Abstract
Background and Aims Plant growth-promoting bacteria (PGPB) are soil micro-organisms able to interact with plants and stimulate their growth, positively affecting plant physiology and development. Although ethylene plays a key role in plant growth, little is known about the involvement of ethylene sensitivity in bacterial inoculation effects on plant physiology. Thus, the present study was pursued to establish whether ethylene perception is critical for plant–bacteria interaction and growth induction by two different PGPB strains, and to assess the physiological effects of these strains in juvenile and mature tomato (Solanum lycopersicum) plants.
Methods An experiment was performed with the ethylene-insensitive tomato never ripe and its isogenic wild-type line in which these two strains were inoculated with either Bacillus megaterium or Enterobacter sp. C7. Plants were grown until juvenile and mature stages, when biomass, stomatal conductance, photosynthesis as well as nutritional, hormonal and metabolic statuses were analysed.
Key Results Bacillus megaterium promoted growth only in mature wild type plants. However, Enterobacter C7 PGPB activity affected both wild-type and never ripe plants. Furthermore, PGPB inoculation affected physiological parameters and root metabolite levels in juvenile plants; meanwhile plant nutrition was highly dependent on ethylene sensitivity and was altered at the mature stage. Bacillus megaterium inoculation improved carbon assimilation in wild-type plants. However, insensitivity to ethylene compromised B. megaterium PGPB activity, affecting photosynthetic efficiency, plant nutrition and the root sugar content. Nevertheless, Enterobacter C7 inoculation modified the root amino acid content in addition to stomatal conductance and plant nutrition.
Conclusions Insensitivity to ethylene severely impaired B. megaterium interaction with tomato plants, resulting in physiological modifications and loss of PGPB activity. In contrast, Enterobacter C7 inoculation stimulated growth independently of ethylene perception and improved nitrogen assimilation in ethylene-insensitive plants. Thus, ethylene sensitivity is a determinant for B. megaterium, but is not involved in Enterobacter C7 PGPB activity.
Keywords: Bacillus megaterium, Enterobacter, ethylene, plant growth-promoting bacteria (PGPB), plant nutrition, Solanum lycorpersicum (tomato)
INTRODUCTION
The aim of the sustainable intensification of agriculture is to provide food security to a growing global population while minimizing any harmful environmental impacts of cropping systems (Tilman et al., 2011). Consequently, the resource use efficiency of crops needs to be increased without sacrificing current yields (Dodd and Ruiz-Lozano, 2012). A wide range of soil micro-organisms are able to establish associations with plants (Gray and Smith, 2005), and dozens of reports of plant growth stimulation by beneficial soil micro-organisms can be found (Lucy et al., 2004; Adesemoye and Kloepper, 2009; Nadeem et al., 2014). Management of microbial populations living in the rhizosphere is a flexible, low-cost and environmentally friendly method to enhance plant growth (Berg, 2009; Singh et al., 2011). The root system growth and physiology are modulated by these micro-organisms (Barea et al., 2005) and some of these can increase plant stress tolerance (Glick, 2004; Aroca and Ruiz-Lozano, 2009; Dimkpa et al., 2009).
Among soil micro-organisms, plant growth-promoting bacteria (PGPB) have been studied in detail (Lugtenberg and Kamilova, 2009; Pii et al., 2015; Santoyo et al., 2016). The PGPB concept has been confined to bacterial strains with at least two of the three criteria such as aggressive colonization, plant growth stimulation or biocontrol (Weller et al., 2002; Vessey, 2003). The root colonization process is influenced by bacterial traits, root exudates and abiotic and biotic factors (Benizri et al., 2001; el Zahar Haichar et al., 2014). PGPB can act either directly or indirectly (Glick, 1995; Ortíz-Castro et al., 2009). Direct promotion includes increasing supply of nutrients, phytohormone modulation and induction of systemic resistance. Indirect stimulation is basically related to biocontrol (van Loon, 2007; Lugtenberg and Kamilova, 2009). Several mechanisms may be active simultaneously, promoting plant growth as a net result (Martínez-Viveros et al., 2010) and, in consequence, the use of PGPB can be described as an attractive way to replace use of chemicals in agriculture (Bhattacharyya and Jha, 2012). Plant-beneficial microbe associations are thought to be ancient and shaped during coevolution so that bacteria could have significant effects on plant physiology (Lambers et al., 2009). In fact, the action mechanism of some PGPB suggests a simple interaction and responses between the two partners.
Plant–bacterial interactions and their environment are essentials for better uptake of water and nutrients by plants (Ryan et al., 2009). Although nutrient availability is limited in most soils, a constant level of essential mineral nutrients needs to be maintained and rhizosphere microbial communities are associated with nutrient biogeochemical cycles (Barea et al., 2005). To cope with nutrient limitation, several physiological and developmental responses can be triggered which produce modifications on whole-plant morphology and metabolism (López-Bucio et al., 2002). In this regard, plant nutritional and hormonal homeostasis are closely inter-related and co-ordinated for the fine regulation of growth and development (Krouk et al., 2011).
Ethylene plays a prominent role in plant physiology and, like most other phytohormones, can inhibit or promote growth depending on the cell type and plant species (Pierik et al., 2006). Its production is typically upregulated in response to environmental stresses (F. Wang et al., 2013). Although the interactions among ethylene, nutrients and plant responses has been reviewed, our knowledge of these interactions is incomplete (Iqbal et al., 2013). Several bacterial strains can produce ethylene (Primrose and Dilworth, 1976; Saleem et al., 2007) or reduce its levels. The latter have been called stress controllers (Lugtenberg and Kamilova, 2009) and induce plant growth by decreasing the content of 1-aminocyclopropane-1-carboxylic acid (ACC), the immediate precursor of ethylene in all higher plants (Glick et al., 2007; Glick, 2014). However, relatively little is known about bacterial inoculation effects on plant physiology and its relationship with ethylene sensitivity of mature compared with juvenile plants.
In order to shed light on plant–bacteria interactions, various omics approaches have been used (Cheng et al., 2010; Stearns et al., 2012; van de Mortel et al., 2012; Couillerot et al., 2013; Su et al., 2016). Metabolites play a key role in regulatory mechanisms because they are in many cases the end-products. Thus, metabolic information is very useful to understand a plant’s interaction with its environment (Feussner and Polle, 2015) and could be interpreted as a snapshot of inoculation effects on plant physiology. Metabolomic analyses in arabidopsis showed modified amino acid and sugar levels due to bacterial inoculation (Su et al., 2016). Also, bacterial root colonization can produce modifications in physiological status and metabolite content for short- and long-term periods (Su et al., 2016).
The aim of this study was to gain more insights into the role of ethylene sensitivity in plant–microbe interactions, specifically PGPB activity. Tomato is the most important horticultural crop around the world, and the second most consumed vegetable after potato (Schwarz et al., 2010). Moreover, mutants and previous research are available, representing a suitable model for understanding the interaction of PGPB and other plants. The ethylene-insensitive tomato mutant never ripe (nr) and its isogenic wild-type (wt) parental line were used. Previous studies have shown that nr is largely unable to perceive ethylene, with some residual responsiveness (Lanahan et al., 1994) because of a mutation in the sensor domain of the ethylene receptor LeETR3 (Wilkinson et al., 1995). In addition, two PGPB strains isolated from arid soils in southern Spain have been used: Bacillus megaterium strain (Bm) (Marulanda-Aguirre et al., 2008) and Enterobacter sp. [hereafter Enterobacter C7 (C7)]. Bm has shown PGPB features in previous reports (Marulanda et al., 2009, 2010; Armada et al., 2014a; Porcel et al., 2014; Ortiz et al., 2015). However, Enterobacter C7 was evaluated here for the first time. We aimed to determine if ethylene sensitivity is critical for plant–bacteria interaction and growth induction by these two PGPB strains and to evaluate the effects of bacterial inoculation on physiology in both juvenile and mature plants. Since most studies on the role of ethylene in PGPB activity have been focused on bacteria able to reduce ACC contents (Glick et al., 2007; Glick, 2014), we intended to use bacteria without either ACC deaminase activity or ethylene production capacity in order to avoid any perturbation of plant ethylene metabolism caused by the bacteria. Plant growth, bacterial colonization, stomatal conductance, photosynthetic efficiency and chlorophyll contents, nutrients, phytohormones and root metabolite contents were determined at 4 and 8 weeks post-inoculation (wpi).
MATERIALS AND METHODS
Biological material
Seeds of nr tomato (Solanum lycopersicum) (LA0162) (Wilkinson et al., 1995) and its isogenic parental cultivar ‘Pearson’ (LA0012) were obtained from the Tomato Genetics Resource Center at the University of California, Davis, CA, USA. PGPB strains were isolated from soils in southern Spain. Bacillus megaterium was identified and partially characterized in a previous study (Marulanda-Aguirre et al., 2008). Enterobacter C7 was isolated and identified by E. Armada as described in Armada et al. (2014b).
Experimental design and growth conditions
The experiment consisted of a randomized complete block design with two plant lines: tomato ‘Pearson’ wt and its ethylene-insensitive mutant (nr), and three inoculation treatments: (1) non-inoculated control plants; (2) B. megaterium-inoculated plants; and (3) Enterobacter C7-inoculated plants. Experiments consisted of 18 replicates per treatment, and two different harvests were established: 4 and 8 wpi, using nine plants at each harvest (n = 9).
Seeds were sterilized (70 % ethanol 5 min, 5 % sodium hypochlorite 10 min and three washing steps with sterile water to remove any trace of chemicals), kept at 4 ºC overnight and placed on sterile vermiculite at 25 ºC until germination. Ten-day-old seedlings were transferred to 1 L plastic pots containing sterile peat moss:perlite (1:1, v/v, autoclaved twice at 120 °C for 20 min). Bacteria were grown in Luria broth (LB) medium with shaking (200 rpm) at 28 °C overnight. The culture optical density was measured at 600 nm (OD600), the cells were centrifuged (2655 g, 10 min) and the pellet was resuspended in sterile distilled water until OD600 = 1·5, corresponding to a cell density of 107 CFU mL−1. A 1 mL aliquot of distilled water (control plants), or a bacterial suspension: Bm or C7 (inoculated plants), was added to each root seedling at transplantation. Plants were grown for 4 or 8 weeks in a greenhouse under controlled climatic conditions (18–24 °C, 50–60 % relative humidity, 16 h:8 h light (600 μmol m−2 s−1):dark). In order to maintain constant soil water content close to water-holding capacity during the whole experiment, water was supplied every 2 d.
Biomass production
Shoots were separated from root systems at each harvest time, samples were dried in a forced draught oven (70 ºC, 3 d) and dry weights were determined. The relative growth rate (RGR) was calculated using the classical approach (Hunt, 1982) following the equation: RGR = (ln W2 – ln W1)/(t2 – t1) where W1 and W2 are dry weights at times t1 an t2, respectively.
Colonization of tomato root system
Sterilized seeds were germinated on filter paper soaked with sterile distilled water on Petri plates in darkness for 3 d. Ten-day-old seedlings were transferred to sterilized glass bottles containing the same substrate as above. At transplantation, plants were inoculated as mentioned above. Eight replicates of each were performed (n = 8). The glass bottles were closed and kept for 1 week in a climate-controlled growth chamber (18–24 °C, 50–60 % relative humidity, 16 h daylight). A 1 cm long intermediate root segment was carefully cut and suspended in 1 mL of sterile water. Tubes were incubated for 1 h on an orbital shaker (35 rpm) with vibration. Suspensions were serially diluted (10−2–10−9). Dilutions were plated on LB agar medium and cultivated overnight at 28 ºC. Finally, colonies were counted and CFU cm−1 root values were calculated.
Bacterial ACC deaminase activity bioassay
The PGPB strains were tested for the ability to use ACC as a sole N source in order to evaluate their possible effect on plant ACC levels. ACC deaminase activity of cell-free extracts was determined by estimating α-ketobutyrate production (nmol mg−1 protein h−1) according to the procedure described by Penrose and Glick (2003).
Ethylene production by bacterial strains
Bacterial ethylene production was measured by gas chromatography (GC) in order to determine possible effects of microbially derived ethylene in plant–bacteria interaction and/or growth promotion. Bacteria were grown in LB medium with shaking (200 rpm) at 28 ºC overnight. Culture OD600 was measured and new sub-cultures (LB, 6 mL, OD600 = 0·01) were started in sterile 20 mL vials (Supelco Analytical, Pennsylvania, USA). Vials were closed and incubated at 28 ºC with shaking (200 rpm). Samples of 1 mL were withdrawn from each vial with a syringe, and ethylene was quantified using a Hewlett Packard model 5890 gas chromatograph equipped with a Poropak-R column and a hydrogen flame ionization detector at 3, 6, 9 and 24 h after starting the culture. Six replicates per bacterium and LB without inoculum were analysed (n = 6).
Physiological parameters
Stomatal conductance.
Stomatal conductance was measured 3 h after sunrise with a porometer system (Porometer AP4, Delta-T Devices Ltd, Cambridge, UK).
Photosynthetic efficiency.
A FluorPen FP100 (Photon Systems Instruments, Brno, Czech Republic) was used to measure photosystem II efficiency according to Oxborough and Baker (1997).
Leaf chlorophyll concentration.
Photosynthetic pigments were extracted from leaf samples (0·5 cm2) in 100 % methanol at 4 ºC for 24 h. Pigment concentration was spectrophotometrically determined according to Lichtenthaler (1987). Samples were taken from the last expanded leaf for stomatal conductance, photosynthetic efficiency and chlorophyll content (n = 9).
Nutrient measurement.
Mineral analysis was determined in shoots and roots (n = 4). C and N concentration (% d. wt) were determined by mass spectrometry (ELEMENTAL LECO TruSpec CN) and were performed by the Analytical Service of the Instituto de Nutrición Animal (CSIC), Granada, Spain. Ca, K, Mg, Na, P, S and Si concentration (% d. wt) as well as Cu, Fe, Mn, and Zn concentration (ppm) analyses were determined by inductively coupled plasma-optical emission spectrometry (ICP-OES; Varina ICP 720-ES) and were performed by the Instrumentation Service of the Estación Experimental del Zaidín (CSIC), Granada, Spain.
Phytohormone analysis.
Indole acetic acid (IAA), abscisic acid (ABA), salicylic acid (SA), jasmonic acid (JA) and jasmonoyl isoleucine (JA-Ile) were analysed using high-performance liquid chromatography-electrospray ionization-high-resolution accurate mass spectrometry (HPLC-ESI-HRMS). The extraction and purification were performed using the following method: 250 mg of frozen tissue (previously ground to a powder in a mortar with liquid N2) was homogenized with 2·5 mL of pre-cooled methanol:water:HCOOH (90:9:1, v/v/v with 2·5 mm Na-diethyldithiocarbamate) and 25 μL of a stock solution of 1000 ng mL−1 of deuterium-labelled internal standards D-IAA, D-ABA, D-SA and D-JA, and 200 ng mL−1 of D-JA-Ile in methanol. The mixture was shaken for 60 min at room temperature before being centrifuged (20 000 g, 10 min), shaken again for 20 min and centrifuged. A 2 mL aliquot of pooled supernatants was taken and dried at 40 ºC. The residue was dissolved in 500 μL of methanol:0·133 % acetic acid (40:60, v/v) and centrifuged (20 000 g, 10 min) before being injected in an HPLC-ESI-HRMS system.
Hormones were quantified using a Dionex Ultimate 3000 UHPLC device coupled to a Q Exactive Focus Mass Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA), equipped with an HESI(II) source, a quadrupole mass filter, a C-Trap, a HCD collision cell and an Orbitrap mass analyzer (Orbitrap-Focus, Thermo Scientific). A reverse-phase column (Synergi 4 mm Hydro-RP 80A, 150 × 2 mm; Phenomenex, Torrance, CA, USA) was used. A linear gradient of methanol (A), water (B) and 2 % acetic acid in water (C) was used: 38 % A for 3 min, 38 % to 96 % A in 12 min, 96 % A for 2 min and 96 % to 38 % A in 1 min, and kept for 4 min. C remains constant at 4 %. The flow rate was 0·30 mL min−1, the injection volume was 40 μL and column and sample temperatures were 35 and 15 ºC, respectively. The detection and quantification were performed using a Full MS experiment with tandem mass spectrometry (MS/MS) confirmation in the negative-ion mode. Instrument control and data processing were performed by TraceFinder 3.3 EFS software. Compounds as well as instrumental parameters are reported in the Supplementary Data (reagents and internal standards in Table S1, ionization source in Table S2 and compound accurate masses in Table S3).
