Abstract
MicroRNA-27b (miR-27b) is frequently upregulated in pressure-overloaded hypertrophic hearts. The clinical implications of aberrant circulating miR-27b in the diagnosis and management of left ventricular hypertrophy warrant study. We investigated whether serum miR-27b is a biomarker for left ventricular hypertrophy (LVH).
We used stem-loop reverse-transcription quantitative polymerase chain reaction techniques to analyze serum miR-27b levels in 200 hypertensive patients with LVH, 100 hypertensive patients without LVH, and 100 healthy volunteers. We found that serum miR-27b levels were significantly higher in the hypertensive patients with LVH than in the hypertensive patients without LVH and in the healthy volunteers. Upon receiver operating characteristic curve analysis, serum miR-27b had an area under the curve of 0.885 with 91% sensitivity and 73% specificity in distinguishing hypertensive patients with LVH from healthy volunteers (P=0.021), and an area under the curve of 0.818 with 79.1% sensitivity and 70.3% specificity in distinguishing hypertensive patients with LVH from those without LVH (P=0.036). We conclude that circulating miR-27b might serve as a specific, noninvasive biomarker in screening for LVH.
Keywords: Biomarkers/blood; cardiomyopathy, hypertrophic/genetics; cardiovascular physiologic phenomena/genetics; gene expression regulation; hypertrophy, left ventricular/diagnosis; microRNAs/blood/isolation & purification/physiology; real-time polymerase chain reaction; ROC curve; sensitivity and specificity
Cardiac hypertrophy can be a response to diverse forms of injury and stress. Its typical features on a cellular level are reactivation of fetal genes, assembly of sarcomeres, and increase in cardiomyocyte size.1 Although physiologic hypertrophy indicates an improvement in cardiac function, hypertrophic growth in response to pathophysiologic stimuli such as neurohumoral activation or hypertension is pathologic and increases the risk of heart failure or sudden death. Moreover, cardiac hypertrophy—a major risk factor for stroke and coronary artery disease—is associated with a risk of cardiovascular events 2 to 4 times greater than that in patients without cardiac hypertrophy.2 Therefore, early-stage screening and diagnosis of cardiac hypertrophy is important in minimizing the impact of cardiovascular disease.
Methods for diagnosing cardiac hypertrophy include electrocardiography, echocardiography, computed tomography, and magnetic resonance imaging. However, their effectiveness can be hindered by low sensitivity and specificity, lengthy diagnostic time, and technical complexity. Therefore, novel methods for the accurate, uncomplicated detection of cardiac hypertrophy are needed.
MicroRNAs (miRNAs) are small (~22 nucleotides in length), endogenous, noncoding RNAs that inhibit the expression of specific genes by degrading the target messenger RNA or by direct translational inhibition.3 MicroRNAs play fundamental roles in diverse biological and pathologic processes, including cell development, differentiation, proliferation, and apoptosis.4,5 Furthermore, miRNAs are identified not only in tissue, but also in serum and plasma. With their stability, abundant circulation, and relative convenience of detection, extraction, and quantification, circulating miRNAs can be used effectively as clinical biomarkers.6 For example, plasma miR-141 can distinguish prostate cancer patients from healthy subjects,7 and circulating miR-196a can be used to identify chronic hepatitis C.8
MicroRNAs play important roles in cardiac hypertrophy and dysfunction.9–11 Their expression is altered in cardiac hypertrophy by a series of high-throughput miRNA microarray analyses.12–14 The miR-27 family influences many cellular processes, and the b isoform of miR-27 functions as an angiogenic switch by promoting endothelial tip cell fate and sprouting.15,16 MicroRNA-27b is upregulated in the Smad4-deficient hypertrophic heart, and miR-27b overexpression is sufficient to induce cardiac hypertrophy in vitro and in vivo.17
The clinical implications of aberrant miR-27b expression in the diagnosis and management of cardiac hypertrophy warrant study. Because cardiac hypertrophy is a major risk factor for cardiovascular disease, we evaluated whether serum miR-27b is a novel biomarker in screening for cardiac hypertrophy.
