Abstract
Background:
A rapid, accurate, flexible and reliable diagnostic method may significantly decrease the costs of diagnosis and treatment. Designing an appropriate microarray chip reduces noises and probable biases in the final result.
Objective:
The aim of this study was to design and construct a DNA Microarray Chip for a rapid detection and identification of 10 important bacterial agents.
Method:
In the present survey, 10 unique genomic regions relating to 10 pathogenic bacterial agents including Escherichia coli (E.coli), Shigella boydii, Sh.dysenteriae, Sh.flexneri, Sh.sonnei, Salmonella typhi, S.typhimurium, Brucella sp., Legionella pneumophila, and Vibrio cholera were selected for designing specific long oligo microarray probes. For this reason, the in-silico operations including utilization of the NCBI RefSeq database, Servers of PanSeq and Gview, AlleleID 7.7 and Oligo Analyzer 3.1 was done. On the other hand, the in-vitro part of the study comprised stages of robotic microarray chip probe spotting, bacterial DNAs extraction and DNA labeling, hybridization and microarray chip scanning. In wet lab section, different tools and apparatus such as Nexterion® Slide E, Qarraymini spotter, NimbleGen kit, TrayMixTM S4, and Innoscan 710 were used.
Results:
A DNA microarray chip including 10 long oligo microarray probes was designed and constructed for detection and identification of 10 pathogenic bacteria.
Conclusion:
The DNA microarray chip was capable to identify all 10 bacterial agents tested simultaneously. The presence of a professional bioinformatician as a probe designer is needed to design appropriate multifunctional microarray probes to increase the accuracy of the outcomes.
Keywords: DNA Microarrays, DNA Microchips, Bioinformatics, DNA Probes, Bacteria
1. INTRODUCTION
Food and water borne pathogenic bacteria including E. coli, Shigella, Salmonella, Vibrio cholera, Brucella and Legionella are considered as the most important causes of life-threatening diseases throughout the world. The infections caused by these bacterial pathogens are endemic now in many countries including Iran [1-7].
The conventional methods for detection of bacterial infections may take several days to be completed. In recent years, DNA-based approaches have become potentially powerful in microbiological diagnostics and molecular epidemiology of infections because of their higher rapidity, reproducibility, accuracy, and affordability [8, 9].
DNA microarray technology is a proper and high-throughput diagnostic tool for detection and identification pathogenic microorganisms. It is flexible, sensitive, specific and very sharp technique. The process of this technology is divided into two sections of dry laboratory (in-silico) (Bioinformatic practices) and wet laboratory (in-vitro). The dry laboratory of microarray technique includes the processes of probe designing and finalizing data analysis which are known as in-silico study. Other stages of DNA microarray technology such as probe spotting, DNA labeling, and hybridization are recognized as wet laboratory (in-vitro) practices [10-18].
Traditional microbiological, immunological and biochemical assays are generally time consuming and they normally give some false +ve or –ve results. Moreover, the application of advanced molecular techniques such as Polymerase Chain Reaction (PCR) is useful when the number of samples is low and limited. In the presence of a huge number of clinical/environmental samples, DNA microarray technology is a suitable option as a rapid and reliable diagnosis [16, 19-26]. To construct a DNA microarray chip, the designed single stranded probes are immobilized on the solid surface of a chip by an automated and robotic spotting machine (spotter/printer). The immobilization of DNA microarray long oligo probes is done when the probes are modified by different types of active chemical groups such as NH2, Succinyl, Disulfide, and Hydrazide. Originally, the microarray chip is made of glass; but, the surface of a chip may be treated with different types of chemical materials including Epoxy silane, Isothiocyanate, Aminophenyl, Mercaptosilan, Aldehyde or Epoxide to improve the stability of the covalent bonds between a single stranded DNA probe and the surface of a chip. The modified probes and the related treated chips guarantee the occurrence of a stable, powerful and optimized bond. Each chemical group in a modified probe needs its specific chemical substance on the treated chip slides. For example, the NH2 group in a modified probe binds best with the Epoxy silane covered chip and they create powerful covalent bonds [10, 16, 17, 27-30].
