Abstract
Background
Infants are at greatest risk for severe pertussis. In 2006, the Advisory Committee on Immunization Practices recommended that adolescents and adults, especially those with infant contact, receive a single dose of Tdap (tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine). To assess the effectiveness of cocooning, we conducted a case-control evaluation of infant close contacts.
Methods
Pertussis cases aged <2 months with onset between 1 January 2011 and 31 December 2011 were identified in Emerging Infections Program Network sites. For each case, we recruited 3 controls from birth certificates and interviewed identified adult close contacts (CCs) or parents of CCs aged <18 years. Pertussis vaccination was verified through medical providers and/or immunization registries.
Results
Forty-two cases were enrolled, with 154 matched controls. Around enrolled infants, 859 CCs were identified (600 adult and 259 nonadult). An average of 5.4 CCs was identified per case and 4.1 CCs per control. Five hundred fifty-four (64.5%) CCs were enrolled (371 adult and 183 non-adult CCs); 119 (32.1% of enrolled) adult CCs had received Tdap. The proportion of Tdap-vaccinated adult CCs was similar between cases and controls (P = .89). The 600 identified adult CCs comprised 172 potential cocoons; 71 (41.3%) potential cocoons had all identified adult CCs enrolled. Of these, 9 were fully vaccinated and 43.7% contained no Tdap-vaccinated adults. The proportion of fully vaccinated case (4.8%) and control (10.0%) cocoons was similar (P = .43).
Conclusions
Low Tdap coverage among adult CCs reinforces the difficulty of implementing the cocooning strategy and the importance of vaccination during pregnancy to prevent infant pertussis.
Keywords: cocooning, pertussis, Tdap
Reported pertussis incidence has been increasing in the United States since the 1990s, with significant epidemic peaks occurring in recent years. The greatest morbidity and mortality from pertussis is observed among infants, especially during the first few months of life. Approximately half of infants <1 year of age who are diagnosed with pertussis will be hospitalized, and 1 in 100 hospitalized infant cases will die as a result of their infection [1]. Between 2000 and 2011, approximately 14 604 pertussis hospitalizations and 215 pertussis deaths were reported in the United States among infants <1 year of age [1]. When a source of pertussis infection can be identified, parents, siblings, or other infant caregivers are typically responsible for transmitting the disease to vulnerable infants [2–5].
In the United States, the recommended childhood immunization schedule includes 5 doses of diphtheria toxoid, tetanus toxoid, and acellular pertussis (DTaP) vaccine beginning at 2 months of age, which leaves young infants unprotected against disease during the first months of life [6]. In 2005, 2 tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccines were licensed for routine use among adolescents and adults. The Advisory Committee on Immunization Practices (ACIP) recommended in 2006 that all adolescents and adults be vaccinated with a single dose of Tdap, emphasizing the importance of vaccinating adults in close contact with young infants [7, 8]. This strategy targets mothers, fathers, and other infant caregivers for vaccination to create a protective “cocoon” around vulnerable infants, with the goal of interrupting pertussis transmission from infected adults.
While recommendations for Tdap cocooning have been in place since 2006, implementation of the strategy has posed many challenges [9–11]. Logistically, infant close contacts are usually targeted during the postpartum hospital stay, a period typically lasting no more than a few days. This may require additional hospital staffing resources for patient education and Tdap administration. Additionally, hospitals may be unable to provide vaccine to nonpatients, forcing the referral of family members to outside locations for vaccination and decreasing the likelihood of compliance. The cost of vaccines is also an important consideration. Cocooning programs offering free vaccine have had better success, but must ensure continued funding; for programs that do not offer free vaccine, billing and reimbursement challenges are a reality [11]. When cocooning has been successfully implemented, it has generally been limited to single institutions where there is an immunization champion and where a substantial investment of resources has been made [11, 12].
We conducted a case-control evaluation among infant pertussis cases <2 months of age to evaluate the ACIP-recommended Tdap cocooning strategy.
