Abstract
“Tumor-associated macrophages” (TAMs) form a significant cell population in malignant tumors and contribute to tumor growth, metastasis, and neovascularization. Gliomas are characterized by extensive neo-angiogenesis, and knowledge of the role of TAMs in neovascularization is important for future anti-angiogenic therapies. The phenotypes and functions of TAMs are heterogeneous and more complex than a classification into M1 and M2 inflammation response types would suggest. In this review, we provide an update on the current knowledge of the ontogeny of TAMs, focusing on diffuse gliomas. The role of TAMs in the regulation of the different processes in tumor angiogenesis is highlighted and the most recently discovered mechanisms by which TAMs mediate resistance against current antivascular therapies are mentioned. Novel compounds tested in clinical trials are discussed and brought in relation to different TAM-related angiogenesis pathways. In addition, potential therapeutic targets used to intervene in TAM-regulated tumor angiogenesis are summarized.
Keywords: angiogenesis, glioma, therapy, tumor-associated macrophages
The Origin and Action of Tumor-Associated Macrophages
Initiation of malignant tumors is associated with a sustained state of chronic inflammation.1 In the initial stage of tumorigenesis, the first activated immune cells are usually the tissue-resident macrophages. In glioma, these cells are mainly represented by microglial cells.2 Activated microglial cells generate high levels of O2 radicals that induce genomic mutations and enhance interleukin (IL)-6 and tumor necrosis factor α production supporting tumor cell survival.3 Recruitment of peripheral monocytes enhances this initial response. Various tumor cell–derived factors work together with hypoxia and trigger monocyte recruitment.4 For example, glioma stem cells produce periostin, an extracellular matrix component that provides interactive binding sites to αVβ3 integrins on the cell surface of peripheral monocytes and M2-like TAMs to promote extravasation and migration in the glioma environment (Fig. 1A). Hypoxia can also trigger activation of vascular endothelial growth factor receptor 1 (VEGFR1) and neuropilin-1 (NRP1) in monocytes, leading to chemotaxis in, for instance, glioma and breast cancer.5 Likewise VEGFA and semaphorin 3A (Sema 3A)6 released by the tumor cells, including glioma,7 can activate NRP1, triggering the activation of VEGFR1 and subsequent recruitment of TAMs in glioma8–10 (Fig. 1B).
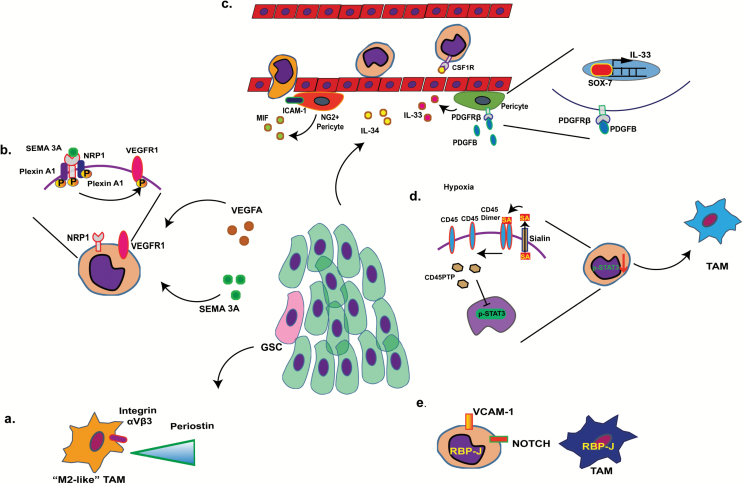
Fig. 1.
Routes of TAM recruitment and processes of monocyte maturation. (A) Glioma stem cells (pink) deposit periostin in the tumor stroma (green cells represent glioma cells), where it serves as a chemoattractant to infiltrating (M2-like) TAMs. (B) VEGFA and semaphorin 3A (Sema 3A) released by the tumor cells activate neuropilin-1 (NRP1), triggering the activation of VEGFR1 and subsequent recruitment of TAMs. (C) IL-34 derived from tumor cells mediates attachment of monocytes to the endothelial layer of blood vessel via CSF-1R binding on the surface of peripheral monocytes. IL-33, released from perivascular pericytes via PDGF-BB-PDGFRβ-Sox7 signaling also serves as a chemoattractant to peripheral monocytes. Likewise, macrophage inhibitory factor (MIF) and intercellular adhesion molecule 1 (produced by neural glial antigen 2+ pericytes) provide guiding signals to monocytes during extravasation. (D) Hypoxic stress enhances tumor microenvironment levels of sialic acid (SA), which decreases CD45 dimer signaling, increases CD45PTP (CD45 phosphatase), and inhibits STAT3 signaling in recruited monocytes, triggering differentiation of the TAM phenotype. (E) Notch signaling triggers maturation of vascular cell adhesion molecule 1+ monocytes.