In parallel with the probe spotting, the bacterial cells isolated from samples must be handled in the process of DNA extraction. If the bacterial cells are few in clinical samples, there are two different ways to prepare an appropriate amount of bacterial DNA molecules: 1. Performance of PCR, 2. Utilization of culture medium. Regarding E.coli, both ways are possible but not for fastidious or unculturable bacteria. Then, the extracted DNAs must be melted into single strands and labeled with fluorescent dyes such as Cy3, Cy5, Tamro, and Texas Red. In the field of microbial diagnosis, the use of one color (one-channel microarray) is acceptable while, regarding gene expression profiling the use of two fluorescent dyes is needed (two-channel microarray). In the third step, labeled bacterial DNAs (target sequences) and immobilized probes go through the hybridization process. Finally, the hybridized DNA strands (probes and labeled targets) result in fluorescent spots which are detectable by the microarray scanner. In this survey, the authors have designed and constructed a DNA microarray chip for detection and identification of 10 bacterial pathogens.
2. MATERIALS AND METHODS
2.1. Bacterial Samples and Culture Medium (Wet Lab)
In the present investigation, clinical bacterial samples including E. coli, Sh. boydii, Sh. dysenteriae, Sh. flexneri, Sh. sonnei, Salmonella typhi, S. typhimurium, Brucella sp., L. pneumophila, and V. cholera were aerobically inoculated in Luria-Bertani (LB) agar and incubated at 37°C for 48 hours. After a suitable growth of bacterial colonies, the total DNA molecules were extracted. The accuracy of the bacterial samples was confirmed by biochemical tests [31-34].
2.2. DNA Extraction Procedure (Wet Lab)
The process of total DNA extraction was performed in accordance with the modified boiling water protocol suggested by Peng et al. [35]. So, the bacterial colonies were scratched from LB agar and put into a microtube containing 100 μl double distilled water (ddw). The microtube was shaken for dissolving bacterial cells and then was put into the boiling water vertically for 10 minutes. The tube was floating in the boiling water via the cork. In the next step, the microtube containing bacterial cells and ddw was centrifuged for 10 minutes at 13000 rpm. Finally, the supernatant containing DNAs was put into a new sterile microtube for the following steps and the pellet was omitted. The DNA purity was checked via nanodrop spectrophotometer. The appropriate OD range is reported from 1.7 to 2.0. These processes were done for each bacterial sample, separately [36].
2.3. Microarray Long Oligo Probe Designing (Dry Lab)
Each of the long oligo microarray probe was designed and checked by the authors. In brief, the complete genome data for each bacterial sample was downloaded from NCBI ftp and RefSeq complete genome. Then, the related .gbk and .fna files pertaining to each bacterial sample and its close phylogenetic microorganisms were compared by GView server. In this study the PanSeq Server, BLASTn tool, AelleID 7.7 software, and OligoAnalyzer tool were also employed for designing and analyzing the long oligo microarray probe [13, 16-18, 37].
In accordance with the aforementioned methodology, we just got a single one microarray long oligo microarray probe with the Best Quality (Table 1).
Table 1.
A microarray probe designed by AlleleID 7.7 for unique regions.
| Related Gene | Length | Designed Long Oligo Microarray Probes o (5'→3') | Microorganisms |
|---|---|---|---|
| NC_010740.1; 564401-564458/ twin-arginine translocation pathway signal protein | 58 | GTCCTCATGCGAGCGTTTCATCGTTGCGGTCAGGTCTTCTAAAGAATAGTTCAACTGG | Brucella sp. |
| NC_010468.1; 4619941-4619998/ TetR family transcriptional regulator | 58 | AAATAGCCGCCAGTTGTGTATTATTCATATTTGAACTTATTTCGCCTGAAGATAACGC | E. coli |