Ethylene production was analysed using GC. Completely expanded leaflets from the last developed leaf, and entire roots were chosen for shoot and root ethylene determination, respectively. Samples were introduced in 20 mL vials (Supelco Analytical, PA, USA) with 200 μL of MilliQ water to avoid tissue drying. After 15 min to let the ethylene produced from injury escape, vials were closed and incubated for 1 h (leaflets) and 4 h (roots) at room temperature. Samples of 1 mL were withdrawn from each vial with a syringe, and ethylene was quantified using a Hewlett Packard model 5890 gas chromatograph equipped with a Poropak-R column and a hydrogen flame ionization detector. Six and four replicates per treatment were used in 4 and 8 wpi harvests, respectively.
Metabolite analysis.
Metabolite analysis by GC-MS was performed according to Roessner et al. (2000). A 100 mg aliquot of frozen tissue (previously ground to a powder in a mortar with liquid N2) was extracted with 1 mL of methanol containing internal standard (ribitol 9 μg mL−1 in methanol). The mixture was extracted for 20 min at 4 °C, and 400 μL of water were added and mixed before centrifugation (18 626 g, 5 min). Two aliquots of supernatant were taken for analysis of major components (10 μL) and minor components (200 μL) after being dried overnight under vacuum. The residue was derivatized in two steps, methoxymation, and silylation (60 μL of methoxyamine hydrochloride in pyridine, 37 °C 90 min and BSTFA + TMCS, 37 ºC 30 min). A Varian (now Bruker) 450GC 240MS system was used for GC-MS analysis. All samples were analysed twice. A 1 μL aliquot was injected at 230 °C in split 1/50 mode with Pressure Pulse (30 psi 0·2 min). Helium flux at 1 mL min−1 and temperature ramp started at 70 °C for 5 min, increased at 5 °C a time until 245 °C, increased at 20 °C a time until 310 °C and kept for 1 min. The column was a DB-5ms 30 m, 0·25 mm, 0·25 μm. Ionization was by electronic impact and mass analysis in TIC Full Scan mode acquiring masses in the range 50–600 m/z. Identification was by comparison with the NIST08 spectra library and retention time of pure compounds. For comparative purposes, within each chromatogram, the compound peak areas were normalized by the sample fresh weight and by the internal standard peak area, resulting in relative response ratios. Six and four replicates per treatment were used for 4 and 8 wpi harvests, respectively.
Statistical analysis
Data were processed by two-way analysis of variance (ANOVA) with plant genotype (G) and inoculum (I) as sources of variation. The significance of sources of variation as well as their interaction (G × I) was evaluated by P-value (P < 0·05). In the case of significant interaction between factors, all treatments were compared against each other by least significant difference (LSD) test (P < 0·05). In the case of no interaction between factors, inoculum effects were evaluated analysing wt and nr plants separately using ANOVA followed by LSD test (P < 0·05). Plant genotype effect was evaluated by analysing wt and nr plants under the same inoculation treatment (No, Bm or C7) by Student t-test (P < 0·05). Plant dry weight, RGR, bacterial colonization, stomatal conductance, photosynthetic efficiency, photosynthetic pigments, nutrient, phytohormone and metabolite contents were analysed by this method using R software (v3.2.2 Open Source; http://www.r-project.org/). Relationships between total, shoot and root dry weights and nutrients, and metabolites were examined using the Pearson correlation coefficient (P < 0·05). Principal component analysis (PCA) was also used to compare nutrient and metabolite profiles with those obtained under different experimental conditions using Excel add-in the Multibase package (v_2015 Open source; http://www.numericaldynamics.com/).
RESULTS
Colonization of the tomato root system by PGPB strains
A bioassay for bacterial colonization was performed to test the ability of the PGPB strains that were used to colonize wt and nr root systems. As expected, no bacterial growth was observed in non-inoculated plants. Bm and C7 were able to colonize roots independently of plant genotype. Moreover, no significant differences were noticed in colonization rates, reaching values of around 2 × 106 CFU cm−1 root (Table 1). Furthermore, the PGPB strains did not show either ethylene production or the ability to cleave ACC, the direct precursor of ethylene (data not shown).
Table 1.
Bacterial root colonization
| Treatment | CFU root cm−1 | s.e. |
|---|---|---|
| Bm | ||
| wt | 2·55 × 106 | ± 0·68 × 106 |
| nr | 2·02 × 106 | ± 0·41 × 106 |
| C7 | ||
| wt | 1·85 × 106 | ± 0·39 × 106 |
| nr | 2·69 × 106 | ± 0·76 × 106 |
Colony-forming units (CFU) per root centimetre of Bacillus megaterium (Bm) and Enterobacter C7 (C7) in wild type ‘Pearson’ (wt) and never ripe (nr) tomato plants.
Data are means ± s.e. (n = 8).
No significant differences were seen (P < 0·05) according to two-way ANOVA with plant genotype (G) and inoculum (I) as sources of variation as well as their interaction.
Biomass production of wt and nr plants inoculated with two PGPB strains
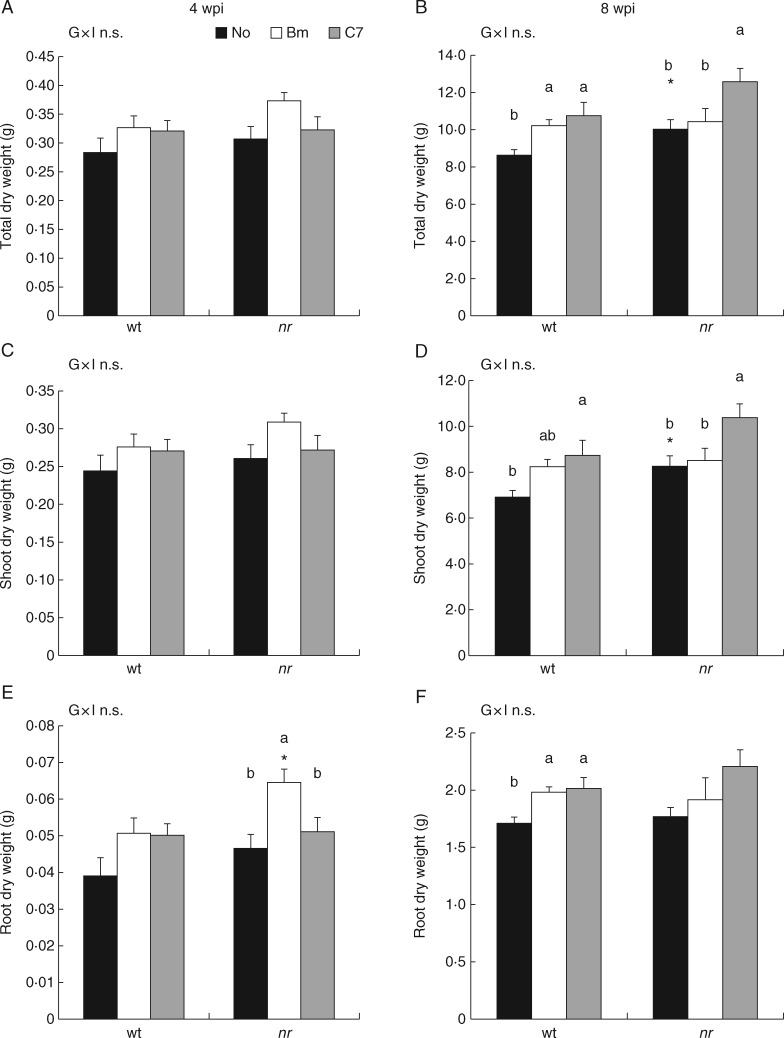
The dry weight of wt and nr plants inoculated with PGPB strains [B. megaterium (Bm) and Enterobacter C7)] showed different growth patterns at 4 and 8 wpi, but no interaction between factors was seen (Fig. 1). At 4 wpi, total and shoot dry weight showed no differences due to bacterial inoculation in wt and nr plants or due to plant genotype under different inoculations (Fig. 1A, C). However, root dry weight was increased by Bm inoculation in nr plants, while no growth promotion in wt roots was observed. Moreover, a significant difference between wt and nr plants was only observed in Bm-inoculated roots (Fig. 1E). At 8 wpi, total dry weight showed an increase due to PGPB inoculation in wt plants (18·4 and 24·6 % for Bm and C7, respectively). Nevertheless, in nr plants, total dry weight was only increased by C7 inoculation (25·5 %), showing no significant differences due to Bm inoculation (Fig. 1 B). Shoot dry weight was increased by C7 inoculation in both plant genotypes (26·3 % and 25·7 % in wt and nr plants, respectively). However, Bm inoculation did not produce a significant effect on shoot growth (Fig. 1D). Moreover, significant difference between plant genotypes was only seen in non-inoculated plants in total and shoot dry weight (Fig. 1B, D). In addition, root dry weight was increased by PGPB inoculation in wt plants (15·8 % and 18·1 % for Bm and C7, respectively), with no significant differences seen in nr plants (Fig. 1F).
Fig. 1.
Effects of bacterial inoculation on plant dry weights at 4 and 8 weeks post-inoculation (wpi). Total (A), shoot (C) and root (E) dry weights of wild-type ‘Pearson’ (wt) and never ripe (nr) tomato (Solanum lycopersicum) plants at 4 wpi. Total (B), shoot (D) and root (F) dry weights of wt and nr tomato plants at 8 wpi. Treatments are designed as non-inoculated controls (No, black bars), Bacillus megaterium-inoculated plants (Bm, white bars), and Enterobacter C7-inoculated plants (C7, grey bars). Data are means ± s.e. (n = 9). Data were analysed by two-way ANOVA, with plant genotype (G) and inoculum (I) as sources of variation. Significance of sources of variation as well as their interaction (G × I) were evaluated by P-value; n.s., not significant; *P ≤ 0·05; **P ≤ 0·01; ***P ≤ 0·001. In the case of a significant interaction between factors, all treatments were compared with each other. In the case of a non-significant interaction between factors, inoculum effects were evaluated, analysing wt and nr plants separately using ANOVA. Means followed by different lower case letters are significantly different (P < 0·05) according to LSD test. Plant genotype effect was evaluated by analysing wt and nr plants under the same inoculation treatment (No, Bm or C7) by Student t-test, and a significant difference (P < 0·05) is shown as (*) above nr means.
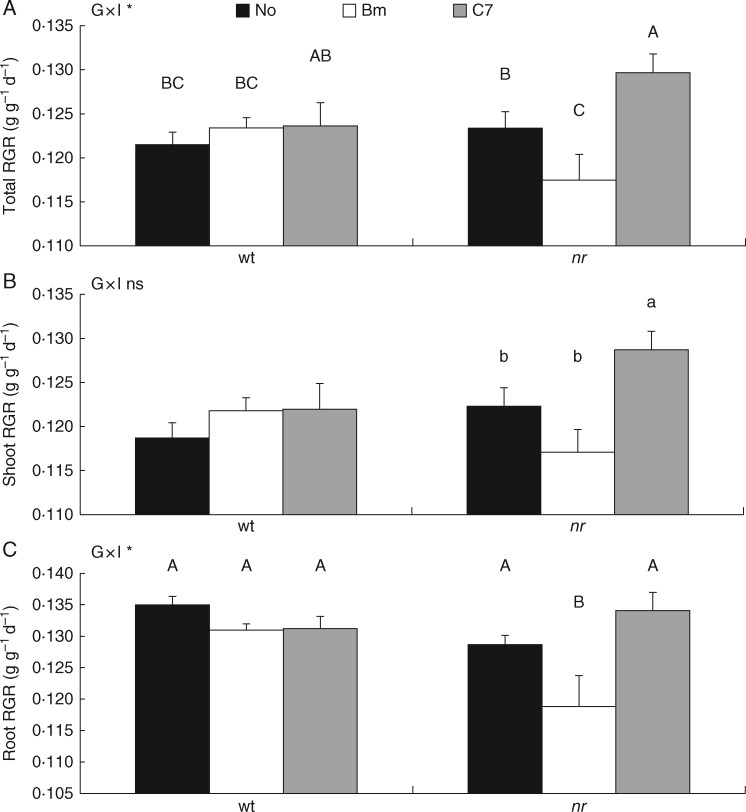
In order to analyse plant growth responses between the two harvests, RGR was calculated, showing an interaction between factors in total and root dry weights (Fig. 2). In wt plants, RGRs were unaffected. However, in nr plants, RGR changed according to inoculated bacteria and plant tissue (Fig. 2A–C). Total RGR showed the highest value in C7-inoculated nr plants and the lowest value in Bm-inoculated nr plants, both of them showing significant differences from control plants. Furthermore, no differences due to plant genotype were noticed (Fig. 2A). In shoots, C7-inoculated nr plants increased RGR, but Bm inoculation did not affect shoot RGR compared with control plants (Fig. 2B). Furthermore, root RGR was only decreased by Bm inoculation in nr plants and a significant difference between plant genotypes was exclusively seen under Bm inoculation (Fig. 2 C).
Fig. 2.
Effects of bacterial inoculation on relative growth rates (RGRs). Total (A), shoot (C) and root (E) RGRs of wild-type ‘Pearson’(wt) and never ripe (nr) tomato (Solanum lycopersicum) plants at 8 weeks post-inoculation (wpi). Treatments are designed as non-inoculated controls (No, black bars), Bacillus megaterium-inoculated plants (Bm, white bars) and Enterobacter C7-inoculated plants (C7, grey bars). Data are means ± s.e. (n = 9). Data were analysed by two-way ANOVA with plant genotype (G) and inoculum (I) as sources of variation. Significance of sources of variation as well as their interaction (G × I) was evaluated by P-value; n.s., not significant; *P ≤ 0·05; **P ≤ 0·01; ***P ≤ 0·001. In the case of a significant interaction between factors, all treatments were compared with each other. Means followed by different upper case letters are significantly different (P < 0·05) according to LSD test. In the case of a non-significant interaction between factors, inoculum effects were evaluated by analysing wt and nr plants separately using ANOVA. Means followed by different lower case letters are significantly different (P < 0·05) according to LSD test. Plant genotype effect was evaluated by analysing wt and nr plants under the same inoculation treatment (No, Bm or C7) by Student t-test, and a significant difference (P < 0·05) is shown as (*) above nr means.
PGPB effects on stomatal conductance, photosynthetic efficiency and chlorophyll content
Factor interaction was not significant in stomatal conductance, photosynthetic efficiency and chlorophyll content (Table 2). Stomatal conductance was affected by bacterial inoculation in both plant genotypes only at 4 wpi, with C7 inoculation showing increased stomatal conductance independently of plant genotype (27·1 and 36·6 % in wt and nr plants, respectively). Moreover, Bm decreased stomatal conductance by 17·3 % in wt plants, with no effect in nr plants. In addition, significant differences between wt and nr plants in stomatal conductance were only seen under Bm inoculation at 4 wpi (higher in nr plants) and under all inoculation treatments at 8 wpi (higher values in wt plants) (Table 2). C7 did not affect photosynthetic efficiency, maintaining similar values to control plants. However, Bm inoculation decreased this parameter independently of plant genotype (25·8 and 10·9 % in wt and nr plants, respectively). Significant differences between wt and nr plants were only seen in Bm-inoculated plants, with higher values in nr plants. Although photosynthetic efficiency was not modified by bacterial inoculation at 8 wpi, significant plant genotype differences were seen in non- and C7-inoculated plants, with higher values in wt plants (Table 2). Chlorophyll content was unaltered by PGPB inoculation in both plant genotypes at both harvests. Moreover, a significant plant genotype difference was only seen under Bm inoculation at 4 wpi (Table 2).
Table 2.
