Abstract
Background and Aims Climate change is expected to have major impacts on high alpine and arctic ecosystems in the future, but empirical data on the impact of long-term warming on lichen diversity and richness are sparse. This study report the effects of 18 years of ambient and experimental warming on lichens and vascular plant cover in two alpine plant communities, a dry heath with sparse canopy cover (54 %) and a mesic meadow with a more developed (67 %) canopy cover, in sub-arctic Sweden.
Methods The effects of long-term passive experimental warming using open top chambers (OTCs) on lichens and total vascular plant cover, and the impact of plant cover on lichen community parameters, were analysed.
Key Results Between 1993 and 2013, mean annual temperature increased about 2 °C. Both site and experimental warming had a significant effect on cover, species richness, effective number of species evenness of lichens, and total plant canopy cover. Lichen cover increased in the heath under ambient conditions, and remained more stable under experimental warming. The negative effect on species richness and effective number of species was driven by a decrease in lichens under experimental warming in the meadow. Lichen cover, species richness, effective number of species evenness were negatively correlated with plant canopy cover. There was a significant negative impact on one species and a non-significant tendency of lower abundance of the most common species in response to experimental warming.
Conclusions The results from the long-term warming study imply that arctic and high alpine lichen communities are likely to be negatively affected by climate change and an increase in plant canopy cover. Both biotic and abiotic factors are thus important for future impacts of climate change on lichens.
Keywords: Arctic, climate change, effective number of species, global warming, plant–climate interactions, species richness, tundra
Introduction
Climate change is predicted to have a large impact on a wide range of ecosystems and ecosystem services (Shen and Ma, 2014; Wu et al., 2014; Zhang et al., 2014; Hao et al., 2017). Polar and high-elevation alpine ecosystems are likely to experience rapid climate change (Chapin et al., 1995; Mack et al., 2004; Stocker et al., 2013). Changes in species composition in alpine and arctic plant communities have already been recorded (Capers and Stone, 2011; Erschbamer et al., 2011; Callaghan et al., 2013), and a meta-analysis incorporating 1367 species responses provided evidence of a rapid latitudinal and elevational range shift in species across a large geographical range (Chen et al., 2011). However, it is not always possible to identify the cause of range shifts, and they may sometimes be due to other changes brought about by human activities (Groom, 2013). The loss of habitat due to climate change is predicted to increase the extinction risks on mountain ranges worldwide (Colwell et al., 2008; Raxworthy et al., 2008; Dirnböck et al., 2011; Engler et al., 2011). However, as glaciers retreat, this may uncover new habitats to be colonized, e.g. a number of studies have shown that lichen diversity and abundance is correlated with increasing time since glacier retreat (Bilovitz et al., 2014a, b, 2015a). However, the ice-free glacial tills exposed sometimes require several hundred years to reach the climax stage of alpine grassland (Raffl and Erschbamer, 2004). In polar regions and high alpine areas, lichens tend to be more important in terms of cover and biomass for N2 fixation and as a food source for herbivores as vascular plants become smaller (Longton, 1984; Heggberget et al., 2002; Wirtz et al., 2003; Nash, 2008; Rees et al., 2008; Rai et al., 2014). Thus, lichens are a very important part of high-altitude/latitude ecosystems but, despite this, the majority of climate change studies to date focus on vascular plants (Alatalo and Totland, 1997; Arft et al., 1999; Dumais et al., 2014; Wheeler et al., 2016; Zhang and Wang, 2016). Long-term studies have shown that vascular plants have increased in abundance in response to warming (Sturm et al., 2001; Myers-Smith et al., 2011; Hobbie et al., 2017). Most studies on lichens lack information about species-level responses, and only a few incorporate species-level data to study the impact on different species or on lichen diversity and richness (Molau and Alatalo, 1998; Klanderud and Totland, 2005; Lang et al., 2012; Alatalo et al., 2014a, 2015a). However, studies on lichens are currently being initiated worldwide to study potential impacts of climate change and other anthropogenic disturbances on lichen communities (Maphangwa et al., 2012; Rai et al., 2012a, b; Darnajoux et al., 2015; Shukla et al., 2015; Upreti et al., 2015; Piercey-Normore et al., 2016). Existing long-term studies (9–20 years) show that lichen biomass and/or cover is sensitive to long-term warming at alpine and arctic sites (Chapin et al., 1995; Van Wijk et al., 2003; Elmendorf et al., 2012; Lang et al., 2012; Sistla et al., 2013), while shorter term studies (2–7 years) report contrasting results (Press et al., 1998; Klanderud and Totland, 2005; Biasi et al., 2008; Alatalo et al., 2014a, 2015a). Lichen diversity has also been shown to decrease due to warming in arctic Alaska (16 years of warming) and sub-arctic Sweden (9 years of warming) (Lang et al., 2012). Furthermore, the response may be context dependent, depending on potential competition with vascular plants (Alatalo, 1998; Cornelissen et al., 2001). Modelling studies on the potential impact of climate change on lichens suggest that many lichen species are potentially threatened (Allen and Lendemer, 2016; Nascimbene et al., 2016; Rubio-Salcedo et al., 2017).
Here we examined lichen communities following 18 years of experimental warming in two contrasting alpine sub-arctic plant communities (mesic meadow and dry, poor heath) in Sweden. The hypotheses tested were that (1) lichen cover and diversity are negatively affected (decreasing) by long-term warming; (2) the negative impacts of warming are greater for a meadow community with a more developed vascular plant community (67 % canopy cover) than a poor heath with a less developed vascular plant community (54 % canopy cover); and (3) more species are lost than gained owing to long-term warming.
MATERIALS AND METHODS
Study area
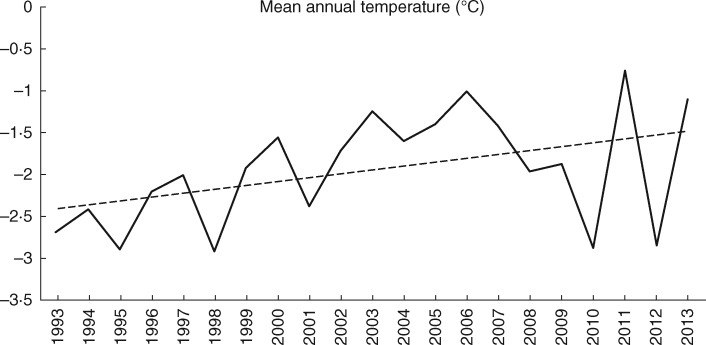
Fieldwork took place at Latnjajaure field station, which is located in the Latnjavagge valley (68°21′N, 18°29′E; 1000 m above sea level) in northern Sweden. Climate parameters were measured daily from early spring 1992 onwards (by data loggers that collect hourly data on temperature, and by a manned climate station during summers). The climate at the site is classified as sub-arctic (Polunin, 1951), with snow cover for most of the year, cool summers and relatively mild, snow-rich winters. The growing season starts in late May and ends in early September (Molau et al., 2005). Mean annual air temperature ranged from -0·76 to -2·92 °C between 1993 and 2013 (Fig. 1; Supplementary Data Fig. S1). The mean temperature was highest in July, with mean temperature ranging from 5·9 °C in 1995 to 13·1 °C in 2013 (Fig. S1). Mean annual precipitation during that period was 846 mm, but in individual years it ranged from a low of 607 mm (1996) to a high of 1091 mm (2003) (Fig. S1). Climate data are collected throughout the year at the weather station at Latnjajaure field station, with hourly means, maxima and minima. Physical conditions in the soils in the valley vary from dry to wet and poor and acidic to base rich, with an associated variation in plant communities (Molau and Alatalo, 1998; Lindblad et al., 2006; Björk et al., 2007; Alatalo et al., 2014b). The mesic meadow community is dominated by Carex vaginata, C. bigelowii, Festuca ovina, Salix reticulata, S. polaris, Cassiope tetragona, Polygonum viviparum and Thalictrum alpinum (Molau and Alatalo, 1998; Alatalo et al., 2014b). The more sparsely vegetated poor heath community is dominated by Betula nana, S. herbacea and Calamagrostis lapponica (Molau and Alatalo, 1998; Alatalo et al., 2015b).