IL-34 derived from tumor cells mediates attachment of monocytes to the endothelial layer of blood vessels via colony stimulating factor 1 receptor (CSF-1R) binding on the surface of peripheral monocytes. IL-33, released from perivascular pericytes via platelet derived growth factor (PDGF)–BB–PDGF receptor beta (PDGFRβ)–Sox7 signaling also serves as a chemoattractant to peripheral monocytes. Likewise, macrophage inhibitory factor (MIF) and intercellular adhesion molecule 1 (produced by neural glial antigen 2+ pericytes) provide guiding signals to monocytes during extravasation11 (Fig. 1C).
In addition to recruitment signals, the tumor environment can also provide different maturation signals to the recruited monocytes. As reported in, for instance, breast cancer and melanoma, hypoxia can enhance tumor microenvironment levels of sialic acid, leading to decreased cluster of differentiation (CD)45 dimerization, increase of CD45PTP (CD45 phosphatase), and inhibition of signal transducer and activator of transcription 3 (STAT3) signaling in recruited monocytes, providing the initial trigger for differentiation of the TAM phenotype12 (Fig. 1D). In mammary tumors, recruited vascular cell adhesion molecule 1+ monocytes have been reported to be stimulated to undergo maturation via Notch upregulation of the transcription factor RBPJ (recombination signal binding protein for immunoglobulin kappa J region)13 (Fig. 1E).
Phenotypes of TAMs: Beyond the M1/M2 Spectrum
The classification into M1/M2 profiles is a way to separate macrophage functions into pro-inflammatory (type 1 T helper cell [Th1]) and anti-inflammatory (Th2) responses of T lymphocytes.14 M1 and M2 profiles represent the extremes of a continuum of phenotypical characteristics marked by sets of cytokines and surface markers. M1 macrophages regulate the acute inflammatory response and are induced by lipopolysaccharide and interferon-γ stimulation. M1 macrophages are characterized by expression of CD40, CD80, CD16/32, CD86, C-C chemokine receptor 7, and human leukocyte antigen D related (HLA-DR),15 and are capable of phagocytosis and antigen presentation to T cells. M2 macrophages reduce acute inflammation and promote tissue repair. The M2 profile is induced by IL-4, IL-13, and macrophage colony stimulating factor (M-CSF). Studies on murine and human cancers, including glioblastomas, show that TAMs can express M1 and M2 markers, depending on tumor stage and area.16–18 Glioma-associated macrophages may resemble naïve macrophages more than classic M1 or M2 macrophage subtypes,19 illustrative of the complex nature of TAMs in glioma.
Analysis of TAMs in glioblastomas in our laboratory showed coexpression of the M1 surface markers HLA-DR and CD16 with M2 CD204 and CD163 (unpublished data), which is in line with previous findings demonstrating that the M2 marker CD204 colocalized with the M1 marker HLA-DR in perivascular macrophages in glioblastoma.20 Similarly, in other malignant tumors like ovarian cancer, the M2 macrophage surface marker CD163 was coexpressed with classical Th1 cytokines as IL-6 and IL-8.21 Cytokines from the M1 profile like IL-6, chemokine C-C ligand 5 (CCL5), and CCL2 are typically tumor supportive and are associated with poor prognosis.22 The mixed phenotypes of TAMs encountered in tumors may well result from simultaneous stimulation by both pro-inflammatory and anti-inflammatory factors in the tumor microenvironment.