Effects of bacterial inoculation on stomatal conductance, photosynthetic efficiency and chlorophyll content
| Stomatal conductance (mmol m−2 s−1) |
Photosynthetic efficiency (r_u) |
Total clorophyll (mg cm−2) |
||||
|---|---|---|---|---|---|---|
| 4 wpi | 8 wpi | 4 wpi | 8 wpi | 4 wpi | 8 wpi | |
| wt No | 484·125 ± 30·503b | 131·636 ± 13·199 | 0·641 ± 0·017a | 0·727 ± 0·004 | 5·486 ± 0·506 | 2·830 ± 0·464 |
| wt Bm | 400·222 ± 27·720c | 136·455 ± 13·644 | 0·476 ± 0·026b | 0·720 ± 0·005 | 5·446 ± 0·420b | 2·682 ± 0·379 |
| wt C7 | 615·313 ± 25·015a | 134·636 ± 13·304 | 0·590 ± 0·029a | 0·732 ± 0·005 | 5·146 ± 0·481 | 2·937 ± 0·389 |
| P-value | *** | n.s. | *** | n.s. | n.s. | n.s . |
| nr No | 470·833 ± 22·065b | 97·910 ± 4·157* | 0·664 ± 0·018a | 0·699 ± 0·006* | 5·263 ± 0·371 | 2·498 ± 0·389 |
| nr Bm | 499·833 ± 31·429b* | 100·273 ± 8·552* | 0·592 ± 0·025b* | 0·713 ± 0·008 | 5·011 ± 0·233* | 2·598 ± 0·580 |
| nr C7 | 643·333 ± 32·067a | 105·500 ± 5·973* | 0·660 ± 0·017a | 0·701 ± 0·005* | 5·228 ± 0·367 | 2·831 ± 0·582 |
| P-value | *** | n.s. | * | n.s. | n.s. | n.s. |
| Significance of source of variation interaction | ||||||
| G × I | n.s. | ns | ns | ns | ns | ns |
Data were analysed by two-way ANOVA with plant genotype (G) and inoculum (I) as sources of variation.
Data are means ± s.e. (n = 9).
The significance of sources of variation as well as their interaction (G × I) was evaluated by P-value. In the case of significant interaction between factors, all treatments were compared with each other. Means followed by different lower case letters are significantly different (P < 0·05) according to LSD test. In the case of a non-significant interaction between factors, inoculum effects were evaluated by analysing separately wt and nr plants using ANOVA. Plant genotype effect was evaluated by analysing wt and nr plants separately under the same inoculation treatment (No, Bm or C7) by Student t-test and significant difference (P < 0·05) is shown as (*) next to nr means.
Treatments: non-inoculated, Bacillus megaterium-inoculated and Enterobacter C7-inoculated wild-type plants (wt No, wt Bm and wt C7, respectively) and non-inoculated, Bacillus megaterium-inoculated and Enterobacter C7-inoculated never ripe plants (nr No, nr Bm and nr C7. respectively).
PGPB effects on plant nutritional status
In order to throw some light on PGPB inoculation effects on plant nutrition regarding ethylene insensitivity, macro- and micronutrients were quantified in roots and shoots (Supplementary Data Tables S4 and S5, respectively).
In the case of root nutrients, factor interaction was only significant for Ca concentration at 8 wpi (Table S4). Root nutrients showed no significant differences due to PGPB inoculation in wt plants at 4 wpi. However, the Na level was decreased by C7 inoculation in nr plants at 4 wpi. Moreover, Zn concentration was higher in C7, with Bm-inoculated nr plants showing no differences compared with control plants. Furthermore, Mn concentration showed significant differences between wt and nr plants, with nr plants showing higher values than the wt under all inoculation treatments (Table S4). In contrast, several changes were seen due to PGPB inoculation and ethylene insensitivity at 8 wpi. In wt plants, C concentration was increased by both bacterial inoculations (1·8 and 3·2 % by Bm and C7, respectively) and Ca concentration was increased only by Bm inoculation. Most changes were found in nr plants, showing a general reduction by bacterial inoculation. C7 inoculation decreased Cu, Mg, S and Si concentrations in nr plants. In the case of Bm-inoculated nr plants, only Cu, Mg and Si concentrations were decreased compared with control plants. Moreover, some differences between inocula were observed in Cu and Si concentrations, decreasing to a greater extent after C7 inoculation than after Bm inoculation. Furthermore, significant differences between plant genotypes were noticed for several nutrients. Ca concentration showed higher values in wt plants under all inoculations. C concentration was higher in nr than wt plants only in non-inoculated plants. Exclusively under Bm inoculation, Na concentration was higher in wt plants. Under C7 inoculation, Mn showed a higher level in nr plants, while Si showed higher levels in wt plants. Finally, Cu, Mg and S concentrations were significantly lower in nr than in wt plants under both bacterial inoculations (Table S4).
Regarding shoot nutrients, several changes were seen in wt and nr plants at 4 and 8 wpi. Moreover, factor interaction was only significant for Cu, Fe and P concentrations at 8 wpi (Table S5). At 4 wpi, C7 inoculation did not affect shoot nutrients, while Bm inoculation modified nutrients in both plant genotypes. The C concentration was increased by Bm inoculation of wt plants. Moreover, Bm inoculation decreased N and Mn concentrations in wt plants, and Zn and Fe concentrations in nr plants. In addition, significant differences between wt and nr plants were noted for Cu, Mn and Zn under all inoculation treatments, with nr plants showing lower values than wt plants. Moreover, significant differences between plants genotypes were seen in non-inoculated plants only for the Mg level and under both bacterial inoculations for Ca concentration, with a higher concentration in wt plants in all cases (Table S5). At 8 wpi, Bm only increased the C level in wt plants, showing no effect on shoot nutrition of nr plants. However, C7 inoculation increased Cu and Fe concentrations in wt plants and decreased Cu, Na and P concentrations in nr plants. Furthermore, significant differences between plant genotypes were noted for several nutrients. Ca and Mg concentrations showed higher values in wt than in nr plants under all inoculation treatments. In the case of Na, genotype differences were observed in non- and Bm-inoculated plants, with higher values in nr plants. Moreover, significant differences between wt and nr plants were seen for K and Mn in non- and C7-inoculated plants, with both showing higher values in wt plants. Finally, significant differences were exclusively observed under C7 inoculation for C, Cu, Fe, P and Zn concentrations, showing higher values for C in nr plants and for Cu, Fe, P and Zn in wt plants (Table S5).
Nutrient concentrations and plant dry weights were assessed by Pearson correlation analysis (Table 3). A positive correlation was observed between root S concentration and total, shoot and root dry weights at 4 wpi. Shoot Fe concentration showed a strong negative correlation with all dry weights at this time. At 8 wpi, dry weights were correlated with several nutrients. Indeed, a positive correlation was found between the root C concentration and all dry weights. Moreover, negative correlations were obtained between total dry weight and root Cu, K and S concentrations. Shoot K concentration was also negatively correlated with total dry weight. In addition, shoot dry weight showed the same correlations as total dry weight (Table 3).
Table 3.
Significant Pearson correlations between plant dry weights and nutrient contents
| 4 weeks post-inoculation |
8 weeks-post-inoculation |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Root |
Shoot |
Root |
Shoot |
|||||||||||||||||
| C (% d. wt) | Cu (ppm.) | Fe (ppm.) | K (% d. wt) | S (% d. wt) | C (% d. wt) | Cu (ppm) | Fe (ppm) | K (% d. wt) | S (% d. wt) | C (% d. wt) | Cu (ppm) | Fe (ppm) | K (% d. wt) | S (% d. wt) | C (% d. wt) | Cu (ppm) | Fe (ppm) | K (% d. wt) | S (% d. wt) | |
| Total d. wt | ||||||||||||||||||||
| r | 0·376 | 0·177 | –0·155 | 0·523 | 0·809 | 0·396 | –0·371 | –0·973 | –0·540 | –0·078 | 0·916 | –0·848 | –0·502 | –0·836 | –0·849 | 0·715 | –0·479 | –0·016 | –0·901 | –0·687 |
| P-value | 0·449 | 0·732 | 0·764 | 0·266 | 0·033 | 0·421 | 0·455 | 0·000 | 0·247 | 0·881 | 0·004 | 0·018 | 0·290 | 0·023 | 0·018 | 0·086 | 0·316 | 0·975 | 0·006 | 0·107 |
| Shoot d. wt | ||||||||||||||||||||
| r | 0·359 | 0·179 | –0·148 | 0·510 | 0·805 | 0·407 | –0·355 | –0·971 | –0·535 | –0·063 | 0·921 | –0·868 | –0·541 | –0·854 | –0·865 | 0·714 | –0·489 | –0·043 | –0·920 | –0·714 |
| P-value | 0·472 | 0·729 | 0·774 | 0·281 | 0·035 | 0·407 | 0·476 | 0·000 | 0·252 | 0·903 | 0·003 | 0·013 | 0·246 | 0·017 | 0·014 | 0·087 | 0·305 | 0·935 | 0·003 | 0·087 |
| Root d. wt | ||||||||||||||||||||
| r | 0·418 | 0·172 | 0·172 | –0·172 | 0·818 | 0·368 | –0·411 | –0·974 | –0·549 | –0·116 | 0·810 | –0·661 | –0·225 | –0·661 | –0·686 | 0·669 | –0·385 | 0·149 | –0·714 | –0·465 |
| P-value | 0·393 | 0·745 | 0·740 | 0·739 | 0·030 | 0·459 | 0·402 | 0·000 | 0·237 | 0·823 | 0·033 | 0·129 | 0·661 | 0·128 | 0·108 | 0·122 | 0·436 | 0·773 | 0·087 | 0·334 |
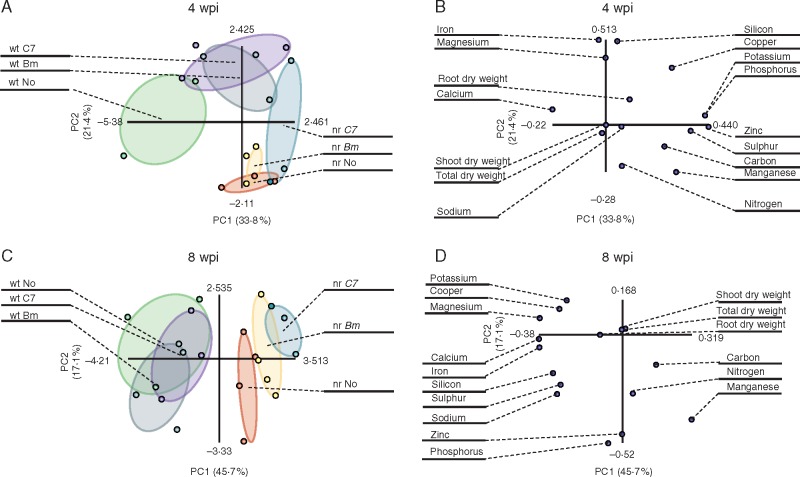
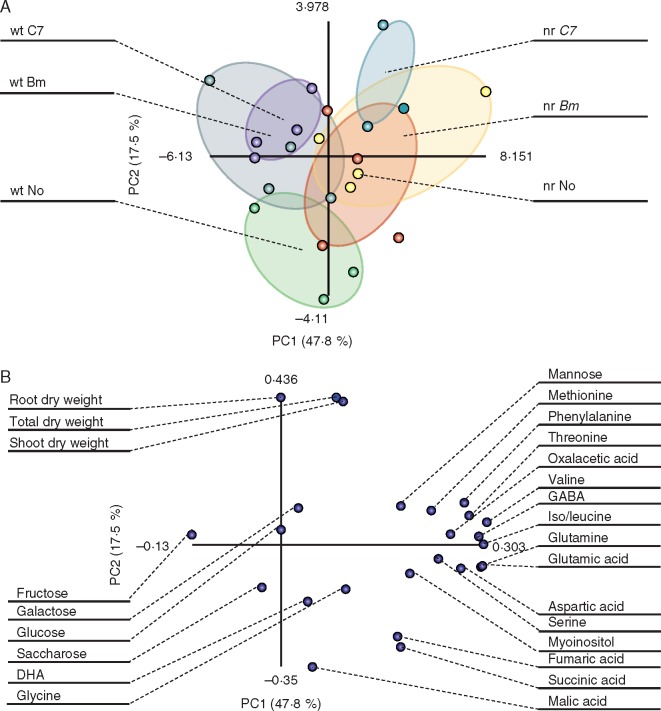
Root nutrient concentrations and total, shoot and root dry weight values were used to build a data matrix in order to perform a PCA to compare inoculation treatments in both plant genotypes (Fig. 3). Axes PC1 and PC2, represented in the factorial plan, explained 55·2 % of data variability at 4 wpi. PCA separated treatments by plant genotype. In wt plants, Bm inoculation produced separation from control plants; however, C7-inoculated plants shared a tiny area with control plants. However, both bacterial effects mostly overlapped. Also, both bacterial inoculations shared a small area with non-inoculated nr plants without overlapping each other (Fig. 3A). The nutrients with a greater contribution to the observed variability were K, P, S and Zn for PC1, and Fe, Mg and Si for PC2 (Fig. 3B). At 8 wpi, analysis (62·8 % variability along axes PC1 and PC2) showed a clear difference between genotypes. In the case of wt plants, there was no separation due to bacterial inoculation. In contrast, both bacterial inoculations were completely separated from control nr plants, sharing a small area between Bm- and C7-inoculated areas (Fig. 3C). Nutrients with a higher contribution to variability were Ca, Fe and Mg for PC1, and P and Zn for PC2 (Fig. 3D).
Fig. 3.
Principal component analysis (PCA) of root nutrient concentrations and dry weights. Analyses were performed based on nutrient concentration and dry weight data obtained from tomato (Solanum lycopersicum) plants. Score plot at 4 weeks post-inoculation (wpi) (A) and 8 wpi (C). Treatments: non-inoculated, Bacillus megaterium-inoculated and Enterobacter C7-inoculated wild-type plants (wt No, wt Bm and wt C7, respectively) and non-inoculated, Bacillus megaterium-inoculated and Enterobacter C7-inoculated never ripe plants (nr No, nr Bm and nr C7, respectively). Each point represents one plant, and points of the same treatment are enclosed in a different coloured ellipse: green for wt No, grey for wt Bm, purple for wt C7, red for nr No, yellow for nr Bm and blue for nr C7. Loading plot at 4 wpi (B) and 8 wpi (D). Each point represents one nutrient or dry weight.
PGPB effects on plant phytohormonal status
PGPB inoculation effects on phytohormones were studied regarding ethylene sensitivity in roots and shoots, evaluating ethylene, IAA, ABA, SA, JA and JA-Ile concentrations at 4 and 8 wpi (Tables 4 and 5, respectively). Factor interaction was only noticed in root ABA concentration at 4 wpi.
Table 4.
Effects of bacterial inoculation on phytohormone concentrations at 4 weeks post-inoculation
| Root |
Shoot |
|||||||
|---|---|---|---|---|---|---|---|---|
| Ethylene (nmol g−1 h−1) | IAA (pmol g−1) | ABA (pmol g−1) | SA (pmol g−1) | Ethylene (nmol g−1 h−1) | IAA (pmol g−1) | ABA (pmol g−1) | SA (pmol g−1) | |
| wt No | 3·05 ± 0·24 | 88·52 ± 4·38 | 37·65 ± 4·91BC | 80·33 ± 6·30 | 0·88 ± 0·05 | 42·07 ± 3·19 | 669·22 ± 36·47 | 224·89 ± 43·23 |
| wt Bm | 3·89 ± 0·28 | 79·13 ± 2·16 | 30·18 ± 1·96C | 67·65 ± 3·53 | 1·03 ± 0·12 | 38·53 ± 2·34 | 676·00 ± 12·40 | 216·23 ± 6·69 |
| wt C7 | 3·55 ± 0·24 | 82·34 ± 3·62 | 32·32 ± 2·89C | 68·03 ± 8·60 | 1·21 ± 0·15 | 37·64 ± 1·67 | 655·35 ± 17·35 | 240·28 ± 25·36 |
| P-value | n.s. | n.s. | n.a. | n.s. | n.s. | n.s. | n.s. | n.s. |
| nr No | 6·70 ± 0·49* | 108·66 ± 7·21* | 42·00 ± 1·04B | 68·20 ± 4·60 | 2·02 ± 0·32* | 41·95 ± 1·15 | 710·53 ± 28·63 | 226·43 ± 15·23 |
| nr Bm | 5·91 ± 0·53* | 96·64 ± 6·83 | 37·05 ± 1·51BC | 72·13 ± 8·08 | 1·38 ± 0·20 | 44·20 ± 2·53 | 862·62 ± 86·44 | 265·35 ± 15·01* |
| nr C7 | 5·40 ± 1·07 | 119·92 ± 3·69* | 51·42 ± 3·23A | 87·75 ± 11·59 | 2·23 ± 0·31* | 46·17 ± 1·60* | 693·38 ± 13·18 | 226·86 ± 16·30 |
| P-value | n.s. | n.s. | n.a. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Significance of source of variation interaction | ||||||||
| G × I | n.s. | n.s. | * | n.s. | n.s. | n.s. | n.s. | n.s. |
Data were analysed by two-way ANOVA with plant genotype (G) and inoculum (I) as sources of variation.