METHODS
Infants <2 months of age who were diagnosed with confirmed or probable pertussis according to the Council of State and Territorial Epidemiologists (CSTE) pertussis case definition and had cough onset between 1 January 2011 and 31 December 2011 were identified through routine surveillance in 6 Emerging Infections Program Network sites including Connecticut (statewide), Minnesota (statewide), New Mexico (statewide), New York (33 counties), and Oregon (3 counties); case identification in California (statewide) was limited to those cases with cough onset between 1 July 2011 and 31 December 2011. At the time of the evaluation, CSTE defined a confirmed pertussis case as cough illness of any duration with isolation of Bordetella pertussis from a clinical specimen, or cough illness of ≥ 2 weeks with at least 1 pertussis symptom (paroxysmal cough, inspiratory “whoop,” posttussive vomiting) and polymerase chain reaction (PCR) positive for pertussis, or epi-linkage to a laboratory-confirmed case and cough illness of ≥ 2 weeks with at least 1 pertussis symptom [13]. A probable case was defined as cough illness of ≥ 2 weeks with at least 1 pertussis symptom (paroxysmal cough, inspiratory “whoop,” posttussive vomiting) in the absence of laboratory confirmation. Infants who were PCR positive for pertussis and had a cough illness of any duration were also eligible for enrollment in the evaluation.
Identified cases were eligible for enrollment if they were at least 2 days of age and resided in the catchment area on date of cough onset, were born in a hospital in their state of residence, were ≥ 37 weeks gestational age at birth, were not adopted or in foster care, and did not reside in an institution or nonresidential facility. For each eligible case, project personnel attempted to recruit 3 controls from birth certificate records that were born at the same hospital as the case and were <2 months of age on the date of the matched case’s cough onset. Control infants had the same eligibility criteria as cases, but were additionally considered ineligible if they had been diagnosed with pertussis prior to the corresponding case cough onset date.
Mothers of enrolled case and control infants were interviewed by phone to collect information on demographic characteristics, relationship to and amount of time spent with the case or control infant, recent vaccinations and medical providers, and to identify household contacts and caregivers of the case or control infant. The reference period was defined as the 30-day period prior to case cough onset date. Infant close contacts were defined as people who stayed overnight in the same household as the case or control infant ≥ 50% of time during the reference period or provided care to the infant including changing diapers and clothing, feeding, bathing, taking on walks, playing, etc. Identified close contacts who were <18 years of age at time of interview and whose parent/guardian was not the same as the case or control infant’s parent/guardian, who had no contact with the infant during the reference period, or who resided outside the United States at the time of interview were not eligible for enrollment. Eligible adult close contacts were interviewed to collect demographic characteristics, relationship to and amount of time spent with the case or control infant, medical providers, and Tdap vaccination history; identified close contacts <18 years of age were not interviewed, but their relationship to and the amount of time spent with the case or control infant and information on pertussis vaccination history was collected through interview of their parent/guardian.
Project personnel collected pertussis vaccination status of all eligible close contacts through medical providers and/or state immunization registries. When complete pertussis vaccine history was not available in the state immunization registry, all medical providers identified by the close contact during the interview were contacted. Birth hospitals of the case or control infant were also contacted to obtain maternal Tdap history. A dose of Tdap was considered valid if received at least 2 weeks before the case infant’s cough onset date; for controls, the date of cough onset for the matched case infant was used. A close contact was considered enrolled if both the interview and vaccine history follow-up were completed. Complete enrollment around an infant was achieved when all identified adult close contacts were enrolled; because Tdap is routinely recommended for adolescents at 11–12 years of age and the cocooning strategy’s primary focus is vaccinating adults who will have close contact with an infant, close contacts aged <18 years were not considered for completeness.
Data analysis was performed using SAS software version 9.3. Differences between proportions were evaluated using Co-chran-Mantel-Haenszel statistics; differences between means were tested using Student t test. A P value of <.05 was considered statistically significant.
RESULTS
During the evaluation period, 90 cases were identified, resulting in 42 (46.7%) enrolled cases and 154 matched controls. Of the 48 cases that were not enrolled, 5 (10.4%) were ineligible, 7 (14.6%) refused, and 36 (75.0%) could not be reached. Among the 196 enrolled case and control infants, 859 individual close contacts were identified (Figure 1). Overall, an average of 5.4 (range, 1–10) and 4.1 (range, 1–11) close contacts was identified per enrolled case and per enrolled control, respectively (P = .0018). When identified close contacts were stratified by age group, significant differences were observed between case and control infants in the average number of identified close contacts <18 years of age (2.3 close contacts per case vs 1.7 close contacts per control; P = .0037), but not close contacts ≥ 18 years of age (3.4 close contacts per case vs 3.0 close contacts per control; P = .0616).
