Abstract
Background
Emerging data have established links between systemic metabolic dysfunction, such as diabetes and metabolic syndrome (MetS), with neurocognitive impairment, including dementia. The common gene signature and the associated signaling pathways of MetS, diabetes, and dementia have not been widely studied.
Material/Methods
We exploited the translational bioinformatics approach to choose the common gene signatures for both dementia and MetS. For this we employed “DisGeNET discovery platform”.
Results
Gene mining analysis revealed that a total of 173 genes (86 genes common to all three diseases) which comprised a proportion of 43% of the total genes associated with dementia. The gene enrichment analysis showed that these genes were involved in dysregulation in the neurological system (23.2%) and the central nervous system (20.8%) phenotype processes. The network analysis revealed APOE, APP, PARK2, CEPBP, PARP1, MT-CO2, CXCR4, IGFIR, CCR5, and PIK3CD as important nodes with significant interacting partners. The meta-regression analysis showed modest association of APOE with dementia and metabolic complications. The directionality of effects of the variants on Alzheimer disease is generally consistent with previous observations and did not differ by race/ethnicity (p>0.05), although our study had low power for this test.
Conclusions
Our novel approach showed APOE as a common gene signature with a link to dementia, MetS, and diabetes. Future gene association studies should focus on the association of gene polymorphisms with multiple disease models to identify novel putative drug targets.
MeSH Keywords: Alzheimer Disease; Apolipoproteins E; Metabolic Syndrome X; Polymorphism, Genetic
Background
Dementia is the leading cause of dependence and disability among elderly population worldwide [1]. Dementia is an umbrella term for an array of symptoms that are often associated with the cognitive decline of aging [2]. Alzheimer disease is a common cause of dementia causing as many as 50% to 70% of all dementia cases [3]. According to the latest World Alzheimer Report, the aging of the global population will make the economic impact of dementia far greater than that of other non-communicable diseases such as cancer, heart disease, and stroke combined. Globally, the number of people now living with dementia is expected to rise from the current 46 million to 131.5 million by 2050 [3]. Alzheimer disease is the leading cause of dementia in the elderly, causing slow irreversible loss of cognitive functions, which precedes dementia and death. According to the latest systematic review, the incidence of dementia was 9.87 cases per 1,000 person-years, Alzheimer disease was 6.25 cases per 1,000 person-years, vascular dementia was 2.42 cases per 1,000 person-years, and other rare forms of dementia was 0.46 cases per 1,000 person-years [4].
The increasing prevalence of dementia might be due to the parallel increase in other chronic diseases, such as diabetes and metabolic syndrome (MetS), in an aging population [5]. According to the latest estimates, China had more cases of dementia in 2010 than any other country in the world (9.19 million (range 5.92–12.48 million) [4]. MetS is a collection of biochemical and physiological abnormalities that are associated with the development of cardiovascular disease and type II diabetes [6]. Recent epidemiological and prospective studies have revealed an association between dementia and MetS due to the presence of common risk factors, including increasing age, obesity status, and environmental factors (sleep, diet, and physical inactivity and television viewing, etc.) [7–9]. Some studies found familial co-aggregation of diabetes and dementia [10]. These overlapping features suggest that dementia and MetS, especially diabetes mellitus, may share common genetic, epigenetic modifiers. Thus, we must take a step back in our understanding of shared molecular mechanisms between dementia and MetS. A landmark genetic meta-analysis study pinpointed the association of several genetic polymorphisms such as ACE, CHRNB2, CST3, ESR1, GAPDHS, IDE, MTHFR, NCSTN, PRNP, PSEN1, TF, TFAM, and TNF with Alzheimer disease [11]. A recent epidemiological study showed that the association between PICALM, BIN1, CLU, and MS4A4E variants and Alzheimer disease susceptibility in Han Chinese populations [12]. Most of these markers are also causative of insulin resistance and MetS. Therefore, we hypothesized that this common gene signature and its associated signaling pathways would not only reveal its shared molecular mechanism, but also would serve as a potential early biomarker for diagnosis of dementia.
