Fleas play critical roles in transmitting some infections among animals and from animals to humans. Detection of pathogens in fleas is important to determine human risks for flea-borne diseases and can help guide diagnosis and treatment. Our findings of high prevalence rates of B. elizabethae and R. felis in fleas in the Arua and Zombo districts of Uganda implicate these agents as potential causative agents of undiagnosed febrile illnesses in this area.
KEYWORDS: Bartonella, Rickettsia, Uganda, Yersinia, fleas, off-host, rodents
ABSTRACT
Fleas (n = 407) were collected from small mammals trapped inside huts and surroundings of homesteads in five villages within the Arua and Zombo districts of Uganda. The most common flea species were Dinopsyllus lypusus (26%) and Xenopsylla cheopis (50%). Off-host fleas (n = 225) were collected inside huts by using Kilonzo flea traps. The majority of the off-host fleas were Ctenocephalides felis (80%). All fleas were examined for the presence of Bartonella spp., Rickettsia spp., and Yersinia spp. Bartonella DNA was detected in 91 fleas, with an overall prevalence of 14%. Bartonella prevalence was significantly higher in rodent or shrew fleas than in off-host fleas (22% versus 1%). The majority of Bartonella-positive fleas were of the species D. lypusus (61%), X. cheopis (20%), and Ctenophthalmus calceatus (14%). Sequencing analysis identified 12 Bartonella genetic variants, 9 of which belonged to the zoonotic pathogen B. elizabethae species complex. Rickettsia DNA was detected in 143 fleas, giving an overall prevalence of 23%, with a significantly higher prevalence in off-host fleas than in rodent or shrew fleas (56% versus 4%). The majority (88%) of Rickettsia-positive fleas were C. felis and were collected from Kilonzo traps, while a small portion (10%) were X. cheopis collected from rodents. Sequencing analysis identified six Rickettsia genogroups that belonged either to zoonotic R. felis or to the closely related “Candidatus Ricksettia asemboensis” and “Candidatus Ricksettia sengalensis.” Yersinia DNA was not detected in the fleas tested. These observations suggested that fleas in northwestern Uganda commonly carry the zoonotic agents B. elizabethae and R. felis and potentially play an important role in transmitting these infections to humans.
IMPORTANCE Fleas play critical roles in transmitting some infections among animals and from animals to humans. Detection of pathogens in fleas is important to determine human risks for flea-borne diseases and can help guide diagnosis and treatment. Our findings of high prevalence rates of B. elizabethae and R. felis in fleas in the Arua and Zombo districts of Uganda implicate these agents as potential causative agents of undiagnosed febrile illnesses in this area.
INTRODUCTION
Fleas are well-known vectors that can transmit infectious pathogens to humans, including the etiologic agents of plague, bartonellosis, and rickettsiosis. Plague, caused by the highly pathogenic bacterium Yersinia pestis, was responsible for three great pandemics that killed millions of people around the world. Currently, plague is still endemic in Africa, Central Asia, and South and North America. According to WHO, more than 2,500 cases are reported each year from different regions, with nearly 80% of cases from Africa. Northwestern Uganda, often referred to as the West Nile region, is a region where plague is endemic, with more than 2,400 suspect plague cases reported between 1999 and 2011 (1). Multiple studies on fleas have been conducted in this area (2–5).
The genus Bartonella, with more than 30 species identified, has drawn increased attention as more and more reported clinical illnesses have been associated with Bartonella infections (6–11). Rickettsia felis, the causative agent of flea-borne spotted fever, is considered an important neglected agent in sub-Saharan Africa (12). Both bartonelloses and rickettsioses are widely regarded as emerging/reemerging diseases (13). Many studies have reported Bartonella spp. in a variety of mammalian species and their ectoparasites, all over the world (14–20). In the Democratic Republic of Congo, Sackal et al. (21) reported the detection of Bartonella spp. and R. felis in Ctenocephalides felis and Pulex irritans collected from flea traps. Billeter et al. (22) identified Bartonella species in invasive and indigenous rodents and their fleas from Uganda. Surprisingly, the prevalence of Bartonella infections in invasive rats (Rattus rattus) reported in that study was very low (1.3%), a finding that stands in stark contrast to results of a study conducted in neighboring Kenya, in which the Bartonella prevalence was 13% in Rattus spp. (19). In addition, R. felis and “Candidatus Rickettsia asemboensis” were detected in fleas collected in Kenya, Tanzania, Ethiopia, and Uganda (13, 23–25).
Although Bartonella spp. and Rickettsia spp. have been reported in countries neighboring Uganda, information regarding the distribution of Bartonella spp., Rickettsia spp., and Yersinia spp. in fleas from Uganda is very limited and requires a better understanding, as each of these agents is likely to cause disease in humans. In this study, we evaluated the prevalence rates and characterized the genetic identities of Bartonella spp., Rickettsia spp., and Yersinia spp. in fleas collected from the West Nile region of Uganda.
RESULTS
Fleas.
A total of 632 fleas belonging to 10 species were collected (Table 1). Of these, 407 fleas were collected from eight species of rodents (Aethomys hindei, Aethomys kaiseri, Arvicanthis niloticus, Lophuromys sikapusi, Mastomys spp., Mus spp., Rattus rattus, and Tatera valida) and from shrews (Crocidura spp.) (Table 2). The fleas belonged to eight species, including Ctenocephalides felis (n = 1), Ctenophthalmus calceatus (n = 44), Dinopsyllus longifrons (n = 2), D. lypusus (n = 106), Stivalius torvus (n = 11), Xenopsylla brasiliensis (n = 40), X. cheopis (n = 202), and Xenopsylla nubica (n = 1) (Table 1). The other 225 fleas were collected from Kilonzo traps and belonged to seven species, including C. felis (n = 181), D. lypusus (n = 1), Echinophaga gallinacea (n = 30), S. torvus (n = 1), Tunga penetrans (n = 3), X. brasiliensis (n = 3), and X. cheopis (n = 6) (Table 1).
TABLE 1 .
