [Google Scholar]
- 83.Song YG, Won YH, Park SH, Ko MH, Seo JH. Changes in Body Temperature in Incomplete Spinal Cord Injury by Digital Infrared Thermographic Imaging. Ann Rehabil Med. 2015;39:696–704. doi: 10.5535/arm.2015.39.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koeppen AH. Subnormal body temperatures in spinal cord injury. Neurology. 1985;35:1259–1260. doi: 10.1212/wnl.35.8.1259-c. [DOI] [PubMed] [Google Scholar]
- 85.Menard MR, Hahn G. Acute and chronic hypothermia in a man with spinal cord injury: environmental and pharmacologic causes. Arch Phys Med Rehabil. 1991;72:421–424. [PubMed] [Google Scholar]
- 86.Guttmann L, SILVER J, Wyndham CH. Thermoregulation in spinal man. J Physiol. 1958;142:406–419. doi: 10.1113/jphysiol.1958.sp006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Downey JA, Chiodi HP, Darling RC. Central temperature regulation in the spinal man. J Appl Physiol. 1967;22:91–94. doi: 10.1152/jappl.1967.22.1.91. [DOI] [PubMed] [Google Scholar]
- 88.Downey JA, Huckaba CE, Myers SJ, Darling RC. Thermoregulation in the spinal man. J Appl Physiol. 1973;34:790–794. doi: 10.1152/jappl.1973.34.6.790. [DOI] [PubMed] [Google Scholar]
- 89.Attia M, Engel P. Thermoregulatory set point in patients with spinal cord injuries (spinal man) Paraplegia. 1983;21:233–248. doi: 10.1038/sc.1983.37. [DOI] [PubMed] [Google Scholar]
- 90.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 91.Cinti S. The adipose organ. Prostaglandins Leukot Essent Fatty Acids. 2005;73:9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 92.Cinti S. The role of brown adipose tissue in human obesity. Nutr Metab Cardiovasc Dis. 2006;16:569–574. doi: 10.1016/j.numecd.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 93.Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes (Lond) 2010;34(Suppl 1):S36–S42. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Foster DO, Depocas F, Zaror-Behrens G. Unilaterality of the sympathetic innervation of each pad of rat interscapular brown adipose tissue. Can J Physiol Pharmacol. 1982;60:107–113. doi: 10.1139/y82-018. [DOI] [PubMed] [Google Scholar]
- 95.Dulloo AG, Miller DS. Energy balance following sympathetic denervation of brown adipose tissue. Can J Physiol Pharmacol. 1984;62:235–240. doi: 10.1139/y84-035. [DOI] [PubMed] [Google Scholar]
- 96.Bartness TJ, Wade GN. Effects of interscapular brown adipose tissue denervation on body weight and energy metabolism in ovariectomized and estradiol-treated rats. Behav Neurosci. 1984;98:674–685. doi: 10.1037//0735-7044.98.4.674. [DOI] [PubMed] [Google Scholar]
- 97.Nedergaard J, Bengtsson T, Cannon B. Three years with adult human brown adipose tissue. Ann NY Acad Sci. 2010;1212:E20–E36. doi: 10.1111/j.1749-6632.2010.05905.x. [DOI] [PubMed] [Google Scholar]
- 98.Cannon B, Nedergaard J. Yes, even human brown fat is on fire! J Clin Invest. 2012;122:486–489. doi: 10.1172/JCI60941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cohade C, Mourtzikos KA, Wahl RL. “USA-Fat”: prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. J Nucl Med. 2003;44:1267–1270. [PubMed] [Google Scholar]
- 100.Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J Nucl Med. 2003;44:170–176. [PubMed] [Google Scholar]
- 101.Lee P, Greenfield JR, Ho KK, Fulham MJ. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2010;299:E601–E606. doi: 10.1152/ajpendo.00298.2010. [DOI] [PubMed] [Google Scholar]