Fig. 1.
Mean annual temperature (°C), 1993–2013, at Latnjajaure, Northern Sweden. Solid line, mean annual temperature; dotted line, trend line.
Experimental design and measurements
In July 1995, 1 × 1 m plots with homogeneous vegetation cover were marked out in an alpine mesic meadow plant community and in a heath plant community, and randomly assigned to treatments (control and warming) in a factorial design. At the start of the experiment there were eight control (CTR) plots and four plots with warming in each plant community (a total of 12 in each plant community). However, as we could not identify all initial control plots in 2013, in that year we only made measurements in four control and four warming plots in each community. Warming was applied by open top chambers (OTCs), and we monitored the temperature in control and plots with OTCs in the initial years with Delta™ and Tinytag™ loggers (Molau and Alatalo, 1998). As found in other studies (Marion et al., 1997; Molau and Alatalo, 1998; Hollister and Webber, 2000), OTCs increased the air temperature by 1·5–3 °C compared with control plots with ambient temperature. OTCs have also been shown to decrease canopy moisture (Hollister and Webber, 2000), causing earlier snow melt and prolonging the growing season (Molau and Alatalo, 1998; Hollister and Webber, 2000). The OTCs were then left on plots with warming treatment all year around. The majority of lichens in the plots were identified to species level. When necessary, we collected a specimen of the same species outside the experimental plots to be determined in the laboratory. In the case of Cladonia, when we were not able to determine the specimen to species level, we labelled it Cladonia spp. Coverage of each species was assessed by point-intercept using a 1 ×1 m frame with 100 grid points (Walker, 1996) in the peak of the 1995, 1999, 2001 and 2013 growing seasons. Due to their hexagonal shape, the OTCs reduced the number of points per plot to 77–87, and thus warmed plots had fewer pin-point intercepts than control plots.
Statistical analyses
The following community parameters were calculated for comparison of the lichen assemblages: cover, species richness, Shannon’s evenness and effective number of species (expH = exponential of Shannon entropy), which is the number of equally abundant species needed for the average proportional abundance of the species to equal that observed in the data set (where all species may not be equally abundant) (Jost, 2006). For vascular plants, we calculated total canopy cover. All data were checked for normality assumptions and homogeneity of variance by the Kolmogorov–Smirnov test and Levene’s test of equality of error variances, respectively. We then applied a univariate analysis of variance (ANOVA) with treatment (control or warming) and vegetation type (meadow or heath) as fixed factors, and the ratio of the value in 2013 to the value in 1995 (relative change of the community parameters mentioned above) on each plot as the response variable. The use of the ratio as the response variable was due to the fact that the number of hits per plot differed between treatments, and that plots differed in their starting values of cover, richness and species composition. Thus we opted to analyse relative changes between 1995 and 2013 instead of actual numbers. The ANOVA included the initial value in 1995 of each variable as covariate, which may potentially have affected the response variable. To check for the effect of time