Material and Methods
Identification of common gene signature
We exploited the translational bioinformatics approach to choose the common gene signatures for both dementia and MetS. For this we employed “DisGeNET discovery platform” [13] with the search terms “dementia”, “metabolic syndrome”, and “diabetes mellitus” to download the list of genes associated with the respective diseases. DisGeNET is a discovery platform integrating information on gene-disease associations (GDAs) from several public data sources and the literature pertaining to gene expression, biomarker, variant-disease association, single nucleotide polymorphisms, and clinical phenotype association with the corresponding diseases [13]. The current DisGeNET v4.0 contains about 429,036 associations, between 17,381 genes and 15,093 diseases, disorders, and clinical or abnormal human phenotypes, and 72,870 variant-disease associations, between 46,589 SNPs and 6,356 diseases and phenotypes. The GDAs were mined from MEDLINE via a NLP-based approach. Furthermore, the DisGeNET discovery platform allowed prioritizing of GDAs on the basis of the evidence supporting the data. The inbuilt statistical algorithm was used to score the association of genes with the disease (DisGeNet score). Venn diagram was plotted using online Jvenn [14] tool to visualize common gene signature.
Functional enrichment analysis
Gene enrichment analysis was carried out using FunRich 3.0 stand-alone software tool [15]. FunRich 3.0 was used to predict enrichment of clinical phenotype and biological pathways. Specific biological pathways gene overrepresentation, transcription factor association, and disease gene association or among any of the gene signatures was calculated by a hypergeometric test. Given a set of “n” signature genes of which “m” was contained in a certain pathway and provided that the union of all pathways contains “N” genes of which “M” were in the considered pathway, the p-values were computed according to the following formula:
The corresponding p-values were for multiple testing using the false discovery rate (FDR) correction and Benjamin and Hochberg method (BH) [16]. As a criterion for significant enrichment,we used a p-value <0.05 cutoff as significant.
Candidate gene prioritization, network analysis, and meta-analysis
Candidate gene prioritization is done using the ToppGene suite [17]. The common gene signatures between dementia-MetS-diabetes mellitus were used as the test set and all dementia associated gene lists from DisGeNet were used as the training set. FDR multiple testing corrections (BH) and p-value <0.05 was applied as the cutoff for significance. NetworkAnalyst online tool [18] was used to visualize common disease gene lists as a network. The common gene signatures between dementia-MetS-diabetes mellitus were used as input and default options were used to construct minimum interaction networks. AlzGene database [19] was queried for meta-analysis of the top ranked gene list. AlzGene database is comprised of comprehensive, unbiased, publicly available and regularly updated field synopsis of published genetic association studies performed on AD phenotypes. For more details on methods and statistical analysis for meta-analysis please check AlzGene database (http://www.alzgene.org/methods.asp).
Results
Dementia, metabolic syndrome, and diabetes mellitus share several common genes
In order to find the common genes (or gene signature) associated with dementia, MetS, and diabetes mellitus, we collected a list of disease GADs using DisGeNET database v4.0 and compared their similarity by plotting a Venn diagram (Figure 1). We focused on the common genes observed between dementia-MetS (100 genes) and dementia-diabetes mellitus (159 genes). There were a total of 173 genes (86 genes common in all three diseases) which were approximately 43% of the total genes associated with dementia. These results were in consensus with previous studies reporting common risk factors involved in dementia and MetS, including diabetes mellitus. Furthermore, it provided the motivation for functional characterization of these common gene signatures.
Figure 1.

Venn diagram showing common genes associated with dementia-metabolic syndrome-diabetes mellitus. Gene list from DisGeNET database was used to find the common gene association. Nearly 43% of the genes associated with dementia are also associated with either diabetes or metabolic syndrome and both diseases.
Gene set enrichment reveals shared molecular mechanisms between dementia, metabolic syndrome, and diabetes mellitus
Gene associations with diseases are meaningful only if they contribute towards disease development and progression. We decided to investigate the effect of a common gene signature that was common to dementia-MetS-diabetes (obtained from DisGeNet) across clinical phenotype physiology, biological process, and biological pathways. This top-down approach we hypothesized would aid in the stepwise understanding of the biological significance of the common gene signature.