Occurrence of Bartonella spp. and Rickettsia spp. in fleas collected from Kilonzo traps and from small mammals, 2002 to 2013, Uganda
| Flea species | No. collected (% of total) |
From Kilonzo traps |
From small mammals |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n |
Bartonella |
Rickettsia |
n |
Bartonella |
Rickettsia |
||||||
| No. (%) positive |
% prevalence |
No. (%) positive |
% prevalence |
No. (%) positive |
% prevalence |
No. (%) positive |
% prevalence |
||||
| Ctenocephalides felis | 182 (29) | 181 | 1 (33) | 0.6 | 125 (99) | 69.1 | 1 | 0 (0) | 0 | 1 (6) | 100 |
| Ctenophthalmus calceatus | 44 (7) | 0 | 0 (0) | NAa | 0 (0) | NA | 44 | 12 (14) | 27 | 1 (6) | 2 |
| Dinopsyllus longifrons | 2 (0) | 0 | 0 (0) | NA | 0 (0) | NA | 2 | 2 (2) | 100 | 0 (0) | 0 |
| Dinopsyllus lypusus | 107 (17) | 1 | 0 (0) | 0 | 0 (0) | 0 | 106 | 54 (61) | 51 | 1 (6) | 0 |
| Echinophaga gallinaceae | 30 (5) | 30 | 0 (0) | 0 | 0 (0) | 0 | 0 | 0 (0) | NA | 0 (0) | NA |
| Stivalius torvus | 12 (2) | 1 | 1 (33) | 100 | 0 (0) | 0 | 11 | 1 (1) | 9 | 0 (0) | 0 |
| Tunga penetrans | 3 (0) | 3 | 0 (0) | 0 | 0 (0) | 0 | 0 | 0 (0) | NA | 0 (0) | NA |
| Xenopsylla brasiliensis | 43 (7) | 3 | 0 (0) | 0 | 0 (0) | 0 | 40 | 1 (1) | 3 | 0 (0) | 0 |
| Xenopsylla cheopis | 208 (33) | 6 | 1 (33) | 16.7 | 1 (1) | 16.7 | 202 | 18 (20) | 9 | 14 (97) | 7 |
| Xenopsylla nubica | 1 (0) | 0 | 0 (0) | NA | 0 (0) | NA | 1 | 0 (0) | 0 | 0 (0) | 0 |
| Total | 632 | 225 | 3 | 1.3 | 126 | 56 | 407 | 88 | 22 | 17 | 4 |
NA, not applicable.
TABLE 2 .
Flea species found on small mammals and detection of Bartonella spp. in the fleas
| Order and species of small mammal |
No. of fleas found (no. positive for Bartonella) |
||||||
|---|---|---|---|---|---|---|---|
| Total | C. cabirus | D. lypusus | S. torvus | X. brasiliensis | X. cheopis | Other speciesa |
|
| Order Rodentia | |||||||
| Aethomys hindei | 9 (6) | 1 (1) | 8 (5) | ||||
| Aethomys kaiseri | 4 (2) | 2 (1) | 2 (1) | ||||
| Arvicanthis niloticus | 72 (32) | 28 (10) | 35 (21) | 2 | 6 (1) | 1 | |
| Lophuromys sikapusi | 3 (1) | 3 (1) | |||||
| Mastomys natalensis | 44 (26) | 4 | 25 (17) | 1 | 1 (1) | 11 (6) | 2 (2) |
| Mus spp. | 1 (0) | 1 (0) | |||||
| Rattus rattus | 208 (10) | 2 | 19 (6) | 37 | 148 (3) | 2 (1) | |
| Tatera valida | 9 (6) | 8 (6) | 1 | ||||
| Order Insectivora | |||||||
| Crocidura spp. | 57 (5) | 5 | 16 (2) | 9 (1) | 26 (2) | 1 | |
| Total | 407 (88) | 42 (11) | 106 (54) | 11 (1) | 40 (1) | 202 (18) | 6 (3) |
The other species included C. felis, C. bacopus, D. longifrons, and X. nubica. These were combined due to the very small number of each species collected.
Among all tested fleas, X. cheopis, C. felis, and D. lypusus were the most common species, accounting for 33% (208/632), 29% (182/632), and 17% (107/632), respectively. Both X. cheopis and D. lypusus fleas were mainly collected from small mammals (97% [202/208] and 99% [106/107], respectively) rather than from Kilonzo traps (3% [6/208] and 1% [1/107], respectively). The majority of X. cheopis fleas (73% [148/202]) were collected from R. rattus, while the D. lypusus fleas were mainly collected from A. niloticus (33% [35/106]), Mastomys natalensis (24% [25/106]), R. rattus (18% [19/106]), and Crocidura spp. (15% [16/106]). All but one C. felis flea (181 of 182) were collected from Kilonzo traps. All C. calceatus fleas (n = 44) were collected from multiple species of rodents, and all E. gallinacea fleas (n = 30) were collected from Kilonzo traps (Table 1).
Bartonella infections.
Bartonella DNA was detected in 91 of the 632 tested fleas, with an overall prevalence of 14%. Among the positive fleas, 88 (97%) were collected from small mammals; only 3 were collected from Kilonzo traps. The Bartonella prevalence was 22% (88/407) in fleas collected from small mammals and 1% (3/225) in fleas collected from Kilonzo traps, showing a significant difference between the two sources (χ2 = 38.4, P < 0.01). The Bartonella-infected fleas belonged to eight species (Table 1). Compared to other flea species, D. lypusus and C. calceatus fleas had a higher rate of Bartonella infection, with 51% (54/107) and 27% (12/44) of individuals infected, respectively. Of the most prevalent flea species found on rodents, X. cheopis, only 9% (18/202) of fleas of this species were found to be infected with Bartonella spp. Interestingly, only 3 of the 18 positive X. cheopis fleas were collected from black rats, which were the main hosts of X. cheopis in this study (Table 2). In other words, only 3 of 148 X. cheopis fleas (2%) collected from black rats were infected with Bartonella spp. X. brasiliensis fleas also had a very low infection rate for Bartonella species, with only 1 positive flea among 43 tested fleas (2%). The three Bartonella-positive fleas that were collected from Kilonzo traps were of the species C. felis, S. torvus, and X. cheopis. Notably, only 1 of 182 C. felis fleas was infected with Bartonella. None of the 30 E. gallinacea fleas, all of which were collected from Kilonzo traps, was infected (Table 1).
Rickettsia infections.
