Abstract
Social cognition abilities are severely impaired in schizophrenia (SZ). The current meta-analysis used foci of 21 individual studies on functional abnormalities in the schizophrenic brain in order to identify regions that reveal convergent under- or over-activation during theory of mind (TOM) tasks. Studies were included in the analyses when contrasting tasks that require the processing of mental states with tasks which did not. Only studies that investigated patients with an ICD or DSM diagnosis were included. Quantitative voxel-based meta-analyses were done using Seed-based d Mapping software. Common TOM regions like medial-prefrontal cortex and temporo-parietal junction revealed abnormal activation in schizophrenic patients: Under-activation was identified in the medial prefrontal cortex, left orbito-frontal cortex, and in a small section of the left posterior temporo-parietal junction. Remarkably, robust over-activation was identified in a more dorsal, bilateral section of the temporo-parietal junction. Further abnormal activation was identified in medial occipito-parietal cortex, right premotor areas, left cingulate gyrus, and lingual gyrus. The findings of this study suggest that SZ patients simultaneously show over- and under-activation in TOM-related regions. Especially interesting, temporo-parietal junction reveals diverging activation patterns with an under-activating left posterior and an over-activating bilateral dorsal section. In conclusion, SZ patients show less specialized brain activation in regions linked to TOM and increased activation in attention-related networks suggesting compensatory effects.
Keywords: fMRI, mentalizing, psychosis
Introduction
Poor social cognition is one of the defining characteristics of schizophrenia (SZ).1,2 A wealth of studies show that schizophrenic patients are impaired in tasks that require mental state reasoning, perspective taking or an understanding of others’ intentions and beliefs.3,4 These abilities are subsumed under the term theory of mind (TOM), which is defined as the ability to reason about mental states of others and to interpret and predict behavior based on an understanding of their minds.5,6
Decreased TOM abilities are also evident in patients during remission,3 in relatives of schizophrenic patients and in individuals who bear a risk of developing a psychosis.7,8 Therefore, abnormal TOM is not a mere side effect of chronic SZ or an acute psychotic break but may rather be trait-dependent.3,9 Recent studies found that social cognition abilities like TOM are reliable predictors of global social functioning in schizophrenic patients.10,11 Furthermore, social cognition is able to explain a unique amount of variance in abnormal social functioning.12,13 Thus, although neurocognitive measures can account for up to 60% of variance in functional outcome,14 TOM is a strong additional factor to predict functional outcome in SZ.
In healthy participants, TOM tasks lead to activation in several brain areas like medial-prefrontal cortex (MPFC), superior temporal sulcus (STS), bilateral temporo-parietal junction (TPJ), and precuneus.15–17 Investigations on SZ patients are inconsistent and reveal decreased and increased activation of TOM-related regions. Several studies provide evidence for an increased activation in MPFC, left STS, left temporo-parietal junction, and precuneus cortex.18–20 Other studies reveal decreased activation in similar regions21–24 and some studies show increased as well as decreased activation in TOM-related brain regions.20,25 The diversity in neuroimaging findings is often discussed as being (at least partly) driven by the different tasks used to assess TOM processes and by heterogeneous patient groups.3,4,26 Heterogeneous findings may also be due to the relatively small amount of participants in the individual studies. Therefore, meta-analytic studies are indispensable to gain an accurate insight in TOM abnormalities of SZ.
Sugranyes27 provides a first meta-analytic overview of neuroimaging studies on TOM in SZ. Their findings indicate convergent under-activation for schizophrenic patients in the MPFC, middle temporal gyrus, posterior cingulate cortex, and the thalamus. Over-activation was identified in right parietal lobe and posterior cingulate cortex. Due to the lack of a sufficient amount of studies available at that time (n = 9), the range of possible analyses was limited. Meta-analyses on TOM in healthy controls commonly provide separate analyses for task subgroups16 since a pooled meta-analysis alone does not reveal possible task effects and therefore bears artificially increased variance. However, analyses of subtasks are difficult in samples where the number of available studies is limited, since a division in several smaller groups diminishes the power even more. Furthermore, the number of included studies per se is relatively small and a greater amount of data would lead to more reliable results.
Since the last meta-analysis,27 more than 10 neuroimaging articles (meeting the criteria for Seed-based d Mapping meta-analyses) on TOM in SZ were published, thus providing an enlarged data pool which clearly enhances the statistical power. Furthermore, Molenberghs et al26 put forward an alternative way of separating TOM tasks by means of 3 bivariate TOM task parameters: instructional focus (implicit vs. explicit), inference type (cognitive vs. affective), and modality of presentation (verbal vs. visual). Our meta-analysis aims to estimate a composite effect size of 21 individual studies assessing brain activation differences in TOM tasks between schizophrenic patients and control groups. Analyses of task subgroups (modeled after Molenberghs et al26) are provided.
Heterogeneous patient samples represent a significant challenge for a unified interpretation of meta-analytic findings.3,4 We consider illness-specific parameters like the duration of illness and the severity of positive and negative symptoms as possible regressors in analyses which are provided in the supplementary material. Meta-analytic calculations are done using Seed-based d Mapping,28 which was successfully used in meta-analyses on patient samples like obsessive-compulsive disorder29 and autism.30
Materials and Methods
Included Studies and Characteristics
To identify appropriate studies, we performed several MEDLINE searches using the keywords “schizophrenia,” “psychosis,” “theory of mind,” “mentalizing,” “perspective taking,” “fMRI,” “functional magnetic resonance imaging,” “PET,” “positron emission tomography,” and “neuroimaging.” Criteria for the selection of relevant fMRI and PET studies were as follows:
Studies included patients with a diagnosis of SZ (according to DSM or ICD criteria).
Whole brain group comparisons (SZ vs. healthy controls) were reported in a standard stereotactic space (MNI or Talairach).
All activation patterns clearly referred to TOM processes. We merely included studies that contrasted tasks requiring the processing of mental states with tasks which did not (eg, studies that contrast false belief vs. false photo conditions). If several contrasts were reported in a study, we selected the one that matched best the contrasts of the other studies. To avoid biases toward certain brain regions, only studies that used the same threshold throughout the brain (within their study) were included in the analysis. The thresholds do not need to be the same between all 21 included studies.
On this basis, 21 studies with a total of 623 participants (308 schizophrenic patients, 315 healthy controls) and 133 activation foci were included in the meta-analysis. The demographic and clinical characteristics of the participants are shown in table 1. Tasks descriptions of the individual studies are provided in supplementary figure SUP1.
Table 1.