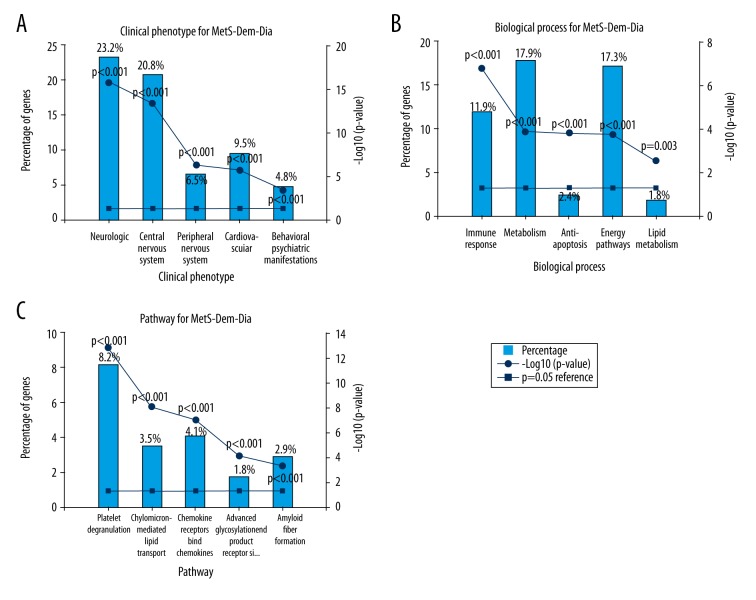
Physiology level clinical phenotype prediction results showed (Figure 2A) statistically significant (p<0.001) enrichment of genes associated with dysregulation in neurological (23.2%) and central nervous system (20.8%) phenotypes. Genes associated with the mutational phenotypes of peripheral nervous system (6.5%), cardiovascular system (9.5%), and behavioral and psychiatric manifestations (4.8%) were the other top five categories enriched for dysregulated clinical phenotypes.
Figure 2.
Gene set enrichment reveals shared molecular mechanisms between dementia, metabolic syndrome and diabetes mellitus. The gene set enrichment analysis was done using FunRich 3.0 software and human FunRich annotation was used as a database. The common gene signature between dementia-MetS-diabetes was used as input. Percentage of input genes associated with a total number of genes associated was represented along with −log (p-value) trend line. A p-value <0.05 was used as significant after applying Benjamin and Hochberg multiple testing method. (A) Clinical phenotype association of common gene signature. The mutational landscape of genes leads to dysregulated clinical phenotype which is an important determinant of its association with disease outcome. (B) Biological process association of common gene signature. Understanding the gene ontology biological process would provide the top level biological functions commonly affected in all three disease. (C) Biological pathways association of common gene signature. Understanding Reactome annotated biological pathways would provide the downstream biological signaling networks that are commonly affected in all three diseases.
Next, we checked the biological process (Figure 2B) and pathway (Figure 2C) associated with the common gene signature. Immune response (11.9%), metabolism (17.9%), and energy pathways (17.3%) were the top dysregulated biological processes significantly enriched. A similar trend was reflected in the biological pathway association with dementia, including platelet degranulation (8.2%), chemokine receptor interaction (4.1%), cholesterol and lipid transport (3.5%), and amyloid fiber formation (2.9%) indicating a strong association between MetS and inflammation with dementia. Interestingly, type II diabetes associated with advanced glycation end product signaling, and platelet degranulation linked cardiovascular disease and diabetes mellitus with dementia.
Candidate gene prioritization, network analysis, and meta-analysis reveal metabolic syndrome and diabetes associated genes APOE, IGF1R, and CCR5 were associated with dementia
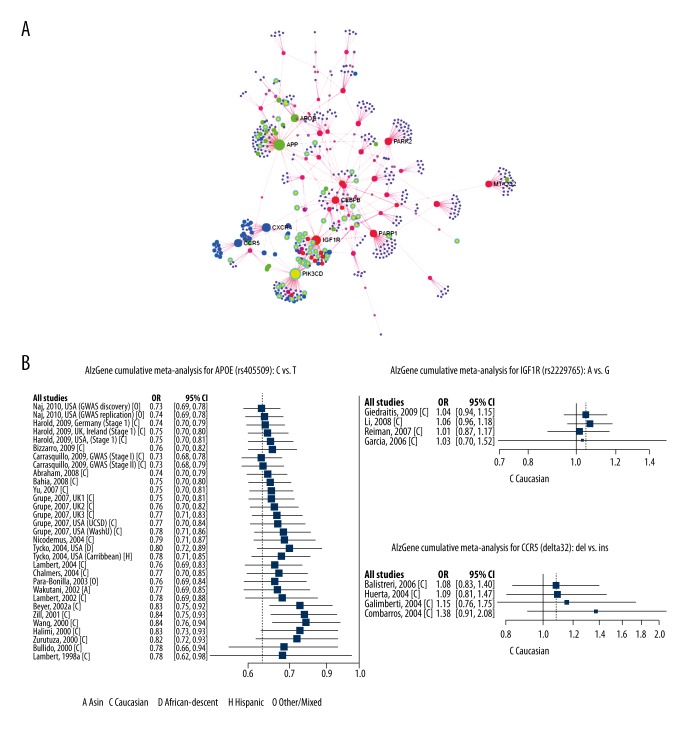
The gene set enrichment analysis using common gene signature comprising dementia, MetS, and diabetes mellitus revealed shared molecular mechanisms across three top-down levels: clinical phenotype, biological process, and biological pathway. Next, we set out to identify the most important genes that were truly relevant to the observed biological property. Hence, we did candidate gene prioritization analysis using ToppGene tool, which ranks the candidate genes by profiling across multiple genomic data sources and integrate this heterogeneous information into a global ranking. All 173 common signature genes were ranked and their protein-protein interaction based on gene ontology, literature, experimental observation, gene neighborhood was created using the network analyst tool (Figure 3A). The network analysis revealed APOE, APP, PARK2, CEPBP, PARP1, MT-CO2, CXCR4, IGFIR, CCR5, and PIK3CD as important nodes with significant interacting partners. This result also coincided with the gene rank prioritization as these set of genes also featured in top 50 ranks.
Figure 3.
Candidate gene prioritization, network analysis, and meta-analysis reveal metabolic syndrome and diabetes-associated genes APOE, IGF1R, and CCR5 were associated with dementia. (A) Network analysis of common disease signature genes. The node with the highest number of connections is labeled. (B) Forest plot showing APOE, IGFIR, and CCR5 gene polymorphism association with Alzheimer disease.