Patients and Methods
The study period was July 2014 through June 2015. We searched our hospital's clinical databases and prospectively recruited 200 patients diagnosed with hypertension-related cardiac hypertrophy (group I), and 100 hypertensive patients who did not have cardiac hypertrophy (group II). All patients were at least 40 years old and had at least one year's history of hypertension. Left ventricular hypertrophy (LVH) was echocardiographically defined as a left ventricular (LV) mass/body surface area >95 g/m2 in women and >115 g/m2 in men.18 We then recruited 100 healthy volunteers (group III), with normal LV mass/height (m2) and no history of hypertension, as a control group. No study participant had been diagnosed with cancer, peripheral arteriosclerosis obliterans, autoimmune disease, osteoarthropathy, or psychosis. Persons who had been given heparin therapy within the previous 2 weeks were excluded. All participants supplied written informed consent in accordance with guidelines from our institutional research board. Our university's Clinical Research Ethics Committee approved this study.
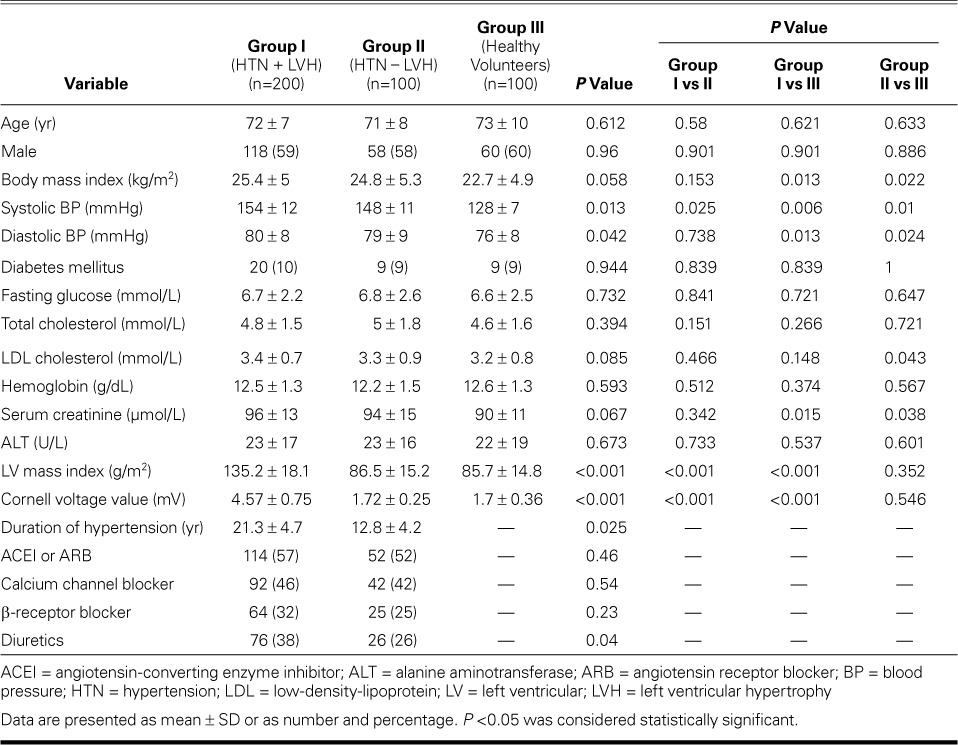
Table I shows the characteristics of the study population. Age and sex did not differ significantly between the 3 groups. Groups I and II had significant differences in systolic blood pressure, duration of hypertension, LV mass index, and Cornell voltage value.
TABLE I.
Clinical and Laboratory Findings in the Study Population
Serum Collection. Blood samples were collected from all participants. After 2-step centrifugation (7 min at 3,400 g, then 8 min at 15,000 g), the supernatant was transferred to RNase/DNase-free tubes and immediately stored at −80 °C. The mean storage times were 36.8 ± 18.7 d in group I, 35.2 ± 15.3 d in group II, and 28.7 ± 12.3 d in group III.