Clinical Description of Patients With Schizophrenia Included in Theory of Mind Studies
| Reference | Mean Age (SD) Patients/Controls | Gender M/F Patients; M/F Controls | Symptom Scales Mean Scores (SD) | Diagnosis/Recruitment/ Illness Duration (SD) | Medication Dose (mg): Mean (SD) |
|---|---|---|---|---|---|
| Brüne et al19 | 27.9 (6.6)/26.5 (5.3) | 3/6; 4/9 | PANSS + 16.67 (5.7); PANSS − 15.67 (8.8) | First episode (n = 3); duration: 3 (4.23) | NLP: 244.44 (173) |
| Walter et al23 | 29.5 (6.0)/24.7 (2.6) | 6/6; 6/6 | PANSS + 17.75 (5.0); PANSS − 19.41 (3.9) | Inpatients; duration: 6.3 (5.2) | N/A |
| Benedetti et al25 | 37.2 (10.23)/35.1 (9.95) | 14/10; 7/13 | PANSS + 16 (4.58); PANSS − 21.66 (5.42) | Stable outpatients; first episode (n = 3); duration:12.7 (6.96) | Clozapine n = 9; Risperidone n = 1;, Aripi- prazole n = 2; Haloperidol n = 2 |
| Lee et al31 | 26 (4.3)/25.8 (2.2) | 7/8; 9/9 | PANSS + 13.1 (5.1); PANSS − 15.4 (4.1) | Stable outpatients; inpatients; duration: 4.6 (3.4) | NLP: 422.1 (237) |
| Lee et al24 | 38.3 (10.7)/42.5 (7.7) | 10/2; 10/3 | N/A | DSM-IV (SCID-P); stable outpatients | N/A |
| Pedersen et al18 | 29 (8.2)/29.9 (8.8) | 9/6; 9/5 | PANSS + 10.9 (2.8); PANSS − 14.9 (5.4) | Stable outpatients; duration: 5.5 (6.3) | NLP: 629.6 (395) |
| Eack et al32 | 27.8 (6.61)/26.50 (5.82) | 14/6; 13/7 | N/A | DSM-IV; stable outpatients; duration: 4.85 (3.18) | NLP: 308.08 (235.89) |
| Varga et al33 | 37.95 (9.06)/33.96 (8.51) | 9/12; 10/14 | PANSS + 13.81 (3.2); PANSS − 17.00 (5.4) | DSM-IV; remission; duration: 11.95 (8.45) | Amisulpiride n = 1; Aripiprazole n = 1; clozapine n = 4; Olanzapine n = 2; Quetiapine n = 2; Risperidone n = 2; Sertindole n = 1; Ziprazidone n = 1; Flupentixol n = 3; Haloperidol n = 1; |
| Harvey et al34 | 42.4 (11.8)/42.9 (8.6) | 13/2; 13/2 | SANS 27.1 (9.7) | DSM-IV (SCID-P); psychiatrically stable | NPL: 210 (142) |
| Bedford et al35 | 39 (11)/31 (9) | 7/4; 3/5 | N/A | DSM-IV-TR; inpatients = 4, outpatients = 7; remission; duration: 12 (8) | Anti-psychotic medication |
| Rapp et al36 | 28.1 (N/A)/32.9 (N/A) | 0/15 | PANSS + 17.4 (N/A); PANSS − 16.00 (N/A) | DSM-IV; inpatients; clinically stable | NPL: 516.0 (237) |
| Russell et al37 | 36 (9)/ | 5/0; 7/0 | N/A | DSM-IV; inpatients = 4, outpatients = 7; duration: 13 (7) | N/A |
| Brüne et al38 | 6.8 (5.5)/8.8 (4.1) | 15/7; 16/9 | PANSS + 18.2 (4.8); PANSS − 21.2 (7.1) | DSM-IV; inpatients; duration: 3.3 (3.7) | NPL: 475 (429) |
| Brunet et al21 | 31 (6.5)/23.3 (1.68) | 7/0; 8/0 | N/A | DSM-IV; outpatients | Loxapine n = 1; Oxazepam n = 1; Tropatenine n = 2; Olanzapine n = 3; Haloperidol n = 2; Dipo- tassic n = 1; Tropatepine n = 1; Risperidone n = 2; Paroxetine n = 1; Citalopram n = 1 |
| Mier et al39 | 34.25 (6.95)/37.0 (8.18) | 11/5; 11/5 | SAPS 1.5 (1.37); SANS 7.94 (2.86) | DSM-IV; outpatients | NPL: 901.59 (647.1) |
| Andreasen et al20 | 32.5 (11.0)/26.5 (6.4) | 14/4; 6/7 | SAPS 2.8 (N/A); SANS 2.1 (N/A) | DSM-IV; outpatients; duration: 8.96 (9.3) | No medication |
| Ciaramidaro et al40 | range: 14–32/ range:15–27 | 14/4; 19/4 | N/A | DSM-IV; inpatients; duration: 6.25 (3.5) | N/A |
| Das et al22 | 34.5 (8.4)/33.5 (8.4) | 20/0; 19/0 | PANSS + 10.1 (3.0); PANSS − 18.2 (5.2) | DSM-IV; duration: 9.4 (6.5) | Lithium n = 4; Sertraline n = 9; 12 were on antipsychotic medications. |
| Dodell et al41 | 38.8 (9.7)/32.4 (12.1) | 12/8; 12/6 | SAPS 3.1 (N/A); SANS 1.7 (N/A) | DSM-IV; inpatients; duration: 17.1 (12.2) | NPL: 501.6 (402.8) |
| Pauly et al42 | 36.23 (9.46)/34.46 (8.58) | 7/6; 7/6 | SAPS 4.3 (N/A); SANS 7.0 (N/A) | DSM-IV; remission; duration: 12 (6.93) | N/A |
| Lee et al43 | 31.7 (7.3)/30.5 (8.8) | 13/1; 13/1 | SAPS 6.9 (2.6); SANS 7.6 (2.8) | DSM-IV; inpatients | NPL: 354.3 (N/A) |
Note: Unless otherwise specified, neuroleptic dose (NPL) is expressed in chloropormazine equivalents and duration of illness is reported in years. N/A, data not available; PANSS, Positive and Negative Symptom Scale; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms.
Meta-Analytic Method
The current voxel-based meta-analysis was conducted using Seed-based d Mapping (SDM; formerly Signed Differential Mapping) software (http://www.sdmproject.com) version 4.31.28,29,44,45 Based on given foci of under- and over-activation, their respective statistical values and the sample size, SDM recreates maps of effect-sizes (Hedge’s d) for each included study. The meta-analytic maps were thresholded using the recommended voxel-level (height) threshold of P < .005 (uncorrected) and a cluster-level (extent) threshold of 10 voxels, which is found to be an approximate equivalent to a corrected threshold of P < .05 in original neuroimaging studies.28
Systematic whole-brain voxel-based jackknife sensitivity analysis was used to evaluate the replicability of the meta-analytic findings.
