Abstract
Background
There is limited information on rates of STIs in Jamaica due to syndromic management and limited aetiological surveillance. We examined the prevalence of Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG) and Trichomonas vaginalis (TV) and characteristics associated with STIs among sexually active women who participated in a randomised trial of a progestin implant initiation in Jamaica (the Sino-Implant Study (SIS)).
Methods
SIS was a randomised trial conducted in Kingston, Jamaica, from 2012 to 2014 to evaluate whether initiation of the Sino-Implant (II) led to more unprotected sex among women ages 18–44 years. Data collected included self-reported demographic, sexual behaviour information; and vaginal swabs collected at baseline, 1-month and 3-month follow-up visits for a biomarker of recent semen exposure (prostate-specific antigen (PSA)) and for STIs. We examined associations between STIs and PSA, demographics, sexual behaviour and insertion of an implant, with a repeated-measures analysis using generalised estimating equations (SAS Institute, V.9.3).
Results
Remnant vaginal swabs from 254 of 414 study participants were tested for STIs. At baseline, 29% of participants tested for STIs (n=247) had laboratory-confirmed CT, 5% NG, 23% TV and 45% any STI. In a repeated-measures analysis adjusted for study arm (immediate vs delayed implant insertion), those with PSA detected did not have an increased prevalence of any STI (prevalence ratio (PR)=1.04 (95% CI 0.89 to 1.21)), whereas prevalence decreased for each 1-year increase in age (PR=0.98 (95% CI 0.97 to 0.99)). Immediate implant insertion was not associated with increases in any STI in subsequent visits (PR=1.09 (95% CI 0.94 to 1.27)).
Conclusions
Although the prevalence of laboratory-confirmed STIs was high, the immediate initiation of a contraceptive implant was not associated with higher STI prevalence rates over 3 months.
Trial registration number
INTRODUCTION
STIs are a major public health problem worldwide. The WHO estimates that there were approximately 125 million new cases of chlamydia, gonorrhoea, syphilis and trichomoniasis combined in 2008 in the region of the Americas.1–4 In Jamaica, the STI rates are highest in sexually active adolescents, with at least one in four having had an STI.3 These infections are often asymptomatic and could lead to serious complications if left untreated.
Correct and consistent use of condoms reduces STI risk.5,6 Self-reported condom use can be, however, affected by measurement error5 and several types of bias including recall bias and social desirability bias.6–10 In addition, incorrect use of condoms or a condom breakage unbeknownst to the user can lead to inadvertent unprotected sexual exposure and an incorrect self-report of condom use.11 Demonstrating an association between biomarkers of semen exposure and STI risk would be useful, given limitations of self-report of sensitive sexual behaviours.5,8–17 Semen biomarkers have been recently used in reproductive health studies to objectively assess recent vaginal semen exposure,6–16 the efficacy of barrier methods of contraception,11,18,19 self-reported condom use14,15,20,21 and study protocol compliance (when it requires avoidance of unprotected sex).8,21,22 Both Y chromosome–DNA and prostate-specific antigen (PSA) are biomarkers of semen exposure,6,7,11,19,23 with PSA more widely studied and readily available for use in low-resource settings.8–10,19–21,24 PSA is detectable in vaginal fluids for up to 48 hours after vaginal exposure to semen.11,19 Further evaluation of biomarkers as objective indicators of semen exposure and STI prevalence and incidence is needed.
There is currently only limited information about the correlation of semen biomarkers with STI prevalence and incidence7,10,12,21 or pregnancy outcomes.13 There is also no information with regard to initiation of long-acting reversible contraceptives (LARCs) and their effects on STI for sexually active Jamaican women. For this study, PSA, used as a bio-marker of semen exposure, was evaluated for association with laboratory-confirmed STI results (Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG) and Trichomonas vaginalis (TV)) among women enrolled in Sino-Implant Study (SIS) (a randomised trial)25 of early versus delayed contraceptive implant initiation (LARC) in Kingston, Jamaica. We examined the prevalence and risk factors associated with STIs in this population.