TAMs and Tumor Neovascularization
TAMs promote proliferation, invasion, and metastasis of cancer cells but also stimulate neo-angiogenesis.1 Macrophages contribute to angiogenesis under a variety of conditions such as tissue regeneration/wound healing,23 immune-mediated diseases like rheumatoid arthritis,24 and neoplasia.25 The number of macrophages present around blood vessels in healthy tissues is significantly increased in tumors such as glioblastoma,26,27 particularly during proliferative microvessels.28–30 In glioblastoma, CD163+ TAMs are found in parenchymal and perivascular areas.31 In addition, tunica interna endothelial cell kinase 2–positive (Tie-2+) TAMs are found at perivascular sites in various malignant cancers, including glioblastoma.32 Perivascular TAMs in glioblastomas are positively correlated with microvascular density and higher expression of VEGFA, heme oxygenase 1,33 and thymigen phosphorylase,34 which is not detected in anaplastic oligodendroglioma or ependymoma.35 The secretion of inflammatory cytokines by TAMs promotes neovascularization along various molecular pathways. For example, TAMs actively contribute to the process of vasculogenesis, or de novo synthesis of mature endothelial cells from (circulatory) endothelial progenitor cells. Macrophage release of IL-6 and subsequent activation of Janus kinase/signal transducers and activators of transcription (JAK-STAT) pathways in recruited endothelial progenitor cells promote the vasculogenic process in these cells36 (Fig. 2A). Similarly, various in vitro and in vivo experiments have indicated that monocytes themselves are capable of undergoing “vasculogenesis” and transform into cordlike structures that express endothelial markers like von Willebrand factor, CD31, vascular endothelial (VE)–cadherin, and CD105, following exposure to pro-angiogenic factors such as VEGFA and insulin-like growth factor 1.37,38 In multiple myeloma, TAMs that express multiple endothelial markers cooperate with endothelial cells in the formation of the endothelial lining of tumor blood vessels.39 The expression of endothelial lineage markers like VE-cadherin, VEGFR2, factor VIII-related antigen, and von Willebrand factor and the formation of capillary-like structures in vitro can also be induced in macrophages/monocytes in response to VEGFA, basic fibroblast growth factor,40 or pleiotrophin41 stimulation (Fig. 2B). In glioma, TAMs have been indicated to be able to enhance the vascular mimicry of glioma cells, a process mediated via cyclooxygenase 2 (COX2) and IL-6.42,43
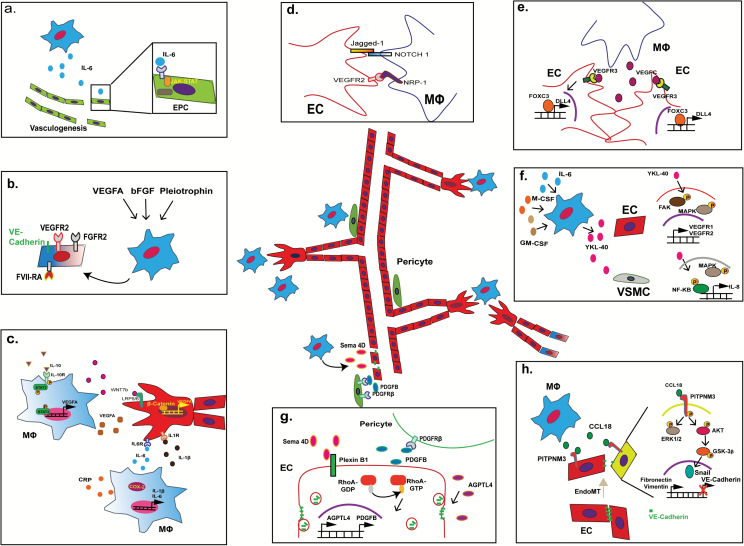
Fig. 2.
Various roles of macrophages in angiogenesis. (A) IL-6 released by TAMs recruits and promotes vasculogenesis of endothelial progenitor cells via JAK-STAT signaling. (B) Stimulation of TAMs by VEGFA, basic fibroblast growth factor (FGF), and pleiotrophin triggers TAMs to undergo vasculogenesis, as shown by upregulation of endothelial markers VEGFR2, FGF receptor 2, factor VIII-related antigen, and VE-cadherin in affected TAMs. (C) Paracrine interaction mechanisms between TAMs and endothelial cell sprouting: IL-10 upregulates VEGFA expression by TAMs via STAT3 signaling. Likewise, glioma-derived C-reactive protein (CRP) promotes IL-6 and IL-1β expression in COX2+ TAMs. In particular IL-1β promotes endothelial proliferation and enhances vascular sprouting. WNT7b release by TAMs can also enhance VEGFA expression in tip and stalk cells during sprouting, with similar effects. (D) Putative direct contact interaction mechanisms between TAMs and endothelial cells during sprouting: ligand-receptor binding of Jagged1 (on tip cell) with Notch 1 (MФ) as well as VEGFR2 (tip cell) with NRP1 (MФ) could help activate sprouting and determine sprouting direction. (E) VEGFC released by macrophages stimulates expression of delta-like protein 4 in tip cells via VEGF receptor C–FoxC3 signaling and promotes fusion of vascular sprouts (anastomosis). (F) Under the stimulation of GM/M-CSF and IL-6, TAM releases YKL-40 that triggers the signaling of focal adhesion kinase–MAPK in endothelial cells leading to increased expression of VEGFR1 and VEGFR2. Similarly, YKL-40 activates MAPK-NFκB in vascular smooth muscle cells (VSMCs), which leads to IL-8 secretion. (G) Semaphorin-4D (Sema 4D) from TAMs stimulates Plexin B1-RhoA signaling, which increases the expression of PDGFB and AGPTL4 in the endothelial cells. Increased expression of PDGFB promotes the recruitment of pericytes via PDGFRβ signaling, which enhances perivascular coverage and neovessel stabilization. In contrast, increased expression of AGPTL4 leads to the endocytosis of VE-cadherin in endothelial cells, which enhances the permeability of the blood vessels. (H) CCL18 released by TAMs aids the endoMT process via interaction with the PITPNM3 receptor on the endothelial cell surface, triggering activation of the extracellular signal-regulated kinase 1/2 and Akt/glycogen synthase kinase 3β signaling pathway. This results in decreased expression of VE-cadherin and upregulation of vimentin as well as fibronectin, which is indicative of endoMT.