| NC_006369.1; 2041551-2041612/ putative Apyrase | 62 | AAATGCGGAGATTAGCAAACATAACCAGGTAGAACTCAATATCTATGGACAGAATATCAATC | L. pneumophila |
| 1877374-1877430/ exported phage protein | 57 | TTTGAGTAACTAATTGAAACCTTGGTATATCCAGACTGCTGCCCTTTCTTTCTTAAA | S. enterica |
| NC_003198.1; 4534547-4534606/ hypothetical protein | 60 | CGACCATTGAACCGACAATCTTGCTTATTCCATTACGACAATCACATTCATAGGATTCTT | S. typhi (37) |
| NC_003197.1; 2866027-2866084/ hypothetical protein | 58 | CGGACTCTAACTCTCTAGGGCTCTTATATATTCCTGTGGCAACATATTTAGCGGTAAA | S. typhimurium |
| NC_007613.1; 3805070-3805132/233 bp at 5' side: single-stranded DNA-binding protein80 bp at 3' side: hypothetical protein | 63 | CCCTGTTGTCTCAAAATGGCTAAATGTTGTCTCGGTGTTGTCTCAGAAATTAAAATAAAATCC | Sh. boydii |
| NC_007606.1; 3769778-3769835/ hypothetical protein | 58 | AATATGATAATACCGTTTCCGTGGGTAGCAGCACTCTACAGCGTAAAGTCGTCAATAT | Sh. dysentriae |
| NC_004741.1; 3520449-3520506/chaperone,Capsule protein fraction 1,pili assembly chaperone protein SafB,Gram-negative pili assembly chaperone, N-terminal domain | 58 | GCCAGGATATTAAATGGTCTGTGATTACTGATGAAGGTGGTGAGAGTCGTTTGTTTAT | Sh. flexneri |
| NC_007384.1; 1664092-1664150 / putative methylase | 59 | GCTTGGATGAGTTTGGTATTGCTCGAATCTGTACTAATGCCACTATGTGTAAAGGTAAA | Sh. sonnei |
|
NC_012578.1; 15648-15704/ 10 bp at 5' side: _nteric oxidoreductase, YhdH/YhfP family protein182 bp at 3' side: threonine ammonia-lyase, biosynthetic |
57 | CCCTCTGTTATGCCTTTTCTACCTGAATGGACAAGTAGTCGGGTATGGATGGTAAAG | V. cholerae |
The designed probes were ordered by Bioneer Company (South Korea) for synthesis. Besides, the probes were modified by its 5' end with a six-carbon-spacer and a NH2 group [27, 38].
2.4. DNA (Target Sequence) Labeling (Wet Lab)
The extracted total DNAs were amplified and labeled in accordance with NimbleGen (Roche) protocol [39].A single color of Cy3 as one-channeled DNA microarray was used in the present study. The concentration of each bacterial DNA (including E. coli, Sh. boydii, Sh. dysenteriae, Sh. flexneri, Sh. sonnei, V. cholerae, S. typhi, S. typhimurium, Brucella sp., and L. pneumophila,) was adjusted. So, the related amounts were taken from each bacterial DNA respectively, put into an RNase and DNase free microtube and mixed with each other. 40 μl Cy3 (the cyanine dyes) was added to the mixed bacterial genomes. Finally, the PCR grade water was added to the mixture of Cy3 and bacterial genomes. The total volume of the mixture was reached up to 80 μl. This process was performed in a dark room for avoiding Cy3 destruction. The final mixture was completely mixed through up-and-down performance and incubated in PCR thermocycler at 98°C for 10 minutes. Right after, the incubated mixture was put into the ice for 2 minutes.
Then, a mixture of 8 μl PCR grade water, 10 μl dNTP and 2 μl Klenow fragment (with a total volume of 20 μl) was added to the fluorescent bacterial genomic DNA. The final mixture with the volume of 100 μl was completely dissolved by up-and-down technique. Next, it was incubated in PCR thermocycler at 37°C for 2h and in follow, 21.5 μl stopsolution and 110 μl isopropanol were respectively added to the incubated mixture. The latter was incubated at lab temperature (25°C) for 10 minutes. After incubation, the mixture was centrifuged at 12000 rpm for 10 minutes. The supernatant was discharged and 500 μl from cold ethanol 80% was added to the pellet. The pellet was suspended within the ethanol and then centrifuged at 12000 rpm for 2 minutes. Again, the supernatant was discharged and the microtube was put opened. The opened and dried microtube was centrifuged by the DNA vacuum concentrator at 3000 rpm for 5 minutes. Finally, 25 μl PCR grade water was added to the bacterial DNA mixture pellet and its OD was measured by nanodrop spectrophotometer as 543 nm.
