Abstract
BACKGROUND:
Administrative claims data are used for a wide variety of research and quality assurance purposes; however, they are prone to medication exposure misclassification if medications are purchased without using an insurance benefit. Low-cost generic drug programs (LCGPs) offered at major chain pharmacies are a relatively new and sparsely investigated source of exposure misclassification. LCGP medications are often purchased out of pocket; thus, a pharmacy claim may never be submitted, and the exposure may go unobserved in claims data. As heavy users of medications, Medicare beneficiaries have much to gain from the affordable medications offered through LCGPs. This use may put them at increased risk of exposure misclassification in claims data. Many high-risk medications (HRMs) and medications tracked for adherence and utilization quality metrics are available through LCGPs, and exposure misclassification of these medications may impact the quality assurance efforts reliant on administrative claims data. Presently, there is little information regarding the use of these programs among a geriatric population.
OBJECTIVES:
To (a) quantify the prevalence of LCGP users in a nationally representative population of Medicare beneficiaries; (b) compare clinical and demographic characteristics of LCGP users and nonusers; (c) assess determinants of LCGP use and medications acquired through these programs; and (d) analyze patterns of LCGP use during the years 2007-2012.
METHODS:
This study relied on data from the Medical Expenditure Panel Survey (MEPS) from 2007 to 2012. The first 3 objectives were completed with a cohort of individuals in the most recent MEPS panel, while the fourth objective was completed with a separate cohort composed of individuals who participated in MEPS from 2007 to 2012. Inclusion in either study cohort required that individuals were Medicare beneficiaries aged 65 years or greater, used at least 1 prescription drug during their 2-year panel period, and participated in all 5 rounds of data collection during their panel period. MEPS captures medication utilization by surveying individuals on current and previous medication use and verifies this information at the pharmacy level, so prescription fills can be observed irrespective of payment by an insurer or a filed claim. Pharmaceutical utilization was assessed at the individual level for each year of the study period, and LCGP use was recorded as a binary variable for each individual. An LCGP medication fill was identified if the total cost of the drug was paid out of pocket and matched the cost of medications listed on LCGP formularies available from major pharmacy retailers during these years. Cohort demographics and characteristics of interest included age, gender, race, employment status, marital status, family income level, education level, residence in a metropolitan statistical area, geographic region, prescription drug coverage, Medicare type, comorbidities, number of unique medications used, and number of medication fills. Comparisons were made between users and nonusers using chi-square and t-tests. Multivariable logistic regression was used to identify factors associated with LCGP use.
RESULTS:
From the most recent MEPS panel, 1,861 individuals were included in the study cohort, of which 53.5% were observed to be LCGP users. The 995 LCGP users in this cohort represented over 20 million Medicare beneficiaries who used LCGPs from 2011 to 2012. Significant differences between LCGP users and nonusers existed in terms of race, educational attainment, comorbidity burden, type of Medicare insurance, number of unique medications used, and number of medication fills. Each additional unique medication filled increased the odds of LCGP use by 12% (95% CI = 1.09-1.14). Individuals with insurance in addition to Medicare (i.e., Tricare/Veteran’s Affairs or Medicaid) had less than half the odds of using LCGPs compared with those with Medicare or Medicare managed care insurance coverage only. The proportion of LCGP users and the proportion of LCGP fills out of all medications available through LCGPs increased from 2007 to 2012.
CONCLUSIONS:
There is a high rate of LCGP use among Medicare beneficiaries aged 65 years or greater. Claims-based research and quality assurance programs focusing on the benefits and harms of medications available through these programs are at risk of underestimating the true medication exposure in this population and should account for this possibility in sensitivity analyses. Managed care organizations should incentivize the reporting of LCGP medication use or make adjustments to generic medication benefit structures to more effectively capture true medication exposure.
What is already known about this subject
Administrative claims data maintained by managed care organizations are used for a variety of research and quality assurance purposes, but a major limitation of claims data is unobserved medication use that may result in medication exposure misclassification.
When a medication is purchased through low-cost generic drug programs (LCGPs), a claim may never be submitted, and the medication use may go unobserved in administrative claims data.
LCGPs have been estimated by previous surveys to be used by approximately 16% of Medicare beneficiaries and include a variety of medication classes used to treat many common acute and chronic conditions.
What this study adds
In a nationally representative cohort of Medicare beneficiaries, this study found that 54.5% of individuals filled a prescription through an LCGP and that 8.0% of all prescription fills from 2007 to 2012 that were available through LCGPs were actually filled through these programs.
The proportion of Medicare beneficiaries using these programs has increased from 30.0% in 2007 to 38.0% in 2012. Significant predictors of LCGP use included educational attainment, insurance type, and number of unique medications filled.
Over 15% of all fills for cephalosporins, levothyroxine, angioten-sin-converting-enzyme inhibitors, and metformin were obtained through LCGPs, while over 10% of beta blockers and over 5% of statins were filled through LCGPs. Additionally, nearly 10% of all fills for high-risk medications were purchased through LCGPs.
The usefulness of administrative claims data for drug utilization studies, pharmacovigilance, health policy research, benefit design, and quality assurance are well established.1-7 A notable limitation of prescription claims data is the potential for exposure misclassification, which may occur when individuals acquire medication outside of their prescription drug benefits. Common sources of misclassifica-tion include individuals not ingesting filled prescriptions, free samples provided by physicians, medications obtained but not covered under a drug benefit, paying for drugs out of pocket, and over-the-counter medications.1,2,8-11 Low-cost generic programs (LCGPs) are a relatively recent and sparsely investigated source of exposure misclassification.