on cover, species richness, effective number of species and Shannon’s evenness, we applied a repeated measurement ANOVA on relative changes between 1995 and 1999, 1995 and 2001, and 1995 and 2013. As the species-level data did not meet the assumption of normal distribution after transformation, we used the conservative non-parametric Mann–Whitney U-test to analyse the effect of treatment on the relative change between 1995 and 2013 of the most common species. Only species with >100 pin-point intercept hits in total from the years 1995, 1999, 2001 and 2013 were included (Alatalo et al., 2015a). Two-tailed Pearson correlation was used to analyse correlations between lichen community parameters (total lichen cover, species richness, Shannon’s evenness and effective number of species) and vascular plant canopy cover. All analyses were performed in IBM© SPSS© Statistics Version 23.0.0.2.
Results
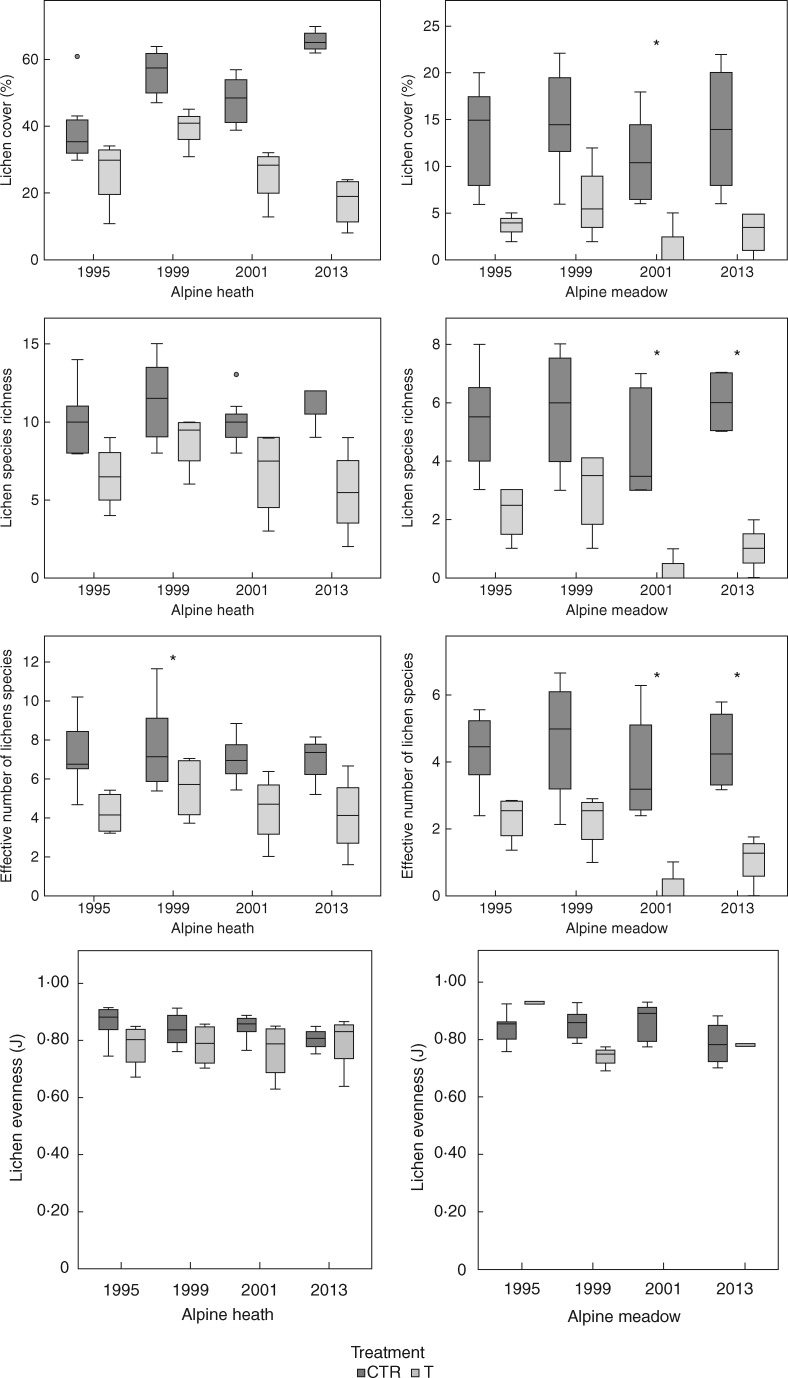
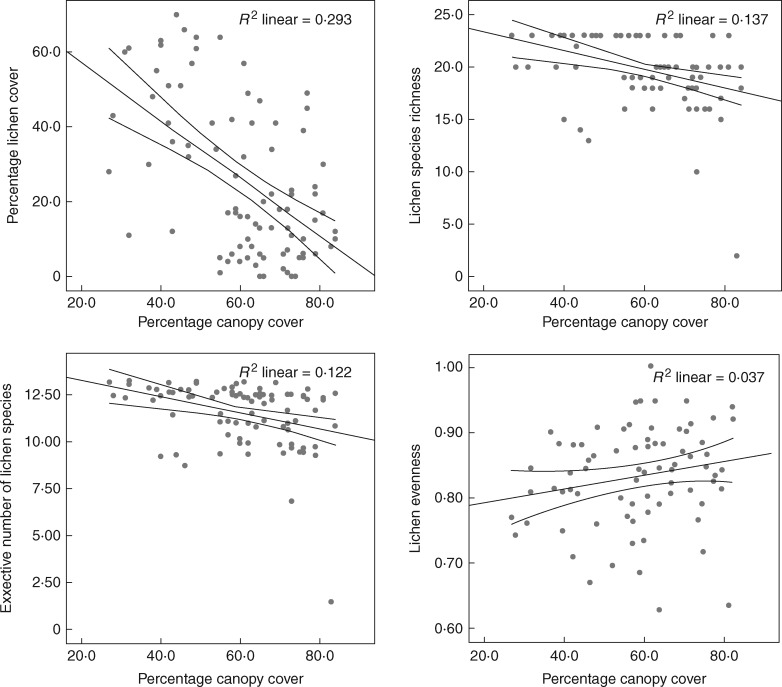
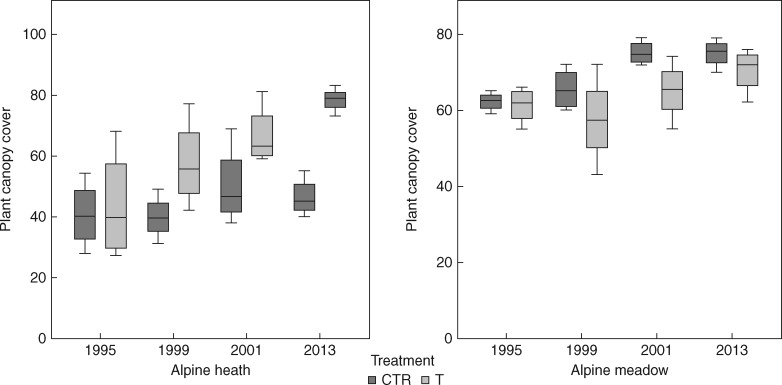
Initial lichen cover in the heath and meadow control plots was 35·75 % (s.d. ±5·44) and 11·75 % (±5·56), respectively. There was a significant effect of time on lichen total cover (P < 0·001), species richness (P = 0·009) and effective number of species (P = 0·004), but not on Shannon’s evenness (P = 0·3160). Cover of lichens increased by 87 % over the 18 year study period in the heath control plots. Over the same period, lichen cover in control plots in the meadow increased by 21 %. Eighteen years of experimental warming and site both had significant effects on cover, species richness, effective number of species and evenness of lichens (Fig. 2; Table 1). Lichen cover decreased significantly in response to the long-term warming in both heath and meadow communities (P < 0·001); there was an 8 % decline in lichen cover in the heath and a 31 % decline in the meadow compared with the starting year. A total of 22 lichen species were recorded in the study plots during the course of the study, with 19 in 1995 and 16 in 2013 (three new species recorded and six species lost in 2013) (Table 2). Lichen richness and, effective number of species, and evenness declined significantly (P < 0·001, P < 0·014, P < 0·035, respectively) in response to long-term warming, an effect mainly driven by a decline in the meadow, while species richness and effective number of species both remained more stable in the heath community (Fig. 2). Total lichen cover (-0·542, P < 0·001), species richness (-0·370, P < 0·001), effective number of species (-0·350, P = 0·001), but not Shannon's evenness (0·070, P = 0·545), were negatively correlated with vascular plant canopy cover (Fig. 3). Eighteen years of experimental warming and site both had significant effects on plant canopy cover (Table 3); warming having a positive impact on plant cover, with the largest increase found in the heath (Fig. 4).
Fig. 2.
Boxplots of changes in lichens during an 18 year period (1995–2013) of experimental warming in an alpine heath and an alpine meadow community at Latnjajaure, sub-arctic Sweden. Total cover of lichens (percentage), species richness of lichens, effective number of lichen species (exponential of Shannon’s entropy) and Shannon evenness. Boxplots show the 10th–90th percentile of the data. Treatments: CTR, control; T, temperature warming. Number of plots: n = 4 for CTR and T at each site. Asterisks (*) indicate significant differences in relative changes between treatments.
Table 1.