Comparisons of subgroups and meta-regressions were conducted using the built-in function of SDM. The reported findings were thresholded at values recommended for group comparisons (P < .005 (uncorrected), cluster-extent threshold: 10 voxels) and regressions (P < .0005 (Recommendation taken from AES-SDM tutorial from Joaquim Radua (Version May 2015), which can be retrieved from http://www.sdmproject.com/software/tutorial.pdf.) (uncorrected), cluster-extent threshold: 10 voxels).
Separate meta-analyses for patients and healthy controls are provided in the supplementary material. Details concerning methodological aspects are also shown in the supplements.
Results
Meta-Analysis
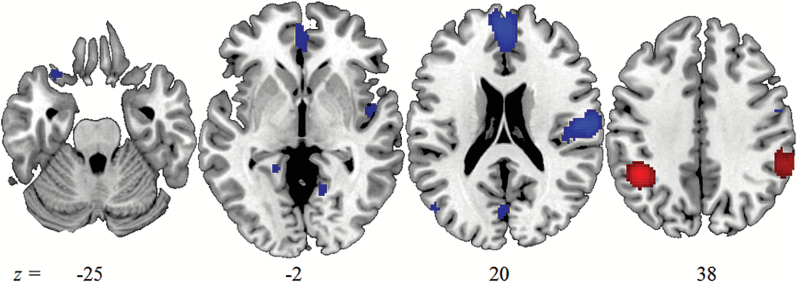
The pattern of abnormal neural activation in SZ compared to healthy controls is depicted in figure 1. The peak MNI coordinates, SDM Z-values, and cluster extent are listed in table 2.
Fig. 1.
Slices illustrate convergent under-activation (slices -25, -2, 20) and over-activation (slice 38) in patients compared to healthy controls at a voxel-level (height) threshold of P < .005 (uncorrected) and a cluster-level (extent) threshold of 10 voxels.
Table 2.
Results of the meta-analysis of functional brain abnormalities during theory of mind
| Tasks in Schizophrenia | ||||||
|---|---|---|---|---|---|---|
| Label | x | y | z | SDM-Z | Voxels | JK |
| Under-activation | ||||||
| Medial prefrontal cortex | −2 | 52 | 18 | 1.612 | 1573 | 20/21 |
| Frontal medial cortex | −4 | 40 | −10 | |||
| Paracingulate gyrus | −2 | 42 | −6 | |||
| R premotor cortex | 54 | −14 | 18 | 1.728 | 1101 | 21/21 |
| Central opercular cortex | 48 | −6 | 4 | |||
| Postcentral gyrus | 48 | −10 | 28 | |||
| Precentral gyrus | 56 | −4 | 34 | |||
| Medial occipitoparietal | −4 | −76 | 14 | 1.158 | 128 | 19/21 |
| R lingual gyrus | 12 | −58 | 2 | 1.221 | 99 | 19/21 |
| L orbito-frontal cortex | −30 | 22 | −24 | 1.109 | 30 | 14/21 |
| L lateral occipitotemporal | −48 | −72 | 22 | 1.124 | 27 | 18/21 |
| L cingulate gyrus | −18 | −44 | −2 | 1.084 | 24 | 18/21 |
| Over-activation | ||||||
| L inferior parietal cortex | −42 | −48 | 38 | −1.697 | 486 | 21/21 |
| R inferior parietal cortex | 58 | −40 | 40 | −1.114 | 405 | 19/21 |
Note: L, left; R, right; JK, jackknife analysis (number of subsamples that replicate the finding).
Subclusters are reported for cluster sizes above 1000 voxels only.
The meta-analysis identified 9 clusters that revealed deviant brain activation for SZ patients. Convergent under-activation for patients was found in a widespread dorsal to ventral MPFC cluster, mostly located in the anterior rostral section of the medial frontal cortex.46 Further under-activation was detected in a left orbito-frontal cluster. Right premotor cortex, medial occipito-parietal cortex, lingual gyrus, and the cingulate gyrus also revealed under-activation.
Our meta-analysis revealed convergent under-activation in a left lateral occipito-temporal cluster and convergent over-activation for patients compared to controls in bilateral inferior parietal lobe (IPL). These clusters lie within a region which is often referred to as temporo-parietal junction.47–49 Left occipito-temporal and bilateral IPL are mapped against common TPJ atlases in supplementary figure SUP2.
Replicability of meta-analytic findings was tested by means of a jackknife sensitivity analysis. In sum, our data reveal strong robustness against changes in individual samples (lowest replicability in left orbito-frontal cluster in 14 out of 21 recalculations). We checked for publication bias by means of funnel plots and Egger test.50 No evidence for publication bias could be found in the current dataset. Details concerning replicability and publication bias analyses are provided in the supplementary material.
Evaluation of Different TOM Tasks
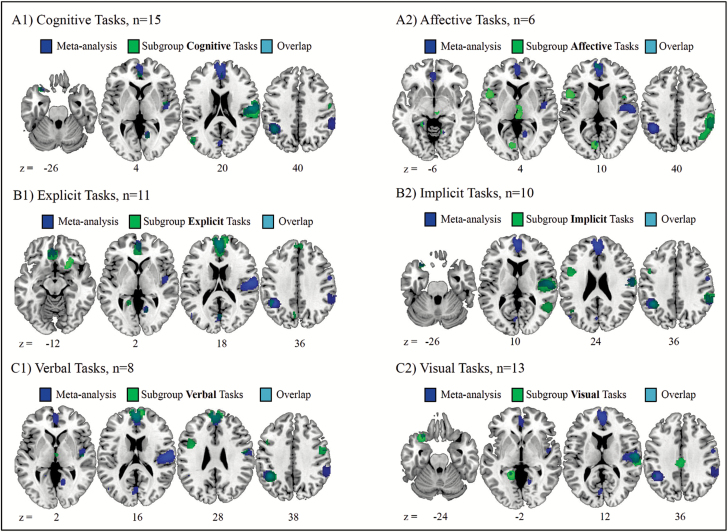
To evaluate possible effects of different task types on the convergence of meta-analytic regions, the included studies were classified according to 3 criteria used by Molenberghs et al.26 Therefore, each study was rated in the categories cognitive (15 out of 21 studies)/affective (6/21), implicit (10/21)/explicit (11/21), and verbal (8/21)/visual (13/21). Classification details are listed in supplementary table 1. The reported findings describe the differences between patients and healthy controls for each subgroup (cognitive, affective, implicit, explicit, verbal, and visual) separately. Findings are illustrated in figure 2A1–C2. Note that the seemingly small overlap between our subgroup analyses and the main analysis depicted in table 3 is caused by the way we calculated the percentage of overlap (for details see supplementary material).