Paracrine interaction between TAMs and mature endothelial cells also governs the sprouting process, which is the initial step in the formation of a new tubule structure from preexisting vasculature.44 Rymo et al showed a VEGFA-independent involvement of microglial cells in the process of vessel sprouting and branching in vivo.45 In relation to TAMs, IL-10 stimulation of TAMs/macrophages promotes VEGFA release via STAT3 signaling, as reported for both tumoral and nontumoral conditions.46,47 Likewise, glioblastoma-derived C-reactive protein promotes IL-6 and IL-1β production by COX2+ TAMs.48 IL-1β is known to promote the proliferation of endothelial cells and enhances endothelial expression of pro-angiogenic factors including IL-8, VEGFA, and hypoxia-inducible factor (HIF)-1α,49,50 which stimulate tip and stalk cell activity during the sprouting process (Fig. 2C). Tip cells are endothelial cells at the tip of newly formed blood vessels that determine the direction of growth. Tip cells are trailed by stalk cells, which are endothelial cells that form the new blood vessel lining (creating an open lumen for blood flow). IL-6 is able to promote epithelial-mesenchymal transition of cancer cells, which is typically operative in high-grade gliomas.51
Other paracrine factors that influence the sprouting process include TAM-derived WNT7b and M-CSF in breast cancer and glioma, which can activate the canonical WNT/β-catenin and insulin-like growth factor binding protein pathways, respectively, in endothelial cells, thereby increasing the expression of VEGFA and contributing to vascular sprouting52,53 (Fig. 2C). In a model of retinal vascular development, direct contact stimulation via Notch 1 on macrophages with Jagged1+ tip cells mediated the elongation of vascular sprouts via this molecular mechanism54 (Fig. 2D). In addition, direct binding of pro-angiogenic NRP1 expressed by macrophages to VEGFR2 present on tip cells can contribute to vessel branching during the sprouting process.55,56 Furthermore, macrophages act as cellular chaperons for tip cells to form anastomoses, which are fused junctions composed of 2 neighboring vessel sprouts. In relation to this process, macrophages have been reported to secrete VEGFC, which upregulates delta-like protein 4 in endothelial cells through VEGFR3 signaling. This leads to activation of transcription factor FoxC2, which mediates conversion of tip cells into stalk cells, contributing to the anastomotic process57 (Fig. 2E). Other paracrine pathways of TAM stimulation of endothelial cells include the release of YKL-40 (chitinase 3-like protein 1) by TAMs in response to granulocyte-macrophage (GM)/M-CSF and IL-6.58 YKL-40 is a secreted protein member of the chitinase gene family and has been demonstrated to be useful as a prognostic marker in various cancers, including glioblastoma.59 YKL-40 expression has previously shown to be associated with angiogenesis.60 YKL-40 release by TAMs triggers focal adhesion kinase–mitogen-activated protein kinase (MAPK) signaling in endothelial cells, leading to increased expression of VEGFR1 and VEGFR2, which promotes vessel sprouting61 (Fig. 2F).
Paracrine pathways of TAM stimulation of non-endothelial vascular cells have also been reported. Newly formed blood vessels require the coverage of perivascular cells called “mural cells” for vascular stabilization and vasomotion control. These mural cells include pericytes and vascular smooth muscle cells (VSMCs). PDGF-BB acts as one of the most potent chemoattractants of mural cells. Although PDGF-BB is produced by endothelial cells during angiogenesis, it is also secreted by TAMs. Perivascular TAMs aid in the recruitment of PDGFRβ+ mural cells, contributing to the coverage of newly formed blood vessels.62 Similarly, YKL-40 release by TAMs activates MAPK–nuclear factor-kappaB (NFκB) in VSMCs, which leads to secretion of the pro-angiogenic factor IL-863 (Fig. 2F). YKL-40 was also reported to promote the cell contacts between glioma stem cell–derived perivascular cells with endothelial cells, resulting in the stabilization of blood vessels in glioma.64 Another TAM-derived factor involved in mural recruitment is semaphorin 4D (Sema 4D), which has been shown to promote pericyte recruitment of neovessels, as demonstrated in a murine breast cancer model. Sema 4D from TAMs stimulates Plexin B1-RhoA signaling, which increases the expression of PDGFB and angiopoietin-like protein 4 (AGPTL4) in the endothelial cells.65 Increased expression of PDGFB promotes the recruitment of pericytes via PDGFRβ signaling, which in turn enhances perivascular coverage and neovessel stabilization (Fig. 2G).