Result of univariate ANOVAs testing the effects of treatment (18 years of experimental warming) and site (alpine meadow and alpine heath) on relative change in: cover, species richness, effective number of species and evenness for lichens
| Source | Type III sum of squares | d.f. | Mean square | F | P-value |
|---|---|---|---|---|---|
| Relative change in cover | |||||
| Initial cover | 1·900 | 1 | 1·900 | 9·815 | 0·010 |
| Treatment | 4·050 | 1 | 4·050 | 20·926 | <0·001 |
| Site | 2·652 | 1 | 2·652 | 13·703 | 0·003 |
| Treatment × Site | 0·269 | 1 | 0·269 | 1·388 | 0·264 |
| Total | 29·178 | 16 | |||
| R2 = 0·704 (Adjusted R2 = 0·596) | |||||
| Relative change in species richness | |||||
| Initial richness | 0·885 | 1 | 0·885 | 8·919 | 0·012 |
| Treatment | 2·142 | 1 | 2·142 | 21·580 | <0·001 |
| Site | 1·088 | 1 | 1·088 | 10·963 | 0·007 |
| Treatment × Site | 0·259 | 1 | 0·259 | 2·613 | 0·134 |
| Total | 17·714 | 16 | |||
| R2 = 0·716 (Adjusted R2 = 0·613) | |||||
| Relative change in effective number of species | |||||
| Initial effective no. sp. | 0·839 | 1 | 0·839 | 5·737 | 0·036 |
| Treatment | 1·256 | 1 | 1·256 | 8·594 | 0·014 |
| Site | 1·196 | 1 | 1·196 | 8·180 | 0·016 |
| Treatment × Site | 0·206 | 1 | 0·206 | 1·411 | 0·260 |
| Total | 17·035 | 16 | |||
| R2 = 0·579 (Adjusted R2 = 0·425) | |||||
| Relative change in evenness | |||||
| Initial evenness | 0·004 | 1 | 0·004 | 0·060 | 0·811 |
| Treatment | 0·397 | 1 | 0·397 | 5·750 | 0·035 |
| Site | 0·677 | 1 | 0·6777 | 9·807 | 0·010 |
| Treatment × Site | 0·660 | 1 | 0·660 | 9·566 | 0·010 |
| Total | 12·352 | 16 | |||
| R2 = 0·700 (Adjusted R2 = 0·591) | |||||
D.f., degrees of freedom; F, F-statistics; P-value, significance level.
Table 2.
List of lichens recorded in the heath (H) and meadow (M) alpine vegetation communities at Latnjajaure, sub-arctic Sweden
| Lichen species | 1995 | 1999 | 2001 | 2013 |
| Alectoria nigricans | H | H | H | Not recorded |
| Alectoria ochroleuca | H | H | H | H |
| Cetrariella delisei | H, M | H, M | H, M | H, M |
| Cladina arbuscula | H, M | H, M | H, M | H, M |
| Cladina rangiferina | H | Not recorded | Not recorded | Not recorded |
| Cladonia spp. | H, M | H, M | H, M | H |
| Cladonia furcata | H | H, M | H | Not recorded |
| Cladonia gracilis | Not recorded | H | H | H, M |
| Cladonia pyxidata | Not recorded | Not recorded | Not recorded | H |
| Cladonia uncialis | H, M | H, M | H, M | H, M |
| Cornicularia divergens | H | H | H | Not recorded |
| Flavocetraria cucullata | H, M | H, M | H, M | H, M |
| Flavocetraria nivalis | H, M | H, M | H, M | H, M |
| Nephroma arctica | H, M | H, M | H, M | H |
| Ochrolechia frigida | H, M | H, M | M | Not recorded |
| Peltigera aphthosa | H, M | H, M | H, M | H, M |
| Peltigera scabrosa | H | H | H | H, M |
| Pertusaria dactylina | Not recorded | Not recorded | Not recorded | H |
| Solorina crocea | H | H | H | Not recorded |
| Sphaerophorus globosus | H, M | H, M | H, M | H, M |
| Stereocaulon alpinum | H | H | H | H |
| Thamnolia vermicularis | H, M | H, M | H, M | H, M |
Species in bold are the eight most common lichen species found.
Fig. 3.
Relationship between lichens and vascular plant canopy cover at Latnjajaure, sub-arctic Sweden: total cover of lichens, species richness of lichens, effective number of lichen species (exponential of Shannon’s entropy) and Shannon evenness. Number of plots: n = 88.
Table 3.
Result of univariate ANOVAs testing the effects of treatment (18 years of experimental warming) and site (alpine meadow and alpine heath) on relative change in vascular plant canopy cover
| Source | Type III sum of squares | d.f. | Mean square | F | P-value |
|---|---|---|---|---|---|
| Relative change in cover | |||||
| Initial cover | 2·247 | 1 | 2·247 | 54·156 | <0·001 |
| Treatment | 0·698 | 1 | 0·698 | 16·831 | 0·002 |
| Site | 0·194 | 1 | 0·194 | 4·664 | 0·054 |
| Treatment × Site | 0·456 | 1 | 1·018 | 24·542 | <0·001 |
| Total | 36·623 | 16 | |||
| R2 = 0·906 (adjusted R2 = 0·872) | |||||
Fig. 4.
Boxplots of changes in vascular plant canopy cover during an 18 year period (1995–2013) of experimental warming in an alpine heath and an alpine meadow community at Latnjajaure, sub-arctic Sweden. Boxplots show the 10th–90th percentile of the data. Treatments: CTR, control; T, temperature warming. Number of plots: n = 4 for CTR and T at each site.