Fig. 2.
Significant cluster revealed by the filtered subgroup analyses of the SDM group-comparison option. (A1-C2) To facilitate the comparison with the main meta-analysis, the results of the overall meta-analysis are indicated. Significant cluster (voxel level threshold P < .005, cluster extent = 10) of the subgroup analyses and the overlap between regions obtained from the subgroup analyses and the overall meta-analysis are shown as indicated by the color legend.
Table 3.
Accordance Between Clusters of Subgroups With Main Meta-Analysis
| Task Type | Medial Prefrontal Cortex | R Premotor Cortex | L Orbito- frontal Cortex | Medial Occipito- parietal | R Lingual Gyrus | L Lateral Occipito- temporal | L Cingulate Gyrus | L Inferior Parietal Cortex | R Inferior Parietal Cortex |
|---|---|---|---|---|---|---|---|---|---|
| Visual | 22.9 | 10.8 | 2.3 | 32.0 | |||||
| Verbal | 20.5 | 11.5 | 21.9 | ||||||
| Cognitive | 13.7 | 16.3 | 69.4 | 20.4 | 4.0 | 28.1 | |||
| Affective | 25.4 | 8.1 | 1.6 | 5.5 | |||||
| Implicit | 19.3 | 26.9 | 24.4 | 14.2 | |||||
| Explicit | 15.0 | 8.0 | 18.6 | 7.4 | 22.1 | ||||
| Σ | 4/6 | 4/6 | 3/6 | 2/6 | 2/6 | 1/6 | 3/6 | 5/6 | 2/6 |
Note: Regions marked with a number revealed significant over-/under-activation in the respective subgroup at a threshold of P < .005, voxel extent 10. The specific number indicates to what extent (percent) the clusters of the task-specific analyses overlap with the clusters of the main analysis.
Replicability of General Meta-Analytic Findings in Task Subgroups
Table 3 indicates which of the clusters identified in the general meta-analysis could be replicated in the 6 task subgroup-analyses. Left inferior parietal over-activation was replicated in 5 subgroups but not for affective tasks. MPFC and right premotor cortex revealed robust under-activation in 4 out of 6 task subgroups. MPFC was not replicated for visual and implicit tasks, right premotor cortex was not replicated for affective and explicit tasks. Left orbito-frontal cortex and left cingulate gyrus show under-activation in 3 out of 6 subgroup analyses. Medial occipito-parietal, right lingual gyrus, and left lateral occipitotemporal show inconsistent replicability as they were replicated in merely 1 to 2 subgroups each.
Meta-Regressions
We calculated meta-regressions with the factors duration of illness, positive symptoms, and negative symptoms. Although all meta-regressions revealed significant findings, visual inspection of the data showed that significant results were caused by several strong outliers in brain activation. Therefore, the meta-regressions are merely reported in the supplementary material and are not interpreted in the Discussion section.
Discussion
The aim of this meta-analysis was to investigate in which brain regions patients with SZ, compared to controls, show altered neuronal response during TOM tasks. Our main findings were (1) SZ patients show convergent under-activation in MPFC, left orbito-frontal cortex, right premotor cortex, and left lateral occipito-temporal cortex (posterior TPJ; TPJp); (2) patients reveal over-activation in a bilateral, dorsal section of the TPJ (TPJd); (3) aberrant activation in medial-prefrontal cortex, premotor cortex, and left TPJd was identified in most TOM task types whereas abnormal activation in the remaining cluster varied stronger with task type.
We meta-analyzed brain activation of 21 individual studies, which is far more than previous meta-analyses were able to include. A greater amount of data significantly increases the reliability of meta-analytic findings.51 The higher number of studies might also explain the diverging findings. To illustrate, although we replicate MPFC under-activation, we do not replicate activation patterns in other regions.27
In line with previous research,26 our task subgroup analyses showed that MPFC under-activation is robust against changes in task type whereas abnormal brain activation in other regions is identified only in several subgroups. This implies the following: (1) robust MPFC under-activation suggests a general dysfunction across TOM tasks in SZ; (2) aberrant activation in other regions could not be identified by previous meta-analyses due to the limited number of studies available at that time.
The Schizophrenic Social Brain
We showed under-activation in the schizophrenic brain during mentalizing in areas often subsumed under the term “social brain.”52 “Social brain” refers to the neuronal processes related to social cue perception, experience sharing, inferring other people’s thoughts/emotions and managing emotional reactions to others.53 It includes activation in prefrontal cortex, orbitofrontal cortex, anterior cingulate cortex, premotor cortex, and inferior parietal cortex.54
Although great effort was made to disentangle the pathophysiology of TOM processes in SZ, our meta-analysis is the first to underpin a general dysfunction of the social brain network. The identified under-activation in these regions derives from decreased differences in brain activation between TOM and control tasks in SZ patients. Until now, this decreased difference between TOM and control task was either interpreted as hypo-mentalizing during TOM or hyper-mentalizing during control tasks.55,56 Accordingly, deficient social functioning is assumed to result from treating social agents like objects or the over-interpretation of social cues.40,57 Further research is urgently needed to expand our understanding of what over- and under-activation means with respect to over and under-mentalizing in SZ and how these effects are related to positive and negative symptoms of SZ.58 Since the current meta-analysis analyzed the relative contrast between mentalizing and control tasks, it is beyond the scope of our analyses to foster one of these hypotheses. In neurophysiological terms, both assumptions result in a decreased difference in activation between TOM and control tasks. This implies that irrespective of whether hyper- or hypo-mentalizing causes the maladaptive performance, the schizophrenic brain differentiates less between TOM and control stimuli than a healthy brain. Regarding our finding of under-activation in SZ patients, we therefore propose the theory that the psychopathology of SZ is due to a less specialized social brain network. A decreased difference in brain response toward TOM stimuli versus control stimuli in SZ thus suggests a deficit in these brain regions to efficiently differentiate under which circumstances the processing of the social meaning of a stimulus is necessary. Such inefficiency might be the result of aberrant brain inter-connectivity59 and decreased specialization is also found in language60 and working memory61 studies in SZ.