LCGPs first appeared in late 2006 with Kmart providing 90-day supplies of certain generics for $15 and Walmart offering 30-day supplies for $4.12 LCGPs are now in place at almost all major pharmacy chains, including 8 of the top 10 largest chain pharmacies in the nation, and include one third of the top 100 generics used by Americans by volume.3,12-17 The out-of-pocket purchase price of LCGP medications is generally lower than the $10 to $20 copays required to receive the medication through a prescription benefit.3,18 In 2008, over 70 million Americans were estimated to have used an LCGP to obtain a prescription medication—a figure that has likely expanded as the number and popularity of these programs has increased.12,17,19
The National Committee for Quality Assurance (NCQA) developed the Healthcare Effectiveness Data and Information Set (HEDIS) as a tool to measure performance across multiple dimensions for health plans.20 The Centers for Medicaid & Medicare Services (CMS) monitors several of these measures as indicators of plan quality and assigns star ratings based on these metrics for Medicare Part D and Medicare Advantage plans.21 One HEDIS metric distinguishes “high risk
medications in the elderly.” A number of medications considered as high-risk medications (HRMs) are available through LCGPs, including certain nonsteroidal anti-inflammatory drugs, antiparkinson agents, antipsychotics, tricyclic antidepr-essents, muscle relaxants, and anti-adrenergics. Additionally, HEDIS quality measures also consider medications for their protective benefits, including antihypertensive medications, adherence to antidiabetes medications, and beta blockers after myocardial infarction as metrics of plan performance. Many of these medications are available through LCGPs and could potentially be unobserved in administrative claims data, which could undermine the impact of quality assurance efforts.
This study sought to characterize and assess the use of LCGPs in a nationally representative Medicare population. This study had 4 primary objectives: (1) quantify the prevalence of LCGP users in a Medicare insured population; (2) compare clinical and demographic characteristics of LCGP users and nonusers; (3) analyze patterns of LCGP use over the years 2007-2012 and medications acquired through LCGPs; and (4) assess determinants of LCGP use.
Methods
Data Source
This study used data from the Medical Expenditure Panel Survey (MEPS) from the years 2007-2012. These years were chosen because 2007 was the first full year after LCGPs were implemented, and 2012 is the most recent year of data available. MEPS is weighted based on race, gender, region, and other demographic information to be a nationally representative survey of individuals living in the United States that collects data regarding demographics and clinical conditions, as well as health care and pharmaceutical utilization. MEPS uses an overlapping panel design with a new panel of participants added each year. Panels are followed for up to 2 years with 5 rounds of data collection occurring during each 2-year period.22 MEPS is a de-identified public use dataset supported by the Agency for Healthcare Research and Quality that is intended for research purposes; therefore, it is exempt from institutional review board approval. Details related to the design and data collection processes of MEPS are detailed elsewhere.23
Study Population and Design
A cross-sectional study design was used to compare differences between LCGP users and nonusers in the 2011-2012 MEPS panel. In a separate analysis, the proportion of LCGP users from 2007 to 2012 was quantified to assess trends in the proportions of LCGP fills and LCGP users over these years. Both analyses had the same inclusion criteria, which required that individuals were aged 65 years or greater, were Medicare beneficiaries, participated in all 5 rounds of data collection, and reported using at least 1 prescription medication during the 2-year panel period (Figure 1).
FIGURE 1.
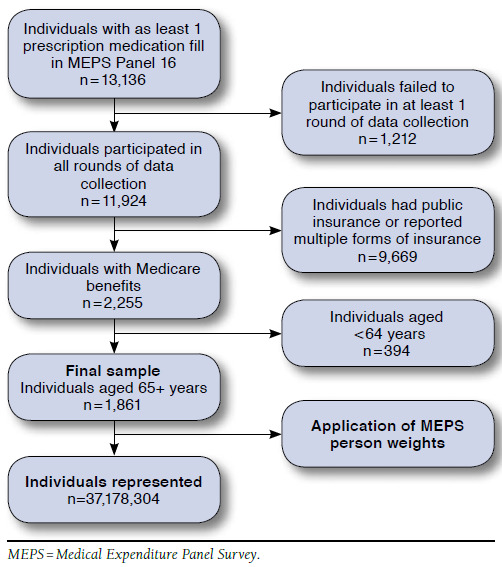
Study Attrition
Pharmaceutical utilization was assessed at the individual level for each year of the study period. Pharmaceutical data in MEPS include drug name, National Drug Code (NDC), MEPS round supplied, strength, quantity dispensed, and days supplied. Each prescription fill included in the MEPS dataset also includes information regarding the amount paid by the individual out of pocket and the amount contributed by other sources. Pharmacy data are collected at the pharmacy level, so prescription fills are captured irrespective of whether an insurance claim was submitted.
LCGP Use
Four stipulations were used to define LCGP use: (1) the total cost of the drug was paid out of pocket; (2) the cost of the drug exactly matched the cost of an LCGP drug as reported by pharmacies; (3) the medication was listed on an LCGP formulary from a major chain pharmacy from 2007 to 2012; and (4) oral medication fills were dispensed for 30- or 90-day supplies of medications, with the exception of anti-infectives and steroids, which were allowed to vary given differential dosing intervals for these classes. LCGP use was coded at the person level as a binary dependent variable for any use during the study period and at the medication level for each medication fill. HRMs were classified according to the NCQA HEDIS measure.20 HRM fills were identified by NDC and classified as high risk by an indicator variable. The number and proportion of HRMs purchased through LCGPs were calculated for the 2011-2012 MEPS panel.
Cohort Characteristics
For the cross-sectional 2011-2012 cohort, demographic variables and characteristics of interest included age, gender, race, employment status, marital status, family income, education level, residence in a metropolitan statistical area (MSA), geographic region, presence of comorbidities, number of unique medications, number of total medication fills, prescription drug coverage, and presence of additional insurance types (Medicare Advantage, Tricare, or Medicaid). Age, employment status, marital status, income level, geographic region, and MSA were all assessed at the last round of data collection.
