Abstract
The interrupted noneverting mattress suture technique is typically used in conventional surgical aortic valve replacement. The continuous suture technique, although faster, has been associated with a higher incidence of paravalvular leak. Using a slightly modified technique to minimize this risk, we investigated whether continuous suturing would shorten aortic cross-clamp time in aortic valve replacement in comparison with interrupted suturing.
We reviewed the cases and compared the perioperative data of 60 consecutive patients in Japan and Australia (35 men and 25 women; median age, 70 yr) who had undergone aortic valve replacement with or without septal myectomy. The continuous suture technique had been used in 41 patients (Group CS) and the standard interrupted suture technique in 19 (Group IS). The groups were similar in age, sex, pathologic valvular conditions, and operative urgency.
In Group CS, aortic cross-clamp time (47 vs 63 min; P=0.0001) and cardiopulmonary bypass time (76 vs 89 min; P=0.04) were significantly shorter. Neither group had early paravalvular leak. Using our continuous suture technique safely shortened aortic cross-clamp time during surgical aortic valve replacement.
Keywords: Aortic valve/surgery, cardiac surgical procedures/methods, heart valve prosthesis implantation/adverse effects/instrumentation/methods, operative time, postoperative complications, retrospective studies, risk factors, suture techniques, time factors
Whereas progress in the transcatheter treatment of aortic valve stenosis has enabled more transcatheter aortic valve replacements (TAVR) from year to year,1 the number of surgical aortic valve replacements (SAVR) has also increased. In SAVR, sutureless valves have shown promise on the basis of early outcomes2; however, implantation with interrupted noneverting mattress suture is performed most often. The continuous suture technique,3,4 although comparatively faster than interrupted suturing, has been associated with a higher incidence of paravalvular leak (PVL),5 possibly because of uneven stitching.
We studied whether our continuous suture technique—including extra attention to precise spacing at commissures and nadirs—compares favorably with interrupted suturing in terms of aortic cross-clamp time and early outcomes after aortic valve replacement (AVR), and we report our findings here.
Patients and Methods
We retrospectively studied the medical records of 60 consecutive patients who had undergone AVR, with or without septal myectomy, in Japan and Australia from July 2006 through April 2016. We analyzed the patients' demographic characteristics, intraoperative data, and early postoperative outcomes. All patients had undergone intraoperative transesophageal echocardiography (TEE) and postoperative transthoracic echocardiography before their discharge from the hospital. This study was conducted in compliance with human studies guidelines at our respective institutions and was approved by the institutional ethical review boards.
Table I shows the patients' preoperative characteristics. The continuous suture technique had been used in 41 patients (Group CS) and the interrupted suture technique in 19 (Group IS). The groups were statistically similar except for a higher EuroScore II in Group CS.
TABLE I.
Preoperative Characteristics of the Study Groups
Surgical Technique
In each patient, after a median sternotomy, cardiopulmonary bypass (CPB) was started along with aortic perfusion and right atrial drainage. A left ventricular vent was inserted through the right upper pulmonary vein. Mild hypothermia was established, the aorta was cross-clamped, and crystalloid cardioplegic solution was administered antegrade, followed by intermittent blood cardioplegic solution retrograde regardless of aortic regurgitation. After the aortic valve was resected and the area thoroughly decalcified, the annulus was sized. Annuli in patients with endocarditis or severe calcification underwent aggressive débridement. Each attending surgeon used one technique—interrupted or continuous—exclusively.
Annular Repair. In continuous suture AVR, we reinforced the annulus when the ventriculoaortic continuity was destroyed. We used 4-0 pledgeted Prolene® polypropylene mattress suture (Ethicon US, LLC, a Johnson & Johnson company; Somerville, NJ).
In interrupted suture AVR, we placed a noneverting mattress suture to repair a damaged annulus, using 2-0 Ethibond Excel® braided polyester sutures (Ethicon).
Continuous Suture Technique
To minimize PVL after continuous suturing, we applied 6 equally spaced stitches in each leaflet attachment for normal-sized valves, and 5 stitches for smaller valves. To achieve adequate depth, we made 4-mm suture bites of 2 mm each on the aortic and ventricular sides. For the first 3 stitches of the right coronary leaflet attachment, we made 3-mm bites (2 mm on the aortic side and 1 mm on the ventricular side), to avoid conduction disturbance.