Data are means ± s.e. (n = 6).
The significance of sources of variation as well as their interaction (G × I) was evaluated by P-value. In the case of significant interaction between factors, all treatments were compared with each other. Means followed by different upper case letters are significantly different (P < 0·05) according to LSD test. In the case of a non-significant interaction between factors, inoculum effects were evaluated by analysing wt and nr plants separately using ANOVA. Plant genotype effect was evaluated by analysing wt and nr plants under the same inoculation treatment (No, Bm or C7) by Student t-test and significant difference (P < 0·05) is shown as (*) next to nr means.
Treatments: non-inoculated, Bacillus megaterium-inoculated and Enterobacter C7-inoculated wild-type plants (wt No, wt Bm and wt C7, respectively) and non-inoculated, Bacillus megaterium-inoculated and Enterobacter C7-inoculated never ripe plants (nr No, nr Bm and nr C7, respectively).
n.a., not-applicable; P-value; n.s., not significant;
P ≤ 0·05;
P ≤ 0·01;
P ≤ 0·001.
Table 5.
Effects of bacterial inoculation on phytohormone concentrations at 8 weeks post-inoculation
| Root |
Shoot |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ethylene (nmol g−1 h−1) | IAA (pmol g−1) | SA (pmol g−1) | JA (pmol g−1) | JA-Ile (pmol g−1) | Ethylene (nmol g−1 h−1) | IAA (pmol g−1) | SA (pmol g−1) | JA (pmol g−1) | JA-Ile (pmol g−1) | |
| wt No | 2·91. ± 0·27 | 22·73 ± 2·09 | 375·79 ± 40·53 | 125·56 ± 21·27 | 5·88 ± 0·38 | 0·85 ± 0·12 | 120·0 ± 2·98 | 2554·80 ± 950·65 | 65·88 ± 10·42 | 1·34 ± 0·24 |
| wt Bm | 2·61 ± 0·55 | 16·20 ± 3·74 | 290·10 ± 35·70 | 91·32 ± 13·19 | 5·11 ± 0·99 | 1·30 ± 0·29 | 133·43 ± 14·14 | 1562·67 ± 375·99 | 50·01 ± 12·65 | 0·99 ± 0·02 |
| wt C7 | 3·09 ± 0·34 | 19·22 ± 4·25 | 350·86 ± 65·99 | 98·85 ± 15·08 | 4·68 ±0·56 | 1·14 ± 0·13 | 144·52 ± 22·89 | 1423·38 ± 393·23 | 75·64 ± 6·03 | 1·21 ± 0·26 |
| P-value | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| nr No | 4·79 ± 0·50* | 30·91 ± 1·82* | 524·44 ± 35·97* | 169·39 ± 14·05 | 6·29 ± 0·25 | 2·09 ± 0·37* | 168·86 ± 10·06* | 2581·40 ± 256·21 | 58·70 ± 9·32 | 1·54 ± 0·27 |
| nr Bm | 4·69 ± 0·75* | 25·66 ± 2·78 | 527·62 ± 78·98* | 154·46 ± 37·03 | 6·53 ± 0·78 | 2·45 ± 0·41* | 146·00 ± 18·12 | 2869·05 ± 277·71* | 95·73 ± 8·34* | 2·49 ± 0·10* |
| nr C7 | 4·68 ± 0·53* | 26·71 ± 4·69 | 549·53 ± 27·92 | 165·45 ± 36·25 | 7·46 ± 0·82 | 2·40 ± 0·51* | 184·87 ± 21·29 | 2585·30 ± 492·29 | 118·31 ± 47·58 | 2·68 ± 0·80 |
| P-value | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Significance of source of variation interaction | ||||||||||
| G × I | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
Data were analysed by two-way ANOVA with plant genotype (G) and inoculum (I) as sources of variation.
Data are means ± s.e. (n = 4).
The significance of sources of variation as well as their interaction (G × I) was evaluated by P-value. In the case of significant interaction between factors, all treatments were compared with each other. In the case of a non-significant interaction between factors, inoculum effects were evaluated by analysing wt and nr plants separately using ANOVA. Plant genotype effect was evaluated by analysing wt and nr plants under the same inoculation treatment (No, Bm or C7) by Student t-test and significant difference (P < 0·05) is shown as (*) next to nr means.
Treatments: non-inoculated, Bacillus megaterium-inoculated and Enterobacter C7-inoculated wild-type plants (wt No, wt Bm and wt C7, respectively) and non-inoculated, Bacillus megaterium-inoculated and Enterobacter C7-inoculated never ripe plants (nr No, nr Bm and nr C7, respectively).
P-value; n.s., not significant;
P ≤ 0·05;
P ≤ 0·01;
P ≤ 0·001.
At 4 wpi, JA and JA-Ile levels were unaffected by PGPB inoculation and by plant genotype (data not shown). Exclusively, C7 inoculation increased root ABA concentration in nr plants, while no changes were noticed in wt plants. Moreover, a significant difference between wt and nr plants was only observed in C7-inoculated roots (higher levels in nr plants) and were not noticeable in shoots (Table 4). In the case of ethylene, genotype differences were observed in non- and Bm-inoculated roots and in non- and C7-inoculated shoots, with nr plants showing higher ethylene levels than wt plants in all cases (Table 4). Moreover, differences between wt and nr plants were seen for IAA concentration in non- and C7-inoculated roots and exclusively under C7 inoculation in shoots, with nr plants showing higher levels than wt plants (Table 4). Finally, a genotype difference in SA concentration was only observed in Bm-inoculated shoots, with higher values in nr plants (Table 4).
At 8 wpi, PGPB inoculation did not affect any phytohormone levels in roots or in shoots. However, plant genotype had a significant effect in both plant tissues (Table 5). ABA levels were unaffected by plant genotype (data not shown). Ethylene concentration showed higher values in nr than in wt plants under all inoculation treatments in roots and shoots (Table 5). In the case of IAA, genotype differences were only seen in non-inoculated plants in root and shoot tissues, with nr plants showing higher levels than wt plants (Table 5). Moreover, SA concentration showed significant differences between plant genotypes in non- and Bm-inoculated roots, with Bm-inoculated shoots always showing higher values in nr plants (Table 5). Furthermore, although genotype differences were not seen for JA and JA-Ile concentration in roots, differences between wt and nr plants were exclusively observed under Bm inoculation in shoots (Table 5).
PGPB effects on root metabolite content
Root metabolites were analysed to determine how PGPB inoculation affects their accumulation regarding ethylene insensitivity in juvenile and mature plants (Tables 6 and 7, respectively). Several root metabolites were identified by GC-MS. These include, carbohydrates (fructose, glucose, galactose, saccharose, mannose and myoinositol), amino acids [glycine, methionine, phenylalanine, threonine, valine, leucine/isoleucine, glutamine, serine, γ-aminobutyric acid (GABA), glutamic acid and aspartic acid] and organic acids [oxalacetic, fumaric, succinic and malic and dehydroascorbic (DHA)]. Factor interaction in root metabolite levels was seen for galactose, aspartic acid, glutamic acid, fumaric acid, glutamine, isoleucine/leucine, phenylalanine, serine threonine and valine at 4 wpi, while no factor interaction was observed at 8 wpi (Tables 6A and 7A).
Table 6.
Root metabolite contents and correlations between plant dry weights at 4 weeks post-inoculation (wpi)
| A | Fructose | Galactose | Glucose | Myoinositol | Aspartic acid | Glutamic acid | Fumaric acid | Succinic acid | Malic acid | Glutamine | Isoleucine/ leucine | Phenylalanine | Serine | Threonine | Valine | GABA | DHA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt No | 7·40 ± 1·41 | 0·025 ± 0·004BC | 4·47 ± 0·90 | 0·55 ± 0·04 | 1·08 ± 0·07BC | 3·70 ± 0·30BC | 0·29 ± 0·02B | 1·12 ± 0·14b | 43·6 ±2·9 | 1·08 ± 0·08B | 0·13 ± 0·01B | 0·086 ± 0·009B | 0·79 ± 0·06BC | 0·059 ± 0·004BC | 0·053 ± 0·010CD | 1·956 ± 0·375 | 0·037 ± 0·008 |
| wt Bm | 8·44 ± 0·74 | 0·033 ± 0·003B | 6·78 ± 0·58 | 0·61 ± 0·08 | 0·93 ± 0·10CD | 3·35 ± 0·41C | 0·33 ± 0·02AB | 1·38 ± 0·22a | 49·7 ± 3·7 | 0·98 ± 0·12BC | 0·14 ± 0·01AB | 0·076 ± 0·013B | 0·72 ± 0·07BC | 0·062 ± 0·005B | 0·066 ± 0·018BC | 2·044 ± 0·287 | 0·031 ± 0·005 |
| wt C7 | 10·71 ± 1·84 | 0·032 ± 0·005B | 5·68 ± 1·04 | 0·61 ± 0·15 | 0·65 ± 0·14D | 1·87 ± 0·42D | 0·37 ± 0·02A | 1·39 ± 0·15a | 47·2 ± 5·2 | 0·67 ± 0·16C | 0·08 ± 0·01C | 0·041 ± 0·005C | 0·33 ± 0·08D | 0·040 ± 0·007C | 0·044 ± 0·019D | 2·154 ± 0·389 | 0·031 ± 0·004 |
| P-value | n.s. | n.a. | n.s. | n.s. | n.a. | n.a. | n.a. | * | n.s. | n.a. | n.a. | n.a. | n.a | n.a. | n.a. | n.s. | n.s. |
| nr No | 9·22 ± 1·01b | 0·032 ± 0·003B | 6·37 ± 0·63a | 0·67 ± 0·10* | 1·31 ± 0·10B | 4·61 ± 0·27B | 0·37 ± 0·01A | 1·36 ± 0·16* | 46·3 ± 6·0 | 1·27 ± 0·07B | 0·16 ± 0·01AB | 0·103 ± 0·007AB | 0·95 ± 0·06AB | 0·087 ± 0·007A | 0·080 ± 0·016AB | 3·305 ± 0·740* | 0·030 ± 0·002 |
| nr Bm | 12·11 ± 1·17a | 0·047 ± 0·003A | 7·88 ± 0·75a | 0·62 ± 0·11 | 0·85 ± 0·12CD | 3·65 ± 0·55BC | 0·29 ±0·03B | 1·28 ±0·43 | 44·9 ±6·3 | 1·03 ± 0·14B | 0·16 ± 0·02AB | 0·093 ± 0·015AB | 0·70 ± 0·11C | 0·062 ±0·007B | 0·070 ±0·02ABC | 2·691 ± 0·534* | 0·026 ± 0·004 |
| nr C7 | 5·73 ± 0·48c | 0·022 ± 0·002C | 4·20 ± 0·41b | 0·66 ± 0·03 | 1·82 ± 0·09A | 5·78 ± 0·26A | 0·36 ± 0·01A | 1·40 ± 0·12 | 41·6 ± 2·4* | 1·59 ± 0·07A | 0·17 ±0·01A | 0·122 ± 0·009A | 1·09 ± 0·04A | 0·102 ± 0·007A | 0·090 ± 0·013A | 3·554 ± 0·686* | 0·026 ± 0·003 |
| P-value | *** | n.a. | ** | n.s. | n.a. | n.a. | n.a. | n.s. | n.s. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.s. | n.s. |
| Significance of source of variation interaction | |||||||||||||||||
| G × I | n.s. | * | n.s. | n.s. | *** | *** | * | n.s. | n.s. | *** | * | * | *** | *** | * | n.s. | n.s. |
| B | Dry weight and metabolite content correlation | ||||||||||||||||
| Total d. wt | |||||||||||||||||
| r | 0·662 | 0·812 | 0·734 | 0·353 | –0·239 | –0·071 | –0·269 | 0·348 | 0·100 | –0·112 | 0·241 | 0·038 | –0·159 | –0·050 | 0·216 | 0·177 | –0·798 |
| P-value | 0·127 | 0·032 | 0·074 | 0·480 | 0·640 | 0·891 | 0·597 | 0·486 | 0·847 | 0·829 | 0·637 | 0·942 | 0·759 | 0·923 | 0·674 | 0·731 | 0·038 |
| Shoot d. wt | |||||||||||||||||
| r | 0·675 | 0·818 | 0·742 | 0·337 | –0·252 | –0·082 | –0·283 | 0·339 | 0·114 | –0·124 | 0·236 | 0·029 | –0·167 | –0·063 | 0·205 | 0·157 | –0·786 |
| P-value | 0·117 | 0·029 | 0·069 | 0·502 | 0·621 | 0·874 | 0·577 | 0·498 | 0·825 | 0·811 | 0·645 | 0·956 | 0·747 | 0·903 | 0·690 | 0·762 | 0·044 |
| Root d. wt | |||||||||||||||||
| r | 0·628 | 0·793 | 0·710 | 0·393 | –0·203 | –0·043 | –0·231 | 0·370 | 0·063 | –0·082 | 0·254 | 0·062 | –0·139 | –0·016 | 0·244 | 0·228 | –0·827 |
| P-value | 0·158 | 0·040 | 0·090 | 0·425 | 0·692 | 0·935 | 0·652 | 0·456 | 0·904 | 0·875 | 0·619 | 0·905 | 0·789 | 0·976 | 0·633 | 0·655 | 0·026 |
Data were analysed by two-way ANOVA with plant genotype (G) and inoculum (I) as sources of variation.
Data are means ± s.e. (n = 4).
In A, the significance of sources of variation as well as their interaction (G × I) was evaluated by P-value. In the case of significant interaction between factors, all treatments were compared with each other. Means followed by different upper case letters are significantly different (P < 0·05) according to LSD test. In the case of a non-significant interaction between factors, inoculum effects were evaluated by analysing wt and nr plants separately using ANOVA. Means followed by different lower letters are significantly different (P < 0·05) according to LSD test. Plant genotype effect was evaluated by analysing wt and nr plants under the same inoculation treatment (No, Bm or C7) by Student t-test and significant difference (P < 0·05) is shown as (*) next to nr means.
In B, significant Pearson correlations between plant dry weights and root metabolites at 4 wpi are shown.
Treatments: non-inoculated, Bacillus megaterium-inoculated and Enterobacter C7-inoculated wild-type plants (wt No, wt Bm and wt C7, respectively) and non-inoculated, Bacillus megaterium-inoculated and Enterobacter C7-inoculated never ripe plants (nr No, nr Bm and nr C7, respectively).
n.a., not applicable; P-value; n.s., not significant;
P ≤ 0·05;
P ≤ 0·01;
P ≤ 0·001.
Table 7.