The presence of comorbidities, as well as the number of prescription fills and number of unique medications, were summed for each individual over his or her entire panel period. For comparison, the cohort was divided by age categories: 65-74 years, 75-84 years, and 85 years and older. Race was divided between white, Hispanic, African American, Asian, and other. Family income in MEPS is recorded as a percentage of the federal poverty level (FPL), which MEPS stratifies as < 100% of FPL, 100%-124% of FPL, 125%-199% of FPL, 200%-399% of FPL, and > 400% of FPL. Comorbidity burden was based on the Charlson Comorbidity Index (CCI), using the adaptation by D’hoore et al. (1996).24 The CCI included 1 point each for past or current history of myocardial infarction, heart failure, peripheral vascular disease, dementia, cerebrovascu-lar disease, chronic pulmonary disease, rheumatic disorders, ulcerative disease, and mild liver disease; 2 points each for hemiplegia, moderate or severe renal disease, diabetes, and cancer; 3 points for severe liver disease; and 6 points for meta-static cancer. The CCI was recorded as the sum of the weighted score for each individual and further categorized for scores of 0-1, 2-4, 5-6, and 7 or greater.
Data Analysis
The proportions of LCGP uses and users were tracked from 2007 to 2012. These proportions were compared with overall pharmaceutical utilization for users and nonusers over the same time period. Comparisons were conducted for each of the studied cohort characteristics between users and nonusers in the 2011-2012 MEPS panel using chi-square or t-tests. Multivariable logistic regression was used to identify factors associated with LCGP use in the 2011-2012 panel. Adjusted odds ratios (AOR) and 95% confidence intervals (CI) were reported. This manuscript was drafted in concordance with the STROBE guidelines for reporting observational research studies.25 All data analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC) implementing SAS procedures (SURVEYMEANS, SURVEYFREQ, and SURVEYLOGISTIC) that take into account the complex survey design of MEPS and use the longitudinal survey weights supplied by MEPS to calculate population estimates over the 2-year period.
Results
Cohort Comparison
In the 2011-2012 MEPS panel, 1,861 Medicare beneficiaries met all inclusion criteria and were included in the study cohort. Of this population, 995 (53.5%) individuals were classified as LCGP users who had at least 1 prescription fill that met the criteria for LCGP use. When weighted to be nationally representative based on demographic information, this sample represents over 37 million individuals with Medicare insurance over the age of 65 with 20 million LCGP users (54.5%). Weighted user and nonuser demographic and clinical characteristics are presented in Table 1.
TABLE 1.
Demographic Characteristics of Study Cohort 2011-2012
| LCGP Users | Nonusers | ||||||||
| n | % | n (weighted) | % (weighted) | n | % | n (weighted) | % (weighted) | ||
| Overall sample: N = 1,861 | 995 | 53.5 | 20,257,085 | 54.5 | 866 | 46.5 | 16,921,219 | 45.5 | |
| Age, years | |||||||||
| 65-74 | 592 | 59.5 | 11,623,826 | 57.4 | 498 | 57.5 | 9,246,366 | 54.7 | |
| 75-84 | 302 | 30.4 | 6,298,540 | 31.1 | 249 | 28.8 | 5,105,807 | 30.2 | |
| 85+ | 101 | 10.2 | 2,334,719 | 11.5 | 119 | 13.7 | 2,569,046 | 15.2 | |
| Gender | |||||||||
| Male | 416 | 41.8 | 8,942,673 | 44.1 | 375 | 43.3 | 7,589,362 | 44.9 | |
| Female | 579 | 58.2 | 11,314,413 | 55.8 | 491 | 56.7 | 9,331,857 | 55.2 | |
| Prescription drug coverage | |||||||||
| Yes | 809 | 81.3 | 16,014,685 | 79.0 | 726 | 83.8 | 13,626,990 | 80.6 | |
| No | 186 | 18.7 | 4,242,400 | 20.9 | 140 | 16.2 | 3,294,229 | 19.5 | |
| Employment | |||||||||
| Unemployed | 808 | 81.2 | 15,993,192 | 78.9 | 727 | 83.9 | 13,422,762 | 79.3 | |
| Employed | 187 | 18.8 | 4,263,893 | 21.0 | 139 | 16.1 | 3,498,457 | 20.7 | |
| Educationa | |||||||||
| Less than high school | 218 | 21.9 | 3,124,521 | 15.4 | 261 | 30.1 | 3,748,149 | 22.2 | |
| High school or equivalent | 594 | 59.7 | 12,960,777 | 64.0 | 475 | 54.8 | 9,985,347 | 59.0 | |