MATERIALS AND METHODS
The results of SIS have previously been described.25 Briefly, SIS was a randomised controlled trial conducted in Jamaica that evaluated whether the initiation of a LARC method, the Sino-Implant (II), is associated with reduced condom use. Women were randomised to receive the Sino-Implant (II) at the beginning (immediate insertion arm, N=208) or at the end (delayed insertion arm, N=206), of a 3-month study period. Vaginal swabs for PSA and STI testing were collected at baseline, and at 1-month and 3-month follow-up study visits. Study participants were recruited through peer-to-peer referrals or from seven maternal and child health and family planning public clinics in Kingston, Jamaica, from September 2012 to October 2013 with follow-up until January 2014.
PSA testing was conducted onsite using published procedures for the ABAcard 30 (Abacus Diagnostics, West Hills, California, USA), a rapid, semiquantitative test kit. The vaginal swab eluate was added to the sample well of the test card, and at 10 min, the results were recorded by local study staff, according to the manufacturer’s instructions. Quality assurance testing was conducted using a quantitative total PSA assay (Abbott Diagnostics, Abbott Park, Illinois, USA) at the US Centers for Disease Control and Prevention.
All participants provided vaginal swab specimens for the study; if swab specimens were not used completely for the primary analysis, they were considered remnant and further evaluated for additional testing, such as for STI. Remnant specimens were available for STI testing from 254 of the 414 (61%) study participants. STI testing was conducted using the Aptima Combo 2 assay for CT/NG and the Aptima Trichomonas vaginalis assay with the Panther system (Hologic, San Diego, California USA) at the US Centers for Disease Control and Prevention. Of the 254 participants, 178 had results available for all three study visits.
Statistical methods
Baseline characteristics for those participants with samples available for STI testing at any study visit were described as medians with an IQR for continuous variables and percentages for categorical variables. Positive results for lab-confirmed CT, NG and TV were combined into a single ‘any STI’ outcome for analysis. Associations between ‘any STI’ at all three study visits and demographic and behavioural characteristics were assessed by calculating prevalence ratios (PRs) adjusted for study arm using repeated measurements models fit by generalised estimating equations. The effect of study arm on STIs was assessed using a similar model, using only results from the two follow-up study visits and adjusting for the baseline STI outcome. Baseline characteristics were compared between study participants with and without specimens available for STI testing using χ2 tests and t-tests, to ensure lack of specimen availability did not introduce bias into the analysis. All analyses were conducted using SAS V.9.3 (SAS Institute).
Ethical review
The Consolidated Standards of Reporting Trials guidelines for conducting and reporting randomised controlled trials were followed.24
RESULTS
Baseline characteristics of the 254 participants with swabs available for STI testing from at least one study visit are shown in table 1. The tested participants did not differ in their characteristics between the two study arms. Most participants were single and had completed high school. About a quarter in each study arm reported that they used hormonal contraception in the past month (table 1). A small proportion of participants reported that they drank four or more drinks in a day (4% in each study arm), ever had transactional sex (5% in the immediate insertion arm; 6% in the delayed insertion arm) or had more than one partner in the past month (7% in the immediate insertion arm; 5% in the delayed insertion arm). At enrolment, unprotected sex in the past two days was reported by 17% of participants in the immediate insertion arm and 15% of participants in the delayed insertion arm, while PSA was detected in 28% of participants in the immediate insertion arm and 23% of participants in the delayed insertion arm. There were no differences found in any baseline participant characteristics between those who had remnant swabs available for STI testing and those who did not.
Table 1.
