Abstract
Anomalous origin of the left coronary artery from the pulmonary artery is rare and typically results in mitral regurgitation, ventricular arrhythmias, heart failure, and sudden death. The condition most often manifests itself in early childhood, but some individuals are diagnosed much later. We describe the case of a 75-year-old woman with heart failure in whom stepwise multimodal imaging revealed anomalous origin of the left coronary artery from the pulmonary artery.
Keywords: Aged; coronary vessel anomalies/diagnosis/diagnostic imaging; diagnostic techniques, cardiovascular; heart defects, congenital/diagnostic imaging; pulmonary artery/abnormalities/diagnostic imaging; tomography, x-ray computed/methods
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA), or Bland-White-Garland syndrome, is a rare defect that typically causes mitral regurgitation, ventricular arrhythmias, heart failure, and sudden death. The condition is most often diagnosed in infants, and if left untreated, it has a high mortality rate. The incidence of ALCAPA is estimated to be 1 in 300,000 infants, and the defect accounts for 0.5% of congenital heart disease.1 Atypically, patients survive into adulthood, and they may present with angina pectoris, heart failure, or sudden death. Multimodal imaging proved helpful in identifying ALCAPA in this case of an elderly woman with heart failure.
Case Report
In September 2014, a 75-year-old woman presented at our institution with a 3-month history of dyspnea on exertion and lower-extremity edema. The patient had longstanding atrial fibrillation.
One month previously, she had been admitted to a local hospital with heart failure exacerbation, which was successfully managed with diuretics. A transthoracic echocardiogram (TTE) during that hospitalization revealed a left ventricular ejection fraction (LVEF) of 0.25, with anterior and anteroseptal wall-motion abnormalities at the mid and basal segments and mild mitral regurgitation.
At the current presentation, the patient reported no symptoms of angina or ischemia. Physical examination revealed coarse bilateral inspiratory crackles and a dilated, nondisplaced apical impulse. An electrocardiogram showed atrial fibrillation, left-axis deviation, a nonspecific intraventricular conduction delay, and lateral T-wave inversion. On review of the patient's medical records, a coronary angiogram from August 2005 (when the patient had presented with chest pain and a positive stress test) revealed a very large and dominant right coronary artery (RCA) with extensive collateral vessels to the left coronary system. Contrast opacification was observed in the main pulmonary artery (PA) (Fig. 1). At the time, surgery was not recommended, and the patient had been lost to follow-up until her recent admission to the hospital.
Fig. 1.
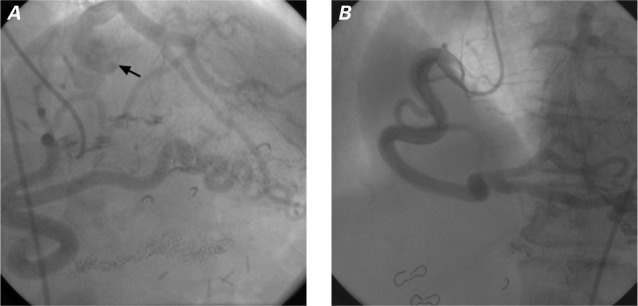
Coronary angiograms from 10 years previously in the A) right and B) left anterior oblique projections. Injection of contrast media into the right coronary artery revealed extensive collateral circulation to the left anterior descending and left circumflex coronary arteries. In A), opacification in the left main coronary artery extends to the pulmonary artery (arrow).
During outpatient evaluation in September 2014, the patient's heart failure medications were optimized, and serial echocardiograms were obtained. In October 2015, a TTE revealed a modest improvement in LVEF to 0.38, mild-to-moderate mitral regurgitation, a dilated RCA (Fig. 2A), and diastolic flow in the main PA (Fig. 2B). A positron emission tomographic adenosine stress test, performed to detect any ischemic causes of persistent left ventricular dysfunction, revealed a large area of apical, anterior, and anterolateral drug-induced ischemia (Fig. 3). A subsequent computed tomographic coronary angiogram revealed that the left main coronary artery (LMCA) originated from the PA, before branching normally into the left anterior descending coronary artery (LAD) and left circumflex coronary artery (Fig. 4). The RCA was large and ectatic.
Fig. 2.