Rickettsia DNA was detected in 143 of the 632 tested fleas, giving an overall prevalence of 23%. Nearly all of the Rickettsia-infected fleas were of the species C. felis (88%; 126/143) or X. cheopis (10%; 14/143) (Table 1). In contrast to Bartonella-infected fleas, the majority (88%; 126/143) of Rickettsia-positive fleas were collected from Kilonzo traps. Only 17 of 173 Rickettsia-positive fleas (12%) were collected from rodents. The prevalence of Rickettsia in off-host fleas was 56% (126/225), whereas it was 4% (17/407) in fleas collected from rodents. The prevalence of Rickettsia in off-host fleas was significantly higher than that in fleas collected from rodents (χ2 = 129.9, P < 0.01). Of the 126 Rickettsia-positive fleas that were collected from Kilonzo traps, 125 were C. felis. The prevalence of Rickettsia in C. felis was also extremely high (69%; 125/182). Of the 17 Rickettsia-positive fleas that were collected from small mammals, 14 were X. cheopis. None of the 30 E. gallinacea fleas collected from Kilonzo traps harbored Rickettsia DNA (Table 1).
Yersinia infections.
Neither Y. pestis nor Y. pseudotuberculosis DNA was detected in any of the fleas tested.
Genetic identification of Bartonella and Rickettsia species.
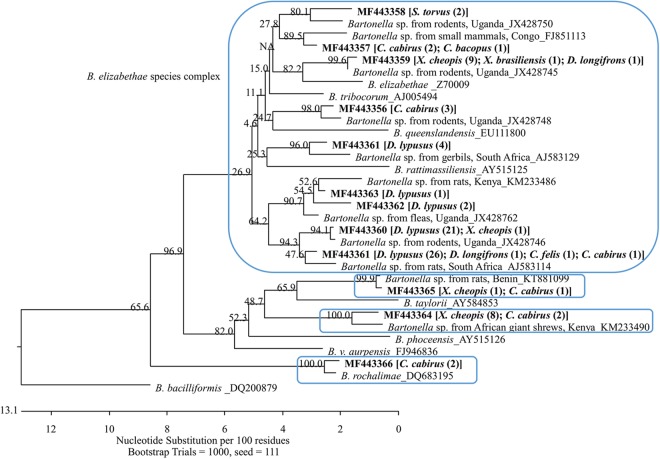
Sequencing analyses were performed based on gltA sequences for both Bartonella and Rickettsia. The 91 Bartonella-positive DNA samples represented 12 distinct genetic variants that have been deposited in GenBank under accession numbers MF443355 to MF443366. These variants were clustered in four major phylogroups (Fig. 1). Nine variants (GenBank accession numbers MF443355 to MF443363) accounted for 77 of the 91 sequences identified, and all of these were relatively close to each other and similar to a few variants previously described in rodents from Uganda (22). These variants are clustered with the so-called Bartonella elizabethae species complex (26). Sequences belonging to this phylogroup were obtained from fleas of all species found positive, with the majority (70%; 54/77) coming from D. lypusus fleas. In fact, all sequences obtained from D. lypusus fleas belonged to this group, showing a specific relationship between this flea species and Bartonella species. Bartonella sequences obtained from the three positive fleas that were collected from Kilonzo traps also belonged to this phylogroup; one variant (GenBank accession number MF443364) was similar to variant KM233490, which was described in an African giant shrew (Crocidura olivieri) from Kenya (19). This variant contained 10 identical sequences that were obtained from X. cheopis (n = 8) and C. calceatus (n = 2) and formed a separate phylogroup. Another variant (GenBank accession number MF443365) was similar to the variant KT881099 from a Mastomys sp. rat in Benin (27). This variant contained only two sequences, which were obtained from one C. calceatus flea (collected from A. niloticus) and one X. cheopis flea (collected from R. rattus), respectively. They are also quite distant from other identified Bartonella genotypes and formed a separate phylogroup. The last variant (GenBank accession number MF443366) contained two sequences, both of which were obtained from C. calceatus fleas that were collected from African grass rats. This variant was similar to Bartonella rochalimae (Fig. 1).
FIG 1 .
Phylogenetic relationships of the 12 Bartonella genetic variants, identified from 91 Bartonella sequences obtained from fleas collected from small mammals or Kilonzo traps in the West Nile region of Uganda, with other Bartonella species based on partial sequences of gltA. Each genetic variant is indicated by its GenBank accession number in boldface, followed by flea species and number of identical sequences obtained from the flea species in brackets and parentheses. The Bartonella variants formed four phylogroups (boxed clades). Nine of the 12 variants fell into the so-called B. elizabethae species complex. The phylogenetic tree was constructed by the neighbor-joining method, and bootstrap values were calculated with 1,000 replicates.
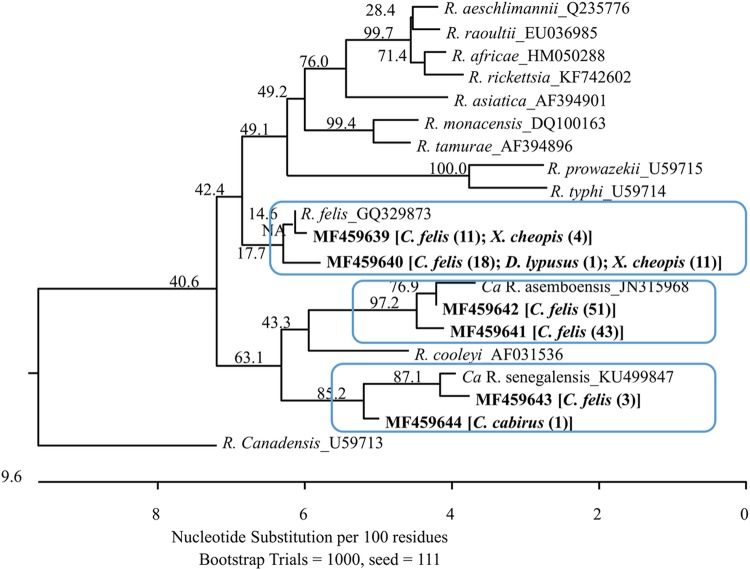
The 143 Rickettsia-positive DNA sequences represented six distinct genetic variants and have been deposited in GenBank under accession numbers MF459639 to MF459644. The six variants were clustered into three phylogroups. Variants MF459639 and MF459640, containing 15 and 30 Rickettsia sequences, respectively, belonged to the zoonotic pathogen R. felis; variants MF459641 and MF459642 were the most common and comprised 43 and 51 of all identified Rickettsia sequences, respectively. These two variants were closely related to “Candidatus R. asemboensis”; the last two variants, MF459643 and MF459644, containing three and one sequence, respectively, were close to “Candidatus Rickettsia sengalensis” (Fig. 2).