Baseline characteristics of Sino-Implant Study participants tested for STIs at one or more study visits (N=254) by study arm, Kingston, Jamaica
| Immediate insertion arm (N=123) n (%) |
Delayed insertion arm (N=131) n (%) |
|
|---|---|---|
| Single, never married | 79 (64.2) | 98 (74.8) |
| Did not complete high school | 41 (33.3) | 33 (25.2) |
| Hormonal contraception in the past month | 29 (23.6) | 36 (27.5) |
| More than one partner in the past month | 8 (6.5) | 7 (5.3) |
| Four or more alcoholic drinks in 1 day in the past week | 4 (3.6) | 5 (4.2) |
| Ever received money or gifts in exchange for sex | 6 (4.9) | 8 (6.1) |
| Positive prostate-specific antigen test | 34 (27.6) | 30 (22.9) |
| Self-reported unprotected sex in the past two days | 21 (17.1) | 19 (14.5) |
|
| ||
| Median (IQR) | Median (IQR) | |
|
| ||
| Age (years) | 25 (21–29) | 25 (21–30) |
| Parity | 2 (1–3) | 2 (1–3) |
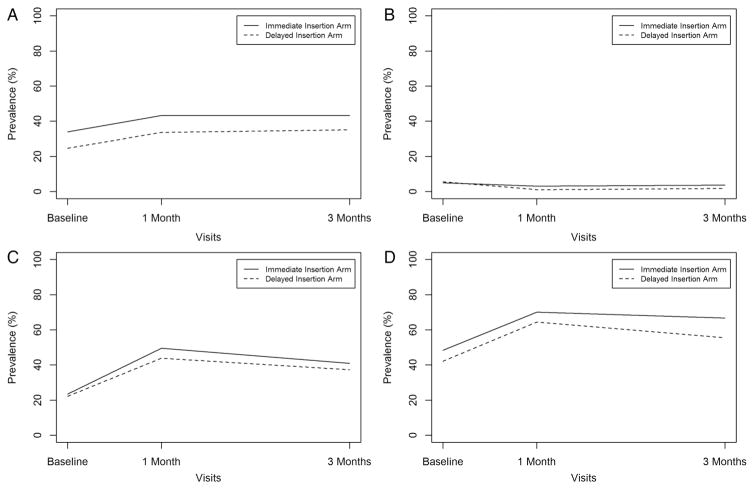
At baseline, 34% of the participants had laboratory-confirmed CT, 5% NG, 24% TV and 48% any STI in the immediate insertion arm, and 25% of the participants had laboratory-confirmed CT, 6% NG, 22% TV and 42% any STI in the delayed insertion arm. At the 3-month study visit, 43% had CT, 4% NG, 41% TV and 67% any STI in the immediate insertion arm, and 35% had CT, 2% NG, 37% TV and 56% any STI in the delayed insertion arm (figure 1).
Figure 1.
Prevalence of test results for (A) Chlamydia trachomatis, (B) Neisseria gonorrhoeae, (C) Trichomonas vaginalis and (D) any STI by study visit and study arm, Sino-Implant Study, Kingston, Jamaica. In total, 254 participants had a swab available for testing for at least one of the three study visits.
PSA detection was not associated with increased prevalence of an STI (PR=1.04 (95% CI 0.89 to 1.21)). The prevalence of an STI was higher for those who self-reported unprotected sex in the past two days (PR=1.16 (95% CI 0.99 to 1.36)), those who reported being single (PR=1.20 (95% CI 0.99 to 1.46)) and those who reported hormonal contraception use in the past month (PR=1.18 (95% CI 0.99 to 1.40)). Prevalence of STI decreased for each 1-year increase in age (PR=0.98 (95% CI 0.97 to 0.99)) (table 2). Randomisation to immediate implant insertion (compared with delayed insertion) was not associated with an increase in prevalence of an STI during follow-up visits (PR=1.09 (95% CI 0.94 to 1.27)), adjusted for baseline STI result.
Table 2.
Associations between participant characteristics and any STI, the Sino-Implant Study, Kingston, Jamaica
| Prevalence ratio* | 95% CI | |
|---|---|---|
| Positive prostate-specific antigen test | 1.04 | 0.89 to 1.21 |
| Self-reported unprotected sex in the past two days | 1.16 | 0.99 to 1.36 |
| Age (per increase of 1 year) | 0.98 | 0.97 to 0.99 |
| Parity (per increase of 1) | 0.97 | 0.91 to 1.05 |
| Single, never married | 1.20 | 0.99 to 1.46 |
| Did not complete high school | 1.10 | 0.93 to 1.30 |
| Four or more alcoholic drinks in 1 day in the past week | 1.14 | 0.77 to 1.69 |
| Ever received money or gifts in exchange for sex | 0.96 | 0.68 to 1.36 |
| Hormonal contraception in the past month | 1.18 | 0.99 to 1.40 |
| More than one partner in the past month | 1.05 | 0.80 to 1.37 |
Adjusted for study arm.