Finally, paracrine stimulation of vascular cells by TAMs can also contribute to endothelial mesenchymal transition (endoMT), a process in which endothelial cells lose their specific vascular phenotype. For example, CCL18 secretion by (M2) type TAMs in breast cancer has been reported to bind to PITPNM3, a membrane-associated phosphatidylinositol transfer domain-containing protein located on endothelial cells. This interaction triggers downstream extracellular signal-regulated kinase and Akt/glycogen synthase kinase 3β/Snail signaling and promotes the endoMT process, thereby increasing the migration ability of endothelial cells51 (Fig. 2H).
Taken together, these data suggest that TAMs participate in all stages of angiogenesis, from vasculogenesis and early sprouting to late neovessel stabilization, under the (paracrine) influence of tumor cells. Since TAMs play such a critical role in the entire angiogenic process in tumors, their role in the resistance to anti-angiogenic drugs deserves to be scrutinized.66
TAMs and Anti-Angiogenic Therapy
Because neo-angiogenesis is an important component of tumorigenesis, it was expected that anti-angiogenic agents would be effective in the treatment of solid tumors. Unfortunately, the results of trials in which anti-angiogenic therapies were tested have been disappointing so far. The agents were designed to interfere with target molecules from common angiogenic pathways. The lack of success of these agents may be partly due to overlooking the critical role of TAMs in tumor angiogenesis.67 The main effect of anti-angiogenic therapy is reduction of the vascular bed, causing hypoxia and raised lactate levels. Subsequent elevation in intracellular HIF-1α levels leads to higher levels of pro-angiogenic factors secreted by the tumor cells that result in the paracrine activation of TAMs.68,69 In experimental mammary tumors, the administration of combretastatin A4 phosphate caused narrowing of the tumor blood vessel diameters and tumor necrosis, but increased levels of C-X-C chemokine ligand 12 (CXCL12), leading to raised numbers of C-X-C chemokine receptor 4–positive (CXCR4+) Tie-2+ monocytes in the tumors, limiting treatment efficiency.70 Over the last decennia, anti-angiogenic drugs targeting the receptor–ligand interactions by monoclonal antibodies like bevacizumab or tyrosine kinase inhibitors were widely applied in clinical practice. Both act as tumor suppressors by promoting TAMs with an antitumor phenotype71–75 or by decreasing monocyte recruitment.76–80 This beneficial effect is partially compromised by activation of HIF-1α pathways that lead to an angiogenic counterresponse.81 In pancreatic adenocarcinoma and murine breast cancers, activation of the Akt/phosphatidylinositol-3 kinase pathway by prolonged exposure to sorafenib triggers TAMs to adapt a tumor supportive and pro-angiogenic phenotype.82 Similar effects are observed in glial tumors—bevacizumab and aflibercept reduce the effects of VEGFA and increase CD206+ and Tie-2+ macrophages in human and murine gliomas by raising levels of endothelial cell–derived angiopoietin-2 (Ang2). The inhibition of Ang2 action together with the administration of bevacizumab or aflibercept results in significant improvement of therapeutic efficiency.83 Recent studies showed that treatment with the Ang2/VEGF bispecific antibody shifted TAMs into an antitumor phenotype, consequently resulting in vascular normalization and tumor regression in a mouse glioblastoma model.84,85 Following treatment with bevacizumab and cetuximab, the numbers of TAMs in glioblastoma and colorectal cancer increased and the cells became activated, respectively.81,86 In biopsies of recurrent gliomas treated with bevacizumab, more Tie-2 expressing monocytes and macrophages were detected. Furthermore, in an orthotopic glioma mouse model, an increase in Tie-2+ TAMs in response to anti-VEGF agents was observed.87 Recently, reduced MIF expression was observed in bevacizumab-resistant glioma cells, which in turn increased M2-like TAM recruitment, promoting tumor progression.88 In experimentally induced primitive neuroectodermal tumors, similar observations were made following drug-induced VEGFR2 blockade.89 Contrasting with these data are the reported effects of the pan-VEGFR inhibitor cediranib that transiently decreases the intratumoral infiltration of macrophages. However, cediranib also increases the VEGFA levels in serum and the number of CXCR4 and CD45 positive circulatory cells in peripheral blood, boosting angiogenesis in murine models of pancreatic neuroendocrine and mammary tumors.82 Cediranib induces JAK-STAT signaling in macrophages, leading to the activation of STAT3 that triggers angiogenesis by VEGFA production. In a murine xenograft glioma model, adverse effects of STAT3 activation in TAMs were counteracted by using the JAK-STAT–specific inhibitor AZD1480.90 Likewise, in a metastatic renal cell carcinoma model, the anti-angiogenic effects of sunitinib were enhanced by simultaneous treatment with STAT3 inhibitors.75 Tyrosine kinase inhibitors have more effects on immune cells. Erlotinib reportedly induces apoptosis of monocytic cell lines in vitro.77 The monoclonal antibodies cetuximab and StemRegenin 1 (SR1) enhance the phagocytic capability of macrophages, contributing to elimination of circulatory tumor cells.73,74 Sunitinib and imatinib change the cytokine secretion profiles of macrophages from pro-inflammatory to anti-inflammatory (IL-12 vs IL-10),72 and following the treatment with sorafenib, increased numbers of TAMs and levels of angiogenic factors including CXCL12 and IL-6 were detected.82 Recruitment of M2-like TAMs or in situ differentiation toward a tumor supportive TAM phenotype was reported after cediranib and axitinib treatment in glioma patients.91,92 A summary of TAM-mediated effects of different tyrosine kinase inhibitors in various therapeutic regimes is provided in Table 1 and Table 2.