| College or higher | 183 | 18.4 | 4,171,788 | 20.6 | 130 | 15.0 | 3,187,723 | 18.8 | |
| Marital status | |||||||||
| Not married | 487 | 48.9 | 9,172,890 | 45.3 | 424 | 49.0 | 7,409,749 | 43.8 | |
| Married | 508 | 51.1 | 11,084,195 | 54.7 | 442 | 51.0 | 9,511,470 | 56.2 | |
| Income category | |||||||||
| < 100% of FPL | 145 | 14.6 | 2,218,614 | 10.9 | 193 | 22.3 | 2,736,051 | 16.2 | |
| 100%-125% of FPL | 71 | 7.1 | 1,144,045 | 5.6 | 63 | 7.3 | 1,090,338 | 6.4 | |
| 125%-200% of FPL | 193 | 19.4 | 3,522,567 | 17.4 | 168 | 19.4 | 2,788,984 | 16.5 | |
| 200%-400% of FPL | 296 | 29.7 | 5,894,264 | 29.1 | 229 | 26.4 | 4,736,978 | 28.0 | |
| > 400% of FPL | 290 | 29.1 | 7,477,595 | 36.9 | 213 | 24.6 | 5,568,867 | 32.9 | |
| Charlson Comorbidity Index (CCI)a | |||||||||
| CCI score < 1 | 488 | 49.0 | 10,644,017 | 52.5 | 497 | 57.4 | 10,154,756 | 60.0 | |
| CCI score 2-4 | 450 | 45.2 | 8,545,011 | 42.2 | 342 | 39.5 | 6,330,457 | 37.4 | |
| CCI score 5-6 | 43 | 4.3 | 797,588 | 3.9 | 21 | 2.4 | 340,155 | 2.0 | |
| CCI score > 6 | 14 | 1.4 | 270,469 | 1.3 | 6 | 0.7 | 95,851 | 0.6 | |
| Racea | |||||||||
| White | 668 | 67.1 | 16,583,240 | 81.8 | 510 | 58.9 | 13,121,132 | 77.6 | |
| Hispanic | 96 | 9.6 | 1,225,657 | 6.0 | 125 | 14.4 | 1,405,772 | 8.3 | |
| Black | 172 | 17.3 | 1,694,785 | 8.4 | 172 | 19.9 | 1,461,105 | 8.6 | |
| Asian | 42 | 4.2 | 474,791 | 2.3 | 49 | 5.7 | 742,601 | 4.4 | |
| Other | 17 | 1.7 | 278,612 | 1.4 | 10 | 1.2 | 190,609 | 1.1 | |
| MSA | |||||||||
| Rural | 170 | 17.1 | 3,951,523 | 19.5 | 145 | 16.7 | 2,892,528 | 17.1 | |
| Urban | 825 | 82.9 | 16,305,562 | 80.5 | 721 | 83.3 | 14,028,691 | 82.9 | |
| Region | |||||||||
| Northeast | 142 | 14.3 | 3,320,603 | 16.4 | 156 | 18.0 | 3,639,734 | 21.5 | |
| Midwest | 237 | 23.8 | 4,878,369 | 24.1 | 168 | 19.4 | 3,691,573 | 21.8 | |
| South | 377 | 37.9 | 7,744,038 | 38.2 | 347 | 40.1 | 5,989,006 | 35.4 | |
| West | 239 | 24.0 | 4,314,076 | 21.3 | 195 | 22.5 | 3,600,906 | 21.3 | |
| Insurance typea | |||||||||
| Medicare | 434 | 43.6 | 8,904,198 | 43.9 | 304 | 35.1 | 7,030,516 | 41.6 | |
| Medicare managed care | 440 | 44.2 | 9,172,375 | 45.3 | 313 | 36.1 | 6,498,501 | 38.4 | |
| Medicare + Tricare | 48 | 4.8 | 1,186,295 | 5.9 | 59 | 6.8 | 1,209,439 | 7.1 | |
| Medicare + Medicaid | 73 | 7.3 | 994,218 | 4.9 | 190 | 21.9 | 2,182,763 | 12.9 | |
| Total number of medication fillsa | |||||||||
| Median (IQR) | 51 (28-95) | 48.8 (28.3-89.1) | 34.5 (14-69) | 31.1 (12.6-64.6) | |||||
| Unique medications useda | |||||||||
| Median (IQR) | 10 (6-14) | 9.1 (5.4-13.3) | 6 (3-11) | 5.8 (2.8-9.5) | |||||
Note: Percentages not adding to 100% are due to rounding errors.
aP < 0.05 between-group comparison.
FPL = federal poverty limit; IQR = interquartile range; LCGP = low-cost generic program; MSA = metropolitan statistical area.
Medication Use
Over the years 2007-2012, 10,230 Medicare beneficiaries met all inclusion criteria and filled a total of 587,732 prescriptions. Out of all prescription fills, 77.3% (n = 454,216) were for medications available through LCGPs. Of all medications available through LCGPs, 8.0% (n = 36,413) were purchased through these programs. Figure 2 displays the proportions of LCGP fills and LCGP users graphed against total prescription fills per person per year in each year from 2007 to 2012. The proportion of LCGP fills out of all medications available through LCGPs increased from 6.2% of fills in 2007 to 9.0% of fills in 2012 — with a peak at 10.3% in 2010. Over the 2007-2012 period, the proportion of LCGP users also increased from 30.0% in 2007 to 38.0% in 2012—again, with a peak in 2010 at 41.5%. While the proportions of LCGP fills and LCGP users increased from 2007-2012, the number of prescription fills per person per year remained relatively stable.
FIGURE 2.
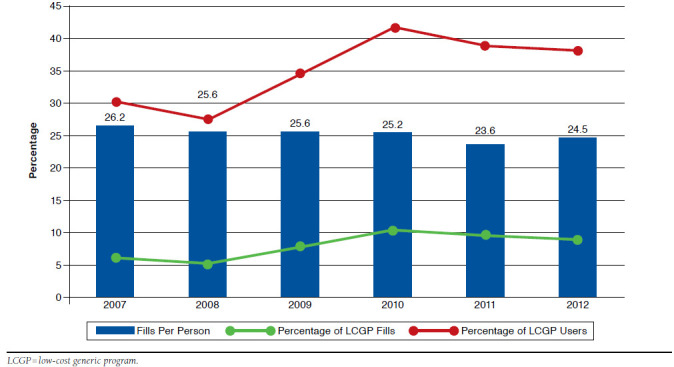
Proportion of LCGP Users and LCGP Fills Versus Total Fills Per Person
Among medications considered in HEDIS quality measures, over 15% of angiotensin-converting enzyme inhibitors and met-formin were acquired through LCGP programs, as well as over 10% of beta blockers and more than 5% of statins (Figure 3).
FIGURE 3.