FIG 2 .
Phylogenetic relationships of the six Rickettsia genetic variants identified from 143 Rickettsia sequences, obtained from the fleas collected from small mammals or Kilonzo traps in the West Nile region of Uganda, with some other Rickettsia species based on partial sequences of gltA. Each genetic variant is indicated by its GenBank accession number in boldface, followed by the flea species and number of identical sequences obtained from the flea species in brackets and parentheses. The Rickettsia variants formed three phylogroups (boxed clades). Two variants fell into the cluster of R. felis; other variants clustered with either “Candidatus R. asemboensis” or “Candidatus R. sengalensis.” The phylogenetic tree was constructed by the neighbor-joining method, and bootstrap values were calculated with 1,000 replicates.
DISCUSSION
Using molecular approaches, we estimated the presence of multiple potential zoonotic pathogens, including Bartonella spp., Rickettsia spp., and Yersinia spp., in fleas collected from small mammals and those that were host seeking in human habitations in northwestern Uganda, where plague is endemic and occasionally causes outbreaks in the local human population (28).
Not surprisingly, Bartonella spp. were prevalent in the fleas, with an overall prevalence of 14%. The prevalence in fleas collected from small mammals was much higher than in off-host fleas (22% versus 1%). In fact, two of the three Bartonella-positive off-host fleas (S. torvus and X. cheopis) commonly parasitize small mammals (Crocidura sp. and Rattus spp. rats, respectively) and are likely to be found only temporarily off their mammal hosts. It is not clear whether these Bartonella spp. cause pathogenic damage in their animal hosts; it is less likely these fleas seek blood meals from humans. Given the context of assessing human risk, studies of vector competence by various flea species might be necessary. The off-host fleas were mostly comprised of C. felis (80%). In the Democratic Republic of the Congo, Sackal et al. (21) similarly found that only 3.6% of off-host fleas (mainly C. felis and Pulex irritans) were infected by Bartonella spp. The absence of Bartonella in the C. felis fleas in the present study might be related to their off-host origin. Bartonellae are known hemotropic parasites (29) and might not be able to survive in fleas that fail to take frequent blood meals, and starving fleas might be quickly cleared of any Bartonella infection. Another possible explanation is that these C. felis fleas may rarely become infected by Bartonella because of the low level of infection in their presumptive hosts. As common ectoparasites of cats and dogs, C. felis has been reported to carry B. henselae (the causative of Cat scratch disease), B. clarridgeiae, and other Bartonella species (30, 31). In northwestern Uganda, however, C. felis feeds primarily on people, goats, and occasionally on chickens, sheep, guinea pigs, or pigs (32). Previous studies have reported that Bartonella bacterium was not cultured in goats (33) and was not detected in chickens from Kenya (Y. Bai, unpublished data).
Among the fleas collected from rodents, D. lypusus demonstrated an extremely high prevalence (>50%) of Bartonella infection, suggesting that this species of flea may play a major role in transmitting Bartonella infection between rodents and potentially from rodents to humans. Again, the vector competence should be studied for this flea species. Although the prevalence of Bartonella in fleas collected from rodents was relatively high, the prevalence in X. cheopis fleas was quite low, with only 9% of this flea species being found infected. Notably, the X. cheopis fleas collected from black rats, which are the main hosts of the fleas, showed an even lower prevalence, with only 2% infected. Typically, high percentages (>40%) of Rattus spp. animals are infected by Bartonella spp. (14, 15). However, Billeter (22) reported that only 2% of R. rattus animals were infected with Bartonella spp. in another study in Uganda. R. rattus invaded the West Nile region of Uganda recently (34) and may have not had ample time to establish a niche in which Bartonella bacterium can amplify. The competence of X. cheopis is not clear, but field investigations have suggested that Bartonella infection is most likely transmitted between rats by X. cheopis (35). In our study, the low number of X. cheopis fleas that were found to be infected with Bartonella spp. might help explain the very low prevalence of this infection in R. rattus from the West Nile region, previously reported by Billeter (22).
Sequencing analysis demonstrated that at least four Bartonella species or phylogroups are circulating in the flea communities in the region of Uganda where plague is endemic. Most of the Bartonella sequences (85%) obtained from the fleas in this study belonged to the B. elizabethae species complex (26), and several species and strains of this complex have been linked to human illnesses recently (6–10). Importantly, B. elizabethae was found in all flea species that were positive for Bartonella in the present study. Our findings suggested that B. elizabethae might be the causative agent for undiagnosed illnesses in local residents, and it is recommended that further investigations be conducted to verify whether B. elizabethae is indeed a human pathogen in this region of Africa. Remarkably, D. lypusus fleas, a very common species found on rodents, were all infected with B. elizabethae, showing a very specific relationship between this flea species and the Bartonella species. D. lypusus is a known vector of Y. pestis in Kenya (36). Our results suggest that they may also transmit B. elizabethae to humans in this region. B. rochalimae, another human pathogen (11) which has been also reported in carnivorous species and their fleas (Pulex simulans) (37–39) and in rodents (40), was also detected in two fleas in this study, suggesting that the presence of this bacterium in local flea communities should not be ignored.
The other two Bartonella species or phylogroups (variants MF443364 and MF443365) were found in small numbers of fleas. Interestingly, variant MF443364 was quite different from other identified genotypes in our study but was similar to the variant KM233490 previously described in an African giant shrew (Crocidura olivieri) from Kenya (12). This variant was found in 10 fleas which were collected from rodents (A. niloticus, Mastomys spp., and R. rattus) but not from shrews (Crocidura spp.) in the current study. Although Bartonella DNA was detected in fleas collected from four Crocidura in the present study, all of the Bartonella genotypes belonged to the B. elizabethae species complex. Further investigations are needed to illustrate the relationship between shrews and Bartonella spp. in Africa, but it should be noted that we have found shrews infested with X. cheopis, X. brasiliensis, D. lypusus, and C. calceatus fleas, providing a possible means for these animals to become infected with B. elizabethae-like bartonellae.
Like many previous reports, we found that Rickettsia infection is commonly circulating in the local flea community, with an overall prevalence of 23%. In contrast to Bartonella, Rickettsia-infected fleas were mainly C. felis fleas collected from Kilonzo traps. Only a very small portion of Rickettsia-infected fleas was obtained from rodents. Such results suggest that Rickettsia infections in the West Nile region are carried primarily by C. felis fleas.