Table 1.
Pro- and antitumor effects of multiple tyrosine kinase inhibitors mediated via TAMs
| Tyrosine Kinase Inhibitor | Molecular Target(s) | Investigation Setting | TAM Effect | Ref. |
|---|---|---|---|---|
| Sunitinib | VEGFR1-3, PDGFR-α/β, c-Kit, CSF-1R, Flt-3 | Inflammatory corneal lymphangiogenesis | Decreases recruitment of F4/80+ macrophages and activity of their secreted factors such as VEGFA, VEGFC | 77 |
| Human renal cell carcinoma | Inhibits myeloid cell proliferation. However, GM-CSF induced resistance of sunitinib was found in intratumor myeloid cells | 105 | ||
| Human recurrent glioblastoma | More severe hypoxia was induced, which increased macrophage infiltration | 80 | ||
| Gastrointestinal stroma tumor (GIST) | Promotes M1 macrophage secretion of IL-10 in vitro | 106 | ||
| Human primary breast cancer | Synergizes with α-GITR to induce antitumor macrophages via inhibition of STAT3 activity | 74 | ||
| Sorafenib | VEGFR1-3, PDGFR-β, Raf-1, B-Raf, CSF-1R | Hepatocellular carcinoma | Converts alternative TAMs to M1-like TAMs and increases secretion of pro-inflammatory cytokines | 70 |
| Triggers macrophage- mediated cytotoxic NK cell activation | ||||
| Murine breast cancer | Increases IL-12 and suppression of IL-10 levels in macrophage | 71 | ||
| Human peripheral CD14+ monocyte derived macrophage | Induces apoptosis and autophagy in macrophages | 108 | ||
| Mouse model for metastatic liver cancer model | Increases peripheral and F4/80+ and CD11b+ macrophage recruitment and infiltration in cancer tissues | 109 | ||
| Mouse breast cancer model and pancreatic β-cell tumor model | Increases angiogenic and immunosuppressive molecules. Can be blocked by PI3K inhibitor IPI145 | 81 | ||
| Classical Hodgkin’s lymphoma | Blocks CSF-1R activity | 107 | ||
| Imatinib | c-Kit | Murine GIST model; human GIST | Shifts TAM into a more M2-like protumor phenotype macrophage | 110 |
| Promotes M1 macrophage secretion of IL-10 in vitro | 106 | |||
| Cediranib | VEGFR1-3 | Alveolar soft part sarcoma (ASPS) | Increases CD68+ and M2-like TAMs and increases CD163, Tie-2, and CCL2 mRNA levels | 111 |
| Transiently decreases macrophage infiltration. Increases plasma VEGFA levels and CXCR4+ CD45+ immune cells. | ||||
| Murine glioma xenograft model | Increases phosphorylation of STAT3 in macrophages. In combination with AZD1480 JAK2 inhibitor, decreases phospho- STAT3+ macrophages | 89 | ||
| Axitinib | VEGFR1-3, PDGFR-β and c-Kit | Murine glioma xenograft model | Inhibits metronomic cyclophosphamide activated antitumor innate immunity and shifts TAM into an M2-like phenotype | 91 |
| Erlotinib | EGFR, ErbB1 | Human U937 cell line induced macrophage | Inhibits monocyte-macrophage differentiation and proliferation | 76 |
| Non small cell lung cancer | Administration of erlotinib induces macrophage and other mononuclear cell infiltration into skin | 112 | ||
| Dasatinib | EGFR, ErbB1 | Murine bone marrow– derived macrophage; RAW264.7 cell line | Induces macrophages with anti-inflammatory function by increasing IL-10 production and suppressing TNF- α and IL-12p40 | 113 |
| Bosutinib | ||||
| PLX3397 | c-Kit, c-Fms inhibitor | Malignant peripheral nerve sheath tumor | Depleting Iba-1+ macrophages combined with rapamycin leads to more severe depletion of TAMs | 78 |
| Recurrent glioblastoma | Depletes CD11b+ cells and potentiates the response of the intracranial tumors to irradiation | 79 | ||
Abbreviations: EGFR, epidermal growth factor receptor; GITR, glucocorticoid-induced tumor necrosis factor (TNF) receptor; NK, natural killer; PI3K, phosphatidylinositol-3 kinase.