Figure 1.
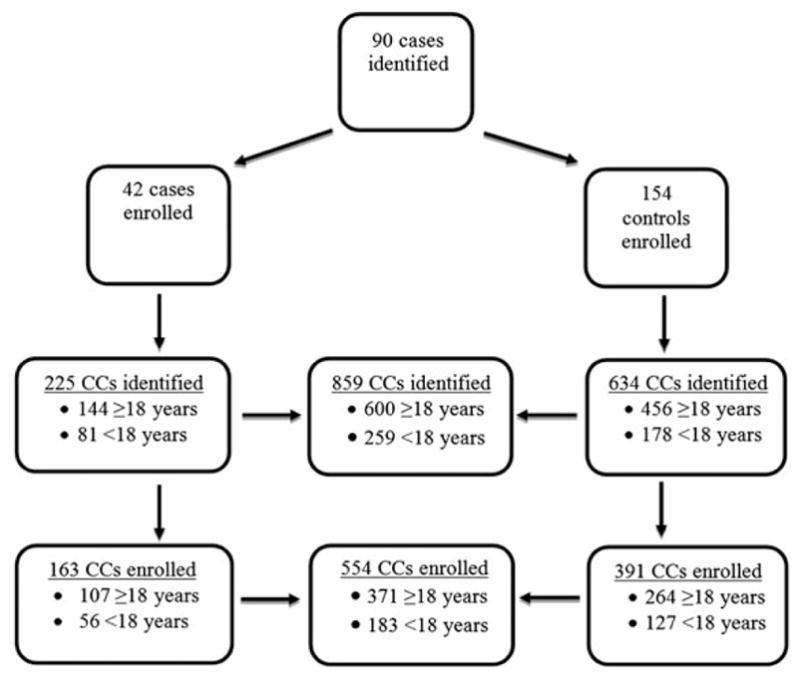
Study enrollment flowchart. Abbreviation: CC, close contact.
Overall, the relationship of identified close contacts to the infant was most frequently siblings (24.7%) or mothers (23.1%) (Figure 2). No differences were observed in the proportion of close contacts identified as siblings (P = .85) or mothers (P = .11) between case and control infants. Close contacts were more frequently identified as fathers among control infants (21.3% for controls vs 14.7% for cases; P = .0327), whereas aunts/uncles (11.6% for cases vs 6.5% for controls; P = .0161) and cousins (6.7% for cases vs 1.6% for controls; P < .0001) were more frequently identified around case infants.
Figure 2.
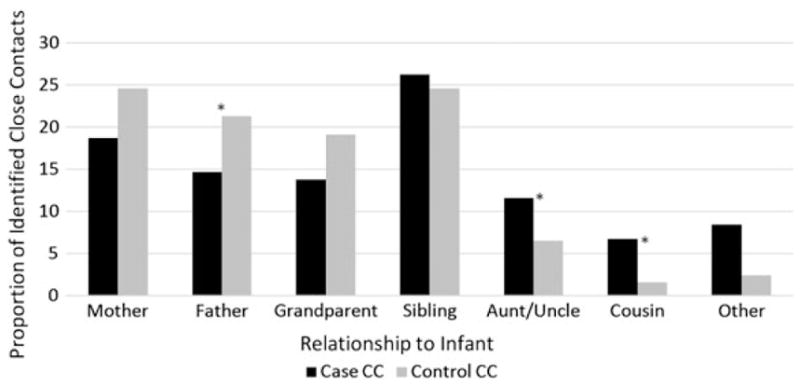
Relationship of identified close contacts (CCs) to infant cases and controls.
A total of 554 of 859 (64.5%) close contacts, comprised of 163 case close contacts and 391 control close contacts, were enrolled (Figure 1). Of identified close contacts who were not enrolled (n = 305), 165 (54.1%) were ineligible, 62 (20.3%) could not be reached, 65 (21.3%) refused or did not consent, 9 (3.0%) spoke a language other than English or Spanish, and 4 (1.3%) were incompetent or deceased at the time of enrollment; the proportion of close contacts who refused or did not consent was significantly higher among control infants than among case infants (3.1% for cases vs 12.2% for controls; P < .0001). The 554 enrolled close contacts included 371 adult close contacts ≥ 18 years of age and 183 close contacts <18 years of age.