- 102.Richard D, Monge-Roffarello B, Chechi K, Labbe SM, Turcotte EE. Control and physiological determinants of sympathetically mediated brown adipose tissue thermogenesis. Front Endocrinol(Lausanne) 2012;3:36. doi: 10.3389/fendo.2012.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carey AL, Formosa MF, Van EB, Bertovic D, Eikelis N, Lambert GW, Kalff V, Duffy SJ, Cherk MH, Kingwell BA. Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia. 2013;56:147–155. doi: 10.1007/s00125-012-2748-1. [DOI] [PubMed] [Google Scholar]
- 104.Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J, Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–3120. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- 105.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 106.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 107.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972;112:35–39. [PMC free article] [PubMed] [Google Scholar]
- 109.Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab. 2010;11:268–272. doi: 10.1016/j.cmet.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 110.Nedergaard J, Bengtsson T, Cannon B. New powers of brown fat: fighting the metabolic syndrome. Cell Metab. 2011;13:238–240. doi: 10.1016/j.cmet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 111.Landsberg L, Krieger DR. Obesity, metabolism, and the sympathetic nervous system. Am J Hypertens. 1989;2:125S–132S. doi: 10.1093/ajh/2.3.125s. [DOI] [PubMed] [Google Scholar]
- 112.Orava J, Nuutila P, Noponen T, Parkkola R, Viljanen T, Enerback S, Rissanen A, Pietilainen KH, Virtanen KA. Blunted Metabolic Responses to Cold and Insulin Stimulation in Brown Adipose Tissue of Obese Humans. Obesity(Silver Spring) 2013 doi: 10.1002/oby.20456. [DOI] [PubMed] [Google Scholar]
- 113.Vijgen GH, Bouvy ND, Teule GJ, Brans B, Schrauwen P, van Marken Lichtenbelt WD. Brown adipose tissue in morbidly obese subjects. PLoS One. 2011;6:e17247. doi: 10.1371/journal.pone.0017247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Admiraal WM, Holleman F, Bahler L, Soeters MR, Hoekstra JB, Verberne HJ. Combining 123I-metaiodobenzylguanidine SPECT/CT and 18F-FDG PET/CT for the assessment of brown adipose tissue activity in humans during cold exposure. J Nucl Med. 2013;54:208–212. doi: 10.2967/jnumed.112.111849. [DOI] [PubMed] [Google Scholar]
- 115.Blondin DP, Labbe SM, Christian TH, Noll C, Kunach M, Phoenix S, Guerin B, Turcotte EE, Carpentier AC, Richard D, Haman F. Increased brown adipose tissue oxidative capacity in cold-acclimated humans. J Clin Endocrinol Metab. 2014:jc20133901. doi: 10.1210/jc.2013-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen KY, Brychta RJ, Linderman JD, Smith S, Courville A, Dieckmann W, Herscovitch P, Millo CM, Remaley A, Lee P, Celi FS. Brown fat activation mediates cold-induced thermogenesis in adult humans in response to a mild decrease in ambient temperature. J Clin Endocrinol Metab. 2013;98:E1218–E1223. doi: 10.1210/jc.2012-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cypess AM, Chen YC, Sze C, Wang K, English J, Chan O, Holman AR, Tal I, Palmer MR, Kolodny GM, Kahn CR. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci USA. 2012;109:10001–10005. doi: 10.1073/pnas.1207911109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gilsanz V, Smith ML, Goodarzian F, Kim M, Wren TA, Hu HH. Changes in brown adipose tissue in boys and girls during childhood and puberty. J Pediatr. 2012;160:604–609. doi: 10.1016/j.jpeds.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hadi M, Chen CC, Whatley M, Pacak K, Carrasquillo JA. Brown fat imaging with (18)F-6-fluorodopamine PET/CT, (18)F-FDG PET/CT, and (123)I-MIBG SPECT: a study of patients being evaluated for pheochromocytoma. J Nucl Med. 2007;48:1077–1083. doi: 10.2967/jnumed.106.035915. [DOI] [PubMed] [Google Scholar]