DISCUSSION
Although the prevalence of laboratory-confirmed STIs was high in this population of sexually active women in Kingston, Jamaica, the immediate initiation of a hormonal contraceptive implant (a LARC) did not lead to higher STI rates over 3 months. This finding is similar to that of the primary SIS study findings, which found that unprotected sex, as measured by detection of PSA in vaginal secretions, did not increase over the first three months of implant use.25 Longer follow-up studies would be needed to confirm this finding, but it nevertheless is reassuring. As efforts to promote use of LARC for prevention of unintended pregnancy are being scaled up, it will be important to monitor rates of STIs, identify markers of higher risk and target STI prevention efforts accordingly.
Our findings seem to be in contrast to a recent cross-sectional study of US high school students, which found that condom use was less likely among LARC users compared with the students who were taking oral contraceptives.26 Our study is notably different; study participants were adults seeking a LARC and Jamaican, we used a longitudinal design and we also used bio-markers such as laboratory confirmation of STIs and a marker of vaginal semen exposure rather than exclusive reliance on self-report of condom use.
Younger age was associated with increased prevalence of an STI, as has been previously reported.1–4,6 The high prevalence of STIs in this population corroborates the high rate of unprotected sex, both self-reported and detected by PSA, in this study. It also confirms previous indications of high rates of STIs and unmet contraceptive needs in Jamaica: >85% of national survey respondents who were sexually active reported not using any barrier method (ie, condoms) to prevent STIs,3 and >3 million adolescents in Latin America and the Caribbean have unmet contraception needs.2 Our own study found that only 25% of respondents used hormonal contraception in the past month. Although there are not recent country-specific rates of STIs for Jamaica, the high rates among study participants indicate a pressing need for surveillance, risk reduction strategies and the promotion of dual protection.
Even though significant progress has been made with the use of biomarkers of semen exposure in reproductive and sexual health studies, there are still many unanswered questions about their utility. In particular, very limited information is available about whether their detection correlates with outcomes such as pregnancy or STI acquisition. A study which examined the accuracy of self-reported sexual behaviour assessed by detection of Y chromosome found that under-reporting semen exposure (positive Y chromosome) predicted pregnancy in 6 months but not 12 months longitudinally in a cohort of adolescents.13 Two previous studies addressed STIs: a cross-sectional study of 1166 women in West Africa showed a strong association between detected PSA in vaginal secretions and the presence of NG, CT and Mycoplasma genitalium among female non-sex workers.21 A recent study linked PSA positivity to increased recurrence of bacterial vaginosis (BV), while notably finding no significant association between self-reported unprotected sex and recurrent BV.12
In our study, neither positive biomarker (PSA) nor self-reported unprotected sex in the past two days was associated with higher prevalence of STIs. Using the observed rate of any STI, this analysis had adequate power (with 80% power to detect a minimum absolute difference in STI rate of 11.2% between those testing positive for PSA and those testing negative for PSA). One of the limitations of the PSA assay is the short 2-day time frame during which PSA is detectable after semen exposure.12,19 This short window of time around the clinic visits may not be reflective of the behaviours or exposures of the participants throughout the entire study period for SIS. It will be valuable to further investigate semen biomarkers, particularly those with longer window of detection after unprotected exposure(s), and determine whether the detection correlates with STIs. Additionally, we used a rapid qualitative PSA assay, and quality assurance testing indicated that its sensitivity was 84% and specificity 81% compared with the ARCHITECT quantitative assay,25 which may also account for our findings.
The research regarding LARCs and decreased condom use is limited and has not been consistently demonstrated.25,26 Our study adds to the existing literature by specifically evaluating LARC users and their rates of STIs for SIS study participants. Although the prevalence of laboratory-confirmed STIs was high among study participants, the immediate initiation of a contraceptive implant was not associated with higher STI rates over 3 months. However, we observed exceptionally high rates of STIs in our study population. Efforts to meet unmet contraceptive needs must be coupled with enhanced STI risk reduction strategies for sexually active Jamaican women.