Of enrolled close contacts ≥ 18 years of age who were eligible to receive a dose of Tdap (n = 371), 119 (32.1%) had record of a valid, provider-verified Tdap. Of the remaining 252 contacts with no reported Tdap, 162 (64.3%) had follow-up completed with all listed providers, 60 (23.8%) reported no providers for follow-up during the interview, 14 (5.6%) had follow-up completed with some but not all listed providers (exhausted follow-up attempts with remaining providers), and 16 (6.3%) had no providers who responded after all attempts to contact were exhausted.
Among the 119 close contacts aged ≥ 18 years with a record of Tdap, the proportion vaccinated did not differ significantly between cases and controls; this finding remained consistent when stratified by relationship to the infant (Table 1). Overall, the median time since Tdap vaccination was 76 days (range, 14 days–5.8 years), and this was similar between cases and controls (P = .27) (Table 1). Enrolled close contacts ≥ 18 years of age who had received a dose of Tdap were most commonly identified as mothers of the case or control infant (n = 74 [62.2%]). Of these 74 vaccinated mothers, 10 control mothers and no case mothers were vaccinated during pregnancy. Among enrolled close contacts 11–17 years of age, 5 of 12 (41.7%) case close contacts and 4 of 22 (18.2%) control close contacts were vaccinated with Tdap (P = .31). The median time since Tdap vaccination among adolescent close contacts was 156 days (range, 14 days–1.7 years), and was also similar between cases and controls (P = .76). All 9 close contacts 11–17 years of age with a dose of Tdap were identified as siblings of the enrolled case or control infant.
Table 1.
Tetanus-Diphtheria-Pertussis Vaccination of Infant Close Contacts ≥ 18 Years of Age
| Contact | Received Tdap, No. (%) | P Value | Time Since Tdap Receipt, d, Median (Range) | P Value | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Case CCs (n = 107) | Control CCs (n = 264) | Case CCs (n = 107) | Control CCs (n = 264) | |||
| Mother | 19/42 (45.2) | 55/126 (43.7) | .91 | 43.5 (14–741) | 52 (15–1674) | .66 |
|
| ||||||
| Father | 4/26 (15.4) | 11/68 (16.2) | .71 | 802 (86–1678) | 109 (28–1895) | .26 |
|
| ||||||
| Grandparent | 6/20 (30.0) | 12/48 (25.0) | .32 | 518.5 (35–1026) | 174 (15–1447) | .70 |
|
| ||||||
| Aunt/Uncle | 3/12 (25.0) | 3/15 (20.0) | .15 | 696.5 (60–1333) | 278 (109–2135) | .89 |
|
| ||||||
| Othera | 3/7 (42.9) | 3/7 (42.9) | ... | 1267 (1116–1473) | 58 (20–2009) | .46 |
|
| ||||||
| Overall | 35 (32.7) | 84 (31.8) | .89 | 86 (14–1845) | 75.5 (15–2135) | .27 |
Abbreviations: CC, close contact; Tdap, tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine.
Other includes adult siblings, cousins, and other close contacts.
Among the 149 close contacts <11 years of age, 121 (81%) were up to date on DTaP. The proportion of case close contacts (72.7%) and the proportion of control close contacts (84.8%) up to date on DTaP was not significantly different (P = .09).
The 371 enrolled adult close contacts comprised 172 individual potential cocoons, 71 (41.3%) of which had complete enrollment, with all identified adult members of the potential cocoon enrolled. The 71 completely enrolled potential cocoons had an average of 2.4 adult close contacts, while the remaining 101 potential cocoons with incomplete enrollment had an average of 3.4 adult close contacts identified and 2.0 adult close contacts enrolled. Of the 71 completely enrolled potential cocoons, 6 (8.5%) achieved complete cocooning by being fully vaccinated with Tdap; 2 of these cocoons included only a mother, and the other 4 cocoons were comprised of only 2 adult members. Despite small numbers, there was no significant difference in the proportion of fully vaccinated cocoons between cases (4.8%) and controls (10.0%) (P = .43). Almost half of the completely enrolled potential cocoons contained no adult members with a valid, provider-verified Tdap dose (31/71 [43.7%]); there was also no significant difference in the proportion of fully unvaccinated potential cocoons around cases and controls (P = .19).