Chemistry. Using standard laboratory methods, we analyzed levels of total cholesterol, low-density-lipoprotein cholesterol, hemoglobin, fasting glucose, creatinine, and alanine aminotransferase.
MicroRNA Extraction and Expression Analysis. Total RNA was extracted from serum with use of a mirVana™ miRNA Isolation Kit (Thermo Fisher Scientific; Waltham, Mass) in accordance with the manufacturer's instructions. The RNA concentration was determined by using a NanoDrop™ ND-1000 spectrophotometer (Thermo Fisher). The miR-27b expression levels were defined with use of a SYBR™ Green real-time reverse transcription-polymerase chain reaction (PCR) assay (Thermo Fisher). MicroRNAs were quantified by using a TaqMan™ miRNA RT qPCR assay (Thermo Fisher).
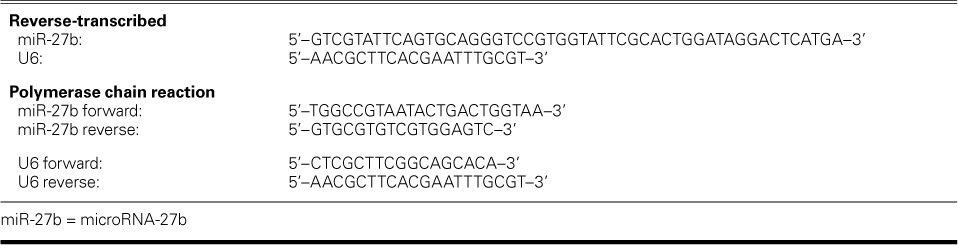
After synthesizing first-strand complementary DNA by using 10 ng of RNA and miRNA-196a–specific stem-loop primer (Thermo Fisher), we performed real-time quantitative PCR amplification with use of a gene-specific forward primer, a reverse primer, and a probe in an ABI Prism® 7500 Real-Time PCR System (Thermo Fisher). A noncoding small nuclear RNA, U6 snRNA, was used as the housekeeping gene. Table II lists the designed primers. Relative miRNA expression was performed in triplicate, in 3 separate experiments. Relative miR-27b production was reported as 2−ΔΔCt.
TABLE II.
Primers Used in the MicroRNA Expression Analysis
Statistical Analysis
Statistical analyses were performed with use of Stata® 10.0 software (StataCorp LLC; College Station, Texas). Data were expressed as mean ± SD or as number and percentage. The t test and analysis of variance were used to compare demographic characteristics. The Mann-Whitney U test was conducted for between-group comparisons. Receiver operating characteristic (ROC) curves were established for distinguishing patients with LVH from those without. Sensitivity and specificity were calculated by means of standard formulas. A 2-tailed P value <0.05 was considered statistically significant.
Results
Examination of MicroRNA Expression
As Figure 1A shows, miR-27b was significantly upregulated in the hypertensive patients with LVH in comparison with the healthy volunteers (P=0.004). Figure 1B shows the significant difference in miR-27b levels between the hypertensive patients with and without LVH (P=0.032). There was a significant positive linear correlation between circulating miR-27b levels and LV mass index (r=0.68; P=0.027).
Fig. 1.
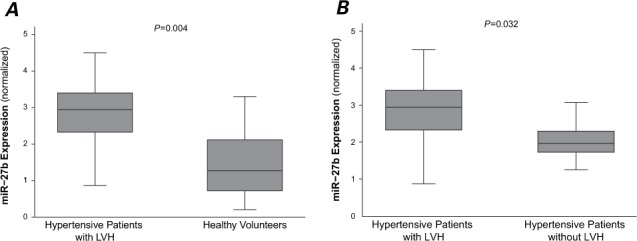
MicroRNA-27b (miR-27b) was significantly upregulated in patients with left ventricular hypertrophy (LVH). Box plots show serum levels of miR-27b in A) healthy volunteers and hypertensive patients with LVH, and B) hypertensive patients with and without LVH. Expression levels of miR-27b are normalized to U6. Lines inside the boxes display the medians. Boxes mark the interval between the 25th and 75th percentiles. Whiskers indicate intervals between the minimum and maximum. P <0.05 was considered statistically significant.