2.5. Probe Spotting on Microarray Chip (Wet Lab)
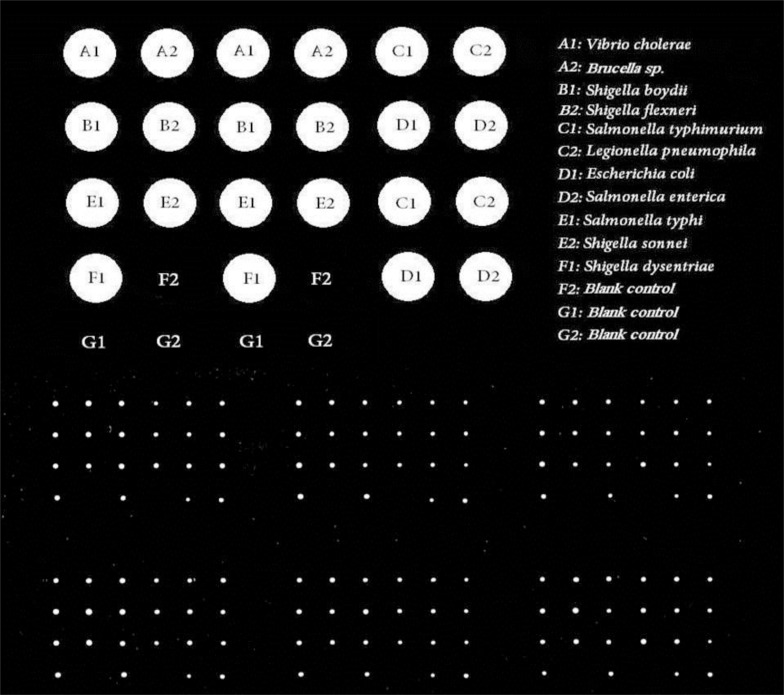
According to the SCHOTT protocol, 17X30 μl containing 15 μl 50 pmol of designed long oligo microarray probes and 15 μl spotting buffer were added to 14 wells (A1-A2, B1-B2, C1-C2, D1-D2, E1-E2, F1-F2, and G1-G2) of a 384-well microplate. The 3 other wells including F2, G1-G2 were filled by spotting buffer as the blank controls in a layout of 2X4. The microplate and epoxy silane covered chip (Nexterion® Slide E, SCHOTT, Germany) were placed into the Qarraymini spotter (Genetix, UK)). The spotter was programmed by Qarray software and the DNA probes were spotted onto Nexterion® Slide E in quadruplicate at 25 °C and 45% humidity. So, the probe was printed in a layout of 4X2 on the chip for quadruplicate [40].
A1-A2, B1-B2, C1-C2, D1-D2, E1-E2, F1-F2, and G1-G2 wells were filled by the designed long oligo microarray probes. The F2, G1 and G2 wells were filled by spotting buffer as blank control samples. The spotted chip was incubated at lab temperature in 30 minutes and then was incubated in humidity chamber with 45% humidity for an overnight. Then, the spotted chip was washed to remove the unchained printed probes. The unfastened probes might be the reason for interference and noise in hybridization process. The chip was rinsed at lab temperature with three different buffer substances including 0.1% Triton® X-100 (once for 5 minutes), 1 mM HCl solution (twice for 2 minutes), 100 mM KCl solution (once for 10 minutes), respectively. The washed printed chip was then incubated in blocking solution at 50°C for 15 minutes to fasten bound probes. The treated chip was then rinsed by deionized water (DIW) at lab temperature for 1 minute. Finally, the spotted Nexterion® Slide E was dried via centrifugation at 1300 rpm for 5 minutes [40-42].
2.6. Hybridization and Analysis Processes (Wet Lab)
The hybridization process was performed by TrayMixTM S4 hybridization station (BioTray). 2 μl of the labeled bacterial DNA mixture was mixed with 8 μl PCR grade water within a microtube. The 10 μl-volume-mixture was then incubated in PCR thermocycler at 95°C for 3 minutes. The incubated mixture was added into TrayMixTM S4 and processed according to the programmed software. The prehybridization process was done at 42°C for 5 minutes. Right after, the hybridization process was performed for 3.5 hours by Nexterion® Hyb at 42°C. By the end of hybridization process, the post hybridization rinsing was achieved by TrayMixTM S4 at 25 °C. The hybridized chip was rinsed by solution I (2XSSC + SDS 0.2%) (once for 10 minutes), solution II (2XSSC) (once for 10 minutes), and then solution III (0.2XSSC) (once for 10 minutes), respectively. At the end, the spotted Nexterion® Slide E was dried via centrifugation at 1300 rpm for 5 minutes [40, 41]. The dried chip was scanned at the resolution of 10μm by Innoscan 710 and Mapix software (Innopsys, Midi-Pyrénées area of France). All the procedures were performed thrice per genome.
3. RESULTS
In this study the best designed microarray probes were successfully worked (Table 1).