In the current analyses, under-activation in regions of the social brain goes along with over-activation in bilateral dorsal TPJ. Our IPL and TPJp cluster correspond to dorsal and posterior TPJ components robustly identified in the literature62–66(for overlapping maps see SUP2). Connectivity and independent component analyses emphasize functional differences between dorsal and posterior TPJ62,63: TPJp is part of a fronto-parietal network63 and is associated with social-cognitive processes66 like mentalizing and the default mode network.67–69 Conversely, IPL (or also TPJd) is related to attention processes62,63 and general cognitive control.70–72 Bilateral TPJd shows convergent over-activation in patients compared to healthy controls during mentalizing tasks. It is possible that TPJd over-activation is associated with a compensatory response that recruits alternative strategies to foster task performance and might reflect the engagement of attention functions and cognitive control. However, these compensatory processes might fail (apparently SZ patients show disturbances in behavioral TOM tasks) for some reasons; First, it is not very likely that the computation applied by the attentional system is appropriate to the mental domain. Second, there is evidence that the attentional network and the regions where we identified under-activation inhibit each other73 which led to the assumption that these networks reflect 2 incompatible modes that do not co-activate.
To date, there is no consensus concerning the pathophysiological mechanisms that cause aberrant neuronal response in SZ. Widespread abnormal activation, as identified in the current analysis, might be due to a general dysconnection in the schizophrenic brain.74 SZ is associated with altered synaptic plasticity, which could modulate functional coupling75 between cortical networks. This dysconnectivity approach is also capable of explaining a range of first rank symptoms in SZ.76 Furthermore, drugs that affect synaptic plasticity have been shown to lead to psychotic symptoms in healthy subjects.77,78
Implications for the Psychopathology of SZ
We showed that SZ patients revealed abnormal brain activity during various mentalizing tasks in a specific set of areas. In the following, we demonstrate how a deficient processing of social meaning is related to pathological outcomes in SZ. In healthy participants, activation in this set of areas is related to social cognition processes including social cue perception, experience sharing, the ability to infer other people’s thoughts and emotions and to manage social reactions.53
Social cue perception refers to the ability to read social cues from others and thus enables us to adequately respond in social interactions. Critically, patients with SZ show impairments in retrieving social cues from biological movements79,80 or faces.81,82 Neuroimaging studies show that these processes are related to activation in lingual gyrus83 but also anterior cingulate, parietal lobe, and cuneus cortex.53 This is in line with our finding of a decreased specialization for TOM stimuli in these regions, which in turn makes it difficult for patients to recognize relevant social meaning in the faces of others.
Experience sharing describes the phenomenon that the observation of another person’s behavior leads to neural activation that normally becomes active when engaging in that behavior oneself.84,85 Such imitation processes are related to activation in premotor cortex which is robustly identified as being part of the mirror neuron system.84–86 It is assumed to play an important role in understanding the actions and intentions of others87 as well as in imitation and empathy.88,89 A decreased differentiation between TOM and control stimuli might make it difficult for patients to empathize or to learn about other’s intentions and actions. In fact, there are studies highlighting problems with spontaneous mimicry and imitation.90,91 Impaired imitation abilities are also found in autistic patients92 and are assumed to determine an abnormal development of social and communicative functioning93 in SZ.94,95 However, neurophysiological findings are mixed53 and further research is needed to explore the link between deficient premotor cortex activation and imitation skills in SZ.
MPFC and TPJ are considered core components of the mentalizing system that co-activate during TOM tasks.96,97 These regions are associated with decoupling mechanisms, referring to the ability to dissociate between one agent’s mental state from one’s own beliefs and to differentiate between belief and reality.98 We showed that the schizophrenic brain differentiates to a weaker extent between TOM tasks and control tasks in both, MPFC and TPJ. Less specialized brain response towards TOM stimuli might implicate a failure of de-coupling mechanisms in SZ. SZ patients therefore might be impaired at identifying the origin of beliefs, intentions, or action and have difficulties to dissociate them from reality. This might be a plausible explanation for delusory perception like paranoia and visual or auditory hallucinations. In fact, there is evidence that patients which tend to hallucinate have greater problems in attributing sources of spoken words.99
Reacting in an adequate manner in social situations requires to (1) successfully identify socially relevant cues; (2) process the meaning of the observed cues; (3) evaluate on an appropriate social response. We already pointed out that SZ is characterized by impaired social cue perception. Processing the facial expression of another person requires the capacity to form complex representation of what others are thinking and to decouple them from own thoughts. Such meta-cognitive abilities are often associated with MPFC cortex,100 which we showed is impaired in SZ. Behavioral symptoms of impaired meta-cognition are also evident in SZ101,102 and are known to be related to social and emotional withdrawal, awareness of illness, and quality of life.102 Choosing social behavior is associated with the personal valence of that situation. To illustrate, neuroimaging studies in learning and gambling tasks found that orbito-frontal cortex is involved in monitoring the reward value of stimuli and responses and therefore guides our behavior regarding the value of possible outcomes of that situation. Recent studies suggest impaired reward processing and motivational impairments in SZ.103 Abnormal activation in orbito-frontal cortex could reflect difficulties in ascribing personal valence to people or social situations and a decreased social motivation to do so.104 It is up to future studies to show how orbito-frontal dysfunction is exactly related to social response behavior in SZ. One way to do so would be to assess brain response in SZ in a task where confederates could either give you helpful or unhelpful advices on how to get rewarded in a game.105 If SZ patients have impairments in ascribing personal valence to others, their task performance (and most probably orbito-frontal brain activation) might differ from healthy controls.
Limitations
A disadvantage of a pooled meta-analysis is the high level of variance produced by combining diverging operationalization of TOM abilities. Since the results of a pooled analysis may obscure possible task effects we provide separate analysis of 6 task groups.26 An additional limitation here is that the distribution is not the same for all task types. For the sake of clarification, we provide an overview of the task classification in the supplementary material.
Another limitation of the meta-analysis is that we were not able to assess the possible influence of medication on neural activation due to an insufficient amount of data. However, decreased neural response in TOM regions during TOM is also found in unaffected siblings of schizophrenic patients and in other relatives who bear an increased genetic risk to develop SZ.7,8 This minimizes the possibility that abnormal brain activation is the mere result of reiterating hospitalization and medication.
Furthermore, we were not able to draw a coherent conclusion about the influence of positive and negative symptoms on neural activation since regressions were carried by several outliers in brain activation estimates which tampered the results. Again, this must await future research in order to gain a sufficient amount of reliable data.
Conclusion
We identified under-activation in schizophrenic patients during TOM tasks in cortical regions normally specialized for social cognition. There was over-activation in attention-related regions which seems to be a failed attempt of the schizophrenic brain to fully compensate for malfunctions in the social domain. We propose that socio-cognitive deficits like an impaired TOM might be explained by less specialized social brain processes for which the brain is not able to compensate. It is now up to future studies to show how these findings can be embedded in targeted psychosocial treatments. We suggest 2 consecutive steps to do so; First, it is necessary to teach patients in which situations the extraction of social meaning is required to understand social situations. A possible way to train adequate processes could be to include training on basic TOM versus control stimuli in the therapy.