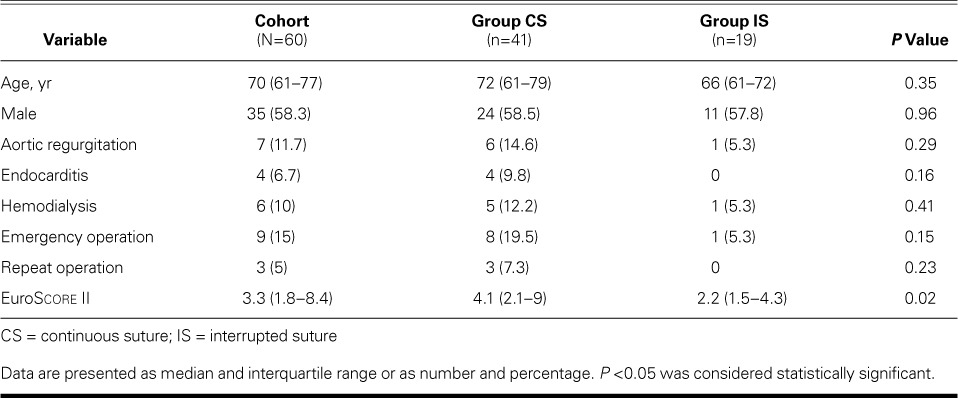
We used one of three 17-mm half-circle needles to pass a 2-0 Prolene suture through the right coronary–noncoronary leaflet commissure from the aortic side to the ventricular side (Fig. 1A), and then through the sewing cuff of the prosthetic valve from bottom to top (Fig. 1B). The suture was run counterclockwise and was passed under the previous suture for the annulus and then over the previous suture for the sewing cuff (Fig. 1C–E). Of the 5 or 6 equidistant stitches for each leaflet attachment, the 3rd stitch was placed just before the nadir and the 4th just beyond it. The suture was twisted as we sutured the right leaflet attachment, but it gradually untwisted as the prosthetic valve was rotated clockwise. After the sewing cuff for the right coronary leaflet was completed, we used rubber-tipped forceps to hold this needle and the needle of the 2nd suture together. The left coronary leaflet attachment was sutured in the same fashion with the 2nd needle (Fig. 1F). The noncoronary leaflet attachment was sutured similarly, and we used another forceps to hold the end of the suture line and the first suture needle together. The prosthetic valve was lowered to the annulus as we pulled the 3 rubber-tipped forceps individually. The suture from the nadir to the commissure was tightened by pulling up the suture at each nadir with a nerve hook (Fig. 1G). Then, 2 strings of each forceps were pulled individually, and the loops at the nadir were tightened (Fig. 1H). We cut off the needles, securely tightened both ends of the sutures, and tied the suture at each commissure (Fig. 2). Finally, we ensured that no sutures were loose on the aortic and ventricular sides.
Fig. 1.
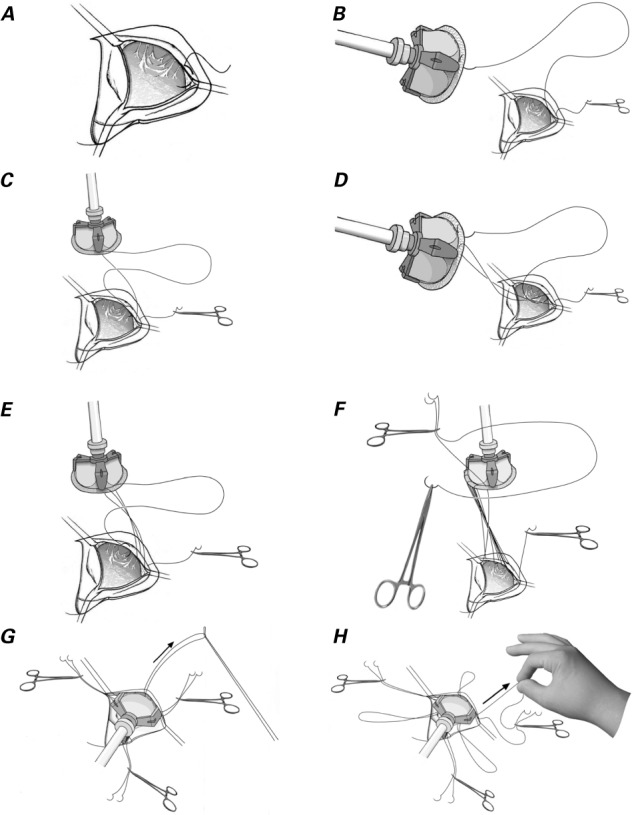
Drawings illustrate the operative technique. A) The first stitch passes through the leaflet attachment at the right coronary and noncoronary leaflet commissure. B) The 2nd stitch passes through the sewing cuff of the prosthesis. C) The 3rd stitch passes under the previous suture and is placed at the leaflet attachment. D) The 4th stitch passes over the previous suture and is placed on the sewing cuff. E) The 5th stitch passes under the previous suture and is placed at the annulus. F) The end of the first suture and one end of the 2nd suture are held with rubber-tipped forceps, which are then placed on the left shoulder of the patient. The left coronary leaflet is sutured in the same manner, with use of the other end of the suture. G) The suture at each nadir is pulled with a nerve hook. H) Six ends of 3 sutures are pulled individually, and the loops at the nadir are tightened.