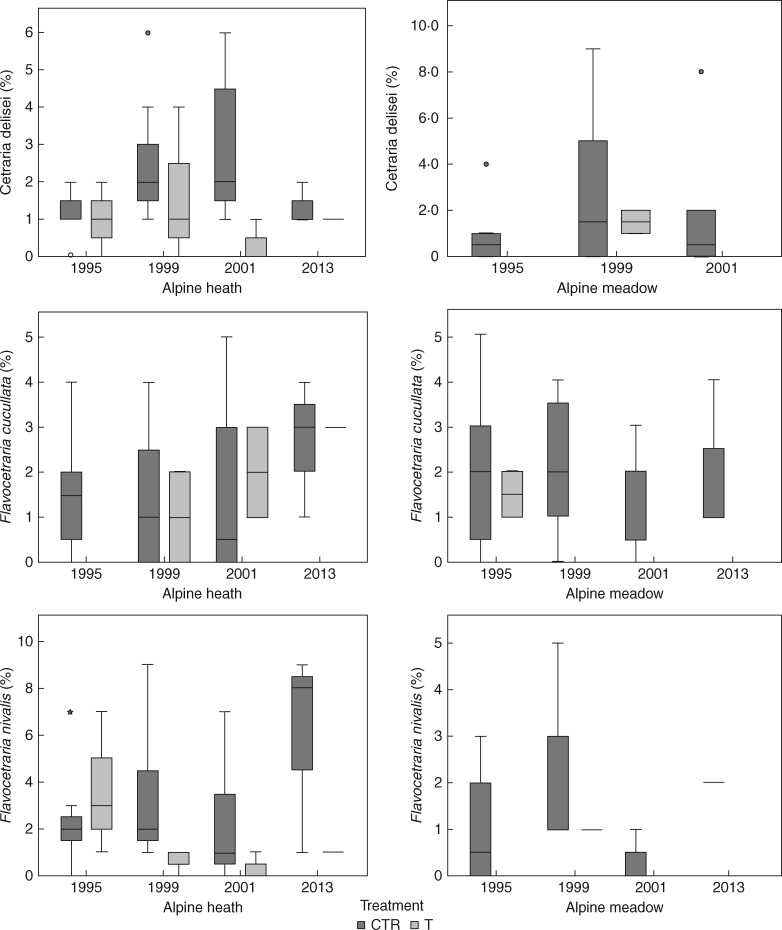
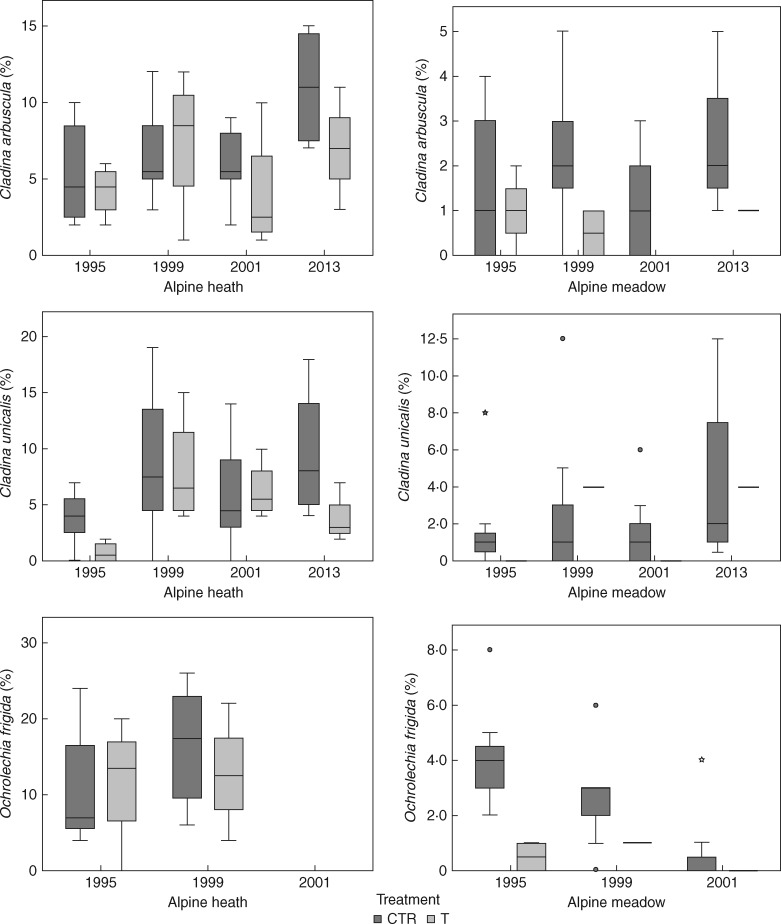
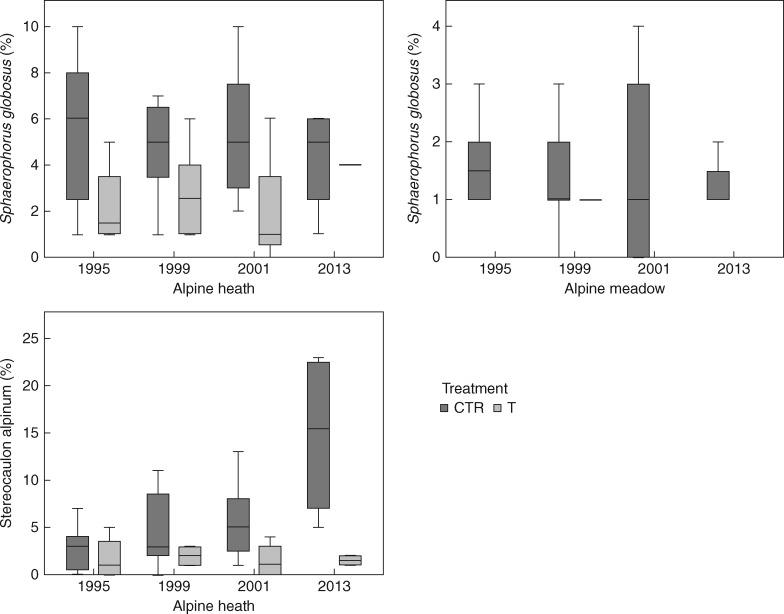
Of the eight most common lichen species, there was a significant negative effect of long-term warming on cover of one species, Flavocetraria cucullata (P = 0·029), in the heath, but not in the meadow (P = 1). For the other seven species, Cetrariella delisei (P = 0·114, P = 1), Flavocetraria nivalis (P = 0·114, P = 0·686), Cladina arbuscula (P = 0·343, P = 0·486), Cladonia unicalis (P = 0·114, P = 0·114), Ochrolechia frigida (P = 1, P = 1), Sphaerophorus globosus (P = 0·20, P = 0·114) and Stereocaulon alpinum (P = 0·114, P = 1), there were no significant effects on cover in either the heath or the meadow (Fig. 5).
Fig. 5.
Boxplots of species-specific responses of lichen cover (percentage) during an 18 year period (1995–2013) of experimental warming in a poor alpine heath and a rich alpine meadow community at Latnjajaure, sub-arctic Sweden. Boxplots show the 10th–90th percentile of the data. Treatments: CTR, control, T, temperature warming. Number of plots: n = 4 for CTR and T at each site.
Discussion
The results from this long-term experiment confirm predictions that lichens may be less vulnerable in plant communities with less developed plant cover than in communities with more developed plant cover (Alatalo, 1998; Cornelissen et al., 2001). As hypothesized, lichens were more vulnerable in the meadow with its more developed plant canopy cover. We found that all lichen community parameters (total lichen cover, species richness, effective number of species and Shannon’s evenness) were negatively correlated with plant canopy cover. Similar to other studies (Sturm et al., 2001; Myers-Smith et al., 2011; Harte et al., 2015; Hobbie et al., 2017), we found that long-term warming caused a significant increase in total plant canopy cover, with canopy expanding most in the heath. As both lichens (this study) and bryophytes (Jägerbrand et al., 2012) have been shown to be negatively correlated with vascular plant canopy, a future increase in vascular plant canopy is therefore likely to have a detrimental effect on these groups. The differences in canopy cover of vascular plant communities may thus help to explain the significant site effect on all response variables. The negative impact on diversity is in line with previous long-term studies in Alaska showing a decrease in lichen diversity under 16 years of warming (Lang et al., 2012). This highlights the importance of long-term studies, as shorter term studies have tended to find contrasting effects on lichens (Klanderud and Totland, 2005; Alatalo et al., 2014a), while longer term studies have typically found negative effects (Chapin et al., 1995; Van Wijk et al., 2003; Elmendorf et al., 2012; Lang et al., 2012; Sistla et al., 2013).