DISCUSSION
At the time of Tdap introduction in the United States, the ACIP recommendation for cocooning was the only recommended strategy for protecting young infants against pertussis in the early months of life. Despite best efforts, cocooning has proven difficult to implement and the effectiveness of the strategy is questionable [10–12], making it a suboptimal model for preventing pertussis-related morbidity and mortality in infancy. Results from our evaluation provide further evidence of the many challenges associated with the cocooning strategy. While overall Tdap coverage among enrolled adult close contacts was higher in our evaluation than national 2011 adult Tdap coverage estimates (12.5% coverage among adults 19–64 years of age) [14], coverage remained low with only 32.1% of enrolled adult close contacts having been vaccinated. Incomplete cocooning around infant cases also occurred frequently in our evaluation population; of the potential cocoons where complete enrollment of adult close contacts was achieved, only 8% were fully vaccinated with Tdap. When complete cocoons could be identified, they were typically very small, comprised of only 1–2 individuals. Unfortunately, low adult Tdap coverage and a high proportion of case and control infants who had no adult close contacts vaccinated left us unable to calculate the effectiveness of the cocooning strategy.
Our data showed that the average number of identified close contacts around infant cases was significantly higher than the number of identified close contacts around infant controls, suggesting that the risk of pertussis increases with an increased number of close contacts. This is consistent with published literature that has highlighted household size as a pertussis risk factor [15–17]. This difference between cases and controls was primarily driven by close contacts <18 years of age, likely mirroring the changing epidemiology of pertussis in the United States and the observed increase in disease among school-aged children and adolescents as a result of waning pertussis vaccine immunity [18, 19]. We did not, however, find a difference in Tdap coverage overall among individual close contacts when comparing the case and control infants (31.8% in case close contacts vs 32.7% in control close contacts). Additionally, we did not observe differences in the proportion of fully vaccinated cocoons (4.8% for case cocoons and 10.0% for control cocoons). Although our data were limited by the proportion of cases enrolled (46.7%) and the small number of potential cocoons where all adult close contacts were enrolled, our findings suggest that cocooning with Tdap had no effect on infant disease, which is consistent with much of the published literature [10, 20, 21].
Waning of vaccine-induced immunity, particularly among acellular-primed cohorts, has been well documented in the years following receipt of both DTaP and Tdap and is a major contributor to the current disease resurgence in the United States [22–27]. While acellular pertussis vaccines remain effective at preventing symptomatic disease, recent animal studies from a nonhuman model suggest that colonization may still occur among acellular-vaccinated individuals [28]. Waning immunity coupled with asymptomatic pertussis transmission in highly vaccinated populations dramatically reduces the likelihood that vaccinating close contacts would provide indirect protection to infants, the basic premise of the cocooning strategy. In light of these challenges, the ACIP has recommended Tdap vaccination during the third trimester of each pregnancy as the preferred strategy for pertussis prevention in infants [29]. Transplacentally transferred maternal antibodies against pertussis resulting from Tdap vaccination during pregnancy have been shown in the United Kingdom to be effective at preventing infant disease in the early months of life, offering important advantages to cocooning [30].
Although cocooning was the only strategy available to protect infants at the time it was recommended, there is now general agreement that the method is costly, is plagued with implementation challenges, and has uncertain effectiveness. Vaccination during pregnancy has been shown to be safe and effective at preventing infant disease in the early months of life and is being adopted by an increasing number of countries as the primary pertussis prevention strategy for young infants [31, 32]. Given the ongoing resurgence of pertussis in the United States, efforts should focus on increasing awareness and implementation of Tdap vaccination during pregnancy to prevent disease in infants too young to be vaccinated themselves.
Acknowledgments
We thank Pam Daily Kirley, Mohammed Khan, Sarah New, Roxanne Ryan, Cynthia Kenyon, Melissa McMahon, Rachel Ostadkar, C. J. Olson, Neeti Sethi, Cheri Denardo, Pam Gahr, Claudia Miller, Karen Scherzinger, and the New Mexico Department of Health Survey Unit, Shelley Zansky, Kathryn Woodworth, Priscela Perez, Salvatore Currenti, Cynthia Schulte, Paul R. Cieslak, Beletshachew Shiferaw, and local and district public health epidemiologists and nurses from California, Connecticut, Minnesota, New Mexico, New York, and Oregon.
Financial support. This work was supported by the Centers for Disease Control and Prevention (cooperative agreement CK12-1202).