It is clear that, the probes which were recognized as the Best Options by AlleleID software were selected. The scanning results confirmed the qualified specificity of the probe. As Fig. (1) indicates, the Cy3 labeled genomic DNAs belonging to bacterial samples had successful hybridization. This result confirms the qualified specificity of the designed microarray long oligo probe. No hybridization has happened in blank controls (Fig. 1).
Fig. (1).
Resolution of 10μm by Innoscan 710. The labeled bacterial DNAs matched with their specific designed probe (successful hybridization); while, the control spots (blank spotting buffer) shows no successful match with the spotted probe. There are 6 arrays of 24-spotted array which is illustrated in Fig. (1).
4. DISCUSSION
A rapid and reliable diagnostic technique is the main goal of the clinical laboratories' experts. Simultaneously, sensitivity, specificity and reproducibility are other preferred options in clinical diagnostics. Among several molecular biology tools, DNA microarray technology is a proper and appropriate technique which enables us to detect and identify a wide range of pathogenic and non-pathogenic microbial agents at once. As the pan-genomic technology of microarray is able to reveal a huge number of genomic content dissimilarities within determined microbial strains, it seems to be an invaluable tool for detection and identification of close microbial agents [43-48]. There are different types of nucleic acid based techniques like conventional PCR, multiplex PCR and etc.; but when there are a huge number of specimens so; PCR techniques will be expensive and time consuming. The sensitivity, specificity and accuracy of the DNA microarray techniques are too sharp. Construction and designing a local microarray chip makes it flexible and effective. Because when a microarray chip is designed in a local lab, it can be useful to be upgraded for genotyping. This idea makes the microarray technology cheap, reliable and accessible. In other words, DNA microarray technology is known as a lab on chip technique which is applicable for several thousand specimens at once [10, 13, 14, 16, 18, 23, 37, 49].
The previous studies report that the sensitivity of microarray is determined by DNA labeling kit. It ranges between 1.0 ng and 10 μg DNA. In our study, the NimbleGen (Roche) kit was used for bacterial DNA labeling; which its sensitivity was 1 μg DNA [39, 43, 50].
By the use of DNA microarray we were able to detect and identify the bacterial samples within 3 days which was significantly shorter than several clinical techniques [43].
The in-silico part of microarray technique which is in association with microarray probe designing has a considerable importance on the quality of the microarray outcomes. In other words, the ability of probe designer in association with microbial phylogenetic relationships and Bioinformatics determines the accuracy and reliability of microarray tool results [10, 14, 16, 18, 37, 38, 41, 46].
The use of microarray long oligo probes increases the specificity of microarray probes which may lead to have a high quality outcome. For this reason we designed a long oligo probe with a length of 58 nucleotides [46, 51].
The pan-genomic diagnostic tool of microarray is based on genes and other genomic elements; thus selection of different unique genes as suitable target sequences increases the sharpness of the work. So, in the present study, the unique genes were significantly selected for each bacterial agent [32, 38, 44].
CONCLUSION
Our chip was capable of identifying all 10 bacterial agents tested simultaneously.The knowledge of Bioinformatics affects directly on the quality of microarray technology. As the result, there is a very interesting possibility for detecting and identifying bacterial agents and even antibiotic resistant strains. Therefore, the art of probe designing makes DNA microarray tool flexible and powerful diagnostic method with high accuracy, sensitivity and specificity within the shortest time.
ACKNOWLEDGEMENT
The present study was financially supported by the grant which was made available by the Health and Medical education Ministry and the Deputy of Research and Technology, Tehran, Iran.