- 120.Matsushita M, Yoneshiro T, Aita S, Kameya T, Sugie H, Saito M. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2013.206. [DOI] [PubMed] [Google Scholar]
- 121.Nedergaard J, Bengtsson T, Cannon B. Three years with adult human brown adipose tissue. Ann NY Acad Sci. 2010;1212:E20–E36. doi: 10.1111/j.1749-6632.2010.05905.x. [DOI] [PubMed] [Google Scholar]
- 122.Nuutila P. Brown adipose tissue thermogenesis in humans. Diabetologia. 2013;56:2110–2112. doi: 10.1007/s00125-013-3005-y. [DOI] [PubMed] [Google Scholar]
- 123.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.van Marken LW. Brown adipose tissue and the regulation of nonshivering thermogenesis. Curr Opin Clin Nutr Metab Care. 2012;15:547–552. doi: 10.1097/MCO.0b013e3283599184. [DOI] [PubMed] [Google Scholar]
- 125.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 126.van Rooijen BD, van der Lans AA, Brans B, Wildberger JE, Mottaghy FM, Schrauwen P, Backes WH, van Marken Lichtenbelt WD. Imaging cold-activated brown adipose tissue using dynamic T2*-weighted magnetic resonance imaging and 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography. Invest Radiol. 2013;48:708–714. doi: 10.1097/RLI.0b013e31829363b8. [DOI] [PubMed] [Google Scholar]
- 127.Vijgen GH, Bouvy ND, Teule GJ, Brans B, Hoeks J, Schrauwen P, van Marken Lichtenbelt WD. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2012;97:E1229–E1233. doi: 10.1210/jc.2012-1289. [DOI] [PubMed] [Google Scholar]
- 128.Virtanen KA, van Marken Lichtenbelt WD, Nuutila P. Brown adipose tissue functions in humans. Biochim Biophys Acta. 2013;1831:1004–1008. doi: 10.1016/j.bbalip.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 129.Yoneshiro T, Aita S, Matsushita M, Kameya T, Nakada K, Kawai Y, Saito M. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring) 2011;19:13–16. doi: 10.1038/oby.2010.105. [DOI] [PubMed] [Google Scholar]
- 130.Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y, Miyagawa M, Tsujisaki M, Saito M. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring) 2011;19:1755–1760. doi: 10.1038/oby.2011.125. [DOI] [PubMed] [Google Scholar]
- 131.Trayhurn P. Brown Adipose-Tissue - from Thermal Physiology to Bioenergetics. Journal of Biosciences. 1993;18:161–173. [Google Scholar]
- 132.Cannon B, Nedergaard J. Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans) Int J Obes(Lond) 2010;34(Suppl 1):S7–16. doi: 10.1038/ijo.2010.177. [DOI] [PubMed] [Google Scholar]
- 133.Bakker LE, Boon MR, van der Linden RA, Arias-Bouda LP, van Klinken JB, Smit F, Verberne HJ, Jukema JW, Tamsma JT, Havekes LM, van Marken Lichtenbelt WD, Jazet IM, Rensen PC. Brown adipose tissue volume in healthy lean south Asian adults compared with white Caucasians: a prospective, case-controlled observational study. Lancet Diabetes Endocrinol. 2014;2:210–217. doi: 10.1016/S2213-8587(13)70156-6. [DOI] [PubMed] [Google Scholar]
- 134.Hanssen MJ, Wierts R, Hoeks J, Gemmink A, Brans B, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD. Glucose uptake in human brown adipose tissue is impaired upon fasting-induced insulin resistance. Diabetologia. 2015;58:586–595. doi: 10.1007/s00125-014-3465-8. [DOI] [PubMed] [Google Scholar]