Second, current psychosocial treatments focused on affect perception and there are already studies showing its efficacy.106,107 A next step toward a better discrimination of social cues in SZ would be to assess how trainings on experience sharing, mentalizing and social reactions would improve functional outcome in SZ. Patients could be trained via imitation exercises or role plays where patients learn how to interpret what others are thinking and how to adequately react in such situations.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
This work was supported by grants provided to Martin Kronbichler by the Austrian Science Fund (FWF grant number: P 23219-B18) and the Scientific Funds of the Paracelsus Medical University (grant number: E-10/12/062-KRO).
Supplementary Material
Acknowledgments
We would like to thank Carys Elizabeth Deeley for help with data collection and preparation, Chris Frith for his helpful comments on our draft manuscript and Kajsa Igelström for kindly providing her TPJ maps for illustration purposes.
References
- 1. Couture SM. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32:S44–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Penn DL, Sanna LJ, Roberts DL. Social cognition in schizophrenia: an overview. Schizophr Bull. 2008;34:408–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sprong M, Schothorst P, Vos E, Hox J, Engeland HV. Theory of mind in schizophrenia: meta-analysis. Br J Psychiatry. 2007;191:5–13. [DOI] [PubMed] [Google Scholar]
- 4. Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. 2009;109:1–9. [DOI] [PubMed] [Google Scholar]
- 5. Frith C, Frith U. Theory of mind. Curr Biol. 2005;15. [DOI] [PubMed] [Google Scholar]
- 6. Perner J, Davies G. Understanding the mind as an active information processor: do young children have a “copy theory of mind”? Cognition. 1991;39:51–69. [DOI] [PubMed] [Google Scholar]
- 7. Janssen I, Krabbendam L, Jolles J, Os JV. Alterations in theory of mind in patients with schizophrenia and non-psychotic relatives. Acta Psychiatr Scand. 2003;108:110–117. [DOI] [PubMed] [Google Scholar]
- 8. Irani F, Platek SM, Panyavin IS, et al. Self-face recognition and theory of mind in patients with schizophrenia and first-degree relatives. Schizophr Res. 2006;88:151–160. [DOI] [PubMed] [Google Scholar]
- 9. Herold R, Tenyi T, Lenard K, Trixler M. Theory of mind deficit in people with schizophrenia during remission. Psychol Med. 2002;32:1125–1129. [DOI] [PubMed] [Google Scholar]
- 10. Roncone R, Falloon IR, Mazza M, et al. Is theory of mind in schizophrenia more strongly associated with clinical and social functioning than with neurocognitive deficits? Psychopathology. 2002;35:280–288. [DOI] [PubMed] [Google Scholar]
- 11. Mehl S, Rief W, Mink K, Lüllmann E, Lincoln TM. Social performance is more closely associated with theory of mind and autobiographical memory than with psychopathological symptoms in clinically stable patients with schizophrenia-spectrum disorders. Psychiatry Res. 2010;178:276–283. [DOI] [PubMed] [Google Scholar]
- 12. Inkham AE, Penn DL. Neurocognitive and social cognitive predictors of interpersonal skill in schizophrenia. Psychiatry Res. 2006;143:167–178. [DOI] [PubMed] [Google Scholar]
- 13. Fett AKJ, Viechtbauer W, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–588. [DOI] [PubMed] [Google Scholar]
- 14. Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. [DOI] [PubMed] [Google Scholar]
- 15. Schurz M, Aichhorn M, Martin A, Perner J. Common brain areas engaged in false belief reasoning and visual perspective taking: a meta-analysis of functional brain imaging studies. Front Hum Neurosci. 2013;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schurz M, Radua J, Aichhorn M, Richlan F, Perner J. Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci Biobehav Rev. 2014;42:9–34. [DOI] [PubMed] [Google Scholar]
- 17. Carrington SJ, Bailey AJ. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum Brain Mapp. 2009;30:2313–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pedersen A, Koelkebeck K, Brandt M, et al. Theory of mind in patients with schizophrenia: is mentalizing delayed? Schizophr Res. 2012;137:224–229. [DOI] [PubMed] [Google Scholar]
- 19. Brüne M, Lissek S, Fuchs N, et al. An fMRI study of theory of mind in schizophrenic patients with “passivity” symptoms. Neuropsychologia. 2008;46:1992–2001. [DOI] [PubMed] [Google Scholar]
- 20. Andreasen NC, Calage CA, O’leary DS. Theory of mind and schizophrenia: a positron emission tomography study of medication-free patients. Schizophr Bull. 2007;34:708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brunet E, Sarfati Y, Hardy-Baylé M-C, Decety J. Abnormalities of brain function during a nonverbal theory of mind task in schizophrenia. Neuropsychologia. 2003;41:1574–1582. [DOI] [PubMed] [Google Scholar]
- 22. Das P, Lagopoulos J, Coulston CM, Henderson AF, Malhi GS. Mentalizing impairment in schizophrenia: a functional MRI study. Schizophr Res. 2012;134:158–164. [DOI] [PubMed] [Google Scholar]
- 23. Walter H, Ciaramidaro A, Adenzato M, et al. Dysfunction of the social brain in schizophrenia is modulated by intention type: an fMRI study. Soc Cogn Affect Neurosci. 2009;4:166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee J, Quintana J, Nori P, Green MF. Theory of mind in schizophrenia: exploring neural mechanisms of belief attribution. Soc Neurosci. 2011;6:569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benedetti F, Bernasconi A, Bosia M, et al. Functional and structural brain correlates of theory of mind and empathy deficits in schizophrenia. Schizophr Res. 2009;114:154–160. [DOI] [PubMed] [Google Scholar]
- 26. Molenberghs P, Johnson H, Henry JD, Mattingley JB. Understanding the minds of others: a neuroimaging meta-analysis. Neurosci Biobehav Rev. 2016;65:276–291. [DOI] [PubMed] [Google Scholar]
- 27. Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S. Autism spectrum disorders and schizophrenia: meta-analysis of the neural correlates of social cognition. PLoS One. 2011;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Radua J, Mataix-Cols D, Phillips M, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry. 2012;27:605–611. [DOI] [PubMed] [Google Scholar]
- 29. Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. [DOI] [PubMed] [Google Scholar]
- 30. Radua J, Via E, Catani M, Mataix-Cols D. Voxel-based meta-analysis of regional white-matter volume differences in autism spectrum disorder versus healthy controls. Psychol Med. 2010;41:1539–1550. [DOI] [PubMed] [Google Scholar]
- 31. Lee SJ, Kang DH, Kim C-W, et al. Multi-level comparison of empathy in schizophrenia: an fMRI study of a cartoon task. Psychiatry Res Neuroimaging. 2010;181:121–129. [DOI] [PubMed] [Google Scholar]