Root metabolite contents and correlations between plant dry weights at 8 weeks post-inoculation (wpi)
| A | Fructose | Galactose | Glucose | Mannose | Myoinositol | Aspartic acid | Fumaric acid | Glutamic acid | Glutamine | Isoleucine/ leucine | Phenylalanine | Serine | Threonine | valine | GABA | Methionine | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt No | 68·91 ± 1·63 | 0·479 ± 0·035 | 41·13 ± 3·33 | 0·30 ± 0·02 | 6·67 ± 1·94 | 0·23 ± 0·13 | 0·083 ± 0·022 | 0·52 ± 0·19 | 0·16 ± 0·06 | 0·17 ± 0·05 | 0·07 ± 0·02 | 0·28 ± 0·05 | 0·023 ± 0·005 | 0·044 ± 0·008 | 2·63 ± 0·85 | 0·12 ± 0·02 | |
| wt Bm | 75·02 ± 4·46 | 0·611 ± 0·068 | 46·37 ± 3·21 | 0·32 ± 0·07 | 5·56 ± 1·48 | 0·18 ± 0·07 | 0·059 ± 0·018 | 0·35 ± 0·12 | 0·11 ± 0·04 | 0·15 ± 0·07 | 0·06 ± 0·02 | 0·25 ± 0·07 | 0·023 ± 0·008 | 0·047 ± 0·024 | 1·90 ± 0·50 | 0·11 ± 0·03 | |
| wt C7 | 79·39 ± 3·51 | 0·710 ± 0·068 | 50·03 ± 3·25 | 0·33 ± 0·05 | 5·96 ± 1·41 | 0·20 ± 0·03 | 0·058 ± 0·003 | 0·40 ± 0·12 | 0·13 ± 0·03 | 0·14 ± 0·03 | 0·07 ± 0·02 | 0·26 ± 0·05 | 0·022 ± 0·005 | 0·041 ± 0·004 | 1·78 ± 0·18 | 0·11 ± 0·03 | |
| P-value | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| nr No | 74·08 ± 4·40 | 0·783 ± 0·076* | 56·67 ± 4·40* | 0·55 ± 0·10* | 9·11 ± 2·70 | 0·33 ± 0·09 | 0·070 ± 0·008 | 0·53 ± 0·09 | 0·17 ± 0·02 | 0·20 ± 0·06 | 0·08 ± 0·01 | 0·49 ± 0·11* | 0·034 ± 0·007 | 0·060 ± 0·014 | 2·73 ± 0·67 | 0·13 ± 0·02 | |
| nr Bm | 74·47 ± 3·03 | 0·781 ± 0·033 | 57·48 ± 3·01* | 0·70 ± 0·20* | 8·85 ± 1·74* | 0·36 ± 0·09* | 0·075 ± 0·008 | 0·57 ± 0·24 | 0·18 ± 0·07 | 0·24 ± 0·09 | 0·10 ± 0·03 | 0·49 ± 0·09* | 0·046 ± 0·013* | 0·083 ± 0·040 | 3·62 ± 1·44 | 0·18 ± 0·05* | |
| nr C7 | 94·26 ± 10·54 | 1·013 ± 0·090 | 67·72 ± 5·26 | 0·74 ± 0·09* | 8·66 ± 1·38 | 0·36 ± 0·12 | 0·075 ± 0·008* | 0·59 ± 0·06* | 0·18 ± 0·02* | 0·24 ± 0·02* | 0·11 ± 0·01* | 0·53 ± 0·07* | 0·044 ± 0·008* | 0·071 ± 0·005* | 3·91 ± 0·26* | 0·20 ± 0·02* | |
| P-value | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Significance of source of variation interaction | |||||||||||||||||
| G × I | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| B | Dry weight and metabolite content correlation | ||||||||||||||||
| Total d. wt | |||||||||||||||||
| r | 0·963 | 0·916 | 0·863 | 0·661 | 0·321 | 0·446 | –0·233 | 0·241 | 0·292 | 0·484 | 0·723 | 0·531 | 0·597 | 0·465 | 0·466 | 0·680 | |
| P-value | 0·000 | 0·004 | 0·014 | 0·128 | 0·523 | 0·358 | 0·649 | 0·638 | 0·563 | 0·312 | 0·082 | 0·257 | 0·187 | 0·334 | 0·333 | 0·113 | |
| Shoot d. wt | |||||||||||||||||
| r | 0·959 | 0·937 | 0·888 | 0·691 | 0·369 | 0·485 | –0·210 | 0·278 | 0·332 | 0·512 | 0·746 | 0·572 | 0·624 | 0·491 | 0·491 | 0·698 | |
| P-value | 0·000 | 0·002 | 0·008 | 0·105 | 0·457 | 0·310 | 0·682 | 0·583 | 0·508 | 0·278 | 0·066 | 0·213 | 0·162 | 0·303 | 0·303 | 0·099 | |
| Root d. wt | |||||||||||||||||
| r | 0·908 | 0·718 | 0·642 | 0·428 | –0·005 | 0·165 | –0·357 | –0·012 | 0·023 | 0·271 | 0·522 | 0·234 | 0·383 | 0·273 | 0·272 | 0·520 | |
| P-value | 0·005 | 0·085 | 0·145 | 0·381 | 0·993 | 0·750 | 0·473 | 0·981 | 0·965 | 0·594 | 0·267 | 0·647 | 0·438 | 0·591 | 0·592 | 0·270 | |
Data were analysed by two-way ANOVA with plant genotype (G) and inoculum (I) as sources of variation.
Data are means ± s.e. (n = 4).
In A, the significance of sources of variation as well as their interaction (G × I) was evaluated by P-value. In the case of significant interaction between factors, all treatments were compared with each other. In the case of a non-significant interaction between factors, inoculum effects were evaluated by analysing wt and nr plants separately using ANOVA. Plant genotype effect was evaluated by analysing wt and nr plants under the same inoculation treatment (No, Bm or C7) by Student t-test and significant difference (P < 0·05) is shown as (*) next to nr means.
In B, significant Pearson correlations between plant dry weights and root metabolites at 8 wpi are shown.
Treatments: non-inoculated, Bacillus megaterium-inoculated and Enterobacter C7-inoculated wild-type plants (wt No, wt Bm and wt C7, respectively) and non-inoculated, Bacillus megaterium-inoculated and Enterobacter C7-inoculated never ripe plants (nr No, nr Bm and nr C7, respectively).
P-value; n.s., not significant;
P ≤ 0·05;
P ≤ 0·01;
P ≤ 0·001.
At 4 wpi, a dozen metabolites showed different levels due to bacterial inoculation in wt and/or nr plants (Table 6A). Carbohydrates were unaffected in wt plants. However, C7 inoculation reduced fructose, galactose and glucose in nr plants compared with controls. In contrast, fructose and galactose levels were increased by Bm inoculation in nr plants. In the case of amino acids, aspartic acid, glutamic acid, glutamine, leucine/isoleucine, phenylalanine and serine levels were reduced in wt plants; meanwhile aspartic acid, glutamic acid and glutamine were increased in nr plants by C7 inoculation. Bm inoculation did not modify amino acid levels in wt plants, but aspartic acid, serine and threonine levels were decreased in nr plants. In addition, different levels between inocula were observed in threonine and serine, showing no changes compared with control plants. Moreover, fumaric acid was reduced by Bm inoculation in nr plants while C7 inoculation increased it in wt plants. Succinic acid was increased by both bacteria in wt plants. No changes due to PGPB inoculation were seen for myoinositol, malic acid, GABA and DHA levels (Table 6A). Furthermore, differences between wt and nr plants were observed in several root metabolites. GABA levels showed higher values in nr than in wt plants under all inoculation treatments. In the case of galactose, a significant difference between plant genotypes was seen in Bm-inoculated plants (a higher level in nr plants) and in C7-inoculated plants (a higher level in wt plants). Moreover, a difference between wt and nr plants was only seen in non-inoculated plants for myoinositol, fumaric acid and succinic acid, with nr plants showing higher values. In the case of malic acid, genotype differences were seen only under C7 inoculation, with higher values in wt plants. In addition, a significant difference between plant genotypes was seen only under C7 inoculation, with aspartic acid, glutamic acid, glutamine, isoleucine/leucine, phenylalanine and serine showing higher values in nr plants. In the case of threonine and valine, significant differences between plant genotypes were observed in non- and C7-inoculated plants, with higher levels in nr plants compared with wt plants (Table 6A).
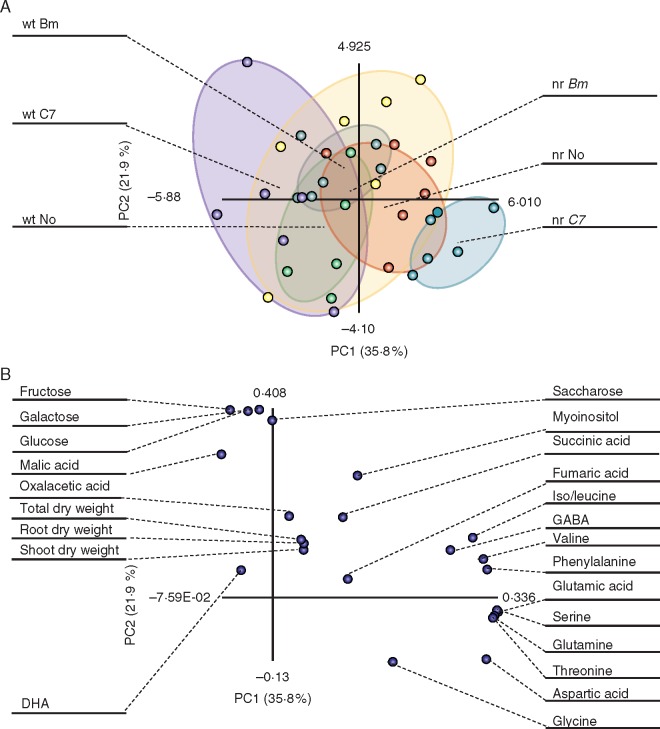
Correlations between plant dry weights and root metabolite levels were evaluated at 4 wpi (Table 6B). The galactose content was positively correlated with total, shoot and root dry weights, while DHA was negatively correlated with all dry weights. PCA was also performed to compare inoculation treatment effects on root metabolite profiles at 4 wpi (Fig. 4). In these analyses, 57·7 % of the variability was explained by the data in axes PC1 and PC2. Separation by plant genotype was only noticed under C7 inoculation. In wt plants, all treatments overlapped, with C7-inoculated plants showing higher variability than Bm-inoculated and control plants. Nevertheless, Bm inoculation in nr plants produced a different profile from C7 inoculation, showing a tiny overlap between them. Moreover, both bacterial inoculations showed a specific overlap with non-inoculated plants. Furthermore, non-inoculated wt and nr profiles showed partial overlapping. The Bm-inoculated nr plant profile enclosed non- and Bm-inoculated wt plant profiles. However, C7 inoculation resulted in a total separation, showing less variation in nr plants (Fig. 4A). The loading plot showed two clear sources of variation; amino acids (glutamine, serine, phenylalanine, valine and threonine) and carbohydrates (fructose, glucose, galactose and saccharose) contributed to the high variability for PC1 and PC2, respectively (Fig. 4B).
Fig. 4.
Principal component analysis (PCA) of root metabolite levels and dry weights at 4 weeks post-inoculation (wpi). Analyses were performed based on metabolite contents and dry weight data obtained from tomato (Solanum lycopersicum) roots. (A) Score plot. Treatments: non-inoculated, Bacillus megaoterium-inoculated and Enterobacter C7-inoculated wild-type plants (wt No, wt Bm and wt C7, respectively) and non-inoculated, Bacillus megaterium-inoculated and Enterobacter C7-inoculated never ripe plants (nr No, nr Bm and nr C7, respectively). Each point represents one plant, and points of the same treatment are enclosed in a different coloured ellipse: green for wt No, grey for wt Bm, purple for wt C7, red for nr No, yellow for nr Bm and blue for nr C7. (B) Loading plot. Each point represents one nutrient or dry weight.
The effects of PGPB inoculation on root metabolite content regarding ethylene sensitivity were also assessed at 8 wpi. Nevertheless, factor interaction was not seen and PGPB inoculation did not affect root metabolite levels in mature plants (Table 7A). Fructose levels were unaffected by plant genotype. However, differences between wt and nr plants were seen for several metabolites. Mannose and serine levels showed higher values in nr than in wt plants under all inoculation treatments. Moreover, a genotype difference was only seen in non-inoculated plant for galactose, with higher levels in nr plants. Glucose showed higher levels in nr plants in non- and Bm-inoculated plants. However, threonine and methionine also showed higher values in nr plants, but under Bm and C7 inoculations. Furthermore, myoinositol and aspartic acid showed higher levels in nr plants exclusively under Bm inoculation. On the other hand, differences between wt and nr plants were only significant under C7 inoculation for fumaric acid, glutamic acid, glutamine, isoleucine/leucine, phenylalanine, valine and GABA levels (Table 7A).
Furthermore, some positive correlations were observed between plant dry weights and root metabolite levels at 8 wpi (Table 7B). Fructose content was positively correlated with all dry weights, and galactose and glucose were correlated positively with total and shoot dry weights. Treatment effects on root metabolites were also evaluated by PCA at 8 wpi (65·3 % variability along axes PC1 and PC2) (Fig. 5). Profiles were not completely separated by plant genotype in Bm-inoculated and non-inoculated plants, but there were differences compared with C7-inoculated plants. In wt plants, both bacterial inoculations showed a totally different root metabolite profile compared with control plants, showing almost complete overlap between Bm- and C7-inoculated plants. In nr plants, Bm inoculation resulted in a profile that overlapped with control plants, while C7 inoculation produced a nearly complete separation from controls (Fig. 5A). PCA also showed that PC1 variability was due to amino acids (glutamine, glutamic acid, leucine/isoleucine, valine and GABA). Unexpectedly, plant dry weights were a source of variation for PC2, minimizing carbohydrate effects. Moreover, malic acid also contributed to the variability of PC2 (Fig. 5B).
Fig. 5.
Principal component analysis (PCA) of root metabolite levels and dry weights at 8 weeks post-inoculation (wpi). Analyses were performed based on metabolite contents and dry weight data obtained from tomato (Solanum lycopersicum) roots. (A) Score plot. Treatments: non-inoculated, Bacillus megaoterium-inoculated and Enterobacter C7-inoculated wild-type plants (wt No, wt Bm and wt C7, respectively) and non-inoculated, Bacillus megaterium-inoculated and Enterobacter C7-inoculated never ripe plants (nr No, nr Bm and nr C7, respectively). Each point represents one plant, and points of the same treatment are enclosed in a different coloured ellipse: green for wt No, grey for wt Bm, purple for wt C7, red for nr No, yellow for nr Bm and blue for nr C7. (B) Loading plot. Each point represents one nutrient or dry weight.
DISCUSSION
Management of rhizospheric micro-organisms is a valuable strategy to induce plant growth (Berg, 2009; Singh et al., 2011) and could decrease chemical inputs in agriculture (Bhattacharyya and Jha, 2012). However, knowledge of PGPB effects on plants is necessary for the proper and effective large-scale use of these bacteria in crop systems. In this research, the outcome of the inoculation of tomato plants with two different PGPB was reported regarding ethylene sensitivity at two different plant developmental stages.
Ethylene sensitivity is essential for growth promotion by Bm but not for C7
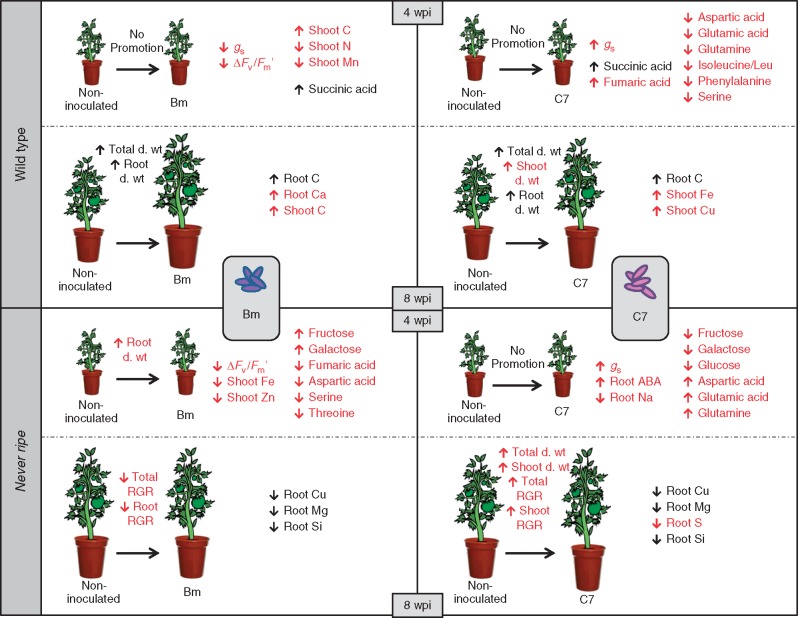
In order for a bacterium to be considered as a PGPB, colonization of the plant root system is a critical trait for plant–bacteria interaction (Benizri et al., 2001). Here, a colonization bioassay confirmed that Bm as well as C7 were able to colonize the roots of ethylene-sensitive and insensitive plants. Moreover, the colonization rates were not significantly different (Table 1), suggesting that plant growth variations are not due to differences in root colonization. In juvenile plants, an increase in root dry weight was only produced by Bm inoculation in nr plants, also resulting in a significant difference between wt and nr plants only under Bm inoculation (Fig. 1E), suggesting that root growth promotion is related to ethylene insensitivity in the case of Bm.