Serum Level of MicroRNA-27b as a Potential Diagnostic Marker
To investigate the usefulness of miR-27b as a diagnostic marker for LVH, we performed ROC curve analysis. The area under the curve (AUC) was 0.885 (95% CI, 0.844–0.926; P=0.021) with 91% sensitivity and 73% specificity in distinguishing the hypertensive patients with LVH from the healthy volunteers, at a cutoff value of 1.98 (Fig. 2A). To determine whether miR-27b elevation was specific for LVH, we examined the serum from the hypertensive patients; the miR-27b level was significantly higher in those who had LVH (P=0.036) (Fig. 2B).
Fig. 2.
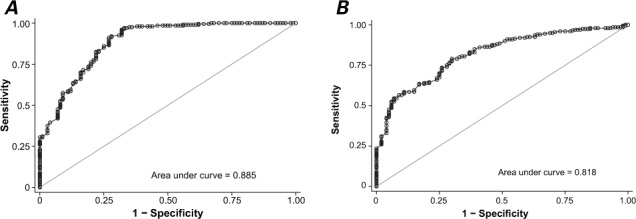
Graphs show receiver operating characteristic curve analyses of serum miR-27b levels. A) In the analysis for distinguishing hypertensive patients with left ventricular hypertrophy (LVH) from healthy volunteers, the area under the curve (AUC) was 0.885 (95% CI, 0.844–0.926; P=0.021) with 91% sensitivity and 73% specificity (cutoff value, 1.98). B) In distinguishing hypertensive patients with LVH from hypertensive patients without LVH, the AUC was 0.818 (95% CI, 0.77–0.866; P=0.036) with 79.1% sensitivity and 70.3% specificity (cutoff value, 2.27). P <0.05 was considered statistically significant.
The results of our ROC curve analyses suggest that the serum miR-27b level is useful in distinguishing hypertensive patients who have LVH from hypertensive patients who do not have LVH. The AUC for distinguishing the former patients from the latter was 0.818 (95% CI, 0.77–0.866). At a cutoff value of 2.27, the 79.1% sensitivity and 70.3% specificity suggested that circulating miR-27b can be used to screen for LVH.
Discussion
Left ventricular hypertrophy is an initial compensatory response to systemic hypertension, cardiac valvular disease, myocardial infarction, and other types of cardiac stress. This adaptation is one facet of cardiac remodeling and subsequent heart failure in an aging worldwide population.19,20 Treatment for heart failure focuses chiefly on symptoms. Therefore, early-stage screening and diagnosis of cardiac hypertrophy is important in minimizing the impact of cardiovascular disease.
Echocardiography has been regarded as a diagnostic gold standard; however, artifactal errors and technical complexities can hinder the diagnosis of cardiac hypertrophy. As an alternative, miRNAs might provide additional insights into the mechanisms involved in LVH. Aberrantly expressed miRNAs have been related to experimental and clinical conditions of cardiac hypertrophy.21
In the current study, we found that serum levels of miR-27b were significantly elevated in group I in comparison with levels in group III. Our ROC analysis yielded an AUC of 0.885, with 91% sensitivity and 73% specificity. Even though hypertension is the chief risk factor for LVH and miR-27b levels may be elevated in hypertensive patients, we found that miR-27b distinguished LVH from hypertension alone, yielding an AUC of 0.818 with 79.1% sensitivity and 70.3% specificity. To our knowledge, this is the first study to show the diagnostic potential of circulating miR-27b in screening for LVH.
