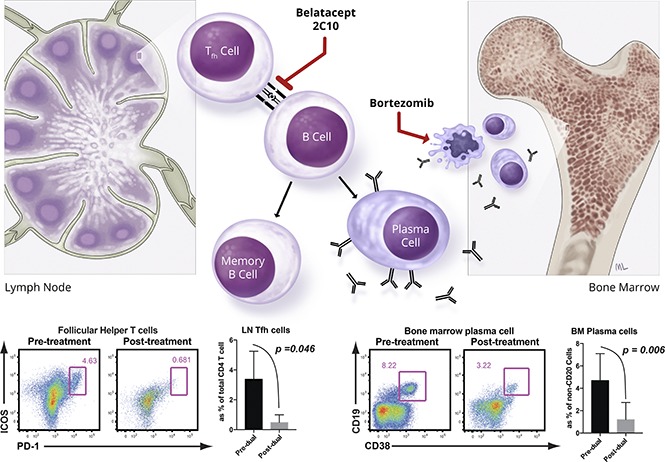
Key Points
Targeting both PCs and GC response reduces donor-specific antibodies and prolongs graft survival in sensitized NHP kidney transplantation.
Abstract
The detrimental effects of donor-directed antibodies in sensitized transplant patients remain a difficult immunologic barrier to successful organ transplantation. Antibody removal is often followed by rebound. Proteasome inhibitors (PIs) deplete antibody-producing plasma cells (PCs) but have shown marginal benefit for desensitization. In an allosensitized nonhuman primate (NHP) model, we observed increased germinal center (GC) formation after PI monotherapy, suggesting a compensatory PC repopulation mediated via GC activation. Here we show that costimulation blockade (CoB) targets GC follicular helper T (Tfh) cells in allosensitized NHPs. Combined PI and CoB significantly reduces bone marrow PCs (CD19+CD20−CD38+), Tfh cells (CD4+ICOS+PD-1hi), and GC B cells (BCL-6+CD20+); controls the homeostatic GC response to PC depletion; and sustains alloantibody decline. Importantly, dual PC and CoB therapy prolongs rejection-free graft survival in major histocompatibility complex incompatible kidney transplantation without alloantibody rebound. Our study illustrates a translatable desensitization method and provides mechanistic insight into maintenance of alloantibody sensitization.
Visual Abstract
Introduction
Kidney transplantation is the preferred treatment of end-stage renal disease with improved patient survival and quality of life compared with dialysis.1,2 However, patients with preformed donor HLA-specific antibodies (DSA) are more difficult to transplant because they require a stringent HLA match for a compatible donor kidney.3 Desensitization treatments reduce DSA in these patients to increase the pool of suitable donors. Desensitization therapies have been limited to combinations of plasmapheresis and IV immunoglobulin.4 Pretransplant, these treatments allow for successful implantation without hyperacute rejection, and posttransplant, they reduce the risk of antibody-mediated rejection5 as a result of antibody rebound.6,7 Desensitization treatments have been most successful in patients with an incompatible living donor; sensitized patients awaiting a compatible deceased donor transplant often have a prolonged wait to obtain a transplant8 and face reduced patient survival.6
It has been suggested that plasma cells (PCs), which are not targeted directly by current desensitization methods, contribute to the rebound in humoral responses seen after desensitization.9,10 Rituximab, a CD20-specific monoclonal antibody (mAb), has also been added to desensitization regimens to deplete B cells, with the hope of reducing PC generation and subsequent antibody production.11 However, B cells lose expression of CD20 upon terminal differentiation to PCs; consequently, rituximab conveys very limited efficacy in depleting PCs.12,13 More recently, proteasome inhibition (PI) targeting PCs was tested in desensitization protocols but has shown marginal benefit.14 We have previously demonstrated that PI with bortezomib for desensitization depleted PCs but did not reduce levels of DSA, possibly because of compensatory upstream germinal center (GC) expansion.15,16 In the present study, we demonstrate that targeting both PCs and follicular helper T (Tfh) cells successfully reduces DSA and prolongs rejection-free graft survival in presensitized nonhuman primate (NHP) kidney transplantation.