The responses to long-term warming differed between the two plant communities studied, with the meadow experiencing a larger decline in lichen cover relative to the initial year of the study than the heath community (Fig. 2). A similar pattern was found for richness and effective number of species, which declined in the meadow but not in the heath. This shows that both abiotic and biotic interactions may play important roles in lichen responses and reveals the necessity of including different plant communities in experimental studies. In our study, of the eight most common species, we only found a significant negative impact on one species, F. cucullata, in the heath, but not in the meadow. However, while there were no significant effects on the other species, there was a general tendency for lower abundance of the most common species in the plots experiencing long-term warming. Thus, it is likely that the increase in evenness observed in our long-term study may have been caused by an overall decline in dominant lichen species over the study period.
The long-term data from our control plots also indicate that bare ground in high alpine areas that has been ice free for a long period (since the retreat of glaciers) can continue to be colonized by lichens, similarly to the plant progression that occurs when glaciers retreat (Raffl and Erschbamer, 2004; Bilovitz et al., 2015a, 2015b).
In fact, Latnjajaure experienced a natural increase in mean annual temperature of about 2 °C between 1995 and 2013. Thus, the lichens in control plots were also exposed to natural climate warming, which may have contributed to the positive effect on lichen cover seen in control plots. It is likely that the main driver for the significant difference between treatments was the increase in lichen cover in the control plots, not the decrease in lichen cover in long-term warming plots. Thus, while the lichens within the warming plots declined less over the study period in the heath compared with the meadow community, the difference compared with the control plots was larger in the heath than in the meadow community. Another potential explanation for the large difference in increase in cover may be differences in initial levels of bare ground and vascular plant cover between the plant communities, with the meadow having less bare ground and a more developed initial plant cover (Molau and Alatalo, 1998). Thus, the heath both offered more bare ground to be colonized by lichens and experienced less competition from vascular plants. The fact that lichen cover, species richness, and effective number of species were all negatively correlated to plant canopy cover indicates that lichens were most probaby outcompeted, or overgrown, by vascular plants.
It should be noted that a constant level of warming is not the most realistic scenario for future climate change, which will most probably increase both the variability and magnitude of climate events (Stocker et al., 2013). For example, increased precipitation during winter could potentially increase snow accumulation, and this in turn may reduce survival and growth of dominant arctic–alpine lichens (Bidussi et al., 2016). However, if an increase in precipitation during winter is accompanied by an earlier onset of spring due to warmer climate, this may be counterbalanced. While we did not measure precipitation or snow accumulation in our study, the experimental long-term warming caused a loss of soil carbon, nitrogen, C/N ratio and soil moisture in the mineral soil layer of the meadow (Alatalo et al., 2017). However, the warming did not have any effect on the soil parameters in the thin organic soil layers in the meadow or heath (Alatalo et al., 2017). As lichens do not have a well-developed root system, drying of deeper soil layers is therefore unlikely to have had a direct effect on lichens. However, OTCs have been shown to decrease moisture in the canopy layer (Hollister and Webber, 2000), which may potentially have had a negative effect on lichens that depend on moisture levels for their photosynthesis and growth. There are very few experimental studies that examine different warming scenarios across years (Jonasson et al., 1999; Alatalo et al., 2014a, 2016), and none is long term. The one existing short-term study (3 years) applying different climate change scenarios to lichens in an alpine meadow found the lichens to be highly resistant to a constant level of warming, a stepwise increase in warming and a single season of high-level pulse warming (Alatalo et al., 2014a). However, as the short-term and longer term responses of lichens have been shown to differ (Alatalo et al., 2015a), there is a need to initiate long-term experiments that incorporate different warming and precipitation scenarios.
Overall, 18 years of experimental warming brought a significant decline in lichen cover, species richness and, effective number of species, and evenness. The results showed that lichen responses are most probably dependent on both biotic and abiotic interactions and that responses differ among communities. Specifically, lichens increased more under ambient conditions in heath with a less developed plant community and decreased more under experimental warming in meadow with a more developed plant community. Species richness and effective number of species remained stable in the heath, but decreased under experimental warming in the meadow. Lichen cover, species richness, effective number of species and evenness were all negatively correlated with plant canopy cover.
Supplementary Data
Supplementary data are available online at https://academic.oup.com/aob and consist of Figure S1: mean, minimum and maximum monthly temperatures (°C), and monthly precipitation (mm) January 1993–December 2013, at Latnjajaure, Northern Sweden.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the staff of Abisko Scientific Research Station for their help and hospitality, and Matthias Molau for assistance in the field. This study was supported by Carl Tryggers stiftelse för vetenskaplig forskning through a grant to J.M.A. The authors thank J. S. Heslop-Harrison, Anne Brysting and two anonymous reviewers whose comments improved the manuscript.
AUTHOR CONTRIBUTIONS
J.M.A and U.M. designed the experiment, J.M.A. and U.M. carried out fieldwork. J.M.A., A.K.J. and S.C. carried out data analyses, J.M.A. and A.K.J. made the figures, and J.M.A. made the tables. J.M.A. drafted the manuscript. All authors read, commented on and approved the final manuscript. The authors declare no competing financial interests.
LITERATURE CITED
- Alatalo J. 1998. Climate change: impacts on structure and biodiversity of subarctic plant communities. PhD Thesis, Göteborg University, Sweden.