Out of the list of top ranked genes, three candidate genes (APOE, IGF1R, and CCR5) were previously known for their association with neurodegenerative disorder including Alzheimer disease, as shown by meta-analysis results from the AlzGene database (Figure 3B). The APOE polymorphism showed modest association with Alzheimer disease. Most importantly, the directionality of effects of the variants on Alzheimer disease was generally consistent with previous observations and did not differ by race/ethnicity (p<0.001), although our study had low power for this test. Altogether our results identified ranked candidate genes that were implicated in MetS and diabetes but also involved in dementia and neurodegenerative disorders.
Discussion
Several epidemiological studies provide a correlation between neurodegenerative diseases and MetS. However, it is still controversial whether metabolic impairments are associated with neurodegenerative diseases [20,21]. Therefore, the current study was intended to explore the relation between MetS and neurodegenerative diseases. Our results (Figure 2B, 2C) revealed several common genes associated with MetS, diabetes, and dementia, which were involved in energy metabolism, metabolic pathways, and also immune responses. Especially, we found APOE polymorphisms modestly associated with increased dementia risk. In neuronal cells, APOE is a major cholesterol carrier that supports lipid transport and injury repair in the brain. APOE isoforms differentially regulate amyloid-β (Aβ) aggregation and clearance in the brain, and have distinct functions in regulating brain lipid transport, glucose metabolism, neuronal signaling, neuroinflammation, and mitochondrial function [22]. The toxic Aβ aggregates in the form of soluble Aβ oligomers, intraneuronal Aβ and amyloid plaques injure synapses and ultimately cause neurodegeneration and dementia. Impairment of insulin signaling in the hypothalamus has been observed in Alzheimer disease-associated metabolic imbalance of carbohydrate, lipid, and amino acids in various organs especially in the liver, adipose tissue [23]. Individuals carrying the ɛ4 allele are at increased risk of Alzheimer disease compared to those carrying the more common ɛ3 allele, whereas those possessing the APO ɛ2 allele have decreased risk. Existence of the APOE ɛ4 allele has also been associated with increased risk for cerebral amyloid angiopathy and age-related cognitive decline during normal ageing. Conversely, numerous studies found an association between MetS and diabetes with APOE allele [24–28]. Association of APOE gene polymorphisms with diabetes, cardiovascular diseases, and dementia is well known. APOE is a multifunctional protein present in all lipoproteins except for low-density lipoprotein cholesterol fraction; it plays a critical role in lipoprotein metabolism [29]. Therefore, it is biologically plausible that individuals carrying abnormal APOE allele might influence an individual’s susceptibility to MetS, especially in terms of both triglyceride and cholesterol dysmetabolism, which are major complications of MetS. A recent genetic association in Chinese ethnicity showed a strong association between MetS components with APOE4, whereas an inverse association was observed with APOE2 [30]. Also, a recent meta-regression analysis of 30 studies involving 5,423 diabetes cases and 8,197 healthy unrelated controls demonstrated a modest association between APOE allele and diabetes (odds ratio: 1.18 [95% CI: 1.02–1.35]) [31]. Conversely, a recent meta-analysis of clinical and autopsy-based studies monotonically showed that, compared with individuals with an ɛ3/ɛ3 genotype, risk of Alzheimer disease was increased in individuals with one copy of the ɛ4 allele (ɛ2/ɛ4, OR 2.6; ɛ3/ɛ4, OR 3.2) or two copies (ɛ4/ɛ4, OR 14.9) among Caucasian patients [32].