Supplement sponsorship. This article appears as part of the supplement “Infant Pertussis Disease Burden in the Context of Maternal Immunization Strategies,” sponsored by the Bill & Melinda Gates Foundation.
Footnotes
Author contributions. A. B. contributed to the design of the analysis, supervised data collection at the participating sites, carried out the initial and subsequent analyses, drafted the initial manuscript, and approved the final manuscript as submitted. M. L. contributed to the design of the analysis; contributed to the analysis, cleaning and interpretation of data; and approved the final manuscript as submitted. E. B., K. K., J. L., S. M., D. S., and J. W. contributed to the design of the analysis, supervised and participated in data collection at participating sites, contributed to interpretation of data, provided critical review of the manuscript for important intellectual content, and approved the final manuscript as submitted. T. H. S. contributed to the design of the analysis, supervised data collection at the participating sites, provided supervision in the analysis and interpretation of data, provided critical review of the manuscript for important intellectual content, and approved the final manuscript as submitted. S. W. M. contributed to the design of the analysis, provided supervision in the analysis and interpretation of data, provided critical review of the manuscript for important intellectual content, and approved the final manuscript as submitted.
Potential conflicts of interest. All authors: no reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Nationally Notifiable Disease surveillance data. 2011. [Google Scholar]
- 2.Wendelboe A, Hudgens MG, Poole C, Van Rie A. Estimating the role of casual contact from the community in transmission of Bordetella pertussis to young infants. Emerg Themes Epidemiol. 2007;4:15. doi: 10.1186/1742-7622-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wendelboe A, Njamkepo E, Bourillon A, et al. Transmission of Bordetella pertussis to young infants. Pediatr Infect Dis J. 2007;26:293. doi: 10.1097/01.inf.0000258699.64164.6d. [DOI] [PubMed] [Google Scholar]
- 4.Bisgard KM, Pascual FB, Ehresmann KR, et al. Infant pertussis: who was the source? Pediatr Infect Dis J. 2004;23:985–9. doi: 10.1097/01.inf.0000145263.37198.2b. [DOI] [PubMed] [Google Scholar]
- 5.Skoff TH, Kenyon C, Cocoros N, et al. Sources of infant pertussis infection in the United States. Pediatrics. 2015;136:635–41. doi: 10.1542/peds.2015-1120. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Pertussis vaccination: use of acellular pertussis vaccines among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1997;46(RR-7):1–25. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR-3):1–43. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine. MMWR Recomm Rep. 2006;55(RR-17):1–37. [PubMed] [Google Scholar]
- 9.Urwyler P, Heininger U. Protecting newborns from pertussis—the challenge of complete cocooning. BMC Infect Dis. 2014;14:397. doi: 10.1186/1471-2334-14-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castagnini L, Healy CM, Rench MA, Wootton SH, Munoz FM, Baker CJ. Impact of maternal post-partum Tdap vaccination on pertussis illness in young infants. 48th Annual Meeting of the Infectious Diseases Society of America; Vancouver, BC. 21–24 October 2010. [Google Scholar]
- 11.Healy CM, Rench MA, Baker CJ. Implementation of cocooning against pertussis in a high-risk population. Clin Infect Dis. 2011;52:157–62. doi: 10.1093/cid/ciq001. [DOI] [PubMed] [Google Scholar]
- 12.Rosenblum E, McBane S, Wang W, Sawyer M. Protecting newborns by immunizing family members in a hospital-based vaccine clinic: a successful Tdap cocooning program during the 2010 California pertussis epidemic. Public Health Rep. 2014;129:245–51. doi: 10.1177/003335491412900306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Council of State and Territorial Epidemiologists. CSTE position statement 97-ID-09: public health surveillance, control and prevention of pertussis. CSTE National Meeting; Saratoga Springs, NY. 1997. [Google Scholar]