Methods
Male, outbred rhesus macaques (Macaca mulatta) were housed in Yerkes National Primate Research Center (Atlanta, GA) or Duke Laboratory Animal Resources (Durham, NC). Donor-recipient pairs were selected based on full major histocompatibility complex class I and maximal major histocompatibility complex class II mismatches by 454 sequencing (supplemental Figure 1). All experiments were compliant with the Emory and Duke Institutional Animal Care and Use Committee. Skin grafts (∼2.5 cm diameter) were swapped between paired animals for allosensitization. Sensitized animals were treated with belatacept (20 mg/kg), anti-CD40 mAb (2C10; 20 mg/kg), and bortezomib (1.3 mg/m2) or carfilzomib (20 mg/m2) twice weekly, IV over 4 weeks for desensitization. Control animals received no treatment prior to kidney transplantation. Renal transplants in these pairs were performed as previously described.17 All recipients received induction with 0.3 mg/kg basiliximab IV on postoperative day (POD) 0 and 4; 0.05 mg/kg tacrolimus intramuscularly twice daily (target trough: 8-12 ng/mL); 15 mg/kg mycophenolate mofetil (MMF) subcutaneously or 30 mg/kg MMF orally; and 125 mg methylprednisolone IV (tapered daily). Peripheral blood, lymph node (LN), and bone marrow (BM) cells were processed and stained with fluorochrome-conjugated antibodies as described in the supplemental Methods. Pathological evaluation was performed by a pathologist (A.B.F.) according to the updated Banff 2007 criteria.18,19 Kaplan-Meier and log-rank tests were used to compare graft survival. Sample comparisons of same animals were achieved by paired t test and Student t test for others. P < .05 was considered statistically significant.
Results and discussion
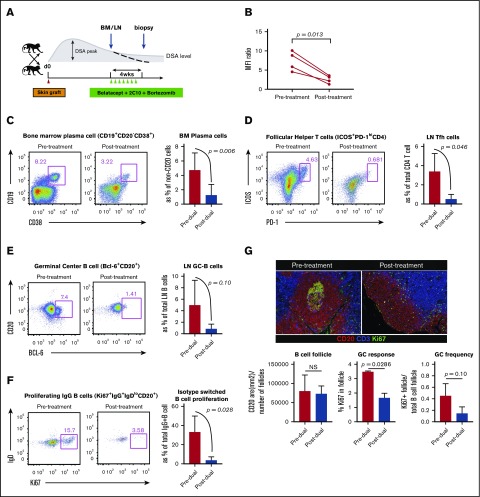
CD28 and CD40 expression on multiple myeloma cells and long-lived PCs has been documented.20-22 Therefore, we hypothesized that targeting PCs with a B7 costimulatory molecule-specific fusion protein (belatacept, Bristol Myers Squibb) and a CD40-specific mAb (2C10, Mass Biologics) could interfere with PC homeostasis and limit PC function. However, we found that DSA level and BM PCs were not significantly affected by combined costimulation blockade (CoB) treatment. Nevertheless, significant reductions in Tfh and GC-B cells, and reduced isotype switched B-cell proliferation, were observed in LNs (supplemental Figure 2). These data suggest that targeting both B7/CD28 and CD40/CD154 signaling does not suppress BM PCs but significantly reduces Tfh cells in the sensitized host. Therefore, we hypothesized that the effect of PI with bortezomib to deplete preformed PCs, when combined with CoB using belatacept and 2C10, would be synergistic,16 controlling both critical T- and B-cell interactions for PC regeneration, avoiding post-PC depletion homeostatic activation, and resulting in desensitization of sensitized NHPs (Figure 1A). We found that this “dual targeting” strategy significantly reduced DSA levels over 4 weeks in sensitized NHPs (Figure 1B). Additionally, we observed a significant reduction in BM PCs (Figure 1C). Tfh, GC-B, and proliferating B cells in LNs were also reduced after treatment (Figure 1D-F). We performed in situ GC staining to confirm the attenuated GC response after dual targeting. The average B-cell follicle size (CD20 area per follicle number per LN) was not significantly different. However, animals treated with dual targeting showed less frequent GC containing follicles and significantly reduced GC size (Ki67+CD20+ area per follicle area) (Figure 1G). Interestingly, the CD4+ Tcm cell levels declined after desensitization (supplemental Figure 3). These data show that dual targeting with CoB and PI modified not only the B-cell and PC compartment but also T-cell components.