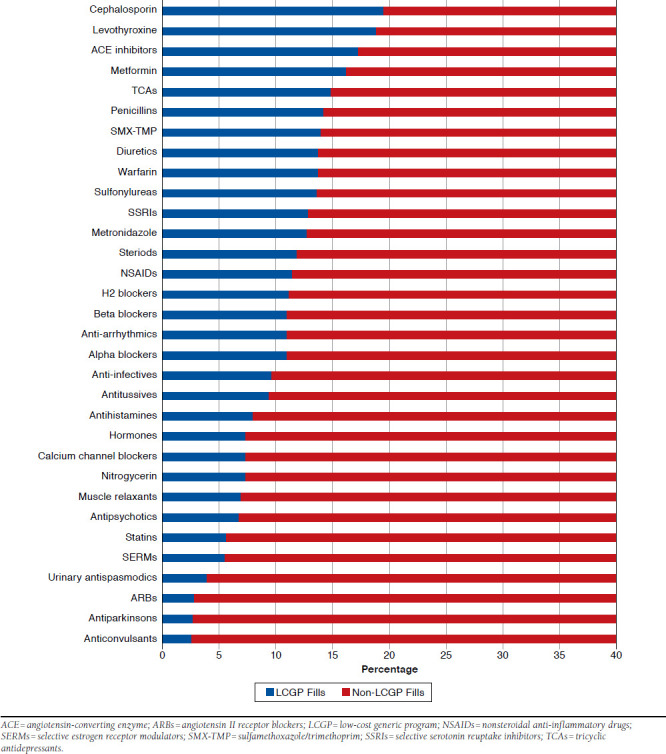
Proportion of Each Drug Class Filled Through LCGPs
There were 4,868 HRM fills during the 2-year panel period, comprising 4.3% of all fills during this period (n = 113,536). Of all HRM fills, 3,642 (74.8%) were available through LCGPs, and 9.6% (n = 348) of these medications were purchased through LCGPs. Figure 4 displays the proportion of LCGP fills for several of the HRMs that were most commonly purchased through LCGPs. Amitriptyline was the only tricyclic antidepressant filled through LCGPs, with 16.5% of its 318 fills purchased through LCGPs. Of 550 total fills for digoxin over the 2-year period, 15.5% were filled through LCGPs, as well as 10.8% of the 564 fills for glyburide. The proportions of other HRMs purchased through LCGPs (Figure 4) included butalbital (10.7%), estrogens (9.4%), indomethacin (9.3%), firstgeneration antihistamines (6.0%), and muscle relaxants (5.9%). In total, 437 (23.5% of the 2011-2012 panel) individuals filled at least 1 HRM during the panel period, and 87 (19.9%) of these individuals used LCGPs to obtain these medications.
FIGURE 4.
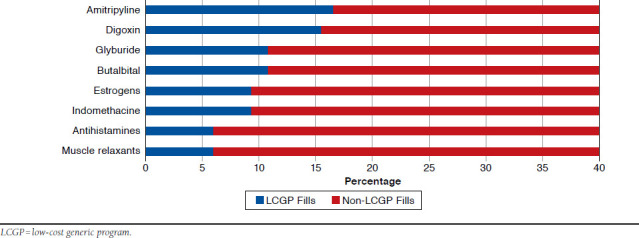
Proportion of High-Risk Medication Fills through LCGPs
Determinants of LCGP Use
Cohort demographics along with the number of unique medications filled were included in a multivariable logistic regression model. AORs and 95% CIs for all variables are presented in Table 2. Education level was the only demographic characteristic that significantly predicted LCGP use. Compared with the reference category of individuals with less than a high school education, those with a high school education or equivalent were 41% more likely to be LCGP users (AOR = 1.41, 95% CI = 1.04-1.92). Each additional unique medication filled also increased the odds of LCGP use by 12% (AOR = 1.12, 95% CI = 1.09-1.14). Those with other insurance types (Tricare/Veteran’s Affairs or Medicaid) had more than 50% lower odds of using LCGPs compared with those with Medicare or Medicare managed care insurance plans alone. Prescription drug coverage was not observed to be a significant predictor of LCGP use. The overall model c-statistic was 0.70, showing acceptable model discrimination between users and nonusers.
TABLE 2.
Multivariable Logistic Regression Results of Predictive Characteristics for LCGP Use
| Characteristic | Adjusted Odds Ratio | 95% Wald Confidence Limits | |
| Lower | Upper | ||
| Age | |||
| 65-74 | Ref. | Ref. | Ref. |
| 75-84 | 0.92 | 0.74 | 1.15 |
| 85+ | 0.77 | 0.53 | 1.12 |
| Gender | |||
| Male | Ref. | Ref. | Ref. |
| Female | 1.04 | 0.84 | 1.29 |
| Employment | |||
| Unemployed | Ref. | Ref. | Ref. |
| Employed | 0.97 | 0.71 | 1.34 |
| Education | |||
| Less than high school | Ref. | Ref. | Ref. |
| High school or equivalent | 1.41 | 1.04 | 1.92 |
| College or higher | 1.44 | 0.97 | 2.14 |
| Marital status | |||
| Not married | Ref. | Ref. | Ref. |
| Married | 0.82 | 0.64 | 1.07 |
| Income category | |||
| < 100% of FPL | Ref. | Ref. | Ref. |
| 100%-125% of FPL | 1.12 | 0.61 | 2.05 |
| 125%-200% of FPL | 1.36 | 0.85 | 2.17 |
| 200%-400% of FPL | 1.20 | 0.82 | 1.76 |
| > 400% of FPL | 1.39 | 0.90 | 2.16 |
| Charlson Comorbidity Index (CCI) | |||
| CCI score < 1 | Ref. | Ref. | Ref. |
| CCI score 2-4 | 0.97 | 0.77 | 1.22 |
| CCI score 5-6 | 1.14 | 0.54 | 2.43 |
| CCI score > 7 | 2.20 | 0.43 | 11.36 |
| Race | |||
| White | Ref. | Ref. | Ref. |
| Hispanic | 1.15 | 0.80 | 1.67 |
| Black | 1.18 | 0.82 | 1.71 |
| Asian | 0.75 | 0.44 | 1.26 |
| Other | 1.14 | 0.48 | 2.68 |
| MSA | |||
| Rural | Ref. | Ref. | Ref. |
| Urban | 0.94 | 0.65 | 1.36 |
| Region | |||
| Northeast | Ref. | Ref. | Ref. |
| Midwest | 1.22 | 0.72 | 2.05 |
| South | 1.31 | 0.79 | 2.18 |
| West | 1.33 | 0.80 | 2.23 |
| Insurance type | |||
| Medicare | Ref. | Ref. | Ref. |
| Medicare managed care | 1.21 | 0.92 | 1.60 |
| Medicare + Tricarea | 0.52 | 0.31 | 0.88 |
| Medicare + Medicaida | 0.33 | 0.21 | 0.52 |
| Prescription drug coverage | |||
| Yes | Ref. | Ref. | Ref. |
| No | 1.29 | 0.85 | 1.96 |
| Number of unique medicationsa | 1.12 | 1.09 | 1.14 |
aSignificant at P < 0.05.