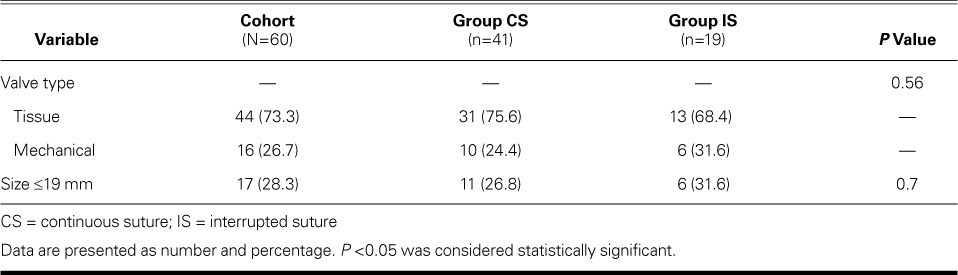
Fig. 2.
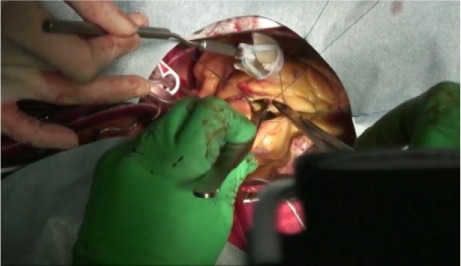
Photograph during aortic valve replacement shows continuous suture passing under the previous suture as the right coronary leaflet attachment is stitched.
Supplemental motion image is available for Figure 2.
When the 3 commissures were not positioned in an equilateral triangle (such as in the case of a bicuspid valve), the sizing replica of the prosthesis was positioned so that it did not interfere with the coronary orifices, and “virtual” commissures were marked on the native valve leaflet attachment. Stitches were placed in accordance with these virtual commissures. Marking the nadirs also helped to ensure equal stitching.
Statistical Analysis
Statistical analysis was performed with use of JMP® version 12 (SAS Institute Inc.; Cary, NC). Continuous data are expressed as median and interquartile range. Continuous variables were analyzed by using the Mann-Whitney U test, and the categorical variables by using the χ2 test. A P value <0.05 was considered statistically significant.
Results
Continuous suturing was performed in 16 of the first 30 patients and in 27 of the next 30 patients (P=0.0003).
The aortic cross-clamp and CPB times were significantly shorter in Group CS (Table II). There was no significant difference between groups in the use of mechanical versus tissue prostheses or in valve size (Table III). No substantial PVL was seen on intraoperative TEE in either group. Postoperatively, one patient in group CS died of bradyarrhythmia within 30 days, one patient in Group IS had atrioventricular block that necessitated pacemaker implantation, and 2 patients in Group CS underwent repeat exploration for hemorrhage. The suture technique, cross-clamp time, and CPB time were not associated with postoperative morbidity or death. Of note, we observed no early PVL in either group.
TABLE II.
Comparison of Aortic Cross-Clamp and Cardiopulmonary Bypass Times
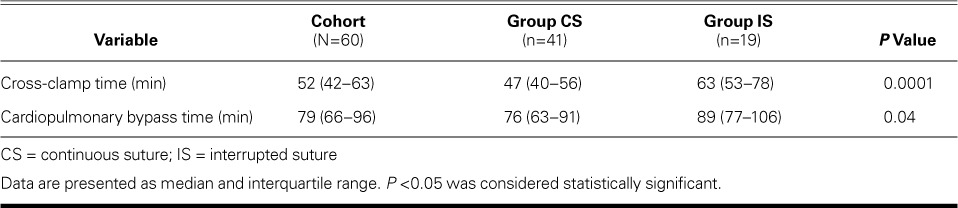
TABLE III.
Characteristics of the Aortic Valve Prostheses
Discussion
Aortic valve replacement to treat aortic stenosis is becoming more prevalent. In 2013, approximately 12,000 AVRs were performed in Japan,6 and the number has continued to increase.7 Technological advances have led to more TAVR procedures.1 However, TAVR is associated with vascular sequelae, postoperative aortic insufficiency, and atrioventricular block.8,9 Moreover, TAVR is not currently indicated purely for aortic regurgitation, so SAVR is performed in most cases of aortic valve disease.
Various minimally invasive surgical approaches for aortic valve disease have been reported. The use of sutureless valves can shorten aortic cross-clamp time2; however, the current-generation devices are associated with the risk of PVL. In addition, a patient who needs annular enlargement will more likely undergo AVR with suturing.