- 135.Raiko J, Holstila M, Virtanen KA, Orava J, Saunavaara V, Niemi T, Laine J, Taittonen M, Borra RJ, Nuutila P, Parkkola R. Brown adipose tissue triglyceride content is associated with decreased insulin sensitivity, independently of age and obesity. Diabetes Obes Metab. 2015;17:516–519. doi: 10.1111/dom.12433. [DOI] [PubMed] [Google Scholar]
- 136.Zhang Q, Ye H, Miao Q, Zhang Z, Wang Y, Zhu X, Zhang S, Zuo C, Zhang Z, Huang Z, Xue R, Zeng M, Huang H, Jin W, Tang Q, Guan Y, Li Y. Differences in the metabolic status of healthy adults with and without active brown adipose tissue. Wien Klin Wochenschr. 2013;125:687–695. doi: 10.1007/s00508-013-0431-2. [DOI] [PubMed] [Google Scholar]
- 137.Lee P, Smith S, Linderman J, Courville AB, Brychta RJ, Dieckmann W, Werner CD, Chen KY, Celi FS. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes. 2014;63:3686–3698. doi: 10.2337/db14-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bray GA. Obesity, a disorder of nutrient partitioning: the MONA LISA hypothesis. J Nutr. 1991;121:1146–1162. doi: 10.1093/jn/121.8.1146. [DOI] [PubMed] [Google Scholar]
- 139.Bray GA. Endocrine disturbances and reduced sympathetic activity in the development of obesity. Infusionstherapie. 1990;17:124–130. doi: 10.1159/000222462. [DOI] [PubMed] [Google Scholar]
- 140.Bray GA. Obesity--a state of reduced sympathetic activity and normal or high adrenal activity (the autonomic and adrenal hypothesis revisited) Int J Obes. 1990;14(Suppl 3):77–91. [PubMed] [Google Scholar]
- 141.Astrup A, Andersen T, Christensen NJ, Bulow J, Madsen J, Breum L, Quaade F. Impaired glucose-induced thermogenesis and arterial norepinephrine response persist after weight reduction in obese humans. Am J Clin Nutr. 1990;51:331–337. doi: 10.1093/ajcn/51.3.331. [DOI] [PubMed] [Google Scholar]
- 142.Astrup A, Buemann B, Christensen NJ, Madsen J. 24-hour energy expenditure and sympathetic activity in postobese women consuming a high-carbohydrate diet. Am J Physiol. 1992;262:E282–E288. doi: 10.1152/ajpendo.1992.262.3.E282. [DOI] [PubMed] [Google Scholar]
- 143.Astrup AV, Christensen NJ, Breum L. Reduced plasma noradrenaline concentrations in simple-obese and diabetic obese patients. Clin Sci (Lond) 1991;80:53–58. doi: 10.1042/cs0800053. [DOI] [PubMed] [Google Scholar]
- 144.Bessard T, Schutz Y, Jequier E. Energy expenditure and postprandial thermogenesis in obese women before and after weight loss. Am J Clin Nutr. 1983;38:680–693. doi: 10.1093/ajcn/38.5.680. [DOI] [PubMed] [Google Scholar]
- 145.Jequier E, Schutz Y. New evidence for a thermogenic defect in human obesity. Int J Obes. 1985;9(Suppl 2):1–7. [PubMed] [Google Scholar]
- 146.Jequier E. Carbohydrate-induced thermogenesis in man. Int J Vitam Nutr Res. 1986;56:193–196. [PubMed] [Google Scholar]
- 147.Jequier E. Energy expenditure in obesity. Clin Endocrinol Metab. 1984;13:563–580. doi: 10.1016/s0300-595x(84)80038-9. [DOI] [PubMed] [Google Scholar]
- 148.Jequier E, Munger R, Felber JP. Thermogenic effects of various beta-adrenoceptor agonists in humans: their potential usefulness in the treatment of obesity. Am J Clin Nutr. 1992;55:249S–251S. doi: 10.1093/ajcn/55.1.249s. [DOI] [PubMed] [Google Scholar]
- 149.Schutz Y, Bessard T, Jequier E. Diet-induced thermogenesis measured over a whole day in obese and nonobese women. Am J Clin Nutr. 1984;40:542–552. doi: 10.1093/ajcn/40.3.542. [DOI] [PubMed] [Google Scholar]
- 150.Kush RD, Young JB, Katzeff HL, Danforth E, Jr, Garrow JS, Scheidegger K, Ravussin E, Howard BV, Sims EA, Horton ES. Effect of diet on energy expenditure and plasma norepinephrine in lean and obese Pima Indians. Metabolism. 1986;35:1110–1120. doi: 10.1016/0026-0495(86)90024-7. [DOI] [PubMed] [Google Scholar]