Series of miRNA arrays have been conducted to investigate changes in miRNAs in clinical and experimentally induced pathologic hypertrophy. Of note, miR-27b is frequently upregulated in pressure-overloaded hypertrophic hearts.22–24 MicroRNA-27b overexpression promotes hypertrophic cell growth, whereas knockdown of miR-27b inhibits hypertrophic cell growth caused by phenylephrine treatment in vitro. MicroRNA-27b is significantly inhibited by transforming growth factor-β1 in cardiomyocytes at the transcriptional level, and miR-27b has shown an overt myocardial expression during heart development.25
The function of miR-27b in cardiac hypertrophy is gradually being clarified. Investigators have shown that miR-27b regulates adipocyte differentiation and modulates inflammatory responses by targeting peroxisome proliferator-activated receptor-γ (PPAR-γ).26,27 Gain-of-function and loss-of-function approaches28 revealed that PPAR-γ was the target of miR-27b and partially mediated cardiac hypertrophy resulting from miR-27b overexpression. Furthermore, PPAR-γ expression was significantly downregulated in hypertrophic hearts of miR-27b–transgenic mice. It is known that PPAR-γ is a crucial regulator during cardiac hypertrophy, and PPAR-γ agonists inhibit stress-induced hypertrophy of cultured neonatal rat ventricular cardiomyocytes.29
Study Limitations. Coronary artery disease, hypertrophic cardiomyopathy, aortic valve stenosis, and other conditions can also cause LVH. However, we did not record these because of our study design. Hypertension was the chief cause of LVH.
Conclusion. Serum miR-27b, which was significantly elevated in our hypertensive patients with LVH, might serve as a novel biomarker to distinguish hypertensive patients who have LVH from those who do not.
References
- 1. Bell D, Campbell M, Wang X, Earle JA, Cosby SL, McDermott BJ.. Adrenomedullin gene delivery is cardio-protective in a model of chronic nitric oxide deficiency combining pressure overload, oxidative stress and cardiomyocyte hypertrophy. Cell Physiol Biochem 2010; 26 3: 383– 94. [DOI] [PubMed] [Google Scholar]
- 2. Vakili BA, Okin PM, Devereux RB.. Prognostic implications of left ventricular hypertrophy. Am Heart J 2001; 141 3: 334– 41. [DOI] [PubMed] [Google Scholar]
- 3. Carrington JC, Ambros V.. Role of microRNAs in plant and animal development. Science 2003; 301 5631: 336– 8. [DOI] [PubMed] [Google Scholar]
- 4. Miska EA. How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev 2005; 15 5: 563– 8. [DOI] [PubMed] [Google Scholar]
- 5. Kutay H, Bai S, Datta J, Motiwala T, Pogribny I, Frankel W, . et al. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J Cell Biochem 2006; 99 3: 671– 8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6. Weiland M, Gao XH, Zhou L, Mi QS.. Small RNAs have a large impact: circulating microRNAs as biomarkers for human diseases. RNA Biol 2012; 9 6: 850– 9. [DOI] [PubMed] [Google Scholar]
- 7. Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Posogova-Agadjanyan EL, . et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 2008; 105 30: 10513– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu B, Xiang Y, Zhang HS.. Circulating microRNA-196a as a candidate diagnostic biomarker for chronic hepatitis C. Mol Med Rep 2015; 12 1: 105– 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen JF, Murchison EP, Tang R, Callis TE, Tatsuguchi M, Deng Z, . et al. Targeted deletion of dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci U S A 2008; 105 6: 2111– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. da Costa Martins PA, Bourajjaj M, Gladka M, Kortland M, van Oort RJ, Pinto YM, . et al. Conditional dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation 2008; 118 15: 1567– 76. [DOI] [PubMed] [Google Scholar]