Aortic cross-clamp time accounts for a substantial portion of CPB time, an independent risk factor in conventional SAVR.10 In comparison with surgical approach and valve selection, less discussion has occurred regarding suturing techniques in relation to cross-clamp and CPB time. In the current series, continuous suturing is associated with significantly shorter cross-clamp time than that of interrupted suturing; in addition, we observed no early PVL. Because postoperative PVL might be due to inaccurate or uneven stitching, we divided our suture line into equidistant segments to achieve consistency.
Another advantage of continuous suturing is that it can be performed through a minimally invasive approach or during aortic root replacement (Fig. 3).
Fig. 3.
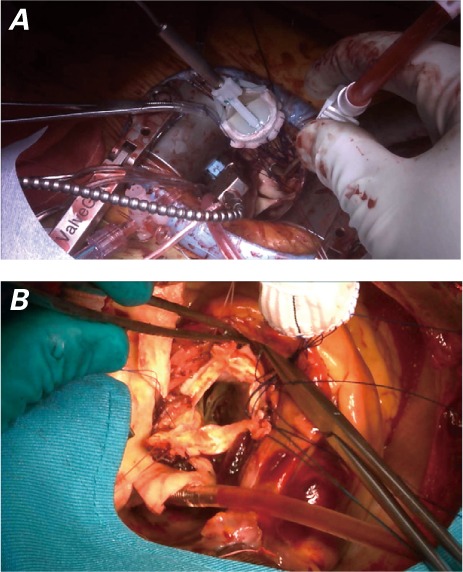
Intraoperative photographs show A) minimally invasive aortic valve replacement and B) aortic root replacement with use of the continuous suture technique.
This retrospective study has some limitations. The small sample size, especially in Group IS, precluded analysis of differences among the attending surgeons in terms of cross-clamp and CPB time. In addition, long-term results were not evaluated, because the patients who underwent AVR in Australia did not undergo regular postoperative echocardiographic evaluation. Nevertheless, we found that our continuous suture technique shortened the aortic cross-clamp time during AVR without increasing the risk of early PVL.
Supplementary Material
References
- 1. Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, . et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol 2013; 62 11: 1002– 12. [DOI] [PubMed] [Google Scholar]
- 2. Vola M, Campisi S, Gerbay A, Fuzellier JF, Ayari I, Favre JP, . et al. Sutureless prostheses and less invasive aortic valve replacement: just an issue of clamping time? Ann Thorac Surg 2015; 99 5: 1518– 23. [DOI] [PubMed] [Google Scholar]
- 3. Cooley DA. Simplified techniques of valve replacement. J Card Surg 1992; 7 4: 357– 62. [DOI] [PubMed] [Google Scholar]
- 4. Doty DB. Aortic valve replacement. : Brown M, Baxter S, . Cardiac surgery: operative technique. St. Louis (MO): Mosby Inc.; 1997. p 216– 7. [Google Scholar]
- 5. Nair SK, Bhatnagar G, Valencia O, Chandrasekaran V.. Effect of valve suture technique on incidence of paraprosthetic regurgitation and 10-year survival. Ann Thorac Surg 2010; 89 4: 1171– 9. [DOI] [PubMed] [Google Scholar]
- 6. Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery, Masuda M, Kuwano H, Okumura M, Arai H, . et al. Thoracic and cardiovascular surgery in Japan during 2013: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2015; 63 12: 670– 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery, Masuda M, Okumura M, Doki Y, Endo S, . et al. Thoracic and cardiovascular surgery in Japan during 2014: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2016; 64 11: 665– 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schymik G, Heimeshoff M, Bramlage P, Herbinger T, Wurth A, Pilz L, . et al. A comparison of transcatheter aortic valve implantation and surgical aortic valve replacement in 1,141 patients with severe symptomatic aortic stenosis and less than high risk. Catheter Cardiovasc Interv 2015; 86 4: 738– 44. [DOI] [PubMed] [Google Scholar]
- 9. Muneretto C, Bisleri G, Moggi A, Di Bacco L, Tespili M, Repossini A, Rambaldini M.. Treating the patients in the ‘grey-zone’ with aortic valve disease: a comparison among conventional surgery, sutureless valves and transcatheter aortic valve replacement. Interact Cardiovasc Thorac Surg 2015; 20 1: 90– 5. [DOI] [PubMed] [Google Scholar]
- 10. Chalmers J, Pullan M, Mediratta N, Poullis M.. A need for speed? Bypass time and outcomes after isolated aortic valve replacement surgery. Interact Cardiovasc Thorac Surg 2014; 19 1: 21– 6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