Sequencing analysis demonstrated three Rickettsia species were circulating within the local flea community in the region of Uganda where plague is endemic. R. felis, a frequently identified zoonotic pathogen that causes cat-flea typhus in humans, was commonly detected in the C. felis fleas we tested. Due to its worldwide distribution, R. felis infection has been considered an emergent threat to human health (13). Because C. felis fleas in Uganda commonly feed on humans in the West Nile region and C. felis is a well-known competent vector of R. felis (41), our results suggest that humans might be exposed to cat flea-transmitted rickettsioses caused by R. felis or another closely related rickettsial species. The other two species identified in this study were “Candidatus R. asemboensis” and “Candidatus R. sengalensis.” In fact, “Candidatus R. asemboensis” was the most common species detected in the study. Although it is not clear whether this species can cause disease in humans or animals, further investigations are needed regarding its wide distribution. The number of E. gallinacea fleas collected from Kilonzo traps was the second largest, after the number of C. felis fleas taken from these traps. Interestingly, neither Rickettsia DNA nor Bartonella DNA was detected in these fleas. Although these fleas occur on a wide range of bird and mammal hosts, they are commonly known as poultry fleas, a fact that could explain the absence of both bartonellae and rickettsiae in populations of these fleas.
Neither Y. pestis nor Y. pseudotuberculosis was detected in the present study. Notably, the villages tested were those lacking a history of plague cases, although all were within the portion of the West Nile region considered at risk for plague. Even in villages where plague has been reported repeatedly, it is very uncommon to find Y. pestis in fleas collected from rodents or to find this bacterium in years when there is no evidence of epizootic activity among rodent populations. Some researchers have suggested that Y. pestis circulates at very low and difficult-to-detect levels in fleas and rodents or is maintained in another reservoir, such as soil, during the interepizootic periods, but the true reservoir is yet to be determined (42, 43).
In summary, the relatively high prevalence rates of different genotypes of Rickettsia and Bartonella in fleas from the West Nile region where plague is endemic suggest that flea-borne diseases other than plague are likely present in this part of Uganda. The potential importance of these flea-borne diseases is also supported by the high abundance of certain species of fleas in this area, including C. felis, X. cheopis, and X. brasiliensis, each of which is known to bite people. Nevertheless, the vector competence of these flea species should be studied in order to gain understanding of whether these fleas pose a risk to humans. In addition, an animal host likely would not die from a Bartonella or Rickettsia infection; therefore, the relevant flea species may not leave their natural hosts. Further research is needed to determine the extent to which humans in this region become infected with not only Y. pestis but also with bacteria of the genera Rickettsia and Bartonella and whether the people infected with these two arthropod-associated bacteria suffer clinically recognizable illnesses.
MATERIALS AND METHODS
Study sites and flea collections.
Study sites and flea and small mammal collection methods were described previously (3). Briefly, 15 villages, including 10 case villages and 5 control villages, were selected within portions of the Arua and Zombo districts that were considered to have increased plague risk, as classified by geographic information system-based statistical models that included variables for elevation (>1,300 m), precipitation, amounts of vegetative growth, and bare soil during the dry month of January (44). In other words, the study villages were believed to have conditions ecologically conducive for plague activity. Within each village, 10 homesteads were randomly selected for sampling. The fleas screened in this study were derived from the five control villages within which no human plague cases had been reported from 1999 to 2011. The fleas were collected either from small mammals that were trapped inside huts and surroundings of the selected homesteads or from modified Kilonzo flea traps (45) that were set inside huts. Collected fleas were stored in 70% ethanol and shipped to the CDC laboratory in Fort Collins, CO, for species identification based on morphological characteristics using appropriate taxonomic keys (46–49) and bacterial testing.
DNA extraction and PCR detection.
DNA was extracted from individual fleas. The fleas were first homogenized using a Bullet Gold blender homogenizer (Next Advance, Averill Park, NY). The homogenates were then transferred to a KingFisher platform (Life Technologies, Inc., Grand Island, NY) for DNA extraction following the tissue protocol provided by the manufacturer. A blank well was included as a negative control to ensure no cross-contamination occurred during the extraction. The DNA was then analyzed for the presence of Bartonella spp., Rickettsia spp., Yersinia pestis, and Y. pseudotuberculosis by using molecular approaches. Real-time PCR was first performed in a CFX96 PCR detection system (Bio-Rad, Hercules, CA) that was set up to target ssrA, gltA, YPO2088, and opgG for Bartonella spp., Rickettsia spp., Yersinia pestis, and Y. pseudotuberculosis, respectively. ssrA for Bartonella spp. and gltA for Rickettsia spp. have been described previously and have been widely applied in many studies (50, 51). The YPO2088 and opgG assays, which were developed for detection of Y. pestis and Y. pseudotuberculosis, respectively, were applied for the first time in this study. Samples showing an amplification curve with a cycle threshold (CT) value of <36 were considered positive. A one-step or nested conventional PCR was further performed in a C1000 Touch thermal cycler (Bio-Rad, Hercules, CA) for real-time PCR-positive samples, targeting Rickettsia-specific gltA (52) and Bartonella-specific gltA (18, 53), respectively. The PCR products were analyzed for the presence of amplicons of the expected sizes via electrophoresis on 1.5% agarose gels containing GelGreen stain (Biotium, Hayward, CA). Positive and negative controls were included in each PCR to evaluate the presence of appropriately sized amplicons and to rule out potential contamination, respectively. The primers and probes used for the targets mentioned in this study are listed in Table 3.
TABLE 3 .