Table 2.
Effects of monoclonal antibodies-based angiogenic therapies on TAMs
| Monoclonal Antibodies- Based Therapeutic Agent | Molecular Target | Tumor Type | TAM Effect | Ref. |
|---|---|---|---|---|
| Bevacizumab | VEGFA | Glioblastoma | Induces Tie-2+ monocyte infiltration and vast infiltration of M2-like macrophages | 86 |
| Increases phosphorylation of STAT3 in macrophages, promoting a tumor supportive phenotype | 89 | |||
| Crossmab,A2V | Angiopoietin-2/ VEGFA bispecific | Transits TAM from M2-like phenotype to M1-like phenotype. Prolonging tumor-bearing mice survival time | 84 | |
| MEDI3617 | Angiopoietin-2 | Promoting tumor vessel normalization and M1-like polarization of TAM when coadministered with cediranib | 83 | |
| Cetuximab | Epidermal growth factor receptor | Colorectal carcinoma | Increases phagocytosis by macrophages to eliminate circulating cancer cells | 72 |
| Activation of M2-like macrophages | 85 | |||
| Heck and neck cancer | Ameliorates suppressive phenotypes of Fcγ receptor bearing myeloid cells in cancer patients | 112 | ||
| Trastuzumab | HER2 | HER2+ breast cancers | Decreases CD68+ macrophage infiltration | 75 |
| SR1 | c-Kit | Imatinib resistant GIST | Increases phagocytosis in macrophages | 73 |
Abbreviations: EGFR, epidermal growth factor receptor; GIST, gastrointestinal stromal tumor; HER2, human epidermal growth factor receptor 2.
Current Perspectives on Targeting Pro-Angiogenic TAMs
Inhibition of Tumor-Related Hematopoiesis and Blocking of Activating Signals for TAMs
Because the majority of TAMs are recruited from bone marrow–derived peripheral monocytes, the inhibition of tumor-related granulo-monocytopoiesis and interference with the monocyte recruitment signals will contribute to tumor suppression and inhibit tumor vascularization.93 Inhibition of CSF-1R to deplete the TAM population increases survival time and decreases tumor microvascular density in animal cancer models.94,95 Patients with tenosynovial giant cell tumors and glioblastomas treated with PLX3397 (CSF-1R blocker) showed a good tolerance to the drug, but only the patients with tenosynovial giant cell tumors showed a significant reduction in tumor size.96 In recurrent glioblastoma, only a trend toward a decrease in the number of Iba-1+ microglia/macrophages and a lower number of CD14dim, CD16+ monocytes in the glioma tissues were observed.97
Inhibition of Recruitment Signals that Guide Circulatory Monocytes into Tumor Tissue
TAMs predominantly originate from recruited peripheral inflammatory monocytes. CCL2-CCR2 chemoattractive signaling is the most studied mechanism in monocyte recruitment. The effectiveness of a CCR2 antagonist in decreasing macrophage infiltration and reducing tumor size was demonstrated in hepatocellular carcinoma,98 and AMD3100, an inhibitor of CXCR4, blocks the infiltration of Tie-2+ monocytes.99 By responding to VEGFA gradients via cell surface expression of Flt-1, infiltrating monocytes are guided into cancer tissues. The agent intravitreal ranibizumab blocks the phosphorylation of VEGFR1 and decreases migration capacity of TAMs in experimental colonic cancer, resulting in reduced numbers of TAMs and reduction in tumor angiogenesis.100
Targeting Macrophage Mediated/Secreted Angiogenic Molecules
Current studies in cancer therapy focus on the development of inhibitors and monoclonal antibodies against central regulatory molecules. Some of these interfere with TAM-mediated tumor angiogenesis and were tested in clinical trials. Monoclonal antibody–based therapy that targets VEGFC has proven to inhibit vascularization and reduce carcinogenesis of skin squamous carcinoma.101 Blocking of VEGFC using the monoclonal antibody VGX-100 also inhibited infiltration of CD11b+ myeloid cells.102 Monoclonal antibodies targeting Sema 4D showed promising results in inhibiting cancer growth and reduced tumor angiogenesis.103 WNT7b activates the VEGFA-mediated angiogenic switch through stimulation of the WNT/β-catenin canonical pathway. In contrast, WNT5a, a Wnt ligand that mainly triggers noncanonical pathways, suppresses vessel sprouting by upregulation of soluble VEGFR1.104 The canonical and noncanonical Wnt signaling pathways may well mediate opposite angiogenic responses. Both inhibition of the Wnt canonical pathway by inhibitors and agonist stimulation of the noncanonical Wnt pathways may become future strategies for anti-angiogenesis. The presence of certain TAM subtypes, as well as circulatory TAM-secreted serum markers, could also be used as parameters to select receptive patients for anti-angiogenic therapy and predict therapeutic efficiency. For example, in a single-arm phase II clinical trial, glioblastoma patients who received the VEGF trapper aflibercept showed elevated serum levels of placenta growth factor (PIGF) and matrix metalloproteinase 9, indicative of a poor prognosis. Patients with lower serum levels of PIGF and VEGFR1+ monocytes and elevated levels of MIF, monocyte chemoattractant protein 3, and CCL27 benefited from anti-VEGF treatment.105
Conclusion
Investigations of the recently identified roles of TAMs in angiogenesis may well lead to the development of biomarkers for assessing the anti-angiogenic therapy efficacy and receptiveness, and could help to identify therapeutic targets for novel anti-angiogenic treatments.