LCGP = low-cost generic program; FPL = federal poverty limit; MSA = metropolitan statistical area.
Discussion
This study found that a significant proportion of Medicare beneficiaries purchase medications through LCGPs and that the prevalence of LCGP use has increased over time. We have previously examined the rate of LCGP use in a population of privately insured adults and the pediatric population, and results of that study indicate that the rate of use is substantially higher among Medicare beneficiaries compared with those populations.26,27 While the proportion of users and uses increased over the study period, the total number of prescriptions filled per person per year remained relatively stable. Thus, the increasing use of LCGPs cannot be attributed to greater pharmaceutical utilization. Rather, it is likely because of increasing popularity and knowledge of these programs, as well as increasing availability of the programs and an expansion of medications offered through them. This is likely driven in part by continuous increases in the cost of health care coverage and increases in the out-of-pocket costs for seniors.28
The results of the present study are consistent with results from previous studies of LCGP use, which indicates that our definition of LCGP use was robust.19,29 Lauffenburger et al. (2013) observed that approximately 10% of individuals with claims for international normalized ratio tests did not have a corresponding prescription claim for warfarin, which is consistent with our observed proportion of LCGP fills for warfarin in the current study.30 We expanded on the previous studies of LCGP use by considering demographic and clinical characteristics, trend of LCGP use over time, and the specific medication classes filled through these programs.19,29 Zhang et al. (2012) evaluated LCGP use in Medicare Part D claims in 2008 and found that about 16% of beneficiaries used a $4 generic medication.31 However, this study may have been limited in that the methods only observed “zero-paid” claims and did not observe those medication fills that occur without any claims adjudication. We included a broader definition of LCGP use and also included topical and other dosage forms that are available through these programs. This accounts for the larger LCGP user group (30%) observed in 2007 in our study, which we believe is closer to the true value, given the concordance with the aforementioned survey studies.19,29 Our measure of LCGP use at the medication level would overestimate an assumption of exposure misclas-sification but is still indicative of the overall level of LCGP utilization in this population.
LCGPs include medication classes with significant risk profiles and medications used to treat serious medical conditions. Based on the results of this study, Medicare Part D and Medicare Advantage plans are likely underestimating the prevalence of HRM use and consequently biasing quality metrics. HRM use is an important metric in the older population, as Pugh et al. (2013) recently found in a Veterans Affairs population where incident use of HRMs was associated with 50%-200% increases in the odds of death, hospitalization, and emergency department visits.32 Although unobserved LCGP HRM use would lead to a higher HRM HEDIS rating for an individual plan, the cost of preventable health care utilization because of adverse drug effects from HRM use may surpass any benefit to a higher plan rating and deferred medication costs. Moreover, HEDIS measures considering beneficial medication classes (e.g., beta blockers after myo-cardial infarction, antidiabetic medication use, and antihy-pertensive medications) will be underestimated, resulting in lower plan ratings for these measures. Thus, it is in a health plan’s best interest to be able to accurately estimate the true metric for these medications. Medicare plans are financially incentivized based on their star ratings, since these ratings have been shown to influence consumer decisions to enroll in particular plans.14 Therefore, it is essential that plans are able to accurately assess metrics that are used to construct these ratings both from a business perspective as well as for the better management of beneficiaries’ health care.