Molecular detection of bacterial zoonotic agents in fleas from Uganda, 2012–2013
| Agent | Target | PCR type | Primer and probe sequences | Reference |
|---|---|---|---|---|
| Bartonella spp. | ssrA | Real time | Forward: GCT ATG GTA ATA AAT GGA CAA TGA AAT AA | 50 |
| Reverse: GCT TCT GTT GCC AGG TG | ||||
| Probe: ACC CCG CTT AAA CCT GCG ACG | ||||
| gltA (outer) | Nested conventional | Forward: GCT ATG TCT GCA TTC TAT CA | 18 | |
| Reverse: GAT CYT CAA TCA TTT CTT TCC A | ||||
| gltA (inner) | Nested conventional | Forward: GGG GAC CAG CTC ATG GTG G | 53 | |
| Reverse: AAT GCA AAA AGA ACA GTA AAC A | ||||
| Rickettsia spp. | gltA | Real time | Forward: GAG AGA AAA TTA TAT CCA AAT GTT GAT | 51 |
| Reverse: AGG GTC TTC GTG CAT TTC TT | ||||
| Probe: CAT TGT GCC ATC CAG CCT ACG GT | ||||
| Conventional | Forward: GGG GGC CTG CTC ACG GCG G | 52 | ||
| Reverse: ATT GC AAA AAG TAC AGT GAA CA | ||||
| Yersinia pestis | YPO2088 | Real time | Forward: TCG GCA ACA GCT CAA CAC CT | This study |
| Reverse: ATG CAT TGG ACG GCA TCA CG | ||||
| Probe: CGC CCT CGA ATC GCT GGC CAA CTG C | ||||
| Yersinia pseudotuberculosis | opgG | Real time | Forward: ACG TGG GCG TGA ATT CTC TCA A | This study |
| Reverse: GCC GTT GGG ATC TCC ACC AA | ||||
| Probe: CCT GCG CCC AAG CGC GTG GG |
Sequencing and phylogenetic analysis for Bartonella and Rickettsia spp.
The PCR amplicons from conventional PCRs for Bartonella and Rickettsia were purified using a QIAquick PCR purification kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions and then sequenced in both directions by using an ABI 3130 genetic analyzer (Applied Biosystems, Foster City, CA). Forward and reverse sequences were assembled using the SeqMan Pro program in Lasergene v.12 (DNASTAR, Madison, WI). A BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) search of the GenBank database was performed with all assembled sequences to confirm their Bartonella or Rickettsia identity. Positive sequences were compared between themselves and with Bartonella and Rickettsia reference sequences available in GenBank, after alignment using the Clustal algorithm in the MegAlign program in Lasergene. Phylogenetic trees were constructed for Bartonella or Rickettsia separately, using the neighbor-joining method. Branch support was estimated using 1,000 bootstrap replicates.
Statistical analyses.
The chi-square test was performed to compare the prevalence of Bartonella and Rickettsia infections between fleas collected from small mammals and fleas collected from Kilonzo traps. Results were considered significant if P was <0.05.
Accession number(s).
The novel Bartonella variants and Rickettsia variants obtained in the study have been deposited in GenBank under accession numbers MF443355 to MF443366 and MF459639 to MF459644, respectively.
ACKNOWLEDGMENTS
We thank Vladimir Motin from the Department of Pathology and Department of Microbiology and Immunology of the University of Texas Medical Branch for designing the primers for detecting Yersinia pestis and Y. pseudotuberculosis.
Y.B. and M.Y.K. designed the experiments, L.M.O. performed the experiments, R.J.E., L.A.A., J.T.M., K.A.B., R.E.E., and K.L.G. performed field work, Y.B. and L.M.O. analyzed the data, and Y.B., M.Y.K., R.J.E., K.L.G., and R.E.E. wrote the paper.
REFERENCES
- 1.Moore SM, Monaghan A, Griffith KS, Apangu T, Mead PS, Eisen RJ. 2012. Improvement of disease prediction and modeling through the use of meteorological ensembles: human plague in Uganda. PLoS One 7:e44431. doi: 10.1371/journal.pone.0044431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amatre G, Babi N, Enscore RE, Ogen-Odoi A, Atiku LA, Akol A, Gage KL, Eisen RJ. 2009. Flea diversity and infestation prevalence on rodents in a plague-endemic region of Uganda. Am J Trop Med Hyg 81:718–724. doi: 10.4269/ajtmh.2009.09-0104. [DOI] [PubMed] [Google Scholar]
- 3.Eisen RJ, MacMillan K, Atiku LA, Mpanga JT, Zielinski-Gutierrez E, Graham CB, Boegler KA, Enscore RE, Gage KL. 2014. Identification of risk factors for plague in the West Nile region of Uganda. Am J Trop Med Hyg 90:1047–1058. doi: 10.4269/ajtmh.14-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones RT, Borchert J, Eisen R, MacMillan K, Boegler K, Gage KL. 2015. Flea-associated bacterial communities across an environmental transect in a plague-endemic region of Uganda. PLoS One 10:e0141057. doi: 10.1371/journal.pone.0141057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisen RJ, Borchert JN, Mpanga JT, Atiku LA, MacMillan K, Boegler KA, Montenieri JA, Monaghan A, Gage KL. 2012. Flea diversity as an element for persistence of plague bacteria in an East African plague focus. PLoS One 7:e35598. doi: 10.1371/journal.pone.0035598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daly JS, Worthington MG, Brenner DJ, Moss CW, Hollis DG, Weyant RS, Steigerwalt AG, Weaver RE, Daneshvar MI, O’Connor SP. 1993. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol 31:872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comer JA, Diaz T, Vlahov D, Monterroso E, Childs JE. 2001. Evidence of rodent-associated Bartonella and Rickettsia infections among intravenous drug users from Central and East Harlem, New York City. Am J Trop Med Hyg 65:855–860. doi: 10.4269/ajtmh.2001.65.855. [DOI] [PubMed] [Google Scholar]
- 8.McGill S, Rajs J, Hjelm E, Lindquist O, Friman G. 2003. A study on forensic samples of Bartonella spp. antibodies in Swedish intravenous heroin addicts. APMIS 111:507–513. doi: 10.1034/j.1600-0463.2003.1110409.x. [DOI] [PubMed] [Google Scholar]