Figure 1.
Dual targeting with proteasome inhibitor and CoB (belatacept and anti-CD40 mAb [2C10]) successfully promoted desensitization. (A) Timing and dosing of bortezomib, belatacept, and anti-CD40 mAb’s for desensitization and biopsy scheme for treated animals. (B) DSA from T-cell flow-cytometric cross-matching of sensitized animals before and after dual targeting treatment. DSA levels are expressed as mean channel fluorescent intensity (MFI) ratio. Serum DSA level was significantly reduced after CoB treatment (pre- vs posttreatment). (C) Visualization of BM PCs. Dual targeting treatment significantly affected CD19+CD20−CD38+ cells in the BM biopsy after dual targeting treatment. Representative flow plot and percentages of the CD19+CD38+ PC population in the BM at the indicated time points (pre- vs posttreatment). (D) Tfh cells were traced with PD-1 and inducible T-cell costimulator (ICOS) from the LN biopsies. LN-Tfh cells showed a significant reduction after dual targeting treatment. Representative flow plot (CD4 gated) and percentage of the ICOS+PD-1high cell population in the LN. (E) A strong trend of reduction of CXCR5+BCL-6+ B cells in the LN biopsy after CoB treatment. Representative flow plot (CD20 gated) and percentage of the CXCR5+BCL-6+ GC B-cell population in the LN. (F) Proliferated isotype switched B cells before and after CoB treatment. Ki67+IgG+IgDloCD20+ B cells in the LNs were greatly reduced after CoB treatment. (G) Immunofluorescent analysis of LN including B-cell follicles and GC staining for Ki67 (green), CD20 (red), and CD3 (blue). Original magnification ×200. Quantification of positive fluorescence signal of CD20 for B-cell follicle, and Ki67/CD20 for proliferating GC. Data represent the mean ± standard deviation (SD) of 4 monkeys per group. NS, nonsignificant.
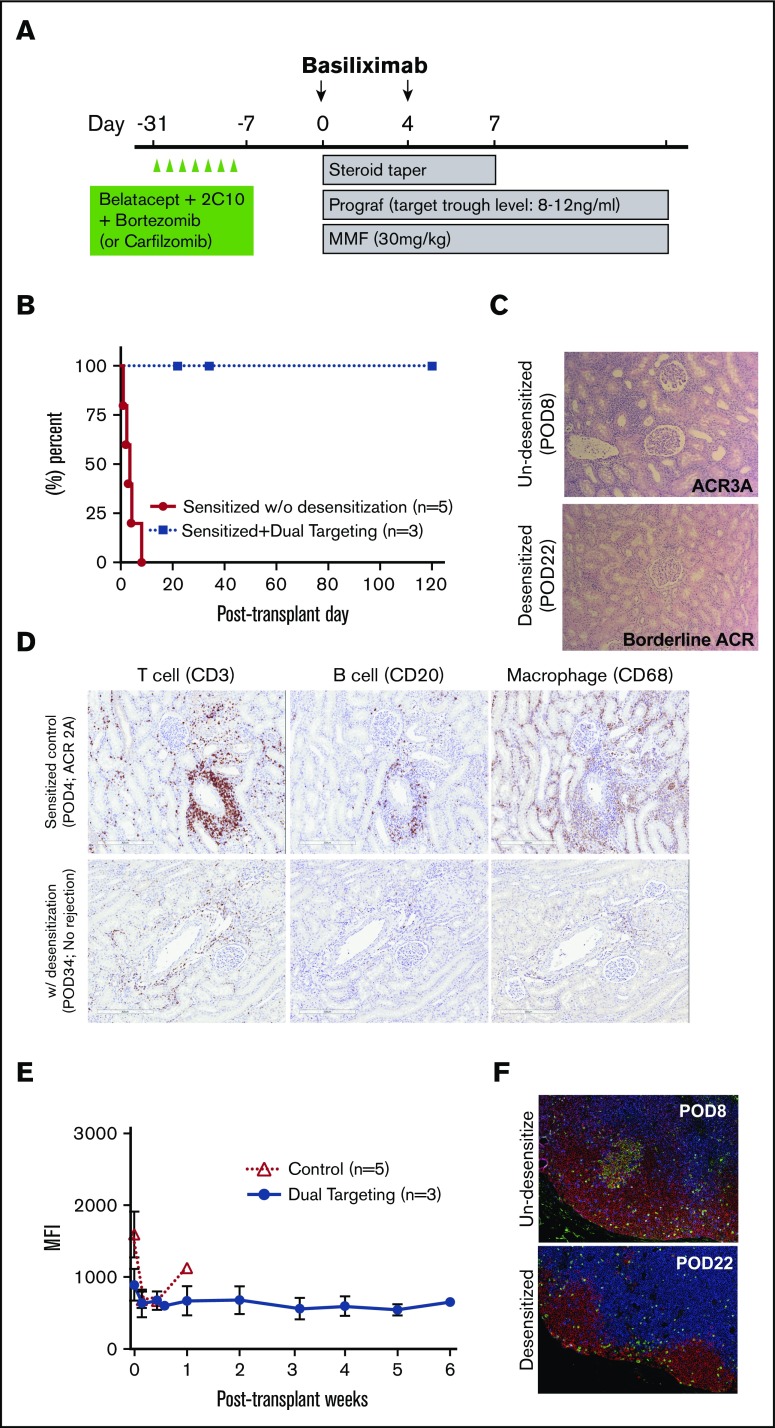