We would like to thank from the “Clinical Research Development Center of Baqiyatallah hospital” for their kindly cooperation. This study was financially supported in part by “Clinical Research Development Center of Baqiyatallah hospital”.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Ranjbar R., Rahbar M., Naghoni A., Farshad S., Davari A., Shahcheraghi F. A cholera outbreak associated with drinking contaminated well water. Arch. Iran Med. 2011;14(5):339–340. [PubMed] [Google Scholar]
- 2.Pourshafie M.R., Bakhshi B., Ranjbar R., Sedaghat M., Sadeghifard N., Zaemi Yazdi J., Parzadeh M., Raesi J. Dissemination of a single Vibrio cholerae clone in cholera outbreaks during 2005 in Iran. J. Med. Microbiol. 2007;56(Pt 12):1615–1619. doi: 10.1099/jmm.0.47218-0. [DOI] [PubMed] [Google Scholar]
- 3.Hosseini M.J., Ranjbar R., Ghasemi H., Jalalian H.R. The prevalence and antibiotic resistance of Shigella sp. recovered from patients admitted to Bouali Hospital, Tehran, Iran during 1999-2001. Pak. J. Biol. Sci. 2007;10(16):2778–2780. doi: 10.3923/pjbs.2007.2778.2780. [DOI] [PubMed] [Google Scholar]
- 4.Ranjbar R., Soltan-Dallal M.M., Pourshafie M.R., Mammina C. Antibiotic resistance among Shigella serogroups isolated in Tehran, Iran (2002-2004). J. Infect. Dev. Ctries. 2009;3(8):647–648. doi: 10.3855/jidc.560. [DOI] [PubMed] [Google Scholar]
- 5.Filliol-Toutain I., Chiou C-S., Mammina C., Gerner-Smidt P., Thong K-L., Phung D.C., Pichel M., Ranjbar R., Sow A.G., Cooper K., Ribot E., Binsztein N., Liang S.Y. Global distribution of shigella sonnei clones. Emerg. Infect. Dis. 2011;17(10):1910–1912. doi: 10.3201/eid1710.101486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranjbar R., Giammanco G.M., Farshad S., Owlia P., Aleo A., Mammina C. Serotypes, antibiotic resistance, and class 1 integrons in Salmonella isolates from pediatric cases of enteritis in Tehran, Iran. Foodborne Pathog. Dis. 2011;8(4):547–553. doi: 10.1089/fpd.2010.0736. [DOI] [PubMed] [Google Scholar]
- 7.Momtaz H, Karimian A, Madani M, et al. Uropathogenic Escherichia coli in Iran: serogroup distributions, virulence factors and antimicrobial resistance properties. Annals Clinical Microbiol. Antimicrob. 2013;12(1):8/1–12. doi: 10.1186/1476-0711-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranjbar R., Naghoni A., Afshar D., Nikkhahi F., Mohammadi M. Rapid molecular approach for simultaneous detection of salmonella spp., Shigella spp., and Vibrio cholera. Osong Public Health Res. Perspect. 2016;7(6):373–377. doi: 10.1016/j.phrp.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranjbar R., Karami A., Farshad S., Giammanco G.M., Mammina C. Typing methods used in the molecular epidemiology of microbial pathogens: a how-to guide. New Microbiol. 2014;37(1):1–15. [PubMed] [Google Scholar]
- 10.Behzadi P., Behzadi E., Ranjbar R. Microarray probe set: Biology, bioinformatics and biophysics. Alban Med J. 2015;2:78–83. [Google Scholar]
- 11.Yoo C., Cooper G.F. A computer-based microarray experiment design-system for gene-regulation pathway discovery. AMIA Annu. Symp. Proc. 2003:733–737. [PMC free article] [PubMed] [Google Scholar]
- 12.Kay E., Wren B.W. Recent advances in systems microbiology. Curr. Opin. Microbiol. 2009;12(5):577–581. doi: 10.1016/j.mib.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Jahandeh N., Ranjbar R., Behzadi P., Behzadi E. Uropathogenic Escherichia coli virulence genes: invaluable approaches for designing DNA microarray probes. Cent. European J. Urol. 2015;68(4):452–458. doi: 10.5173/ceju.2015.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behzadi P., Behzadi E. Uropathogenic Escherichia coli: An ideal resource for DNA microarray probe designing.; International Conference on Bioinformatics and Biomedical Engineering; 2017. pp. 12–9. [DOI] [Google Scholar]
- 15.Rojas I., Ortuño F. Bioinformatics and Biomedical Engineering: 5th International Work-Conference, IWBBIO 2017, Granada, Spain, April 26–28, 2017; Springer: Proceedings; 2017. [Google Scholar]