- 9.Kosoy M, Bai Y, Sheff K, Morway C, Baggett H, Maloney SA, Boonmar S, Bhengsri S, Dowell SF, Sitdhirasdr A, Lerdthusnee K, Richardson J, Peruski LF. 2010. Identification of Bartonella infections in febrile human patients from Thailand and their potential animal reservoirs. Am J Trop Med Hyg 82:1140–1145. doi: 10.4269/ajtmh.2010.09-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vayssier-Taussat M, Moutailler S, Féménia F, Raymond P, Croce O, La Scola B, Fournier PE, Raoult D. 2016. Identification of novel zoonotic activity of Bartonella spp., France. Emerg Infect Dis 22:457–462. doi: 10.3201/eid2203.150269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eremeeva ME, Gerns HL, Lydy SL, Goo JS, Ryan ET, Mathew SS, Ferraro MJ, Holden JM, Nicholson WL, Dasch GA, Koehler JE. 2007. Bacteremia, fever, and splenomegaly caused by a newly recognized Bartonella species. N Engl J Med 356:2381–2387. doi: 10.1056/NEJMoa065987. [DOI] [PubMed] [Google Scholar]
- 12.Angelakis E, Mediannikov O, Parola P, Raoult D. 2016. Rickettsia felis: the complex journey of an emergent human pathogen. Trends Parasitol 32:554–564. doi: 10.1016/j.pt.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Leulmi H, Socolovschi C, Laudisoit A, Houemenou G, Davoust B, Bitam I, Raoult D, Parola P. 2014. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis 8:e3152. doi: 10.1371/journal.pntd.0003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ying B, Kosoy MY, Maupin GO, Tsuchiya KR, Gage KL. 2002. Genetic and ecologic characteristics of Bartonella communities in rodents in southern China. Am J Trop Med Hyg 66:622–627. doi: 10.4269/ajtmh.2002.66.622. [DOI] [PubMed] [Google Scholar]
- 15.Bai Y, Montgomery SP, Sheff KW, Chowdhury MA, Breiman RF, Kabeya H, Kosoy MY. 2007. Bartonella strains in small mammals from Dhaka, Bangladesh related to Bartonella in America and Europe. Am J Trop Med Hyg 77:567–570. [PubMed] [Google Scholar]
- 16.Bai Y, Kosoy MY, Lerdthusnee K, Peruski LF, Richardson JH. 2009. Prevalence and genetic heterogeneity of Bartonella strains cultured from rodents from 17 provinces in Thailand. Am J Trop Med Hyg 81:811–816. doi: 10.4269/ajtmh.2009.09-0294. [DOI] [PubMed] [Google Scholar]
- 17.Tsai YL, Chuang ST, Chang CC, Kass PH, Chomel BB. 2010. Bartonella species in small mammals and their ectoparasites in Taiwan. Am J Trop Med Hyg 83:917–923. doi: 10.4269/ajtmh.2010.10-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gundi VA, Kosoy MY, Makundi RH, Laudisoit A. 2012. Identification of diverse Bartonella genotypes among small mammals from Democratic Republic of Congo and Tanzania. Am J Trop Med Hyg 87:319–326. doi: 10.4269/ajtmh.2012.11-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halliday JEB, Knobel DL, Agwanda B, Bai Y, Breiman RF, Cleaveland S, Njenga MK, Kosoy M. 2015. Prevalence and diversity of small mammal associated Bartonella in rural and urban Kenya. PLoS Negl Trop Dis 9:e0003608. doi: 10.1371/journal.pntd.0003608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malania L, Bai Y, Osikowicz LM, Tsertsvadze N, Katsitadze G, Imnadze P, Kosoy M. 2016. Prevalence and diversity of Bartonella species in rodents from Georgia (Caucasus). Am J Trop Med Hyg 95:466–471. doi: 10.4269/ajtmh.16-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sackal C, Laudisoit A, Kosoy M, Massung R, Eremeeva ME, Karpathy SE, Van Wyk K, Gabitzsch E, Zeidner NS. 2008. Bartonella spp. and Rickettsia felis in fleas, Democratic Republic of Congo. Emerg Infect Dis 14:1972–1974. doi: 10.3201/eid1412.080610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Billeter SA, Borchert JN, Atiku LA, Mpanga JT, Gage KL, Kosoy MY. 2014. Bartonella species in invasive rats and indigenous rodents from Uganda. Vector Borne Zoonotic Dis 14:182–188. doi: 10.1089/vbz.2013.1375. [DOI] [PubMed] [Google Scholar]
- 23.Jiang J, Maina AN, Knobel DL, Cleaveland S, Laudisoit A, Wamburu K, Ogola E, Parola P, Breiman RF, Njenga MK, Richards AL. 2013. Molecular detection of Rickettsia felis and Candidatus Rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis 13:550–558. doi: 10.1089/vbz.2012.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumsa B, Parola P, Raoult D, Socolovschi C. 2014. Molecular detection of Rickettsia felis and Bartonella henselae in dog and cat fleas in Central Oromia, Ethiopia. Am J Trop Med Hyg 90:457–462. doi: 10.4269/ajtmh.13-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palomar AM, Cevidanes A, Portillo A, Kalema-Zikusoka G, Chirife AD, Romero L, Muro J, Mugisha L, Oteo JA, Millán J. 2017. High prevalence of Rickettsia spp. in dog fleas (Siphonaptera: Pulicidae) in rural Uganda. J Med Entomol 54:1076–1079. doi: 10.1093/jme/tjx048. [DOI] [PubMed] [Google Scholar]
- 26.Kosoy M, Hayman DT, Chan KS. 2012. Bartonella bacteria in nature: where does population variability end and a species start? Infect Genet Evol 12:894–904. doi: 10.1016/j.meegid.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Martin-Alonso A, Houemenou G, Abreu-Yanes E, Valladares B, Feliu C, Foronda P. 2016. Bartonella spp. in small mammals, Benin. Vector Borne Zoonotic Dis 16:229–237. doi: 10.1089/vbz.2015.1838. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention 2009. Bubonic and pneumonic plague—Uganda, 2006. MMWR Morb Mortal Wkly Rep 58:778–781. [PubMed] [Google Scholar]
- 29.Anderson BE, Neuman MA. 1997. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev 10:203–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silaghi C, Knaus M, Rapti D, Shukullari E, Pfister K, Rehbein S. 2012. Rickettsia felis and Bartonella spp. in fleas from cats in Albania. Vector Borne Zoonotic Dis 12:76–77. doi: 10.1089/vbz.2011.0732. [DOI] [PubMed] [Google Scholar]