To evaluate the durability of dual targeting desensitization and its application to solid organ transplantation, we performed kidney transplantation after treatment and compared graft survival with nondesensitized controls. Five control animals received kidney transplantation from their previous skin donors. As shown in Figure 2A, 3 animals were treated twice weekly with bortezomib or carfilzomib and belatacept and 2C10 for 1 month before transplantation. Controls and desensitized animals received basiliximab induction with conventional maintenance immunosuppression (tacrolimus, MMF, and steroids). Sensitized animals showed accelerated rejection with mean survival time (MST) of 3.6 days, whereas sensitized animals treated with dual targeting therapy pretransplant had prolonged MST (Figure 2B; MST >58.6 days, P < .05). Two animals treated with bortezomib were euthanized because of weight loss with normal serum creatinine. Carfilzomib was later substituted for bortezomib in 1 subject because of bortezomib-associated weight loss. Although this subject showed transient posttransplant weight loss, at 6 weeks posttransplantation the weight was regained, and prolonged graft survival was observed (supplemental Figure 4). This animal was euthanized at POD 120 with normal graft function. No early graft injury or rejection was observed in biopsies from monkeys desensitized with dual targeting (data not shown) despite the lack of T-cell–depleting induction therapy, which was required in previously reported immunosuppressive protocols to avoid rejection.17 Allograft histology was evaluated at necropsy. Desensitized animals did not show evidence of rejection in contrast to sensitized controls (Figure 2C). Sensitized controls showed profound infiltration of T and B cells and macrophages in the grafts at early time points without desensitization, whereas less infiltration was observed in desensitized animals at later time points (Figure 2D). No significant increases in posttransplant DSA levels, GC responses, or memory T cells were observed in long-term recipients, suggesting a durable effect of dual targeting desensitization (Figure 2E-F). This reflects another missing concept in current desensitization approaches, namely, the need for targeting the reemerging antidonor response. This concept is not limited to PIs and CoBs but extends to agents targeting the effector arm of the humoral response in combination with influencing T-cell help for antidonor B-cell responses.