- 16.Behzadi P., Najafi A., Behzadi E., Ranjbar R. Microarray long oligo probe designing for Escherichia coli: An in-silico DNA marker extraction. Cent. European J. Urol. 2016;69(1):105–111. doi: 10.5173/ceju.2016.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranjbar R., Behzadi P., Mammina C. Respiratory tularemia: Francisella tularensis and microarray probe designing. Open Microbiol. J. 2016;10:176–182. doi: 10.2174/1874285801610010176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behzadi P., Behzadi E., Alavian S.M. DNA microarray technology in HBV genotyping. Minerva Med. 2017;108(5):473–476. doi: 10.23736/S0026-4806.17.05059-5. [DOI] [PubMed] [Google Scholar]
- 19.Parisot N, Peyretaillade E, Dugat-Bony E, Denonfoux J, Mahul A, Peyret P. Probe design strategies for oligonucleotide microarrays. Microarray Technol.: Methods Appli. 2016:67–82. doi: 10.1007/978-1-4939-3136-1_6. [DOI] [PubMed] [Google Scholar]
- 20.Marzancola MG, Sedighi A, Li PC. DNA microarray-based diagnostics. Microarray Technol: Methods and Applications. 2016:161–178. doi: 10.1007/978-1-4939-3136-1_12. [DOI] [PubMed] [Google Scholar]
- 21.Al-Khaldi S.F., Villanueva D., Chizhikov V. Identification and characterization of clostridium perfringens using single target DNA microarray chip. Int. J. Food Microbiol. 2004;91(3):289–296. doi: 10.1016/j.ijfoodmicro.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Bodrossy L., Sessitsch A. Oligonucleotide microarrays in microbial diagnostics. Curr. Opin. Microbiol. 2004;7(3):245–254. doi: 10.1016/j.mib.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Behzadi P., Ranjbar R., Alavian S.M. Nucleic acid-based approaches for detection of viral hepatitis. Jundishapur J. Microbiol. 2014;8(1):e17449. doi: 10.5812/jjm.17449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ranjbar R., Tabatabaee A., Behzadi P., Kheiri R. Enterobacterial repetitive intergenic consensus polymerase chain reaction (ERIC-PCR) genotyping of escherichia coli strains isolated from different animal stool specimens. Iran. J. Pathol. 2017;12(1):25–34. [PMC free article] [PubMed] [Google Scholar]
- 25.Ranjbar R., Behzadi P., Farshad S. Advances in diagnosis and treatment of Helicobacter pylori infection. Acta Microbiol. Immunol. Hung. 2017;64(3):273–292. doi: 10.1556/030.64.2017.008. [DOI] [PubMed] [Google Scholar]
- 26.Behzadi E., Behzadi P., Sirmatel F. Identification of 30-kDa heat shock protein gene in Trichophyton rubrum. Mycoses. 2009;52(3):234–238. doi: 10.1111/j.1439-0507.2008.01561.x. [DOI] [PubMed] [Google Scholar]
- 27.Technologies ID, editor. 5th. Strategies for attaching oligonucleotides to solid supports. pp. 1–19. [Google Scholar]
- 28.Devor E.J., Behlke M.A. Strategies for attaching oligonucleotides to solid supports. IDT DNA Rep; 2005. pp. 1–24. [Google Scholar]
- 29.Kumar A, Larsson O, Parodi D, Liang Z. Silanized nucleic acids: a general platform for DNA immobilization. Nucleic Acids Res. 2000 doi: 10.1093/nar/28.14.e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo Z., Guilfoyle R.A., Thiel A.J., Wang R., Smith L.M. Direct fluorescence analysis of genetic polymorphisms by hybridization with oligonucleotide arrays on glass supports. Nucleic Acids Res. 1994;22(24):5456–5465. doi: 10.1093/nar/22.24.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farshad S., Ranjbar R., Japoni A., Hosseini M., Anvarinejad M., Mohammadzadegan R. Microbial susceptibility, virulence factors, and plasmid profiles of uropathogenic Escherichia coli strains isolated from children in Jahrom, Iran. Arch. Iran Med. 2012;15(5):312–316. [PubMed] [Google Scholar]
- 32.Bekal S., Brousseau R., Masson L., Prefontaine G., Fairbrother J., Harel J. Rapid identification of Escherichia coli pathotypes by virulence gene detection with DNA microarrays. J. Clin. Microbiol. 2003;41(5):2113–2125. doi: 10.1128/JCM.41.5.2113-2125.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behzadi P., Behzadi E. Study of microbial biochemical reactions in bacteriology laboratories. 1st ed. Tehran: Persian Science & Research Publisher; 2012. [Google Scholar]