For our analysis, we used a step-wise top-down approach to characterize shared molecular mechanisms of common gene signatures associated with dementia-MetS-diabetes. Although our study lacked the causality of gene expression and its effect on the cell signaling cascades, this initial evidence provided important insights into the commonly shared mechanism between chronic diseases such as dementia and MetS. Future gene association studies should focus on genetic association of combination of chronic diseases and its comorbidities to identify putative novel genes as therapeutic targets. Also, with ever increasing volumes of public literature databases, genome sequencing studies, and improvement in statistical techniques, it is very important to periodically integrate data sources and re-evaluate our understanding of complex chronic diseases.
Conclusions
With regard to disease development time scale, MetS and diabetes mellitus are chronic diseases that affect individuals during adulthood and persists throughout one’s lifetime, whereas dementia (or associated Alzheimer disease), which affects cognitive impairment, usually occurs in the aged population. Our finding of a common gene signature association between these temporally different diseases indicates genetic predisposition and risk factors are shared by these diseases and could be a useful tool for early diagnosis of dementia.
Footnotes
Source of support: Departmental sources
References
- 1.Agüero-Torres H, Fratiglioni L, Guo Z, et al. Dementia is the major cause of functional dependence in the elderly: 3-year follow-up data from a population-based study. Am J Public Health. 1998;88(10):1452–56. doi: 10.2105/ajph.88.10.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deary IJ, Corley J, Gow AJ, et al. Age-associated cognitive decline. British Medical Bulletin. 2009;92(1):135–52. doi: 10.1093/bmb/ldp033. [DOI] [PubMed] [Google Scholar]
- 3.Alzheimer’s A. 2014 Alzheimer’s disease facts and figures. Alzheimers Dementia. 2014;10(2):e47–e92. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Chan KY, Wang W, Wu JJ, et al. Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: A systematic review and analysis. Lancet. 2013;381(9882):2016–23. doi: 10.1016/S0140-6736(13)60221-4. [DOI] [PubMed] [Google Scholar]
- 5.Jayaraman A, Pike CJ. Alzheimer’s sisease and type 2 diabetes: Multiple mechanisms contribute to interactions. Curr Diab Rep. 2014;14(4):476. doi: 10.1007/s11892-014-0476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yates KF, Sweat V, Yau PL, et al. Impact of metabolic syndrome on cognition and brain: A selected review of the literature. Arterioscler Thromb Vasc Biol. 2012;32(9):2060–67. doi: 10.1161/ATVBAHA.112.252759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ancoli-Israel S, Vitiello MV. Sleep in dementia. Am J Geriatr Psychiatry. 2006;14(2):91–94. doi: 10.1097/01.JGP.0000200973.93494.aa. [DOI] [PubMed] [Google Scholar]
- 8.Ernst E. Diet and dementia, is there a link? A systematic review. Nutr Neurosci. 1999;2(1):1–6. doi: 10.1080/1028415X.1999.11747256. [DOI] [PubMed] [Google Scholar]
- 9.Norton MC, Dew J, Smith H, et al. Lifestyle behavior pattern predicts incident dementia and Alzheimer’s disease. The Cache County Study. J Am Geriatr Soc. 2012;60(3):405–12. doi: 10.1111/j.1532-5415.2011.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sleegers K, Roks G, Theuns J, et al. Familial clustering and genetic risk for dementia in a genetically isolated Dutch population. Brain. 2004;127(7):1641–49. doi: 10.1093/brain/awh179. [DOI] [PubMed] [Google Scholar]
- 11.Bertram L, McQueen MB, Mullin K, et al. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat Genet. 2007;39(1):17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 12.Wang H-Z, Bi R, Hu Q-X, et al. Validating GWAS-identified risk loci for Alzheimer’s disease in Han Chinese populations. Mol Neurobiol. 2016;53(1):379–90. doi: 10.1007/s12035-014-9015-z. [DOI] [PubMed] [Google Scholar]