Figure 2.
The effect of dual targeting with proteasome inhibitor and CoB on renal allograft survival, posttransplant humoral and memory T cell responses in sensitized NHPs. (A) Timing and dosing of desensitization and immunosuppressive regimen with kidney transplantation in the sensitized NHPs. (B) Death censored graft survival shown in days by treatment group. P values were determined by log-rank test comparing sensitized NHPs receiving dual targeting therapy vs without desensitization. NHPs with desensitization had significantly prolonged rejection-free survival compared with sensitized controls without desensitization. (C) Renal allograft histologic examination of necropsy specimens (hematoxylin and eosin) from a control and desensitized macaque. Original magnification ×100. (D) Representative immunohistochemistry for T cells (CD3+), B cells (CD20+) and macrophages (CD68+) of necropsy specimens from controls vs desensitized NHPs. Scanned images were magnified (×10) from the original whole-slide scans. (E) Post–renal transplant serum DSA level from controls vs desensitized NHPs. Values represent mean ± SD and are representative of all separate experiments. (F) Representative post–renal transplant GC response in the LNs from controls vs desensitized NHPs as shown by immunofluorescence for CD3 (blue), CD20 (red), and Ki67 (green). Original magnification ×200. Data represent the mean ± SD of 3 monkeys treated with dual targeting treatment.
These results suggest that dual targeting of PC and GC profoundly alters alloimmunity in sensitized hosts, permitting long-term graft survival and preventing alloantibody rebound, which illustrates the potential of this strategy for treating HLA-sensitized humans and antibody-mediated rejection.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors acknowledge Frank Leopardi (Duke University) for assisting in animal surgeries and procedures, Mingqing Song (Duke University) for supporting tissue preparation and basic histologic support, Drew Roenneburg (University of Wisconsin-Madison) for immunohistochemistry, and the Emory Transplant Center biorepository and Duke Transplant Center substrate core for their weekly viral monitoring. Anti-CD40mAb (2C10) used in this study was produced and provided by the Non-human Primate Reagent Resource (National Institutes of Health grants 5R24OD010976 from the Office of the Director/Office of Research Infrastructure Programs and 1U24AI126683 from the National Institute of Allergy and Infectious Diseases). Belatacept was initially provided by BMS and purchased later. The authors thank veterinary supports from Yerkes National Primate Research Center and Duke Laboratory Animal Resources, especially for the expert assistance of Elizabeth Strobert and Joe Jenkins (Yerkes National Primate Research Center) and Kyha Williams and Felicitas Smith (Duke Laboratory Animal Resources).
This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (U19AI051731) (S.J.K.).
Authorship
Contribution: J.K. designed experiments, performed surgical procedures and cared for experimental macaques, conducted in vitro experiments, analyzed and interpreted data, and prepared the manuscript; C.B. designed experiments, performed surgical procedures and cared for experimental macaques, and conducted in vitro experiments; M.M., B.E., and J.P. participated in surgical procedures and cared for experimental macaques, and prepared the manuscript; J.Y. processed tissue samples, conducted in vitro experiments and performed flow cytometry; J.S.Y. performed flow cytometry; N.I. participated in initial experimental design; A.G. conducted in vitro experiments and performed flow cytometry; J.J.H. performed immunohistochemistry; A.B.F. interpreted data (pathologist); A.D.K. interpreted data and prepared the manuscript; and S.J.K. conceived of experimental design, performed surgical procedures, cared for experimental macaques, interpreted data, and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stuart J. Knechtle, 330 Trent Dr, DUMC Box 3512, Durham, NC 27710; e-mail: stuart.knechtle@duke.edu.