At 8 wpi, C7 inoculation stimulated plant growth independently of sensitivity to ethylene, while Bm inoculation promoted plant growth only in wt plants (Fig. 1B). Furthermore, phenotypic analysis only showed visible differences in plant height and number of flowers, resulting in the same pattern as total dry weight (data not shown). These results indicate that ethylene perception is required for growth promotion by Bm but not for C7. Plant growth promotion by Bm was previously reported in several plant species (Marulanda-Aguirre et al., 2008; Marulanda et al., 2009; Armada et al., 2014b; Porcel et al., 2014). The dependence on ethylene sensitivity of PGPB action was previously reported in arabidopsis, using a Variovorax paradoxus strain and also showing no differences in root colonization rate (Chen et al., 2013). In contrast, other studies using inoculations of Bacillus megaterium UMCV1 in arabidopsis (López-Bucio et al., 2007) and Bacillus sp B55 in Nicotiana attenuata (Meldau et al., 2012) pointed to an ethylene-independent mechanism. As with many plant–bacterial interactions, discrepancies between these studies could be due to strain-specific PGPB mechanisms, plant growth conditions (Ryu et al., 2005; Long et al., 2008) and/or differences between plants in ethylene transduction pathways since functional redundancy was reported for ethylene receptors in arabidopsis (Hua and Meyerowitz, 1998).
Furthermore, the difference between plant genotypes was only significant in non-inoculated plants, indicating less pronounced ethylene growth-inhibitory effects (Pierik et al., 2006) on nr plants due to their mutation (Wilkinson et al., 1995). Under Bm inoculation, this genotype difference was not seen because growth promotion was only produced in wt plants reaching a similar dry weight to nr plants, and suggesting that Bm could modulate ethylene receptor expression, as previously reported in another PGPB strain (Vargas et al., 2012), and needed for the proper establishment of a beneficial plant–bacteria association (Vacheron et al., 2013). Although C7 inoculation promoted growth of wt and nr plants, no significant difference between plant genotypes was observed, probably due to a different intensity of growth promotion. Moreover, IAA is able to induce plant growth (Zhao, 2010), and higher auxin levels were seen exclusively in non-inoculated nr mature plants compared with wt plants.
In addition, total RGR was not affected by ethylene insensitivity, as previously reported (Tholen et al., 2004). However, our data show that bacterial inoculation produced RGR modifications in ethylene-insensitive plants not seen in wt plants. Although few PGPB studies include RGR evaluation, RGRs can be enhanced by PGPB inoculation, but these responses are strain specific (Shishido and Chanway, 2000), as observed in our results (Fig. 2). These results points to deleterious effects of Bm inoculation on mature nr plants, as previously reported in ABA-deficient tomato plants by Porcel et al. (2014). Moreover, it was also noted that short-term growth promotion was not always linked with long-term promotion, as observed previously (Gray and Smith, 2005; Kuan et al., 2016). Thus, further research addressing bacterial inoculation effects on mature plants is necessary, since several studies that propose the use of bacterial strains as PGPB have only evaluated the effects of these bacteria on juvenile plants or seedlings and/or grown in vitro.
Furthermore, several correlations have been found between dry weights and nutrients (Table 3) or metabolites (Tables 6 and 7). In juvenile plants, root sulphur concentration was positively correlated with total, shoot and root dry weights. Levels of S were decreased by C7 inoculation in nr plants, and a previous study reported modulation of S assimilation by PGPB inoculation (Aziz et al., 2016), and ethylene and S nutrition have links at metabolic and regulatory levels (Wawrzynska et al., 2015). In mature plants, C positively correlated with all dry weights. Although C gain is a consequence of photosynthesis, little is known about the impact of carbon availability on plant growth (Smith and Stitt, 2007). Moreover, this correlation could be linked to positive correlations seen between root sugar content and dry weights. Shoot Fe concentration was negatively correlated with all dry weights at 4 wpi. Ethylene is involved in a plant’s response to Fe deficiency (Lucena et al., 2006), resulting in the initiation of root hairs affected by inhibition of ethylene perception (Schmidt, 2001). Thus, lower shoot Fe levels observed in Bm-inoculated nr plants could be due to less Fe uptake and translocation. Competition for Fe uptake between plants and micro-organisms has been reported at the rhizosphere, showing that micro-organisms could be more competitive than plants (Pii et al., 2015). Consequently, our results suggest an interaction failure between Bm and nr plants that leads to competition for Fe at the rhizosphere.
In juvenile plants, galactose and DHA correlated with all dry weights positively and negatively, respectively. DHA is the oxidized form of ascorbic acid, involved in prevention of oxidative damage (J. Wang et al., 2013). Thus, this negative correlation could be due to reduced oxidative damage in Bm-inoculated nr roots which showed increased root dry weight. Moreover, the main ascorbic acid biosynthesis pathway in plants is the l-galactose pathway (Laing et al., 2007), and galactose was increased by Bm inoculation in nr plants. In addition, sugars are immediate substrates for metabolism and signalling molecules, and their availability is linked to plant growth (Rolland et al., 2006; Hanson and Smeekens, 2009). Thus, the observed correlations could be due to sugar availability.
Stomatal conductance and photosynthetic efficiency were affected by PGPB inoculation at the juvenile stage
Effects of PGPB inoculation were only seen in juvenile plants, while differences between plant genotypes showed different patterns at 4 and 8 wpi (Table 2). Stomatal conductance results also suggested a role for ethylene sensitivity in the Bm–tomato interaction. Reduction of stomatal conductance by Bm inoculation was previously reported in clover (Armada et al., 2014a), lavender and salvia (Armada et al., 2014b), although no effect was reported in tomato (Porcel et al., 2014). Moreover, photosynthesis is directly related to chlorophyll content (Richardson et al., 2002), but the chlorophyll content was unaffected by Bm inoculation, suggesting that the decrease in photosynthetic efficiency was not linked to chlorophyll content. Ethylene also modifies photosynthesis by affecting stomatal aperture with a dose-dependent mechanism (Tanaka et al., 2005), and significant differences between plant genotypes were only observed under Bm inoculation for the three parameters at 4 wpi. These results are in accordance with a Bm–tomato interaction mediated by ethylene (and thus dependent on its perception) that could decrease stomatal conductance and then photosynthetic efficiency in wt plants. However, Bm could be misrecognized by nr plants (unable to respond to released ethylene from the interaction), resulting in no change in stomatal conductance and deleterious effects such as reduced photosynthetic efficiency and lower chlorophyll levels in Bm-inoculated nr plants compared with the wt. Nevertheless, stomatal conductance was increased in wt and nr plants, and photosynthetic efficiency and chlorophyll content were unaffected by C7 inoculation, in accordance with a PGPB mechanism independent of ethylene sensitivity.
Furthermore, stomatal conductance was significantly lower in nr than in wt plants under all inoculation treatments, and photosynthetic efficiency was lower in non- and C7-inoculated plants at 8 wpi, when higher ethylene values were observed in nr plants. Although the physiological role of ethylene depends on specific traits of plant species (related in principle to their habitat) and the integrative result of internal and external stimuli (Pierik et al., 2006), these results suggest that nr plants were more stressed than wt plants, probably because ethylene is a key hormone involved in response to environmental stresses (F. Wang et al., 2013; Van de Poel et al., 2015), and some ethylene-insensitive genotypes would fail to produce some adaptive responses (Feng and Barker, 1992; Zhang et al., 2003).
Phytohormonal status was mainly affected by ethylene insensitivity and was altered under bacterial inoculation
The effects of PGPB on phytohormone levels were mainly dependent on ethylene sensitivity, and only C7 inoculation directly affected root ABA (Tables 4 and 5). Most of the previously described action mechanisms in relation to ethylene are mainly related to PGPB containing the enzyme 1-aminocyclopropane-1-carboxylate deaminase (ACCd) that induces plant growth and development by decreasing the immediate ethylene precursor (ACC) and thereby the ethylene levels (Glick et al., 2007; Glick, 2014). However, the PGPB used in this study did not show ACCd activity (data not shown). Furthermore, ethylene production by Bm and C7 was also assessed (data not shown), resulting in no detectable microbially derived ethylene that could affect plant growth or the root colonization process.
Root ABA concentration was exclusively increased by C7 inoculation in nr juvenile plants, as previously reported with others PGPB (Bresson et al., 2013; Cohen et al., 2015). The role of ABA has been historically described as that of a growth inhibitor. However, high ABA levels were reported in young tissues, and ABA-deficient mutants are severely affected in terms of growth (Finkelstein, 2013). Endogenous ABA is crucial in limiting ethylene production, maintaining rather than reducing plant growth (Sharp, 2002). Furthermore, ABA is able to suppress plant resistance mechanisms mediated by JA/ethylene- and SA-dependent immune responses (Anderson et al., 2004; Sánchez-Vallet et al., 2012). Our results also showed higher root ABA levels in C7-inoulated nr plants compared with the wt, but no genotype differences in ethylene, JA and SA concentrations. Thus, although C7 did not promote plant growth at 4 wpi, these hormonal changes predispose plants to further growth. In fact, ethylene, JA, JA-Ile and SA levels were also unaffected by C7 inoculation or plant genotype in mature plants.
However, Bm did not directly affect phytohormone levels. A previous report using Bm-inoculated wt and ABA-deficient tomato lines suggested that normal endogenous ABA levels could be essential for growth promotion by maintaining production of ethylene at low levels (Porcel et al., 2014). Bm only promoted growth in wt plants, and ethylene and ABA levels were unaffected, as reported in wt plants by Porcel et al. (2014). Thus, our results are in concordance with the hypothesis pointing to plant ethylene sensitivity as a new player in Bm–tomato interaction. Indeed, although similar differences between plant genotypes were seen in root ethylene in non- and Bm-inoculated plants, a genotype difference was exclusively not seen in shoot ethylene under Bm inoculation. Furthermore, shoot SA levels exclusively showed higher values in nr plants under Bm inoculation, suggesting that nr plants respond to Bm by activating plant defences since SA is a key factor for establishment of basal defences, effector-triggered inmmunity and both local and systemic acquired response (Vlot et al., 2009). In fact, SA, JA and JA-Ile also showed higher values in nr than in wt shoots only under Bm inoculation in mature plants. The best known role of JA is its regulation of plant immune responses against pathogens (Browse, 2009). Thus, these results also support ‘misinteraction’ resulting in Bm recognition by nr plants as a pathogen-like micro-organism with physiologically deleterious effects. Indeed, ethylene modulates plant resistance and susceptibility to pathogens (van Loon et al., 2006) and even beneficial micro-organisms can be recognized as potential invaders, triggering an immune response (Zamioudis and Pieterse, 2012).
Furthermore, ethylene and IAA affect each other’s synthesis since high IAA levels result in increased ethylene biosynthesis (Muday et al., 2012), and vice versa (Stepanova et al., 2005). The ethylene-insensitive tomato mutant nr is not able to perceive ethylene, but presents some residual responsiveness (Lanahan et al., 1994) and only ETR3 is not functional (Wilkinson et al., 1995). Cross-talk between ethylene and auxins is produced at the level of biosynthesis and thus higher levels of IAA and ethylene were generally seen in non-inoculated nr plants compared with wt plants. Furthermore, microbially derived IAA is a signalling molecule in micro-organisms and interferes with several developmental processes in planta (Spaepen and Vanderleyden, 2011). Although IAA production by these PGPB was not assayed, PGPB inoculation did not directly modify IAA levels and only plant genotype differences were affected under bacterial inoculations, suggesting that both Bm and C7 are unable to produce auxins. Lower IAA levels were seen in Bm-inoculated nr plants compared with wt plants, and IAA signaling downregulation was reported as part of plant defence against bacteria (Spaepen and Vanderleyden, 2011) in agreement with our results. Moreover, root and shoot IAA levels were increased in C7-inocualted nr plants compared with wt plants, predisposing plants to further growth since IAA plays crucial roles in several developmental processes, being identified as a plant growth hormone (Zhao, 2010).
Root metabolites were directly altered by PGPB inoculation at the juvenile stage showing a high dependence on ethylene sensitivity in mature plants
Our results showed changes in root sugars, amino acids and organic acids due to bacterial inoculation. Although performed in shoots, previous studies pointed to modification of those compounds by bacterial inoculation (Weston et al., 2012; Su et al., 2016). Root metabolite contents were affected by bacterial inoculation in juvenile plants, but did not show changes in mature plants when genotype differences were clearly marked (Tables 6 and 7; Figs 4 and 5).
Sugars and amino acids were the main source of variation at 4 wpi, while only amino acids contributed to variability in mature plants, suggesting that modification of root sugar levels rather than amino acid content by bacterial inoculation could be relevant in growth promotion. Furthermore, ethylene perception is related to plant sensitivity to sugars (Paul and Pellny, 2003). Moreover, differences between Bm- and C7-inoculated juvenile plants were observed in nr plants, suggesting strain-specific interactions between bacteria and host plants, as previously reported (Walker et al., 2011; Weston et al., 2012). These results imply that ethylene sensitivity could affect plant–bacteria interaction because similar profiles were observed in wt plants. Root metabolic profiles were in agreement with biomass results at 8 wpi, showing high similarity in profiles of Bm- and non-inoculated nr plants (Figs 1 and 5). Root biomass can be increased by bacterial succinic acid (Yoshikawa et al., 1993), and higher succinic acid levels coupled with higher root biomass were only observed in bacteria-inoculated wt plants. Furthermore, fumaric acid plays a crucial role in biofilm formation necessary for root colonization by Bacillus strains (Zhang et al., 2014; Yuan et al., 2015). Thus, lower fumaric acid levels only seen in Bm-inoculated nr plants could affect functional interaction. Moreover, differences in monosaccharide levels between both inocula were restricted to nr plants. Higher glucose levels were seen in Bm-inoculated nr plants compared with the wt plants at 8 wpi. Ethylene is involved in plant sensitivity to sugars, and it has been reported that ethylene-insensitive plants show a higher response to endogenous glucose, resulting in increased suppression of photosynthesis by carbohydrates (Zhou et al., 1998; León and Sheen, 2003; Paul and Pellny, 2003). Thus, Bm inoculation could enhance this phenomenon only in nr plants, modifying mainly sugar metabolism.
In addition, previous studies have reported changes in plant amino acid levels by bacteria (Curzi et al., 2008). Also, previous reports suggested that amino acid homeostasis could have regulatory functions in maintaining plant growth and development (Walch-Liu et al., 2006; Yu et al., 2013; Ros et al., 2014). Several amino acids were decreased by Bm and increased by C7 in nr plants. These results suggest a strain-specific effect on root amino acid levels that could lead to different growth responses. Aspartic acid is the common precursor of other amino acids in higher plants (Azevedo et al., 2006). Deficiencies in serine and derived molecules have consequences such as altered mineral homeostasis and root development (Muñoz-Bertomeu et al., 2009; Ros et al., 2014). Thus, these specific reductions of amino acid contents in nr plants could affect plant growth under Bm inoculation. Moreover, aspartic acid, glutamic acid and glutamine are involved in plant nitrogen assimilation (Xu and Zhou, 2004), suggesting that C7 inoculation could improve N assimilation in nr juvenile plants. Furthermore, amino acid levels were reduced in wt plants by C7 inoculation. Isoleucine deficiency produces defects in cell proliferation and expansion during development of roots (Yu et al., 2013). Phenylalanine is also used in the phenylpropanoid pathway leading to the biosynthesis of secondary products (Hyun et al., 2011) involved in cell wall structure (Bonawitz and Chapple, 2010), and plant defence or response to stress (Winkel-Shirley, 2001; Fraser and Chapple, 2011). Thus, these results suggest that C7 inoculation could affect plant development though modification of amino acid content. As proposed by Rivero et al. (2015), reduction of amino acids could be due to their use for secondary metabolism. In addition, genotype differences seen exclusively under C7 inoculation were in amino acid levels, also indicating a remodelling of amino acid metabolism.