- 11. Rao PK, Toyama Y, Chiang HR, Gupta S, Bauer M, Medvid R, . et al. Loss of cardiac microRNA-mediated regulation leads to dilated cardiomyopathy and heart failure. Circ Res 2009; 105 6: 585– 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M.. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res 2007; 100 3: 416– 24. [DOI] [PubMed] [Google Scholar]
- 13. Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, . et al. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure [published erratum appears in Circulation 2007;116(3):e135]. Circulation 2007; 116 3: 258– 67. [DOI] [PubMed] [Google Scholar]
- 14. Zhou S, Liu Y, Prater K, Zheng Y, Cai L.. Roles of microRNAs in pressure overload- and ischemia-related myocardial remodeling. Life Sci 2013; 93 23: 855– 62. [DOI] [PubMed] [Google Scholar]
- 15. Biyashev D, Veliceasa D, Topczewski J, Topczewska JM, Mizgirev I, Vinokour E, . et al. miR-27b controls venous specification and tip cell fate. Blood 2012; 119 11: 2679– 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Urbich C, Kaluza D, Fromel T, Knau A, Bennewitz K, Boon RA, . et al. MicroRNA-27a/b controls endothelial cell repulsion and angiogenesis by targeting semaphorin 6A. Blood 2012; 119 6: 1607– 16. [DOI] [PubMed] [Google Scholar]
- 17. Wang J, Song Y, Zhang Y, Xiao H, Sun Q, Hou N, . et al. Cardiomyocyte overexpression of miR-27b induces cardiac hypertrophy and dysfunction in mice. Cell Res 2012; 22 3: 516– 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taylor J. 2013 ESH/ESC guidelines for the management of arterial hypertension. Eur Heart J 2013; 34 28: 2108– 9. [PubMed] [Google Scholar]
- 19. Papoutsidakis N, Deftereos S, Kaoukis A, Bouras G, Giannopoulos G, Theodorakis A, . et al. MicroRNAs and the heart: small things do matter. Curr Top Med Chem 2013; 13 2: 216– 30. [DOI] [PubMed] [Google Scholar]
- 20. Braunwald E. The war against heart failure: the Lancet lecture. Lancet 2015; 385 9970: 812– 24. [DOI] [PubMed] [Google Scholar]
- 21. Bronze-da-Rocha E. MicroRNA s expression profiles in cardiovascular diseases. Biomed Res Int 2014; 2014: 985408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, . et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A 2006; 103 48: 18255– 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen H, . et al. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol 2007; 170 6: 1831– 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M.. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res 2007; 100 3: 416– 24. [DOI] [PubMed] [Google Scholar]
- 25. Chinchilla A, Lozano E, Daimi H, Esteban FJ, Crist C, Aranega AE, Franco D.. MicroRNA profiling during mouse ventricular maturation: a role for miR-27 modulating Mef2c expression. Cardiovasc Res 2011; 89 1: 98– 108. [DOI] [PubMed] [Google Scholar]
- 26. Karbiener M, Fischer C, Nowitsch S, Opriessnig P, Papak C, Ailhaud G, . et al. MicroRNA miR-27b impairs human adipocyte differentiation and targets PPARgamma. Biochem Biophys Res Commun 2009; 390 2: 247– 51. [DOI] [PubMed] [Google Scholar]
- 27. Jennewein C, von Knethen A, Schmid T, Brune B.. Micro-RNA-27b contributes to lipopolysaccharide-mediated peroxisome proliferator-activated receptor gamma (PPARgamma) mRNA destabilization. J Biol Chem 2010; 285 16: 11846– 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang J, Song Y, Zhang Y, Xiao H, Sun Q, Hou N, . et al. Cardiomyocyte overexpression of miR-27b induces cardiac hypertrophy and dysfunction in mice. Cell Res 2012; 22 3: 516– 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamamoto K, Ohki R, Lee RT, Ikeda U, Shimada K.. Peroxisome proliferator-activated receptor gamma activators inhibit cardiac hypertrophy in cardiac myocytes. Circulation 2001; 104 14: 1670– 5. [DOI] [PubMed] [Google Scholar]