- Alatalo JM, Totland Ø.. 1997. Response to simulated climatic change in an alpine and subarctic pollen-risk strategist, Silene acaulis. Global Change Biology 3: 74–79. [Google Scholar]
- Alatalo JM, Jägerbrand AK, Molau U.. 2014a. Climate change and climatic events: community-, functional- and species-level responses of bryophytes and lichens to constant, stepwise, and pulse experimental warming in an alpine tundra. Alpine Botany 124: 81–91. [Google Scholar]
- Alatalo JM, Little CJ, Jägerbrand AK, Molau U.. 2014b. Dominance hierarchies, diversity and species richness of vascular plants in an alpine meadow: contrasting short and medium term responses to simulated global change. PeerJ 2: e406. doi: 10.7717/peerj.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alatalo JM, Jägerbrand AK, Molau U.. 2015a. Testing reliability of short-term responses to predict longer-term responses of bryophytes and lichens to environmental change. Ecological Indicators 58: 77–85. [Google Scholar]
- Alatalo JM, Little CJ, Jägerbrand AK, Molau U.. 2015b. Vascular plant abundance and diversity in an alpine heath under observed and simulated global change. Scientific Reports 5: 10197. doi: 10.1038/srep10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alatalo JM, Jägerbrand AK, Molau U.. 2016. Impacts of different climate change regimes and extreme climatic events on an alpine meadow community. Scientific Reports 6: 21720. doi: 10.1038/srep21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alatalo JM, Jägerbrand AK, Juhanson J, Michelsen A, Luptáčik P.. 2017. Impacts of twenty years of experimental warming on soil carbon, nitrogen, moisture and soil mites across alpine/subarctic tundra communities. Scientific Reports 7: 44489. doi: 10.1038/srep44489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JL, Lendemer JC.. 2016. Climate change impacts on endemic, high-elevation lichens in a biodiversity hotspot. Biodiversity and Conservation 25: 555–568. [Google Scholar]
- Arft AM, Walker MDM, Gurevitch J, et al. 1999. Responses of tundra plants to experimental warming: meta-analysis of the international tundra experiment. Ecological Monographs 69: 491–511. [Google Scholar]
- Biasi C, Meyer H, Rusalimova O, et al. 2008. Initial effects of experimental warming on carbon exchange rates, plant growth and microbial dynamics of a lichen-rich dwarf shrub tundra in Siberia. Plant and Soil 307: 191–205. [Google Scholar]
- Bidussi M, Solhaug KA, Gauslaa Y.. 2016. Increased snow accumulation reduces survival and growth in dominant mat-forming arctic-alpine lichens. The Lichenologist 48: 237–247. [Google Scholar]
- Bilovitz PO, Nascimbene J, Tutzer V, et al. 2014a. Terricolous lichens in the glacier forefield of the Rötkees (Eastern Alps, South Tyrol, Italy). Phyton; annales rei botanicae 54: 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilovitz PO, Wallner A, Tutzer V, et al. 2014b. Terricolous lichens in the glacier forefield of the Gaisbergferner (Eastern Alps, Tyrol, Austria). Phyton; annales rei botanicae 54: 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilovitz PO, Nascimbene J, Mayrhofer H.. 2015a. Terricolous lichens in the glacier forefield of the Morteratsch Glacier (Eastern Alps, Graubünden, Switzerland). Phyton; annales rei botanicae 55: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilovitz PO, Wallner A, Tutzer V, Nascimbene J, Mayrhofer H.. 2015b. Terricolous lichens in the glacier forefield of the Pasterze (Eastern Alps, Carinthia, Austria). Phyton; annales rei botanicae 55: 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk RG, Klemedtsson L, Molau U, Harndorf J, Ödman A, Giesler R.. 2007. Linkages between N turnover and plant community structure in a tundra landscape. Plant and Soil 294: 247–261. [Google Scholar]
- Callaghan T, Jonasson C, Thierfelder T, et al. 2013. Ecosystem change and stability over multiple decades in the Swedish subarctic: complex processes and multiple drivers. Philosophical Transactions of the Royal Society B: Biological Sciences 368: 20120488. doi: 10.1098/rstb.2012.0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capers R, Stone A.. 2011. After 33 years, trees more frequent and shrubs more abundant in northeast US alpine community. Arctic, Antarctic, and Alpine Research 43: 495–502. [Google Scholar]
- Chapin F III, Shaver G, Giblin A, Nadelhoffer K, Laundre J.. 1995. Responses of arctic tundra to experimental and observed changes in climate. Ecology 76: 694–711. [Google Scholar]
- Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD.. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333: 1024–1026. [DOI] [PubMed] [Google Scholar]
- Colwell RK, Brehm G, Cardelús CL, Gilman AC, Longino JT.. 2008. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 322: 258–261. [DOI] [PubMed] [Google Scholar]
- Cornelissen JHC, Callaghan TV, Alatalo JM, et al. 2001. Global change and arctic ecosystems: is lichen decline a function of increases in vascular plant biomass? Journal of Ecology 89: 984–994. [Google Scholar]
- Darnajoux R, Lutzoni F, Miadlikowska J, Bellenger J-P.. 2015. Determination of elemental baseline using peltigeralean lichens from Northeastern Canada (Québec): Initial data collection for long term monitoring of the impact of global climate change on boreal and subarctic area in Canada. Science of the Total Environment 533: 1–7. [DOI] [PubMed] [Google Scholar]
- Dirnböck T, Essl F, Rabitsch W.. 2011. Disproportional risk for habitat loss of high-altitude endemic species under climate change. Global Change Biology 17: 990–996. [Google Scholar]
- Dumais C, Ropars P, Denis M-P, Dufour-Tremblay G, Boudreau S.. 2014. Are low altitude alpine tundra ecosystems under threat? A case study from the Parc National de la Gaspésie, Québec. Environmental Research Letters 9: 94001. [Google Scholar]
- Elmendorf S, Henry G, Hollister R, et al. 2012. Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecology Letters 15: 164–175. [DOI] [PubMed] [Google Scholar]
- Engler R, Randin CF, Thuiller W, et al. 2011. 21st century climate change threatens mountain flora unequally across Europe. Global Change Biology 17: 2330–2341. [Google Scholar]
- Erschbamer B, Unterluggauer P, Winkler E, Mallaun M.. 2011. Changes in plant species diversity revealed by long-term monitoring on mountain summits in the Dolomites (northern Italy). Preslia 83: 387–401. [Google Scholar]
- Groom QJ. 2013. Some poleward movement of British native vascular plants is occurring, but the fingerprint of climate change is not evident. PeerJ 1: e77. doi: 10.7717/peerj.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao R, Yu D, Liu Y, et al. 2017. Impacts of changes in climate and landscape pattern on ecosystem services. Science of the Total Environment 579: 718–728. [DOI] [PubMed] [Google Scholar]
- Harte J, Saleska SR, Levy C.. 2015. Convergent ecosystem responses to 23-year ambient and manipulated warming link advancing snowmelt and shrub encroachment to transient and long-term climate–soil carbon feedback. Global Change Biology 21: 2349–2356. [DOI] [PubMed] [Google Scholar]
- Heggberget TM, Gaare E, Ball JP.. 2002. Reindeer (Rangifer tarandus) and climate change: importance of winter forage. Rangifer 22: 13–31. [Google Scholar]
- Hobbie JE, Shaver GR, Rastetter EB, et al. 2017. Ecosystem responses to climate change at a Low Arctic and a High Arctic long-term research site. Ambio 46: 160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister RD, Webber PJ.. 2000. Biotic validation of small open-top chambers in a tundra ecosystem. Global Change Biology 6: 835–842. [Google Scholar]