Key messages.
Some women who use LARCs may not use condoms or use condoms less frequently, which may increase their risk for STIs.
STI rates were high overall for the Jamaican study participants seeking a contraceptive implant.
The insertion of a contraceptive implant did not lead to higher STI prevalence rates in the short term (3 months).
STI risk reduction strategies should be considered along with the contraceptive needs of sexually active Jamaican women.
Acknowledgments
The authors thank Sophon Bailey and Yanique Wallace for study coordination; Omar Mattis for recruitment and retention coordination; Drs Raquel Gibson and Andrea Cattan for their assistance with patient care; nurses Carol Cooper Smith, Hazel Harris, Sandra Mills and Rachael Bryan for data collection and patient care; Leshawn Mendoza, Shashauna Eastman, Craig Hayes and James Partin for laboratory testing and support; Shemille Samuels for data entry and Sarah Mullins for study monitoring.
Funding Supported by the Centers for Disease Control and Prevention, United States Agency for International Development, Family Health International (FHI 360) cooperative agreement, CA/GPO-A-00-05-00022.
Footnotes
Contributors All authors participated in the interpretation of the study and drafting of the manuscript. All authors have seen and approved the final version. MCS, JW, LF, SE and APK participated in the design and analysis for the manuscript. TH-K, NM, LW, JL-W and BC contributed to the overall study design and concept. CB, JP, CP and MCS participated in acquisition of data and oversight of lab analyses. JW performed the statistical analysis.
Disclaimer The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
Competing interests None declared.
Patient consent Obtained.
Ethics approval The study protocol was approved by the Jamaican Ministry of Health, the CDC and the University of West Indies ethical review boards and was registered with clinicalTrials.gov (NCT01684358). Ethics committee number: 6136.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement For data sharing enquiries, please contact AKourtis@cdc.gov.
References
- 1.WHO. Library Cataloguing-in-Publication Data Global incidence and prevalence of selected curable sexually transmitted infections. 2008. [Google Scholar]
- 2.Darroch JE, Woog Vanessa, Bankole Akinrinola, Ashford Lori S. ADDING IT UP: Costs and Benefits of Meeting the Contraceptive Needs of Adolescents. Guttmacher Institute; 2016. https://www.guttmacher.org/sites/default/files/report_pdf/adding-it-up-adolescents-report.pdf. [Google Scholar]
- 3.Norman LR, Uche C. Prevalence and determinants of sexually transmitted diseases: an analysis of young Jamaican males. Sex Transm Dis. 2002;29:126–32. doi: 10.1097/00007435-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Newman L, Rowley J, Vander Hoorn S, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS ONE. 2015;10:e0143304–e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warner L, Stone KM, Macaluso M, et al. Condom use and risk of gonorrhea and Chlamydia: a systematic review of design and measurement factors assessed in epidemiologic studies. Sex Transm Dis. 2006;33:36–51. doi: 10.1097/01.olq.0000187908.42622.fd. [DOI] [PubMed] [Google Scholar]
- 6.Crosby RA, Diclemente RJ, Wingood GM, et al. Condom failure among adolescents: implications for STD prevention. J Adolesc Health. 2005;36:534–6. doi: 10.1016/j.jadohealth.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Zenilman JM, Weisman CS, Rompalo AM, et al. Condom use to prevent incident STDs: the validity of self-reported condom use. Sex Transm Dis. 1995;22:15–21. doi: 10.1097/00007435-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Carter MW, Bailey A, Snead MC, et al. Exploring discordance between biologic and self-reported measures of semen exposure: a qualitative study among female patients attending an STI clinic in Jamaica. AIDS Behav. 2013;17:728–36. doi: 10.1007/s10461-012-0286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans JL, Couture MC, Stein ES, et al. Biomarker validation of recent unprotected sexual intercourse in a prospective study of young women engaged in sex work in Phnom Penh, Cambodia. Sex Transm Dis. 2013;40:462–8. doi: 10.1097/OLQ.0b013e318286db8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Morisky DE, Lin X, et al. Bias in self-reported condom use: association between over-reported condom use and syphilis in a three-site study in China. AIDS Behav. 