- 32. Eack SM, Wojtalik JA, Newhill CE, Keshavan MS, Phillips ML. Prefrontal cortical dysfunction during visual perspective-taking in schizophrenia. Schizophr Res. 2013;150:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Varga E, Simon M, Tényi T, et al. Irony comprehension and context processing in schizophrenia during remission—a functional MRI study. Brain Lang. 2013;126:231–242. [DOI] [PubMed] [Google Scholar]
- 34. Harvey P-O, Zaki J, Lee J, Ochsner K, Green MF. Neural substrates of empathic accuracy in people with schizophrenia. Schizophr Bull. 2012;39:617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bedford NJ, Surguladze S, Giampietro V, Brammer MJ, David AS. Self-evaluation in schizophrenia: an fRMI study with implications for the understanding of insight. BMC Psychiatry. 2012;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rapp AM, Langohr K, Mutschler DE, Klingberg S, Wild B, Erb M. Isn’t it ironic? Neural correlates of irony comprehension in schizophrenia. PLoS One. 2013;8:e74224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Russell TA, Rubia K, Bullmore ET, et al. Exploring the social brain in schizophrenia: left prefrontal under-activation during mental state attribution. Am J Psychiatry. 2000;157:2040–2042. [DOI] [PubMed] [Google Scholar]
- 38. Brüne M, Özgürdal S, Ansorge N, et al. An fMRI study of “theory of mind” in at-risk states of psychosis: comparison with manifest schizophrenia and healthy controls. NeuroImage. 2011;55:329–337. [DOI] [PubMed] [Google Scholar]
- 39. Mier D, Sauer C, Lis S, et al. Neuronal correlates of affective theory of mind in schizophrenia out-patients: evidence for a baseline deficit. Psychol Med. 2010;40:1607–1617. [DOI] [PubMed] [Google Scholar]
- 40. Ciaramidaro A, Bolte S, Schlitt S, et al. Schizophrenia and autism as contrasting minds: neural evidence for the hypo-hyper-intentionality hypothesis. Schizophr Bull. 2014;41:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dodell-Feder D, Tully LM, Lincoln SH, Hooker CI. The neural basis of theory of mind and its relationship to social functioning and social anhedonia in individuals with schizophrenia. NeuroImage Clin. 2014;4:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pauly KD, Kircher TTJ, Schneider F, Habel U. Me, myself and I: temporal dysfunctions during self-evaluation in patients with schizophrenia. Soc Cogn Affect Neurosci. 2013;9:1779–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee KH, et al. A functional magnetic resonance imaging study of social cognition in schizophrenia during an acute episode and after recovery. Am J Psychiatry. 2006; 163:1926–1933. [DOI] [PubMed] [Google Scholar]
- 44. Radua J, Rubia K, Canales-Rodríguez EJ, et al. Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front Psychiatry. 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Radua J, Heuvel OAVD, Surguladze S, Mataix-Cols D. Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Arch Gen Psychiatry. 2010;67. [DOI] [PubMed] [Google Scholar]
- 46. Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. [DOI] [PubMed] [Google Scholar]
- 47. Dufour N, Redcay E, Young L, et al. Similar brain activation during false belief tasks in a large sample of adults with and without autism. PLoS One. 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saxe R, Kanwisher N. People thinking about thinking people: the role of the temporo-parietal junction in “theory of mind.” NeuroImage. 2003;19:1835–1842. [DOI] [PubMed] [Google Scholar]
- 49. Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–1399. [DOI] [PubMed] [Google Scholar]
- 50. Bora E, Fornito A, Yücel M, Pantelis C. Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biol Psychiatry. 2010;67:1097–1105. [DOI] [PubMed] [Google Scholar]
- 51. Guolo A, Varin C. Random-effects meta-analysis: the number of studies matters. Stat Methods Med Res. 2015; 0962280215583568. [DOI] [PubMed] [Google Scholar]
- 52. Adolphs R. The social brain: neural basis of social knowledge. Annu Rev Psychol. 2009;60:693–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nat Rev Neurosci. 2015;16:620–631. [DOI] [PubMed] [Google Scholar]
- 54. Frith U, Frith C. The social brain: allowing humans to boldly go where no other species has been. Philos Trans R Soc Lond B Biol Sci. 2010;365:165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Frith CD. Schizophrenia and theory of mind. Psychol Med. 2004;34:385–389. [DOI] [PubMed] [Google Scholar]
- 56. Abu-Akel A. Impaired theory of mind in schizophrenia. Pragmat Cogn. 1999;7:247–282. [Google Scholar]
- 57. Langdon R, Brock J. Hypo-or hyper-mentalizing: it all depends upon what one means by “mentalizing”. Behav Brain Sci. 2008;31:274–275. [Google Scholar]
- 58. Abu-Akel AM, Apperly IA, Wood SJ, Hansen PC. Autism and psychosis expressions diametrically modulate the right temporo-parietal junction. Soc Neurosci. 2016;3:1–13. [DOI] [PubMed] [Google Scholar]
- 59. Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 2006;59:929–939. [DOI] [PubMed] [Google Scholar]
- 60. Dollfus S, Razafimandimby A, Delamillieure P, et al. Atypical hemispheric specialization for language in right-handed schizophrenia patients. Biol Psychiatry. 2005;57:1020–1028. [DOI] [PubMed] [Google Scholar]
- 61. Tan HY, Sust S, Buckholtz JW, et al. Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am J Psychiatry. 2006;163:1969–1977. [DOI] [PubMed] [Google Scholar]
- 62. Bzdok D, Langner R, Schilbach L, et al. Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. NeuroImage. 2013;81:381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Igelstrom KM, Webb TW, Graziano MSA. Neural processes in the human temporo-parietal cortex separated by localized independent component analysis. J Neurosci. 2015;35:9432–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Igelstrom KM, Webb TW, Kelly YT, Graziano MSA. Topographical organization of attentional, social, and memory processes in the human temporo-parietal cortex. eNeuro. 2016;3. doi:10.1523/eneuro.0060-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mars RB, Sallet J, Schüffelgen U, Jbabdi S, Toni I, Rushworth MF. Connectivity-based subdivisions of the human right “temporo-parietal junction area”: evidence for different areas participating in different cortical networks. Cereb Cortex. 2012; 22:1894–1903. [DOI] [PubMed] [Google Scholar]
- 66. Bzdok D, Hartwigsen G, Reid A, Laird AR, Fox PT, Eickhoff SB. Left inferior parietal lobe engagement in social cognition and language. Neurosci Biobehav Rev. 2016; 68:319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. [DOI] [PubMed] [Google Scholar]