There are several ways to mitigate the potential for exposure misclassification because of LCGPs. If pharmacists file claims even when patients pay entirely out of pocket, then LCGP use will appear in administrative claims data as zero paid claims. The problem with this solution is that pharmacists do not currently have any reason or incentive to file claims for customers paying out of pocket.3 Pharmacists may be unaware of a patient’s insurance status or may find obtaining this information unnecessary, especially if they believe that submitting a claim to the insurer will result in a copayment that exceeds the LCGP fee. While anecdotally this is a common occurrence, CMS considers the cost of an LCGP-purchased medication in the calculation of “true” out-of-pocket cost (i.e., costs applied towards total yearly benefits for coverage gap), and some plans have covered LCGP costs as “usual and customary” prices, which can then be reimbursed at the typical level (e.g., copay of $1 for a $4 30-day supply).31,33 Managed care organizations providing Part D and Medicare Advantage plans have acknowledged the issue of LCGPs as it pertains to quality measurement, and some have implemented strategies to detect LCGP use. Organizations should continue to look for ways to work directly with pharmacists to ensure reporting of LCGP medications as a means to improve medication use for patients.34
Unless LCGP reporting is incentivized in some way, medication utilization data will almost inevitably be missing from administrative claims datasets.3,14 Thus, efforts to address quality assurance and pharmacovigilance should supplement administrative claims research with data from alternate sources or perform thorough sensitivity analyses to ascertain the level of bias due to exposure misclassification. Exposure misclassification will almost always bias results towards the null, which should always be discussed in the limitations sections of observational studies using administrative prescription claims.35-37
The LCGP estimates of the proportion of fills within a medication class obtained in this study can be used as upward bound estimates in sensitivity analyses to estimate the level of bias or to make estimates for external control of confounders where a medication is not the primary exposure of interest but is important in the study.36-38 Data sources that are not susceptible to missing claims include publicly available research data such as MEPS, proprietary datasets captured at the pharmacy level, or electronic medical records that capture medication prescribing behaviors. All of these data sources, however, have their own inherent strengths and limitations. Future research should compare the exposure profiles of different data sources to determine the completeness of available data and show empiric examples of how this source of exposure misclassification can impact claims-based studies.
Limitations
This study study is subject to several limitations. It remains possible that not all medication use is recorded if all pharmacies used by MEPS participants were not surveyed and because over-the-counter medication is not captured in MEPS. Our study definition of LCGP use may allow for overestimation of use if only pricing is considered; however, this limitation is mitigated by requiring specific quantities supplied for oral medications. Purchasing medications out of pocket is a well-known source of exposure misclassification in administrative claims data. This study relies on the fundamental assumption that medications purchased through LCGPs will be paid for out of pocket and thus will be unobserved in claims data. Even with medication fills that are paid completely out of pocket by Medicare beneficiaries, claims still may be filed, especially in the case where individuals are in the coverage gap. Nevertheless, our findings represent an accurate measure of LCGP use in the population and can provide an upward limit of potential exposure misclassifica-tion. Future studies should assess the proportion of LCGP medications that appear as “zero paid” claims in this population to further assess the robustness of our LCGP metric and to estimate the proportion of LCGP fills that may truly be unobserved in claims data.
Conclusions
This study found that nearly 55% of Medicare beneficiaries followed over a 2-year period used an LCGP to obtain medications, with annual usage of 35.1% in this group. While overall LCGP use as a percentage of all medication fills is relatively low (< 5%), many medication classes purchased through LCGPs are used for quality metrics and rating of Medicare health plan performance. If these medication fills go unobserved in administrative pharmacy claims, plan ratings may be overrated in terms of safety and underrated in terms of effectiveness of care. Adequate reporting of LCGP medication fills should be a priority of managed care organizations so that effective quality assurance and intervention programs can be implemented to improve medication use among beneficiaries.
References
- 1.Schneeweiss S, Avorn J.. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323-37. [DOI] [PubMed] [Google Scholar]
- 2.Gavrielov-Yusim N, Friger M.. Use of administrative medical databases in population-based research. J Epidemiol Community Health. 2014;68(3):283-87. [DOI] [PubMed] [Google Scholar]
- 3.Choudhry NK, Shrank WH.. Four-dollar generics — increased accessibility, impaired quality assurance. N Engl J Med. 2010;363(20):1885-87. [DOI] [PubMed] [Google Scholar]
- 4.Willke R, Mullins D.. “Ten Commandments” for conducting comparative effectiveness research using “real-world data.” J Manag Care Pharm. 2011;17(9 Supp A):S10-S15. Available at: http://www.jmcp.org/doi/abs/10.18553/jmcp.2011.17.s9-a.S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhan C, Miller MR.. Administrative data based patient safety research: a critical review. Qual Saf Health Care. 2003;12(Suppl 2):ii58-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoover KW, Tao G, Kent CK, Aral SO.. Epidemiologic research using administrative databases: garbage in, garbage out. Obstet Gynecol. 2011;117(3):729; author reply 729-30. [DOI] [PubMed] [Google Scholar]
- 7.Grimes DA. Epidemiologic research using administrative databases: garbage in, garbage out. Obstet Gynecol. 2010;116(5):1018-19. [DOI] [PubMed] [Google Scholar]
- 8.Polinski JM, Schneeweiss S, Levin R, Shrank WH.. Completeness of retail pharmacy claims data: implications for pharmacoepidemiologic studies and pharmacy practice in elderly adults. Clin Ther. 2009;31(9):2048-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobus S, Schneeweiss S, Chan KA.. Exposure misclassification as a result of free sample drug utilization in automated claims databases and its effect on a pharmacoepidemiology study of selective COX-2 inhibitors. Pharmacoepidemiol Drug Saf. 2004;13(10):695-702. [DOI] [PubMed] [Google Scholar]