2016;20:1343–52. doi: 10.1007/s10461-015-1269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snead MC, Black CM, Kourtis AP. The use of biomarkers of semen exposure in sexual and reproductive health studies. J Womens Health (Larchmt) 2014;23:787–91. doi: 10.1089/jwh.2014.5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norris Turner A, Carr Reese P, Snead MC, et al. Recent biomarker-confirmed unprotected vaginal sex, but not self-reported unprotected sex, is associated with recurrent bacterial vaginosis. Sex Transm Dis. 2016;43:172–6. doi: 10.1097/OLQ.0000000000000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenbaum JE, Zenilman J, Melendez J, et al. Telling truth from Ys: an evaluation of whether the accuracy of self-reported semen exposure assessed by a semen Y-chromosome biomarker predicts pregnancy in a longitudinal cohort study of pregnancy. Sex Transm Infect. 2014;90:479–84. doi: 10.1136/sextrans-2013-051315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose E, Diclemente RJ, Wingood GM, et al. The validity of teens’ and young adults’ self-reported condom use. Arch Pediatr Adolesc Med. 2009;163:61–4. doi: 10.1001/archpediatrics.2008.509. [DOI] [PubMed] [Google Scholar]
- 15.Mose F, Newman LP, Njunguna R, et al. Biomarker evaluation of self-reported condom use among women in HIV-discordant couples. Int J STD AIDS. 2013;24:537–40. doi: 10.1177/0956462412473892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minnis AM, Steiner MJ, Gallo MF, et al. Biomarker validation of reports of recent sexual activity: results of a randomized controlled study in Zimbabwe. Am J Epidemiol. 2009;170:918–24. doi: 10.1093/aje/kwp219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallo MF, Steiner MJ, Warner L, et al. Self-Reported Condom Use Is Associated With Reduced Risk of Chlamydia, Gonorrhea, and Trichomoniasis. Sex Transm Dis. 2007;34:829–33. doi: 10.1097/OLQ.0b013e318073bd71. [DOI] [PubMed] [Google Scholar]
- 18.Lawson ML, Maculuso M, Bloom A, et al. Objective markers of condom failure. Sex Transm Dis. 1998;25:427–32. doi: 10.1097/00007435-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Mauck CK, Doncel GF Biomarkers of Semen Exposure Clinical Working Group. Biomarkers of semen in the vagina: applications in clinical trials of contraception and prevention of sexually transmitted pathogens including HIV. Contraception. 2007;75:407–19. doi: 10.1016/j.contraception.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Aho J, Koushik A, Diakité SL, et al. Biological validation of self-reported condom use among sex workers in Guinea. AIDS Behav. 2009;14:1287–93. doi: 10.1007/s10461-009-9602-6. [DOI] [PubMed] [Google Scholar]
- 21.Pépin J, Fink GD, Khonde N, et al. Improving second-generation surveillance: the biological measure of unprotected intercourse using prostate-specific antigen in vaginal secretions of West African women. J Acquir Immune Defici Syndr. 2006;42:490–3. doi: 10.1097/01.qai.0000222286.52084.9c. [DOI] [PubMed] [Google Scholar]
- 22.Penrose KJ, Richardson BA, Besson G, et al. Y chromosome and HIV DNA detection in vaginal swabs as biomarkers of semen and HIV exposure in women. Sex Transm Dis. 2014;41:674–9. doi: 10.1097/OLQ.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamshidi R, Penman-Aguilar A, Wiener J, et al. Detection of two biological markers of intercourse: prostate-specific antigen and Y-chromosomal DNA. Contraception. 2013;88:749–57. doi: 10.1016/j.contraception.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;63:834–40. doi: 10.1016/j.jclinepi.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Rattray C, Wiener J, Legardy-Williams J, et al. Effects of initiating a contraceptive implant on subsequent condom use: A randomized controlled trial. Contraception. 2015;92:560–6. doi: 10.1016/j.contraception.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steiner RJ, Liddon N, Swartzendruber AL, et al. Long acting reversible contraception and condom use among female US high school students. JAMA Pediatr. 2016;170:428–34. doi: 10.1001/jamapediatrics.2016.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]