- 151.Lean ME, James WP, Jennings G, Trayhurn P. Brown adipose tissue in patients with phaeochromocytoma. Int J Obes. 1986;10:219–227. [PubMed] [Google Scholar]
- 152.Fukuchi K, Tatsumi M, Ishida Y, Oku N, Hatazawa J, Wahl RL. Radionuclide imaging metabolic activity of brown adipose tissue in a patient with pheochromocytoma. Exp Clin Endocrinol Diabetes. 2004;112:601–603. doi: 10.1055/s-2004-830407. [DOI] [PubMed] [Google Scholar]
- 153.Dundamadappa SK, Shankar S, Danrad R, Singh A, Vijayaraghavan G, Kim Y, Perugini R. Imaging of brown fat associated with adrenal pheochromocytoma. 2007;48:468–472. doi: 10.1080/02841850701199918. [DOI] [PubMed] [Google Scholar]
- 154.Yamaga LY, Thom AF, Wagner J, Baroni RH, Hidal JT, Funari MG. The effect of catecholamines on the glucose uptake in brown adipose tissue demonstrated by (18)F-FDG PET/CT in a patient with adrenal pheochromocytoma. Eur J Nucl Med Mol Imaging. 2008;35:446–447. doi: 10.1007/s00259-007-0538-7. [DOI] [PubMed] [Google Scholar]
- 155.Kuji I, Imabayashi E, Minagawa A, Matsuda H, Miyauchi T. Brown adipose tissue demonstrating intense FDG uptake in a patient with mediastinal pheochromocytoma. Ann Nucl Med. 2008;22:231–235. doi: 10.1007/s12149-007-0096-x. [DOI] [PubMed] [Google Scholar]
- 156.Sekizawa N, Yoshimoto T, Izumiyama H, Hirata Y. Distinct uptake of 18F-fluorodeoxyglucose by brown adipose tissue with a catecholamine-secreting tumor. Intern Med. 2010;49:2363. doi: 10.2169/internalmedicine.49.4293. [DOI] [PubMed] [Google Scholar]
- 157.Wang Q, Zhang M, Ning G, Gu W, Su T, Xu M, Li B, Wang W. Brown adipose tissue in humans is activated by elevated plasma catecholamines levels and is inversely related to central obesity. PLoS One. 2011;6:e21006. doi: 10.1371/journal.pone.0021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wallin BG, Stjernberg L. Sympathetic activity in man after spinal cord injury. Outflow to skin below the lesion. Brain. 1984;107( Pt 1):183–198. doi: 10.1093/brain/107.1.183. [DOI] [PubMed] [Google Scholar]
- 159.Karlsson AK, Attvall S, Jansson PA, Sullivan L, Lonnroth P. Influence of the sympathetic nervous system on insulin sensitivity and adipose tissue metabolism: a study in spinal cord-injured subjects. Metabolism. 1995;44:52–58. doi: 10.1016/0026-0495(95)90289-9. [DOI] [PubMed] [Google Scholar]
- 160.Astrup A. Thermogenesis in human brown adipose tissue and skeletal muscle induced by sympathomimetic stimulation. Acta Endocrinol Suppl (Copenh) 1986;278:1–32. [PubMed] [Google Scholar]
- 161.van Marken Lichtenbelt WD, Schrauwen P. Implications of nonshivering thermogenesis for energy balance regulation in humans. Am J Physiol Regul Integr Comp Physiol. 2011;301:R285–R296. doi: 10.1152/ajpregu.00652.2010. [DOI] [PubMed] [Google Scholar]
- 162.Jequier E. Thermogenesis induced by nutrients in man: its role in weight regulation. J Physiol (Paris) 1985;80:129–140. [PubMed] [Google Scholar]
- 163.Smith DL, Jr, Yang Y, Hu HH, Zhai G, Nagy TR. Measurement of interscapular brown adipose tissue of mice in differentially housed temperatures by chemical-shift-encoded water-fat MRI. J Magn Reson Imaging. 2013;38:1425–1433. doi: 10.1002/jmri.24138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Primeaux SD, Tong M, Holmes GM. Effects of chronic spinal cord injury on body weight and body composition in rats fed a standard chow diet. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1102–R1109. doi: 10.1152/ajpregu.00224.2007. [DOI] [PubMed] [Google Scholar]