- 34.Ranjbar R., Bolandian M., Behzadi P. Virulotyping of Shigella spp. isolated from pediatric patients in Tehran, Iran. Acta Microbiol. Immunol. Hung. 2017;64(1):71–80. doi: 10.1556/030.64.2017.007. [DOI] [PubMed] [Google Scholar]
- 35.Peng X., Yu K-Q., Deng G-H., Jiang Y-X., Wang Y., Zhang G-X., Zhou H.W. Comparison of direct boiling method with commercial kits for extracting fecal microbiome DNA by Illumina sequencing of 16S rRNA tags. J. Microbiol. Methods. 2013;95(3):455–462. doi: 10.1016/j.mimet.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Simona E-S., Diana P., Robertina I., Ionela A., Ileana S., Tatiana V-D. Molecular identification of some yeast strains involved in oral candidosis. Rom. Biotechnol. Lett. 2009;14:4180–4186. [Google Scholar]
- 37.Behzadi P., Ranjbar R. Microarray long oligo probe designing for Bacteria: An in silico pan-genomic research. Alban Med J. 2016;2:5–11. [Google Scholar]
- 38.Najafi A., Ram M., Ranjbar R. Microarray: Principles & Applications. 1st ed. Tehran: Persian Science & Research Publisher; 2012. [Google Scholar]
- 39. NimbleGen Arrays User’s Guide: Gene Expression Arrays. In: Roche N, editor. 6.0 ed 2011. [Google Scholar]
- 40.Nexterion® Slide E DNA application. Protocol. 2010. Available at: http://www.schott.com/d/nextrion/27080efb-c4d9-49a9-a53e-ed2b8dce68bd/1.0/protocol_slide_e_1_3.pdf.
- 41.Lukjancenko O., Ussery D.W. Design of an enterobacteriaceae pan-genome microarray chip. computational systems-biology and bioinformatics. Springer; 2010. pp. 165–179. [DOI] [Google Scholar]
- 42.User Guide - QArraymini - Issue 2, Genetix Ltd. 2006. Available at: http://www.genetix.com.
- 43.Cao B., Liu X., Yu X., Chen M., Feng L., Wang L. A new oligonucleotide microarray for detection of pathogenic and non-pathogenic Legionella spp. PLoS One. 2014;9(12):e113863. doi: 10.1371/journal.pone.0113863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawrence J.G., Hendrickson H. Genome evolution in bacteria: order beneath chaos. Curr. Opin. Microbiol. 2005;8(5):572–578. doi: 10.1016/j.mib.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Welch R.A., Burland V., Plunkett G., III, Redford P., Roesch P., Rasko D., Buckles E.L., Liou S.R., Boutin A., Hackett J., Stroud D., Mayhew G.F., Rose D.J., Zhou S., Schwartz D.C., Perna N.T., Mobley H.L., Donnenberg M.S., Blattner F.R. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA. 2002;99(26):17020–17024. doi: 10.1073/pnas.252529799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salehi T.Z., Tonelli A., Mazza A., Staji H., Badagliacca P., Tamai I.A., Jamshidi R., Harel J., Lelli R., Masson L. Genetic characterization of Escherichia coli O157:H7 strains isolated from the one-humped camel (Camelus dromedarius) by using microarray DNA technology. Mol. Biotechnol. 2012;51(3):283–288. doi: 10.1007/s12033-011-9466-7. [DOI] [PubMed] [Google Scholar]
- 47.Behzadi P., Behzadi E., Ranjbar R. IL-12 family cytokines: General characteristics, pathogenic microorganisms, receptors, and signalling pathways. Acta Microbiol. Immunol. Hung. 2016;63(1):1–25. doi: 10.1556/030.63.2016.1.1. [DOI] [PubMed] [Google Scholar]
- 48.Behzadi E., Behzadi P. The role of toll-like receptors (TLRs) in urinary tract infections (UTIs). Cent. European J. Urol. 2016;69(4):404–410. doi: 10.5173/ceju.2016.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zvara Á., Kitajka K., Faragó N., Puskás L.G. Microarray technology. Acta Biol. Szeged. 2015;59(1):51–67. [Google Scholar]
- 50.Uda A., Tanabayashi K., Fujita O., Hotta A., Yamamoto Y., Yamada A. Comparison of whole genome amplification methods for detecting pathogenic bacterial genomic DNA using microarray. Jpn. J. Infect. Dis. 2007;60(6):355–361. [PubMed] [Google Scholar]
- 51.Relógio A, Schwager C, Richter A, Ansorge W, Valcárcel J. Optimization of oligonucleotide-based DNA microarrays. Nucleic Acids Res. 2002;30(11) doi: 10.1093/nar/30.11.e51. [DOI] [PMC free article] [PubMed] [Google Scholar]