- 68. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network. Ann N Y Acad Sci. 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 69. Allen EA, Erhardt EB, Damaraju E, et al. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci. 2011;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dosenbach NUF, Visscher KM, Palmer ED, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jack AI, Dawson AJ, Begany KL, et al. fMRI reveals reciprocal inhibition between social and physical cognitive domains. NeuroImage. 2013;66:385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Friston K, Brown HR, Siemerkus J, Stephan KE. The dysconnection hypothesis (2016). Schizophr Res. 2016;176:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Friston KJ. The disconnection hypothesis. Schizophr Res.1998;30:115–125. [DOI] [PubMed] [Google Scholar]
- 76. Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Featherstone RE, Kapur S, Fletcher PJ. The amphetamine-induced sensitized state as a model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1556–1571. [DOI] [PubMed] [Google Scholar]
- 78. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. [DOI] [PubMed] [Google Scholar]
- 79. Kim J, Doop ML, Blake R, Park S. Impaired visual recognition of biological motion in schizophrenia. Schizophr Res. 2005;77:299–307. [DOI] [PubMed] [Google Scholar]
- 80. Kim J, Park S, Blake R. Perception of biological motion in schizophrenia and healthy individuals: a behavioral and FMRI study. PLoS One. 2011;6:e19971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Savla GN, Vella L, Armstrong CC, Penn DL, Twamley EW. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr Bull. 2013;39:979–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Li H, Chan RC, McAlonan GM, Gong QY. Facial emotion processing in schizophrenia: a meta-analysis of functional neuroimaging data. Schizophr Bull; 2010;36:1029–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Servos P, Osu R, Santi A, Kawato M. The neural substrates of biological motion perception: an fMRI study. Cereb Cortex. 2002;12:772–782. [DOI] [PubMed] [Google Scholar]
- 84. Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S, Rizzolatti G. Activation of human primary motor cortex during action observation: a neuromagnetic study. Proc Natl Acad Sci U S A. 1998;95:15061–15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cochin S, Barthelemy C, Roux S, Martineau J. Observation and execution of movement: similarities demonstrated by quantified electroencephalography. Eur J Neurosci. 1999;11:1839–1842. [DOI] [PubMed] [Google Scholar]
- 86. Gallese V. The intentional attunement hypothesis the mirror neuron system and its role in interpersonal relations. In: Wermter S, Palm G, Elshaw M, eds. Biomimetic Neural Learning for Intelligent Robots. Berlin: Springer; 2005:19–30. [Google Scholar]
- 87. Kilner JM, Friston KJ, Frith CD. The mirror-neuron system: a Bayesian perspective. Neuroreport. 2007;18:619–623. [DOI] [PubMed] [Google Scholar]
- 88. Gallese V. The roots of empathy: the shared manifold hypothesis and the neural basis of intersubjectivity. Psychopathology. 2003;36:171–180. [DOI] [PubMed] [Google Scholar]
- 89. Rizzolatti G, Craighero L. Mirror neuron: a neurological approach to empathy. In: Changeux JP, Damasio AR, Singer W, Christen Y, eds. Neurobiology of Human Values. Berlin/Heidelberg: Springer; 2005:107–123. [Google Scholar]
- 90. Varcin KJ, Bailey PE, Henry JD. Empathic deficits in schizophrenia: the potential role of rapid facial mimicry. J Int Neuropsychol Soc. 2010;16:621–629. [DOI] [PubMed] [Google Scholar]
- 91. Schwartz BL, Mastropaolo J, Rosse RB, Mathis G, Deutsch SI. Imitation of facial expressions in schizophrenia. Psychiatry Res. 2006;145:87–94. [DOI] [PubMed] [Google Scholar]
- 92. Aldridge MA, Stone KR, Sweeney MH, Bower T. Preverbal children with autism understand the intentions of others. Dev Sci. 2000;3:294–301. [Google Scholar]
- 93. Rogers SJ, Pennington BF. A theoretical approach to the deficits in infantile autism. Dev Psychopathol. 1991;3:137. [Google Scholar]
- 94. Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res. 2008;99:164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Couture SM, Penn DL, Losh M, Adolphs R, Hurley R, Piven J. Comparison of social cognitive functioning in schizophrenia and high functioning autism: more convergence than divergence. Psychol Med. 2009;40:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Decety J, Chaminade T. When the self represents the other: a new cognitive neuroscience view on psychological identification. Conscious Cogn. 2003;12:577–596. [DOI] [PubMed] [Google Scholar]
- 97. Martin A, Weisberg J. Neural foundations for understanding social and mechanical concepts. Cogn Neuropsychol. 2003;20:575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Veluw SJV, Chance SA. Differentiating between self and others: an ALE meta-analysis of fMRI studies of self-recognition and theory of mind. Brain Imaging Behav. 2013;8:24–38. [DOI] [PubMed] [Google Scholar]
- 99. Brébion G, Gorman JM, Amador X, Malaspina D, Sharif Z. Source monitoring impairments in schizophrenia: characterisation and associations with positive and negative symptomatology. Psychiatry Res. 2002;112:27–39. [DOI] [PubMed] [Google Scholar]
- 100. Baird B, Smallwood J, Gorgolewski KJ, Margulies DS. Medial and lateral networks in anterior prefrontal cortex support metacognitive ability for memory and perception. J Neurosci. 2013;33:16657–16665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nicolò G, Dimaggio G, Popolo R, et al. Associations of metacognition with symptoms, insight, and neurocognition in clinically stable outpatients with schizophrenia. J Nerv Ment Dis. 2012;200:644–647. [DOI] [PubMed] [Google Scholar]
- 102. Lysaker PH, Carcione A, Dimaggio G, et al. Metacognition amidst narratives of self and illness in schizophrenia: associations with neurocognition, symptoms, insight and quality of life. Acta Psychiatr Scand. 2005;112:64–71. [DOI] [PubMed] [Google Scholar]
- 103. Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull. 2014;40:S107–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Waltz JA, Kasanova Z, Ross TJ, Salmeron BJ, McMahon RP, Gold JM, Stein EA. The roles of reward, default, and executive control networks in set-shifting impairments in schizophrenia. PLoS One. 2013;8:e57257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Behrens TE, Hunt LT, Woolrich MW, Rushworth MF. Associative learning of social value. Nature. 2008;456:245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Horan WP, Kern RS, Sergi MJ, Shokat-Fadai K, Wynn JK, Green MF. Social cognitive skills training in schizophrenia: an initial efficacy study of stabilized outpatients. Schizophr Res. 2009;107:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Roberts DL, Penn DL. Social cognition and interaction training (SCIT) for outpatients with schizophrenia: a preliminary study. Psychiatry Res. 2009;166:141–147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.