References
- 1.Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA. 1993;270(11):1339-1343. [PubMed] [Google Scholar]
- 2.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725-1730. [DOI] [PubMed] [Google Scholar]
- 3.Vo AA, Sinha A, Haas M, et al. Factors predicting risk for antibody-mediated rejection and graft loss in highly human leukocyte antigen sensitized patients transplanted after desensitization. Transplantation. 2015;99(7):1423-1430. [DOI] [PubMed] [Google Scholar]
- 4.Jordan SC, Pescovitz MD. Presensitization: the problem and its management. Clin J Am Soc Nephrol. 2006;1(3):421-432. [DOI] [PubMed] [Google Scholar]
- 5.Burns JM, Cornell LD, Perry DK, et al. Alloantibody levels and acute humoral rejection early after positive crossmatch kidney transplantation. Am J Transplant. 2008;8(12):2684-2694. [DOI] [PubMed] [Google Scholar]
- 6.Orandi BJ, Luo X, Massie AB, et al. Survival benefit with kidney transplants from HLA-incompatible live donors. N Engl J Med. 2016;374(10):940-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montgomery RA, Lonze BE, King KE, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365(4):318-326. [DOI] [PubMed] [Google Scholar]
- 8.Manook M, Koeser L, Ahmed Z, et al. Post-listing survival for highly sensitised patients on the UK kidney transplant waiting list: a matched cohort analysis. Lancet. 2017;389(10070):727-734. [DOI] [PubMed] [Google Scholar]
- 9.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8(3):363-372. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor BP, Gleeson MW, Noelle RJ, Erickson LD. The rise and fall of long-lived humoral immunity: terminal differentiation of plasma cells in health and disease. Immunol Rev. 2003;194(1):61-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vo AA, Lukovsky M, Toyoda M, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008;359(3):242-251. [DOI] [PubMed] [Google Scholar]
- 12.Martin F, Chan AC. B cell immunobiology in disease: evolving concepts from the clinic. Annu Rev Immunol. 2006;24:467-496. [DOI] [PubMed] [Google Scholar]
- 13.Hoyer BF, Manz RA, Radbruch A, Hiepe F. Long-lived plasma cells and their contribution to autoimmunity. Ann N Y Acad Sci. 2005;1050:124-133. [DOI] [PubMed] [Google Scholar]
- 14.Woodle ES, Shields AR, Ejaz NS, et al. Prospective iterative trial of proteasome inhibitor-based desensitization. Am J Transplant. 2015;15(1):101-118. [DOI] [PubMed] [Google Scholar]
- 15.Moreno Gonzales MA, Gandhi MJ, Schinstock CA, et al. 32 Doses of bortezomib for desensitization is not well tolerated and is associated with only modest reductions in anti-HLA antibody. Transplantation. 2017;101(6):1222-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwun J, Burghuber C, Manook M, et al. Humoral compensation after bortezomib treatment of allosensitized recipients. J Am Soc Nephrol. 2017;28(7):1991-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burghuber CK, Kwun J, Page EJ, et al. Antibody-mediated rejection in sensitized nonhuman primates: modeling human biology. Am J Transplant. 2016;16(6):1726-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8(4):753-760. [DOI] [PubMed] [Google Scholar]
- 19.Sis B, Mengel M, Haas M, et al. Banff ’09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10(3):464-471. [DOI] [PubMed] [Google Scholar]
- 20.Pellat-Deceunynck C, Bataille R, Robillard N, et al. Expression of CD28 and CD40 in human myeloma cells: a comparative study with normal plasma cells. Blood. 1994;84(8):2597-2603. [PubMed] [Google Scholar]
- 21.Robillard N, Jego G, Pellat-Deceunynck C, et al. CD28, a marker associated with tumoral expansion in multiple myeloma. Clin Cancer Res. 1998;4(6):1521-1526. [PubMed] [Google Scholar]
- 22.Bahlis NJ, King AM, Kolonias D, et al. CD28-mediated regulation of multiple myeloma cell proliferation and survival. Blood. 2007;109(11):5002-5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.