- 31.Chandra S, Forsyth M, Lawrence AL, Emery D, Šlapeta J. 2017. Cat fleas (Ctenocephalides felis) from cats and dogs in New Zealand: molecular characterisation, presence of Rickettsia felis and Bartonella clarridgeiae and comparison with Australia. Vet Parasitol 234:25–30. doi: 10.1016/j.vetpar.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Graham CB, Borchert JN, Black WC IV, Atiku LA, Mpanga JT, Boegler KA, Moore SM, Gage KL, Eisen RJ. 2013. Blood meal identification in off-host cat fleas (Ctenocephalides felis) from a plague-endemic region of Uganda. Am J Trop Med Hyg 88:381–389. doi: 10.4269/ajtmh.2012.12-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kosoy M, Bai Y, Enscore R, Rizzo MR, Bender S, Popov V, Albayrak L, Fofanov Y, Chomel B. 2016. Bartonella melophagi in blood of domestic sheep (Ovis aries) and sheep keds (Melophagus ovinus) from the southwestern US: cultures, genetic characterization, and ecological connections. Vet Microbiol 190:43–49. doi: 10.1016/j.vetmic.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Borchert JN, Mach JJ, Linder TJ, Ogen-Odoi A, Angualia S. 2007. Invasive rats and bubonic plague in northwest Uganda, p 282–293. Managing vertebrate invasive species. Proceedings of an international symposium. USDA-APHIS, Fort Collins, CO. [Google Scholar]
- 35.Billeter SA, Colton L, Sangmaneedet S, Suksawat F, Evans BP, Kosoy MY. 2013. Molecular detection and identification of Bartonella species in rat fleas from northeastern Thailand. Am J Trop Med Hyg 89:462–465. doi: 10.4269/ajtmh.12-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oguge NO, Durden LA, Keirans JE, Balami HD, Schwan TG. 2009. Ectoparasites (sucking lice, fleas and ticks) of small mammals in southeastern Kenya. Med Vet Entomol 23:387–392. doi: 10.1111/j.1365-2915.2009.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabriel MW, Henn J, Foley JE, Brown RN, Kasten RW, Foley P, Chomel BB. 2009. Zoonotic Bartonella species in fleas collected on gray foxes (Urocyon cinereoargenteus). Vector Borne Zoonotic Dis 9:597–602. doi: 10.1089/vbz.2008.0134. [DOI] [PubMed] [Google Scholar]
- 38.Bai Y, Gilbert A, Fox K, Osikowicz L, Kosoy M. 2016. Bartonella rochalimae and B. vinsonii subsp. berkhoffii in wild carnivores from Colorado, USA. J Wildl Dis 52:844–849. doi: 10.7589/2016-01-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.López-Pérez AM, Osikowicz L, Bai Y, Montenieri J, Rubio A, Moreno K, Gage K, Suzán G, Kosoy M. 2017. Prevalence and phylogenetic analysis of Bartonella species of wild carnivores and their fleas in northwestern Mexico. Ecohealth 14:116–129. doi: 10.1007/s10393-017-1216-2. [DOI] [PubMed] [Google Scholar]
- 40.Gundi VA, Billeter SA, Rood MP, Kosoy MY. 2012. Bartonella spp. in rats and zoonoses, Los Angeles, California, USA. Emerg Infect Dis 18:631–633. doi: 10.3201/eid1804.110816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawrence AL, Hii SF, Jirsová D, Panáková L, Ionică AM, Gilchrist K, Modrý D, Mihalca AD, Webb CE, Traub RJ, Šlapeta J. 2015. Integrated morphological and molecular identification of cat fleas (Ctenocephalides felis) and dog fleas (Ctenocephalides canis) vectoring Rickettsia felis in central Europe. Vet Parasitol 210:215–223. doi: 10.1016/j.vetpar.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 42.Gage KL, Kosoy MY. 2005. Natural history of plague: perspectives from more than a century of research. Annu Rev Entomol 50:505–528. doi: 10.1146/annurev.ento.50.071803.130337. [DOI] [PubMed] [Google Scholar]
- 43.Ayyadurai S, Houhamdi L, Lepidi H, Nappez C, Raoult D, Drancourt M. 2008. Long-term persistence of virulent Yersinia pestis in soil. Microbiology 154:2865–2871. doi: 10.1099/mic.0.2007/016154-0. [DOI] [PubMed] [Google Scholar]
- 44.Eisen RJ, Griffith KS, Borchert JN, MacMillan K, Apangu T, Owor N, Acayo S, Acidri R, Zielinski-Gutierrez E, Winters AM, Enscore RE, Schriefer ME, Beard CB, Gage KL, Mead PS. 2010. Assessing human risk of exposure to plague bacteria in northwestern Uganda based on remotely sensed predictors. Am J Trop Med Hyg 82:904–911. doi: 10.4269/ajtmh.2010.09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borchert JN, Eisen RJ, Atiku LA, Delorey MJ, Mpanga JT, Babi N, Enscore RE, Gage KL. 2012. Efficacy of indoor residual spraying using lambda-cyhalothrin for controlling nontarget vector fleas (Siphonaptera) on commensal rats in a plague endemic region of northwestern Uganda. J Med Entomol 49:1027–1034. doi: 10.1603/ME11230. [DOI] [PubMed] [Google Scholar]
- 46.Haselbarth E. 1966. Siphonaptera, p 117–212. In Zumpt F (ed), The arthropod parasites of vertebrates in Africa south of the Sahara (Ethiopia region). South African Institute of Medical Research, Johannesburg, South Africa. [Google Scholar]
- 47.Hopkins GHE. 1947. Annotated and illustrated keys to the known fleas of East Africa. Ugandan J 11:133–191. [Google Scholar]
- 48.Smit FGAM. 1973. Siphonaptera (fleas), p 325–371. In Smith KGV (ed), Insects and other arthropods of medical importance. British Museum of Natural History, London, United Kingdom. [Google Scholar]
- 49.Hopkins GHE, Rothschild M. 1966. An illustrated catalogue of the Rothschild collection of fleas (Siphonaptera) in the British Museum (Natural History): Hystrichopsyllidae. Ballantyne and Co, London, United Kingdom. [Google Scholar]
- 50.Diaz MH, Bai Y, Malania L, Winchell JM, Kosoy MY. 2012. Development of a novel genus-specific real-time PCR assay for detection and differentiation of Bartonella species and genotypes. J Clin Microbiol 50:1645–1649. doi: 10.1128/JCM.06621-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guedes E, Leite RC, Prata MC, Pacheco RC, Walker DH, Labruna MB. 2005. Detection of Rickettsia rickettsii in the tick Amblyomma cajennense in a new Brazilian spotted fever-endemic area in the state of Minas Gerais. Mem Inst Oswaldo Cruz 100:841–845. doi: 10.1590/S0074-02762005000800004. [DOI] [PubMed] [Google Scholar]
- 52.Regnery RL, Spruill CL, Plikaytis BD. 1991. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol 173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Norman AF, Regnery R, Jameson P, Greene C, Krause DC. 1995. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J Clin Microbiol 33:1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]