- 14.Centers for Disease Control and Prevention. Noninfluenza vaccination coverage among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2013;62:66–72. [PMC free article] [PubMed] [Google Scholar]
- 15.Iroh Tam PY, Menk JS, Hughes J, Kulasingam SL. An ecological analysis of pertussis disease in Minnesota, 2009–2013. Epidemiol Infect. 2016;144:847–55. doi: 10.1017/S0950268815002046. [DOI] [PubMed] [Google Scholar]
- 16.Levri KM, Reynolds L, Liko J, Dott M, Robinson BF, Cieslak PR. Risk factors for pertussis among Hispanic infants—Metropolitan Portland, Oregon, 2010–2012. Pediatr Infect Dis J. 2016;35:488–93. doi: 10.1097/INF.0000000000001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinn HE, Snelling TL, Habig A, Chiu C, Spokes PJ, McIntyre PB. Parental Tdap boosters and infant pertussis: a case control study. Pediatrics. 2014;134:713–20. doi: 10.1542/peds.2014-1105. [DOI] [PubMed] [Google Scholar]
- 18.Misegades LK, Winter K, Harriman K, et al. Association of childhood pertussis with receipt of 5 doses of pertussis vaccine by time since last vaccine dose, California, 2010. JAMA. 2012;308:2126–32. doi: 10.1001/jama.2012.14939. [DOI] [PubMed] [Google Scholar]
- 19.Acosta AM, DeBolt C, Tasslimi A, et al. Tdap vaccine effectiveness in adolescents during the 2012 Washington State pertussis epidemic. Pediatrics. 2015;135:981–9. doi: 10.1542/peds.2014-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carcione D, Regan AK, Tracey L, et al. The impact of parental postpartum pertussis vaccination on infection in infants: a population-based study of cocooning in Western Australia. Vaccine. 2015;33:5654–61. doi: 10.1016/j.vaccine.2015.08.066. [DOI] [PubMed] [Google Scholar]
- 21.Healy CM, Rench MA, Wootton SH, Castagnini LA. Evaluation of the impact of a pertussis cocooning program on infant pertussis infection. Pediatr Infect Dis J. 2015;34:22–6. doi: 10.1097/INF.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 22.Sheridan SL, McCall BJ, Davis CA, et al. Acellular pertussis vaccine effectiveness for children during the 2009–2010 pertussis epidemic in Queensland. Med J Aust. 2014;200:334–8. doi: 10.5694/mja13.11069. [DOI] [PubMed] [Google Scholar]
- 23.Sheridan SL, Frith K, Snelling TL, Grimwood K, McIntyre PB, Lambert SB. Waning vaccine immunity in teenagers primed with whole cell and acellular pertussis vaccine: recent epidemiology. Expert Rev Vaccines. 2014;13:1081–106. doi: 10.1586/14760584.2014.944167. [DOI] [PubMed] [Google Scholar]
- 24.Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med. 2012;367:1012–9. doi: 10.1056/NEJMoa1200850. [DOI] [PubMed] [Google Scholar]
- 25.Baxter R, Bartlett J, Rowhani-Rahbar A, Fireman B, Klein NP. Effectiveness of pertussis vaccines for adolescents and adults: case-control study. BMJ. 2013;347:f4249. doi: 10.1136/bmj.f4249. [DOI] [PubMed] [Google Scholar]
- 26.Tartof SY, Lewis M, Kenyon C, et al. Waning immunity to pertussis following 5 doses of DTaP. Pediatrics. 2013;131:e1047–52. doi: 10.1542/peds.2012-1928. [DOI] [PubMed] [Google Scholar]
- 27.Koepke R, Eickhoff JC, Ayele RA, et al. Estimating the effectiveness of tetanus diphtheria-acellular pertussis vaccine (Tdap) for preventing pertussis: evidence of rapidly waning immunity and difference in effectiveness by Tdap brand. J Infect Dis. 2014;210:942–53. doi: 10.1093/infdis/jiu322. [DOI] [PubMed] [Google Scholar]
- 28.Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A. 2014;111:787–92. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2013;62:131–5. [PMC free article] [PubMed] [Google Scholar]
- 30.Munoz FM, Bond NH, Maccato M, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. JAMA. 2014;311:1760–9. doi: 10.1001/jama.2014.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dabrera G, Amirthalingam G, Andrews N, et al. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012–2013. Clin Infect Dis. 2015;60:333–7. doi: 10.1093/cid/ciu821. [DOI] [PubMed] [Google Scholar]
- 32.Maertens K, Nadège Caboré R, Huygen K, et al. Pertussis vaccination during pregnancy in Belgium: results of a prospective controlled cohort study. Vaccine. 2015;34:142–50. doi: 10.1016/j.vaccine.2015.10.100. [DOI] [PubMed] [Google Scholar]