- 10.Gamble JM, McAlister FA, Johnson JA, Eurich DT.. Restrictive drug coverage policies can induce substantial drug exposure misclassification in pharmacoepidemiologic studies. Clin Ther. 2012;34(6):1379-86.e3. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Stürmer T, Brookhart MA.. Evidence of sample use among new users of statins: implications for pharmacoepidemiology. Med Care. 2014;52(9):773-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czechowski JL, Tjia J, Triller DM.. Deeply discounted medications: implications of generic prescription drug wars. J Am Pharm Assoc (2003). 2010;50(6):752-57. [DOI] [PubMed] [Google Scholar]
- 13.Patel HK, Dwibedi N, Omajasola A, Sansgiry SS.. Impact of generic drug discount programs on managed care organizations. Am J Pharmacy Benefits. 2011;3(1):45-53. [Google Scholar]
- 14.Shrank WH, Liberman JN, Fischer MA, Girdish C, Brennan TA, Choudhry NK.. Physician perceptions about generic drugs. Ann Pharmacother. 2011;45(1):31-38. [DOI] [PubMed] [Google Scholar]
- 15.Wal-Mart Stores. Retail prescription program drug list. Revised February 6, 2015. Available at: http://i.walmartimages.com/i/if/hmp/fusion/customer_list.pdf. Accessed April 7, 2016. [Google Scholar]
- 16.Walgreen Co. Value-priced medication list. Revised August 8, 2014. Available at: http://www.walgreens.com/images/psc/VPG_List_Update_9-5-2014.pdf. Accessed April 7, 2016. [Google Scholar]
- 17.Rucker NL. $4 generics: How low, how broad, and why patient engagement is priceless. J Am Pharm Assoc (2003). 2010;50(6):761-63. [DOI] [PubMed] [Google Scholar]
- 18.Tungol A, Starner CI, Gunderson BW, Schafer JA, Qiu Y, Gleason PP.. G eneric drug discount programs: are prescriptions being submitted for pharmacy benefit adjudication? J Manag Care Pharm. 2012;18(9):690-700. Available at: http://www.jmcp.org/doi/abs/10.18553/jmcp.2012.18.9.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.C.S. Mott Children’s Hospital, University of Michigan Department of Pediatrics and Communicable Diseases, University of Michigan Child Health Evaluation and Research (CHEAR) Unit. Nearly 70 million Americans using discount generic rx programs. National Poll on Children’s Health. Vol. 3, Issue 2, February 11, 2008. Available at: http://mottnpch.org/sites/default/files/documents/021108GenericRxPrograms.pdf. Accessed April 7, 2016. [Google Scholar]
- 20.National Comittee for Quality Assurance. HEDIS & quality measurement. 2015. Available at: http://www.ncqa.org/hedis-quality-measurement. Accessed April 7, 2016. [Google Scholar]
- 21.Nau D. Executive update on medication quality measures in Medicare Part D plan ratings 2013. Pharmacy Quality Alliance. 2013. Available at: http://adhereforhealth.org/wp-content/uploads/pdf/2013UpdateonMedicarePlanRatings.pdf. Accessed April 7, 2016. [Google Scholar]
- 22.MEPS-HC sample design and collection process. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://meps.ahrq.gov/mepsweb/survey_comp/ic_data_collection.jsp. Accessed April 7, 2016. [Google Scholar]
- 23.Medical Expenditure Panel Survey. Data overview. Agency for Healthcare Research and Quality, Rockville, MD. Revised August 26, 2009. Available at: http://meps.ahrq.gov/mepsweb/data_stats/data_overview.jsp. Accessed April 7, 2016. [Google Scholar]
- 24.D’Hoore W, Bouckaert A, Tilquin C.. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol. 1996;49(12):1429-33. [DOI] [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP.. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pauly NJ, Brown JD.. Prevalence and predictors of low-cost generic program use in a nationally representative cohort of privately insured adults. J Manag Care Spec Pharm. 2015;21(12):1162-70. Available at: http://www.jmcp.org/doi/abs/10.18553/jmcp.2015.21.12.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pauly NJ, Talbert JC, Brown JD.. The prevalence and predictors of low-cost generic program use in the pediatric population. Drugs Real World Outcomes. 2015;2(4):411-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoadley J, Cubanski J, Neuman T.. Medicare Part D at ten years: the 2015 marketplace and key trends, 2006-2015. Kaiser Family Foundation. October 5, 2015. Available at: http://kff.org/medicare/report/medicare-part-d-at-ten-years-the-2015-marketplace-and-key-trends-2006-2015/. Accessed April 7, 2016. [Google Scholar]
- 29.Gatwood J, Tungol A, Truong C, Kucukarslan SN, Erickson SR.. Prevalence and predictors of utilization of community pharmacy generic drug discount programs. J Manag Care Pharm. 2011;17(6):449-55. Available at: http://www.jmcp.org/doi/abs/10.18553/jmcp.2011.17.6.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauffenburger JC, Balasubramanian A, Farley JF, et al. Completeness of prescription information in US commercial claims databases. Pharmacoepidemiol Drug Saf. 2013;22(8):899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Gellad WF, Zhou L, Lin YJ, Lave JR.. Access to and use of $4 generic programs in Medicare. J Gen Intern Med. 2012;27(10):1251-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pugh MJ, Marcum ZA, Copeland LA, et al. The quality of quality measures: HEDIS quality measures for medication management in the elderly and outcomes associated with new exposure. Drugs Aging. 2013;30(8):645-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Medicare & Medicaid Services. Chapter 14: Coordination of benefits. In: Medicare Prescription Drug Benefit Manual. Revised August 23, 2013. Available at: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/downloads/chapter14.pdf. Accessed April 7, 2016. [Google Scholar]
- 34.Owen JA. Medicare star ratings: stakeholder proceedings on community pharmacy and managed care partnerships in quality. J Am Pharm Assoc (2003). 2014;54(3):228-40. [DOI] [PubMed] [Google Scholar]
- 35.Jurek AM, Greenland S, Maldonado G.. How far from non-differential does exposure or disease misclassification have to be to bias measures of association away from the null? Int J Epidemiol. 2008;37(2):382-85. [DOI] [PubMed] [Google Scholar]
- 36.Blair A, Stewart P, Lubin JH, Forastiere F.. Methodological issues regarding confounding and exposure misclassification in epidemiological studies of occupational exposures. Am J Ind Med. 2007;50(3):199-207. [DOI] [PubMed] [Google Scholar]
- 37.Wacholder S, Hartge P, Lubin JH, Dosemeci M.. Non-differential misclassification and bias towards the null: a clarification. Occup Environ Med. 1995;52(2):557-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15(5):291-303. [DOI] [PubMed] [Google Scholar]