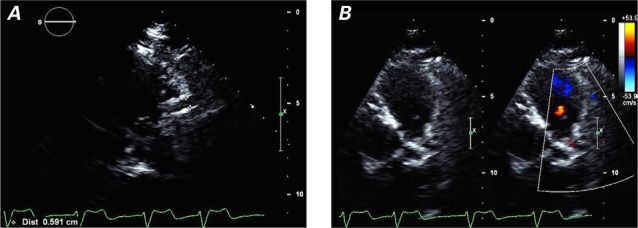
Transthoracic echocardiograms reveal A) a dilated (0.6-cm) right coronary artery (parasternal long-axis view) and B) the pulmonary outflow tract and diastolic flow in the pulmonary artery (parasternal short-axis view with color-flow Doppler).
Supplemental motion images are available for Figure 2A and 2B.
Fig. 3.
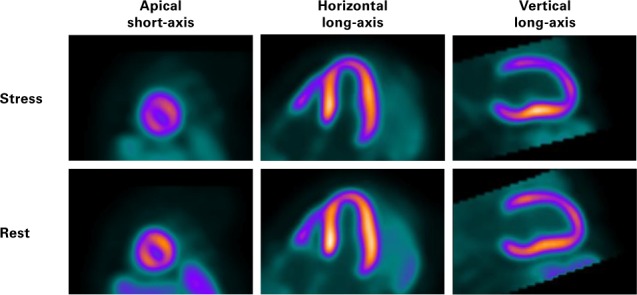
Myocardial perfusion positron emission tomographic images in apical short-axis, horizontal long-axis, and vertical long-axis views show a perfusion abnormality in the mid anterior wall at rest. During stress, apical, anterior, and anterolateral adenosine-induced ischemia is seen in all views.
Fig. 4.
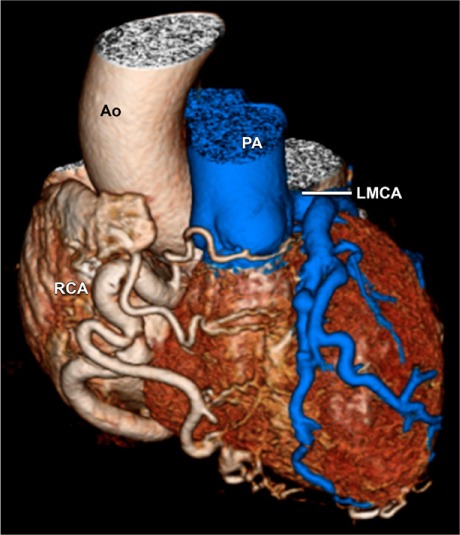
Three-dimensional computed tomogram reveals the left main coronary artery (LMCA) originating from the pulmonary artery (PA), above the left pulmonary valve cusp. A large right coronary artery (RCA) arises from the aorta (Ao).
In December 2015, the patient's myocardium was viable. This finding, as well as the patient's documented left ventricular myocardial ischemia, prompted a surgical consultation, and in March 2016 she underwent successful surgical ligation of the LMCA at its origin, with a subsequent saphenous vein graft from the ascending aorta to the LMCA. Three days postoperatively, an echocardiogram revealed an improved LVEF of 0.46 with only anterior wall-motion abnormalities. In September 2016, the patient's LVEF had improved to 0.50. Her anterior wall-motion abnormalities persisted.
Discussion
The pathophysiology of ALCAPA in both children and adults lies not in delivery of deoxygenated blood to the myocardium, but rather in the coronary steal phenomenon. At birth, high pulmonary pressure causes forward flow through the anomalous coronary artery, thus perfusing the myocardium with poorly oxygenated blood. As pulmonary pressures fall within a few days to weeks after birth, however, flow from the PA to the left coronary system slows and ultimately reverses. The left coronary system then receives its blood supply through collateral circulation from the RCA. This increased flow leads to dilation of the RCA, which we noted on our patient's TTE (Fig. 2A). Blood flow from the RCA travels through small collateral vessels to the distal left system, and then retrograde through the LAD and LMCA into the posterior aspect of the PA. As a result, the myocardial territory supplied by the LMCA is persistently hypoxic because of a mismatch between supply and demand, and this results in subendocardial ischemic changes or infarction (replacement-type fibrosis) in the distribution of the left coronary artery (Fig. 5).1 The development of collateral vessels between the left and right coronary systems and variations in coronary dominance dictate the timing of patient presentation.2 Our patient had clearly developed, substantial collateral vessels and a large right-dominant system, which most likely contributed to her presentation late in adulthood.