PGPB inoculation affected plant nutrition notably at the mature stage with a clear influence of ethylene sensitivity
The effects of PGPB on nutritional status were more prominent in mature plants, showing a high dependence on ethylene sensitivity (Fig. 3; Tables S4 and S5). Indeed, the interaction of ethylene signalling and plant nutrition was reviewed by Iqbal et al. (2013), indicating that nutrient deficiencies are greatly related to ethylene perception and biosynthesis. As observed for metabolites, separation of root nutrient profiles was separated by plant genotype in mature plants, but the strain-specific effect was maintained in nr plants (Fig. 3).
Bm inoculation did not alter nutrients in juvenile roots, but the leaf contents of several nutrients were affected. The C concentration was increased, while N and Mn were decreased in wt plants. Mn plays an important role in protection of photosynthetic tissues (Mehlhorn and Wenzel, 1996), and our results showed a reduction in photosynthetic efficiency of Bm-inoculated wt plants. Moreover, reduced Zn and Fe levels in nr shoots due to Bm inoculation could suggest that Bm affects nutrient translocation in nr plants (Sperotto, 2013). In mature plants, Bm inoculation increased C in roots and shoots of wt plants, but a decrease in several nutrients was seen in nr roots. Thus, the C:N ratio was increased by Bm inoculation in wt plants, suggesting that Bm stimulates plant growth by increasing C assimilation per N unit, leading to an increase in biomass (Lawlor, 2002). Moreover, Ca is a key player conferring structure and rigidity to the cell wall and interacts with ethylene signalling and plant responses to biotic attacks (Iqbal et al., 2013). Thus, the root Ca increase mediated by Bm inoculation could enhance the resistance of wt plants.
Although both PGPB decreased Cu levels in nr roots at 8 wpi, C7 reduced it to a higher degree than Bm. Also, C7 inoculation decreased Cu in nr shoots at 8 wpi. Moreover, root Cu concentration was negatively correlated with total and shoot dry weights. Cu is a structural component of ethylene receptors (Rodríguez, 1999). Thus, lower levels of Cu could result in fewer functional ethylene receptors, and consequentially lower growth inhibition by ethylene (Pierik et al., 2006). These results are in accordance with plant growth promotion observed in nr plants. However, C7 increased shoot Cu levels in wt plants, but the ethylene transduction pathway and regulatory mechanisms are functional, showing no growth inhibitory effects. In addition, both PGPB also affected Si levels in mature nr roots. Moreover, the role of Si in plants has been described as that of a biotic and abiotic protector (Ma and Yamaji, 2008). Thus, lower Si levels seen in bacteria-inoculated plants could indicate that low Si could favour interaction with PGPB, with C7-inoculated nr plants showing lower levels than Bm-inoculated nr plants.
Nevertheless, C7 inoculation only reduced root Na concentration in nr roots, not affecting shoot nutrients in juvenile plants. Although no saline stress was applied in this study, reduction of Na by PGPB was previously reported (Zhang et al., 2008) and could be related to growth promotion because Na is toxic to plant cells and its accumulation within cells is undesirable (Pardo and Quintero, 2002). In contrast, C7 inoculation showed a large impact in plant nutrition at 8 wpi. Root C concentration was increased in wt plants, while root S levels were decreased in nr plants. S reduction by C7 inoculation only in nr plants could be due to modulation of the S assimilation machinery of plants (Aziz et al., 2016) whose regulation is dependent on ethylene perception (Wawrzynska et al., 2015). In addition, C7 inoculation affected shoot nutrients, increasing Fe in wt plants and decreasing P levels in nr plants. Fe is a key factor in photosynthesis and respiration, and its deficiency produced stunted plant growth (Iqbal et al., 2013), suggesting that C7 inoculation of wt plants improves nutrient translocation (Sperotto, 2013) and maintains plant growth. However, P deficiency limits shoot and root dry weighrs (Borch et al., 1999). Ethylene is induced in P-deficient plants and ethylene sensitivity is involved in regulating carbon allocation to adventitious roots (Kim et al., 2008) and root hair development (Zhang et al., 2003) in order to facilitate a rapid recovery of stressed plants. A previous study reported that Bacillus amyloliquefaciens reduced P uptake (Talboys et al., 2014). These results are in agreement with our study but further research is necessary to know how the C7 inoculation affects P nutrition.
Conclusions
In this study, it has been reported that physiological parameters and root metabolites were modified by bacterial inoculation in juvenile plants rather than in mature plants, when plant homeostasis can counteract inoculation effects. However, PGPB effects on nutritional status were more prominent in mature plants, with a high dependence of ethylene sensitivity (Fig. 6).
Fig. 6.
Summary of plant growth-promoting bacteria (PGPB) inoculation effects on wild-type and never ripe tomato (Solanum lycopersicum) plants at 4 and 8 weeks post-inoculation (wpi). Increases or decreases in measured parameters produced by PGPB inoculation are shown with ↑ and ↓ symbols, respectively. Changes induced by PGPB inoculation are shown in black and red letters for common and specific effects, respectively. Bm, Bacillus megaterium; C7, Enterobacter C7; d. wt, dry weight; RGR, relative growth rate; gs, stomatal conductance; ΔFv/Fm′, photosynthetic efficiency; ABA, abscisic acid; C, carbon; N, nitrogen; Mn, manganese; Ca, calcium; Fe, iron; Cu, copper; Zn, zinc; Na, sodium; Mg, magnesium; Si, silicon; S, sulphur.
In conclusion, the inability to perceive ethylene by the ETR3 receptor impairs interaction between tomato plants and B. megaterium, affecting photosynthetic efficiency, plant nutrition and root sugars, that leads to a loss of PGPB activity. Nevertheless, Bm could stimulate plant growth in wt plants by improving carbon assimilation. In contrast, Enterobacter C7 could stimulate plant growth by affecting stomatal conductance, plant nutrition and root amino acids. Moreover, C7 inoculation in ethylene-insensitive plants could improve nitrogen assimilation (Fig. 6). Thus, ethylene sensitivity could be proposed as essential for PGPB activity of B. megaterium in tomato plants, whereas the Enterobacter C7 PGPB mechanism seems to be ETR3 independent.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: reagents and standards used for phytohormone analysis by HPLC-ESI-HRMS. Table S2: instrumental parameters used for HESI (II) ionization. Table S3: accurate masses of the phytohormones and internal standards, and its principal fragments. Table S4: effects of bacterial inoculation on root nutrient concentrations. Table S5: effects of bacterial inoculation on shoot nutrient concentrations.
Supplementary Material
ACKNOWLEDGEMENTS
P.I. was financed by the Spanish Ministry of Economy and Competitiveness (BES-2012-058155). This work was performed in the framework of the project AGL2011-25403. We thank the laboratory of Rosario Azcón for providing PGPB strains. P.I. and R.A. are responsible for conceiving the experimental strategies and analysis of the results. P.I. has collected and organized most of the relevant data, performed general interpretations of results and has written most of the text in the manuscript. P.I. and S.M. have carried out most of the experiments shown throughout the manuscript. R.N. has performed root metabolite analyses. A.M.Z., M.C.O-M. and J.M.G-M. have performed phytohormone analyses. J.M.R-L., B.R.G. and R.A. contributed to the revision of the general sections, and have approved the final version. P.I. and R.A. have co-ordinated general approval of the final version.
LITERATURE CITED
- Adesemoye AO, Kloepper JW.. 2009. Plant–microbes interactions in enhanced fertilizer-use efficiency. Applied Microbiology and Biotechnology 85: 1–12. [DOI] [PubMed] [Google Scholar]
- Anderson JP, Badruzsaufari E, Schenk PM, et al. 2004. Antagonistic interaction between abscisic acid and jasmonate–ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. The Plant Cell 16: 3460–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armada E, Portela G, Roldán A, Azcón R.. 2014a. Combined use of beneficial soil microorganism and agrowaste residue to cope with plant water limitation under semiarid conditions. Geoderma 232–234: 640–648. [Google Scholar]
- Armada E, Roldán A, Azcon R.. 2014b. Differential activity of autochthonous bacteria in controlling drought stress in native Lavandula and Salvia plants species under drought conditions in natural arid soil. Microbial Ecology 67: 410–420. [DOI] [PubMed] [Google Scholar]
- Aroca R, Ruiz-Lozano JM.. 2009. Induction of plant tolerance to semi-arid environments by beneficial soil microorganisms. A review (E) In: Lichtfouse E, ed. Climate change, intercropping, pest control and beneficial microorganisms. Berlin: Springer, 121–135. [Google Scholar]
- Azevedo RA, Lancien M, Lea PJ.. 2006. The aspartic acid metabolic pathway, an exciting and essential pathway in plants. Amino Acids 30: 143–162. [DOI] [PubMed] [Google Scholar]
- Aziz M, Nadipalli RK, Xie X, et al. 2016. Augmenting sulfur metabolism and herbivore defense in arabidopsis by bacterial volatile signaling. Frontiers in Plant Science 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barea JM, Pozo MJ, Azcón R, Azcón-Aguilar C.. 2005. Microbial co-operation in the rhizosphere. Journal of Experimental Botany 56: 1761–1778. [DOI] [PubMed] [Google Scholar]
- Benizri E, Baudoin E, Guckert A.. 2001. Root colonization by inoculated plant growth-promoting rhizobacteria. Biocontrol Science and Technology 11: 557–574. [Google Scholar]
- Berg G. 2009. Plant–microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Applied Microbiology and Biotechnology 84: 11–18. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya PN, Jha DK.. 2012. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World Journal of Microbiology and Biotechnology 28: 1327–1350. [DOI] [PubMed] [Google Scholar]
- Bonawitz ND, Chapple C.. 2010. The genetics of lignin biosynthesis: connecting genotype to phenotype. Annual Review of Genetics 44: 337–363. [DOI] [PubMed] [Google Scholar]
- Borch K, Bouma TJ, Lynch JP, Brown KM.. 1999. Ethylene: a regulator of root architectural responses to soil phosphorus availability. Plant, Cell & Environment 22: 425–431. [Google Scholar]
- Bresson J, Varoquaux F, Bontpart T, Touraine B, Vile D.. 2013. The PGPR strain Phyllobacterium brassicacearum STM196 induces a reproductive delay and physiological changes that result in improved drought tolerance in Arabidopsis. New Phytologist 200: 558–569. [DOI] [PubMed] [Google Scholar]
- Browse J. 2009. Jasmonate passes muster: a receptor and targets for the defense hormone. Annual Review of Plant Biology 60: 183–205. [DOI] [PubMed] [Google Scholar]
- Chen L, Dodd IC, Theobald JC, Belimov AA, Davies WJ.. 2013. The rhizobacterium Variovorax paradoxus 5C-2, containing ACC deaminase, promotes growth and development of Arabidopsis thaliana via an ethylene-dependent pathway. Journal of Experimental Botany 64: 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, McConkey BJ, Glick BR.. 2010. Proteomic studies of plant–bacterial interactions. Soil Biology and Biochemistry 42: 1673–1684. [Google Scholar]
- Cohen AC, Bottini R, Pontin M, et al. 2015. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiologia Plantarum 153: 79–90. [DOI] [PubMed] [Google Scholar]
- Couillerot O, Ramírez-Trujillo A, Walker V, et al. 2013. Comparison of prominent Azospirillum strains in Azospirillum–Pseudomonas–Glomus consortia for promotion of maize growth. Applied Microbiology and Biotechnology 97: 4639–4649. [DOI] [PubMed] [Google Scholar]
- Curzi MJ, Ribaudo CM, Trinchero GD, Curá JA, Pagano EA.. 2008. Changes in the content of organic and amino acids and ethylene production of rice plants in response to the inoculation with Herbaspirillum seropedicae. Journal of Plant Interactions 3: 163–173. [Google Scholar]
- Dimkpa C, Weinand T, Asch F.. 2009. Plant rhizobacteria interactions alleviate abiotic stress conditions. Plant, Cell & Environment 32: 1682–1694. [DOI] [PubMed] [Google Scholar]
- Dodd IC, Ruiz-Lozano JM.. 2012. Microbial enhancement of crop resource use efficiency. Current Opinion in Biotechnology 23: 236–242. [DOI] [PubMed] [Google Scholar]
- Feng J, Barker AV.. 1992. Ethylene evolution and ammonium accumulation by nutrient stressed tomato plants. Journal of Plant Nutrition 15: 137–153. [Google Scholar]
- Feussner I, Polle A.. 2015. What the transcriptome does not tell – proteomics and metabolomics are closer to the plants’ patho-phenotype. Current Opinion in Plant Biology 26: 26–31. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. 2013. Abscisic acid synthesis and response. The Arabidopsis Book 11: e0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Chapple C.. 2011. The phenylpropanoid pathway in Arabidopsis. The Arabidopsis Book 9: e0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BR. 1995. The enhancement of plant growth by free-living bacteria. Canadian Journal of Microbiology 41: 109–117. [Google Scholar]
- Glick BR. 2004. Bacterial ACC deaminase and the alleviation of plant stress. Advances in Applied Microbiology 56: 291–312. [DOI] [PubMed] [Google Scholar]
- Glick BR. 2014. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiological Research 169: 30–39. [DOI] [PubMed] [Google Scholar]
- Glick BR, Cheng Z, Czarny J, Duan J.. 2007. Promotion of plant growth by ACC deaminase-producing soil bacteria. European Journal of Plant Pathology 119: 329–339. [Google Scholar]
- Gray EJ, Smith DL.. 2005. Intracellular and extracellular PGPR: commonalities and distinctions in the plant-bacterium signaling processes. Soil Biology and Biochemistry 37: 395–412. [Google Scholar]
- Hanson J, Smeekens S.. 2009. Sugar perception and signaling – an update. Current Opinion in Plant Biology 12: 562–567. [DOI] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM.. 1998. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94: 261–71. [DOI] [PubMed] [Google Scholar]
- Hunt R. 1982. Plant growth analysis. Journal of Applied Ecology 17: 516. [Google Scholar]
- Hyun MW, Yun YH, Kim JY, Kim SH.. 2011. Fungal and plant phenylalanine ammonia-lyase. Mycobiology 39: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal N, Trivellini A, Masood A, Ferrante A, Khan NA.. 2013. Current understanding on ethylene signaling in plants: the influence of nutrient availability. Plant Physiology and Biochemistry 73: 128–138. [DOI] [PubMed] [Google Scholar]
- Kim H-J, Lynch JP, Brown KM.. 2008. Ethylene insensitivity impedes a subset of responses to phosphorus deficiency in tomato and petunia. Plant, Cell & Environment 31: 1744–1755. [DOI] [PubMed] [Google Scholar]
- Krouk G, Ruffel S, Gutiérrez RA, et al. 2011. A framework integrating plant growth with hormones and nutrients. Trends in Plant Science 16: 178–182. [DOI] [PubMed] [Google Scholar]
- Kuan KB, Othman R, Abdul Rahim K, Shamsuddin ZH.. 2016. Plant growth-promoting rhizobacteria inoculation to enhance vegetative growth, nitrogen fixation and nitrogen remobilisation of maize under greenhouse conditions. PLoS One 11: e0152478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing WA, Wright MA, Cooney J, Bulley SM.. 2007. The missing step of the l-galactose pathway of ascorbate biosynthesis in plants, an l-galactose guanyltransferase, increases leaf ascorbate content. Proceedings of the National Academy of Sciences, USA 104: 9534–9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Mougel C, Jaillard B, Hinsinger P.. 2009. Plant–microbe–soil interactions in the rhizosphere: an evolutionary perspective. Plant and Soil 321: 83–115. [Google Scholar]
- Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ.. 1994. The never ripe mutation blocks ethylene perception in tomato. The Plant Cell 6: 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor d. wt. 2002. Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems. Journal of Experimental Botany 53: 773–787. [PubMed] [Google Scholar]