- Jägerbrand AK, Kudo G, Alatalo JM, Molau U.. 2012. Effects of neighboring vascular plants on the abundance of bryophytes in different vegetation types. Polar Science 6: 200–208. [Google Scholar]
- Jonasson S, Michelsen A, Schmidt I, Nielsen E.. 1999. Responses in microbes and plants to changed temperature, nutrient, and light regimes in the Arctic. Ecology 80: 1828–1843. [Google Scholar]
- Jost L. 2006. Entropy and diversity. Oikos 113: 363–375. [Google Scholar]
- Klanderud K, Totland Ø.. 2005. Simulated climate change altered dominance hierarchies and diversity of an alpine biodiversity hotspot. Ecology 86: 2047–2054. [Google Scholar]
- Lang SI, Cornelissen JHC, Shaver GR, et al. 2012. Arctic warming on two continents has consistent negative effects on lichen diversity and mixed effects on bryophyte diversity. Global Change Biology 18: 1096–1107. [Google Scholar]
- Lindblad KEM, Nyberg R, Molau U.. 2006. Generalization of heterogeneous alpine vegetation in air photo-based image classification, Latnjajaure catchment, northern Sweden. Pirineos 161: 74–79. [Google Scholar]
- Longton R. 1984. The role of bryophytes in terrestrial ecosystems. Journal of the Hattori Botanical Laboratory 55: 147–163. [Google Scholar]
- Mack MC, Schuur EAG, Bret-Harte MS, Shaver GR, Chapin FS.. 2004. Ecosystem carbon storage in arctic tundra reduced by long-term nutrient fertilization. Nature 431: 440–443. [DOI] [PubMed] [Google Scholar]
- Maphangwa KW, Musil CF, Raitt L, Zedda L.. 2012. Experimental climate warming decreases photosynthetic efficiency of lichens in an arid South African ecosystem. Oecologia 169: 257–268. [DOI] [PubMed] [Google Scholar]
- Marion G, Henry GHR, Freckman DW, et al. 1997. Open-top designs for manipulating field temperature in high-latitude ecosystems. Global Change Biology 3: 20–32. [Google Scholar]
- Molau U, Alatalo JM.. 1998. Responses of subarctic–alpine plant communities to simulated environmental change: biodiversity of bryophytes, lichens, and vascular plants. Ambio 27: 322–329. [Google Scholar]
- Molau U, Nordenhäll U, Eriksen B.. 2005. Onset of flowering and climate variability in an alpine landscape: a 10-year study from Swedish Lapland. American Journal of Botany 92: 422–31. [DOI] [PubMed] [Google Scholar]
- Myers-Smith IH, Forbes BC, Wilmking M, et al. 2011. Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environmental Research Letters 6: 45509. doi: 10.1088/1748–9326/6/4/045509. [Google Scholar]
- Nascimbene J, Casazza G, Benesperi R, et al. 2016. Climate change fosters the decline of epiphytic Lobaria species in Italy. Biological Conservation 201: 377–384. [Google Scholar]
- Nash TH., III 2008. Nitrogen, its metabolism and potential contribution to ecosystems In: Lichen biology. Cambridge University Press: Cambridge, 216–233. [Google Scholar]
- Piercey-Normore MD, Brodo IM, Deduke C.. 2016. Lichens on the Hudson Bay Lowlands: a long-term survey in Wapusk National Park, Manitoba. The Lichenologist 48: 581–592. [Google Scholar]
- Polunin N. 1951. The real arctic: suggestions for its delimitation, subdivision, and characterization. Journal of Ecology 39: 308–315. [Google Scholar]
- Press M, Potter J, Burke M, Callaghan T, Lee J.. 1998. Responses of a subarctic dwarf shrub heath community to simulated environmental change. Journal of Ecology 86: 315–327. [Google Scholar]
- Raffl C, Erschbamer B.. 2004. Comparative vegetation analyses of two transects crossing a characteristic glacier valley in the Central Alps. Phytocoenologia 34: 225–240. [Google Scholar]
- Rai H, Khare R, Gupta RK, Upreti DK.. 2012a. Terricolous lichens as indicator of anthropogenic disturbances in a high altitude grassland in Garhwal (Western Himalaya), India. Botanica Orientalis: Journal of Plant Science 8: 16–23. [Google Scholar]
- Rai H, Upreti DK, Gupta RK.. 2012b. Diversity and distribution of terricolous lichens as indicator of habitat heterogeneity and grazing induced trampling in a temperate-alpine shrub and meadow. Biodiversity and Conservation 21: 97–113. [Google Scholar]
- Rai H, Khare R, Upreti DK.. 2014. Lichenological studies in India with reference to terricolous lichens In: Rai H, Upreti FK, eds. Terricolous lichens in India. Berlin: Springer; 1–20. [Google Scholar]
- Raxworthy CJ, Pearson RG, Rabibisoa N, et al. 2008. Extinction vulnerability of tropical montane endemism from warming and upslope displacement: a preliminary appraisal for the highest massif in Madagascar. Global Change Biology 14: 1703–1720. [Google Scholar]
- Rees WG, Stammler FM, Danks FS, Vitebsky P.. 2008. Vulnerability of European reindeer husbandry to global change. Climatic Change 87: 199–217. [Google Scholar]
- Rubio-Salcedo M, Psomas A, Prieto M, Zimmermann NE, Martínez I.. 2017. Case study of the implications of climate change for lichen diversity and distributions. Biodiversity and Conservation 26: 1121. doi:10.1007/s10531-016-1289-1. [Google Scholar]
- Shen Z, Ma K.. 2014. Effects of climate change on biodiversity. Chinese Science Bulletin 59: 4637–4638. [Google Scholar]
- Shukla P, Bajpai R, Singh CP, Sharma N, Upreti DK.. 2015. Lichen diversity in alpine regions of eastern Sikkim with respect to long term monitoring programme of Indian Space Research Organization. Geophytology 45: 57–62. [Google Scholar]
- Sistla SA, Moore JC, Simpson RT, Gough L, Shaver GR, Schimel JP.. 2013. Long-term warming restructures Arctic tundra without changing net soil carbon storage. Nature 497: 615–618. [DOI] [PubMed] [Google Scholar]
- Stocker TF, Qin D, Plattner GK.. 2013. Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge. [Google Scholar]
- Sturm M, Racine C, Tape K.. 2001. Climate change: increasing shrub abundance in the Arctic. Nature 411: 546–547. [DOI] [PubMed] [Google Scholar]
- Upreti DK, Bajpai R, Nayaka S.. 2015. Lichenology: current research in India In: Bahadur B, Rajam MV, Shijram L, Krishnamurthy KV, eds. Plant biology and biotechnology. Berlin: Springer; 263–280. [Google Scholar]
- Van Wijk MT, Clemmensen KE, Shaver GR, et al. 2003. Long-term ecosystem level experiments at Toolik Lake, Alaska, and at Abisko, Northern Sweden: generalizations and differences in ecosystem and plant type responses to global change. Global Change Biology 10: 105–123. [Google Scholar]
- Walker MD. 1996. Community baseline measurements for ITEX studies In: Molau U, Miolgaard P, eds. ITEX Manual, 2nd edn Copenhagen, Denmark: Danish Polar Centre, 39–41. [Google Scholar]
- Wheeler JA, Cortés AJ, Sedlacek J, et al. 2016. The snow and the willows: earlier spring snowmelt reduces performance in the low-lying alpine shrub Salix herbacea. Journal of Ecology 104: 1041–1050. [Google Scholar]
- Wirtz N, Lumbsch HT, Green TG, et al. 2003. Lichen fungi have low cyanobiont selectivity in maritime Antarctica. New Phytologist 160: 177–183. [DOI] [PubMed] [Google Scholar]
- Wu X, Lin X, Zhang Y, Gao J, Guo L, Li J.. 2014. Impacts of climate change on ecosystem in Priority Areas of Biodiversity Conservation in China. Chinese Science Bulletin 59: 4668–4680. [Google Scholar]
- Zhang Y, Wang W.. 2016. Interactions between warming and soil moisture increase overlap in reproductive phenology among species in an alpine meadow. Biology Letters 12: 20150749. doi: 10.1098/rsbl.2015.0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Zhang M, Ma K.. 2014. Climate change threats to protected plants of China: an evaluation based on species distribution modeling. Chinese Science Bulletin 59: 4652–4659. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.