Fig. 5.
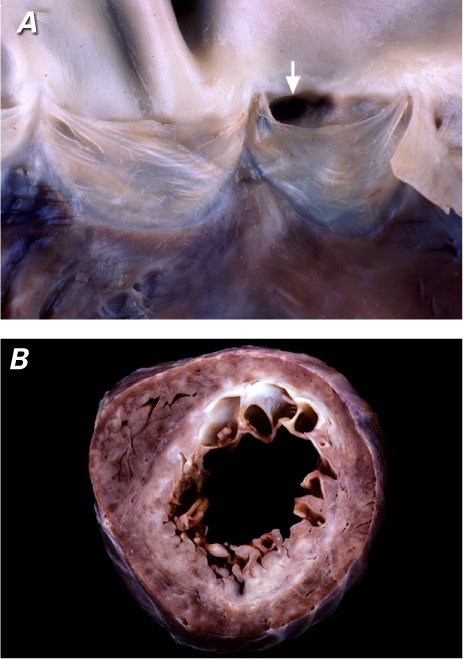
Representative pathologic specimens show A) a coronary ostium (arrow) arising from a left pulmonary arterial sinus, and B) nearly circumferential myocardial scarring (white tissue) in a short-axis section of the subendocardial region with sparing of the inferoseptal region (right coronary artery territory).
In the mid-20th century, coronary angiography emerged as the gold standard for diagnosis of ALCAPA.2 Contrast media injected into the RCA appears in collateral vessels to the left coronary system and ultimately opacifies the PA, as in our patient (Fig. 1). Because of the high incidence of heart failure and ischemic mitral regurgitation associated with ALCAPA, patients frequently undergo echocardiography. Many investigators have published echocardiographic findings that suggest ALCAPA in children and adults. Some of the most typical findings are a dilated RCA, diastolic flow in the main PA, abnormal septal color-flow signals, left ventricular enlargement, and mitral regurgitation.3 Echocardiographic imaging of coronary arteries in adult patients can be challenging; although echocardiographic findings are often not diagnostic, the signs noted above strongly indicate an anomalous coronary artery. Cross-sectional imaging of the coronary arteries can now characterize the coronary anatomy. Coronary computed tomographic angiography is the gold standard for evaluating coronary anomalies, because it can provide information about both anatomy and atherosclerosis.4
The 2008 American College of Cardiology/American Heart Association Guidelines for the Management of Adults with Congenital Heart Disease5 recommend that patients with ALCAPA undergo reconstruction of a dual coronary artery supply, performed by a surgeon with training and expertise in congenital heart disease. Surgical correction involves reimplantation of the anomalous coronary artery, if possible, or coronary artery bypass grafting.
By using TTE, myocardial perfusion scanning, and computed tomography, we were able to diagnose ALCAPA in an elderly woman, and this led to surgical treatment.
Supplementary Material
References
- 1. Pena E, Nguyen ET, Merchant N, Dennie C.. ALCAPA syndrome: not just a pediatric disease. Radiographics 2009; 29 2: 553– 65. [DOI] [PubMed] [Google Scholar]
- 2. Wesselhoeft H, Fawcett JS, Johnson AL.. Anomalous origin of the left coronary artery from the pulmonary trunk. Its clinical spectrum, pathology, and pathophysiology, based on a review of 140 cases with seven further cases. Circulation 1968; 38 2: 403– 25. [DOI] [PubMed] [Google Scholar]
- 3. Yang YL, Nanda NC, Wang XF, Xie MX, Lu Q, He L, Lu XF.. Echocardiographic diagnosis of anomalous origin of the left coronary artery from the pulmonary artery. Echocardiography 2007; 24 4: 405– 11. [DOI] [PubMed] [Google Scholar]
- 4. Bhatt AB, Foster E, Kuehl K, Alpert J, Brabeck S, Crumb S, . et al. Congenital heart disease in the older adult: a scientific statement from the American Heart Association. Circulation 2015; 131 21: 1884– 931 [DOI] [PubMed] [Google Scholar]
- 5. Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, . et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the Ameican College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation 2008; 118 23: e714– 833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