- Leon, P, Sheen, J. 2003. Sugar and hormone connections. Trends in Plants Science 8:110–116. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology 148: 350–382. [Google Scholar]
- Long HH, Schmidt DD, Baldwin IT.. 2008. Native bacterial endophytes promote host growth in a species-specific manner; phytohormone manipulations do not result in common growth responses. PLoS One 3: e2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LC. 2007. Plant responses to plant growth-promoting rhizobacteria. European Journal of Plant Pathology 119: 243–254. [Google Scholar]
- van Loon LC, Geraats BPJ, Linthorst HJM.. 2006. Ethylene as a modulator of disease resistance in plants. Trends in Plant Science 11: 184–191. [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Campos-Cuevas JC, Hernández-Calderón E, et al. 2007. Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana. Molecular Plant-Microbe Interactions 20: 207–217. [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Nieto-Jacobo MF, Simpson J, Herrera-Estrella L.. 2002. Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiology 129: 244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucena C, Waters BM, Romera FJ, et al. 2006. Ethylene could influence ferric reductase, iron transporter, and H+-ATPase gene expression by affecting FER (or FER-like) gene activity. Journal of Experimental Botany 57: 4145–4154. [DOI] [PubMed] [Google Scholar]
- Lucy M, Reed E, Glick BR.. 2004. Applications of free living plant growth-promoting rhizobacteria. Antonie van Leeuwenhoek 86: 1–25. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B, Kamilova F.. 2009. Plant-growth-promoting rhizobacteria. Annual Review of Microbiology 63: 541–556. [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N.. 2008. Functions and transport of silicon in plants. Cellular and Molecular Life Sciences 65: 3049–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Viveros O, Jorquera M., Crowley D., Gajardo G, Mora M.. 2010. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. Journal of Soil Science and Plant Nutrition 10: 293–319. [Google Scholar]
- Marulanda A, Barea J-M, Azcón R.. 2009. Stimulation of plant growth and drought tolerance by native microorganisms (AM fungi and bacteria) from dry environments: mechanisms related to bacterial effectiveness. Journal of Plant Growth Regulation 28: 115–124. [Google Scholar]
- Marulanda A, Azcón R, Chaumont F, Ruiz-Lozano JM, Aroca R.. 2010. Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 232: 533–543. [DOI] [PubMed] [Google Scholar]
- Marulanda-Aguirre A, Azcón R, Ruiz-Lozano JM, Aroca R.. 2008. Differential effects of a Bacillus megaterium strain on Lactuca sativa plant growth depending on the origin of the arbuscular mycorrhizal fungus coinoculated: physiologic and biochemical traits. Journal of Plant Growth Regulation 27: 10–18. [Google Scholar]
- Mehlhorn, H, Wenzel, AA. Manganese deficiency enhances ozone toxicity in bush beans (Phaseolus vulgaris L cv Saxa). Journal of Plant Physiology 148: 155–159. [Google Scholar]
- Meldau DG, Long HH, Baldwin IT.. 2012. A native plant growth promoting bacterium, Bacillus sp. B55, rescues growth performance of an ethylene-insensitive plant genotype in nature. Frontiers in Plant Science 3: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Mortel JE, de Vos RCH, Dekkers E, et al. 2012. Metabolic and transcriptomic changes induced in Arabidopsis by the rhizobacterium Pseudomonas fluorescens SS101. Plant Physiology 160: 2173–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday GK, Rahman A, Binder BM.. 2012. Auxin and ethylene: collaborators or competitors? Trends in Plant Science 17: 181–195. [DOI] [PubMed] [Google Scholar]
- Muñoz-Bertomeu J, Cascales-Miñana B, Mulet JM, et al. 2009. Plastidial glyceraldehyde-3-phosphate dehydrogenase deficiency leads to altered root development and affects the sugar and amino acid balance in Arabidopsis. Plant Physiology 151: 541–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem SM, Ahmad M, Zahir ZA, Javaid A, Ashraf M.. 2014. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnology Advances 32: 429–448. [DOI] [PubMed] [Google Scholar]
- Ortiz N, Armada E, Duque E, Roldán A, Azcón R.. 2015. Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: effectiveness of autochthonous or allochthonous strains. Journal of Plant Physiology 174: 87–96. [DOI] [PubMed] [Google Scholar]
- Ortíz-Castro R, Contreras-Cornejo HA, Macías-Rodríguez L, López-Bucio J.. 2009. The role of microbial signals in plant growth and development. Plant Signaling and Behavior 4: 701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxborough K, Baker NR.. 1997. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components – calculation of qP and Fv'/Fm' without measuring Fo'. Photosynthesis Research 54: 135–142. [Google Scholar]
- Pardo JM, Quintero FJ.. 2002. Plants and sodium ions: keeping company with the enemy. Genome Biology 3: REVIEWS1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Pellny TK.. 2003. Carbon metabolite feedback regulation of leaf photosynthesis and development. Journal of Experimental Botany 54: 539–547. [DOI] [PubMed] [Google Scholar]
- Penrose DM, Glick BR.. 2003. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiologia Plantarum 118: 10–15. [DOI] [PubMed] [Google Scholar]
- Pierik R, Tholen D, Poorter H, Visser EJ, Voesenek LA.. 2006. The Janus face of ethylene: growth inhibition and stimulation. Trends in Plant Science 11: 176–83. [DOI] [PubMed] [Google Scholar]
- Pii Y, Mimmo T, Tomasi N, Terzano R, Cesco S, Crecchio C.. 2015. Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biology and Fertility of Soils 51: 403–415. [Google Scholar]
- Porcel R, Zamarreño ÁM, García-Mina JM, Aroca R.. 2014. Involvement of plant endogenous ABA in Bacillus megaterium PGPR activity in tomato plants. BMC Plant Biology 14: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primrose SB, Dilworth MJ.. 1976. Ethylene production by bacteria. Journal of General Microbiology 93: 177–181. [DOI] [PubMed] [Google Scholar]
- Richardson AD, Duigan SP, Berlyn GP.. 2002. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytologist 153: 185–194. [Google Scholar]
- Rivero J, Gamir J, Aroca R, Pozo MJ, Flors V.. 2015. Metabolic transition in mycorrhizal tomato roots. Frontiers in Microbiology 6: 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez FI. 1999. A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science 283: 996–998. [DOI] [PubMed] [Google Scholar]
- Roessner U, Wagner C, Kopka J, Trethewey RN, Willmitzer L.. 2000. Technical advance: simultaneous analysis of metabolites in potato tuber by gas chromatography–mass spectrometry. The Plant Journal 23: 131–142. [DOI] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J.. 2006. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annual Review of Plant Biology 57: 675–709. [DOI] [PubMed] [Google Scholar]
- Ros R, Muñoz-Bertomeu J, Krueger S.. 2014. Serine in plants: biosynthesis, metabolism, and functions. Trends in Plant Science 19: 564–569. [DOI] [PubMed] [Google Scholar]
- Ryan PR, Dessaux Y, Thomashow LS, Weller DM.. 2009. Rhizosphere engineering and management for sustainable agriculture. Plant and Soil 321: 363–383. [Google Scholar]
- Ryu C-M, Hu C-H, Locy RD, Kloepper JW.. 2005. Study of mechanisms for plant growth promotion elicited by rhizobacteria in Arabidopsis thaliana. Plant and Soil 268: 285–292. [Google Scholar]
- Saleem M, Arshad M, Hussain S, Bhatti AS.. 2007. Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. Journal of Industrial Microbiology and Biotechnology 34: 635–648. [DOI] [PubMed] [Google Scholar]
- Sánchez-Vallet A, López G, Ramos B, et al. 2012. Disruption of abscisic acid signaling constitutively activates Arabidopsis resistance to the necrotrophic fungus Plectosphaerella cucumerina. Plant Physiology 160: 2109–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoyo G, Moreno-Hagelsieb G, del Carmen Orozco-Mosqueda M, Glick BR.. 2016. Plant growth-promoting bacterial endophytes. Microbiological Research 183: 92–99. [DOI] [PubMed] [Google Scholar]
- Schmidt W. 2001. Different pathways are involved in phosphate and iron stress-induced alterations of root epidermal cell development. Plant Physiology 125: 2078–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz D, Rouphael Y, Colla G, Venema JH.. 2010. Grafting as a tool to improve tolerance of vegetables to abiotic stresses: thermal stress, water stress and organic pollutants. Scientia Horticulturae 127: 162–171. [Google Scholar]
- Sharp R. 2002. Interaction with ethylene: changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant, Cell & Environment 25: 211–222. [DOI] [PubMed] [Google Scholar]
- Shishido M, Chanway CP.. 2000. Colonization and growth promotion of outplanted spruce seedlings pre-inoculated with plant growth-promoting rhizobacteria in the greenhouse. Canadian Journal of Forest Research 30: 845–854. [Google Scholar]
- Singh JS, Pandey VC, Singh DP.. 2011. Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development. Agriculture, Ecosystems and Environment 140: 339–353. [Google Scholar]
- Smith AM, Stitt M.. 2007. Coordination of carbon supply and plant growth. Plant, Cell & Environment 30: 1126–1149. [DOI] [PubMed] [Google Scholar]
- Spaepen S, Vanderleyden J.. 2011. Auxin and plant–microbe interactions. Cold Spring Harbor Perspectives in Biology 3: a001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperotto RA. 2013. Zn/Fe remobilization from vegetative tissues to rice seeds: should I stay or should I go? Ask Zn/Fe supply! Frontiers in Plant Science 4: 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns JC, Woody OZ, McConkey BJ, Glick BR.. 2012. Effects of bacterial ACC deaminase on Brassica napus gene expression. Molecular Plant-Microbe Interactions 25: 668–676. [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM.. 2005. A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. The Plant Cell 17: 2230–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su F, Gilard F, Guerard F, et al. 2016. Spatio-temporal responses of Arabidopsis leaves in photosynthetic performance and metabolite contents to Burkholderia phytofirmans PsJN. Frontiers in Plant Science 7: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talboys PJ, Owen d. wt, Healey JR, Withers PJ, Jones DL.. 2014. Auxin secretion by Bacillus amyloliquefaciens FZB42 both stimulates root exudation and limits phosphorus uptake in Triticum aestivium. BMC Plant Biology 14: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Sano T, Tamaoki M, Nakajima N, Kondo N.. 2005. Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiology 138: 2337–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholen D, Voesenek LACJ, Poorter H.. 2004. Ethylene insensitivity does not increase leaf area or relative growth rate in Arabidopsis, Nicotiana tabacum, and Petunia×hybrida. Plant Physiology 134: 1803–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D, Balzer C, Hill J, Befort BL.. 2011. Global food demand and the sustainable intensification of agriculture. Proceedings of the National Academy of Sciences, USA 108: 20260–20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacheron J, Desbrosses G, Bouffaud M-L, et al. 2013. Plant growth-promoting rhizobacteria and root system functioning. Frontiers in Plant Science 4: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Poel B, Smet D, Van Der Straeten D.. 2015. Ethylene and hormonal cross talk in vegetative growth and development. Plant Physiology 169: 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas L, de Carvalho TLG, Ferreira PCG, Baldani VLD, Baldani JI, Hemerly AS.. 2012. Early responses of rice (Oryza sativa L.) seedlings to inoculation with beneficial diazotrophic bacteria are dependent on plant and bacterial genotypes. Plant and Soil 356: 127–137. [Google Scholar]
- Vessey JKJ. 2003. Plant growth promoting rhizobacteria as biofertilizers. Plant and Soil 255: 571–586. [Google Scholar]
- Vlot AC, Dempsey DA, Klessig DF.. 2009. Salicylic acid, a multifaceted hormone to combat disease. Annual Review of Phytopathology 47: 177–206. [DOI] [PubMed] [Google Scholar]
- Walch-Liu P, Liu LH, Remans T, Tester M, Forde BG.. 2006. Evidence that l-glutamate can act as an exogenous signal to modulate root growth and branching in Arabidopsis thaliana. Plant and Cell Physiology 47: 1045–1057. [DOI] [PubMed] [Google Scholar]
- Walker V, Bertrand C, Bellvert F, Moënne-Loccoz Y, Bally R, Comte G.. 2011. Host plant secondary metabolite profiling shows a complex, strain-dependent response of maize to plant growth-promoting rhizobacteria of the genus Azospirillum. New Phytologist 189: 494–506. [DOI] [PubMed] [Google Scholar]
- Wang F, Cui X, Sun Y, Dong C-H.. 2013. Ethylene signaling and regulation in plant growth and stress responses. Plant Cell Reports 32: 1099–1109. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang Z, Huang R.. 2013. Regulation of ascorbic acid synthesis in plants. Plant Signaling and Behavior 8: e24536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrzynska A, Moniuszko G, Sirko A.. 2015. Links between ethylene and sulfur nutrition – a regulatory interplay or just metabolite association? Frontiers in Plant Science 6: 1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller DM, Raaijmakers JM, Gardener BBM, Thomashow LS.. 2002. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annual Review of Phytopathology 40: 309–348. [DOI] [PubMed] [Google Scholar]
- Weston DJ, Pelletier DA, Morrell-Falvey JL, et al. 2012. Pseudomonas fluorescens induces strain-dependent and strain-independent host plant responses in defense networks, primary metabolism, photosynthesis, and fitness. Molecular Plant-Microbe Interactions 25: 765–778. [DOI] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ.. 1995. An ethylene-inducible component of signal transduction encoded by never-ripe. Science 270: 1807–1809. [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley B. 2001. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiology 126: 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Zhou G.. 2004. Research advance in nitrogen metabolism of plant and its environmental regulation. Journal of Applied Ecology 15: 511–516. [PubMed] [Google Scholar]
- Yoshikawa M, Hirai N, Wakabayashi K, Sugizaki H, Iwamura H.. 1993. Succinic and lactic acids as plant growth promoting compounds produced by rhizospheric Pseudomonas putida. Canadian Journal of Microbiology 39: 1150–1154. [Google Scholar]
- Yuan J, Zhang N, Huang Q, et al. 2015. Organic acids from root exudates of banana help root colonization of PGPR strain Bacillus amyloliquefaciens NJN-6. Scientific Reports 5: 13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Zhang F, Wang G, Liu Y, Liu D.. 2013. Partial deficiency of isoleucine impairs root development and alters transcript levels of the genes involved in branched-chain amino acid and glucosinolate metabolism in Arabidopsis. Journal of Experimental Botany 64: 599–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Zahar Haichar F, Santaella C, Heulin T, Achouak W.. 2014. Root exudates mediated interactions belowground. Soil Biology and Biochemistry 77: 69–80. [Google Scholar]
- Zamioudis C, Pieterse CMJ.. 2012. Modulation of host immunity by beneficial microbes. Molecular Plant-Microbe Interactions 25: 139–150. [DOI] [PubMed] [Google Scholar]
- Zhang H, Kim M-S, Sun Y, Dowd SE, Shi H, Paré PW.. 2008. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Molecular Plant-Microbe Interactions 21: 737–744. [DOI] [PubMed] [Google Scholar]
- Zhang N, Wang D, Liu Y, Li S, Shen Q, Zhang R.. 2014. Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant and Soil 374: 689–700. [Google Scholar]
- Zhang YJ, Lynch JP, Brown KM.. 2003. Ethylene and phosphorus availability have interacting yet distinct effects on root hair development. Journal of Experimental Botany 54: 2351–2361. [DOI] [PubMed] [Google Scholar]
- Zhao Y. 2010. Auxin biosynthesis and its role in plant development. Annual Review of Plant Biology 61: 49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Jang JC, Jones TL, Sheen J.. 1998. Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proceedings of the National Academy of Sciences, USA 95: 10294–10299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.