- 13.Pinero J, Queralt-Rosinach N, Bravo A, et al. DisGeNET: A discovery platform for the dynamical exploration of human diseases and their genes. Database (Oxford) 2015;2015:bav028. doi: 10.1093/database/bav028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bardou P, Mariette J, Escudié F, et al. jvenn: An interactive Venn diagram viewer. BMC Bioinformatics. 2014;15(1):293. doi: 10.1186/1471-2105-15-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pathan M, Keerthikumar S, Ang CS, et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics. 2015;15(15):2597–601. doi: 10.1002/pmic.201400515. [DOI] [PubMed] [Google Scholar]
- 16.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 17.Chen J, Bardes EE, Aronow BJ, Jegga AG. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009;37(Web Server issue):W305–11. doi: 10.1093/nar/gkp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia J, Benner MJ, Hancock REW. NetworkAnalyst – integrative approaches for protein-protein interaction network analysis and visual exploration. Nucleic Acids Res. 2014;42(W1):W167–74. doi: 10.1093/nar/gku443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen NC, Bagade S, McQueen MB, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: The SzGene database. Nat Genet. 2008;40(7):827–34. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 20.Kalaria RN, Maestre GE, Arizaga R, et al. Alzheimer’s disease and vascular dementia in developing countries: Prevalence, management, and risk factors. Lancet Neurol. 2008;7(9):812–26. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nambron R, Silajdžić E, Kalliolia E, et al. A metabolic study of Huntington’s disease. PLoS One. 2016;11(1):e0146480. doi: 10.1371/journal.pone.0146480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C-C, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms, and therapy. Nat Rev Neurol. 2013;9(2):106–18. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruiz HH, Chi T, Shin AC, et al. Increased susceptibility to metabolic dysregulation in a mouse model of Alzheimer’s disease is associated with impaired hypothalamic insulin signaling and elevated BCAA levels. Alzheimers Dementia. 2016;12(8):851–61. doi: 10.1016/j.jalz.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y, Wei R, Yan D, et al. Association between APOE polymorphism and metabolic syndrome in Uyghur ethnic men. BMJ Open. 2016;6(1):e010049. doi: 10.1136/bmjopen-2015-010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sima A, Iordan A, Stancu C. Apolipoprotein E polymorphism – a risk factor for metabolic syndrome. Clin Chem Lab Med. 2007;45(9):1149–53. doi: 10.1515/CCLM.2007.258. [DOI] [PubMed] [Google Scholar]
- 26.Cardona F, Morcillo S, Gonzalo-Marin M, Tinahones FJ. The apolipoprotein E genotype predicts postprandial hypertriglyceridemia in patients with the metabolic syndrome. J Clin Endocrinol Metab. 2005;90(5):2972–75. doi: 10.1210/jc.2004-1912. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira CN, Carvalho MG, Fernandes AP, et al. Comparative study of apolipoprotein-E polymorphism and plasma lipid levels in dyslipidemic and asymptomatic subjects, and their implication in cardio/cerebro-vascular disorders. Neurochem Int. 2010;56(1):177–82. doi: 10.1016/j.neuint.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Fall T, Ingelsson E. Genome-wide association studies of obesity and metabolic syndrome. Mol Cell Endocrinol. 2014;382(1):740–57. doi: 10.1016/j.mce.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 29.van den Elzen P, Garg S, Leon L, et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. 2005;437(7060):906–10. doi: 10.1038/nature04001. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Wei R, Yan D, et al. Association between APOE polymorphism and metabolic syndrome in Uyghur ethnic men. BMJ Open. 2016;6(1):e010049. doi: 10.1136/bmjopen-2015-010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anthopoulos PG, Hamodrakas SJ, Bagos PG. Apolipoprotein E polymorphisms and type 2 diabetes: A meta-analysis of 30 studies including 5423 cases and 8197 controls. Mol Genet Metab. 2010;100(3):283–91. doi: 10.1016/j.ymgme.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–56. [PubMed] [Google Scholar]