Funding
C.C. was supported by the Netherlands Foundation for Cardiovascular Excellence and by the Netherlands Cardiovascular Research Initiative, an initiative with support of the Dutch Heart Foundation (CVON2014-11 RECONNECT); VIDI grant 91714302; the ErasmusMC fellowship grant, and the RM fellowship grant of the UMC Utrecht. C.Z. was supported by the Chinese Scholar Council (201206230102).
Conflict of interest statement
None.
References
- 1. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bajenaru ML, Garbow JR, Perry A, Hernandez MR, Gutmann DH. Natural history of neurofibromatosis 1-associated optic nerve glioma in mice. Ann Neurol. 2005;57(1):119–127. [DOI] [PubMed] [Google Scholar]
- 3. Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol. 2013;35(5):585–600. [DOI] [PubMed] [Google Scholar]
- 4. Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19(1):20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murakami M, Zheng Y, Hirashima M, et al. VEGFR1 tyrosine kinase signaling promotes lymphangiogenesis as well as angiogenesis indirectly via macrophage recruitment. Arterioscler Thromb Vasc Biol. 2008;28(4):658–664. [DOI] [PubMed] [Google Scholar]
- 6. Ji JD, Park-Min KH, Ivashkiv LB. Expression and function of semaphorin 3A and its receptors in human monocyte-derived macrophages. Hum Immunol. 2009;70(4):211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sabag AD, Bode J, Fink D, Kigel B, Kugler W, Neufeld G. Semaphorin-3D and semaphorin-3E inhibit the development of tumors from glioblastoma cells implanted in the cortex of the brain. PLoS One. 2012;7(8):e42912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Casazza A, Laoui D, Wenes M, et al. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell. 2013;24(6):695–709. [DOI] [PubMed] [Google Scholar]
- 9. Schuch G, Machluf M, Bartsch G, Jr, et al. In vivo administration of vascular endothelial growth factor (VEGF) and its antagonist, soluble neuropilin-1, predicts a role of VEGF in the progression of acute myeloid leukemia in vivo. Blood. 2002;100(13):4622–4628. [DOI] [PubMed] [Google Scholar]
- 10. Kerber M, Reiss Y, Wickersheim A, et al. Flt-1 signaling in macrophages promotes glioma growth in vivo. Cancer Res. 2008;68(18):7342–7351. [DOI] [PubMed] [Google Scholar]
- 11. Yotsumoto F, You WK, Cejudo-Martin P, Kucharova K, Sakimura K, Stallcup WB. NG2 proteoglycan-dependent recruitment of tumor macrophages promotes pericyte-endothelial cell interactions required for brain tumor vascularization. Oncoimmunology. 2015;4(4):e1001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumar V, Cheng P, Condamine T, et al. CD45 phosphatase inhibits STAT3 transcription factor activity in myeloid cells and promotes tumor-associated macrophage differentiation. Immunity. 2016;44(2):303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Franklin RA, Liao W, Sarkar A, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344(6186):921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mills CD. Anatomy of a discovery: m1 and m2 macrophages. Front Immunol. 2015;6:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177(10):7303–7311. [DOI] [PubMed] [Google Scholar]
- 16. Movahedi K, Laoui D, Gysemans C, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70(14):5728–5739. [DOI] [PubMed] [Google Scholar]
- 17. Laoui D, Van Overmeire E, Movahedi K, et al. Mononuclear phagocyte heterogeneity in cancer: different subsets and activation states reaching out at the tumor site. Immunobiology. 2011;216(11):1192–1202. [DOI] [PubMed] [Google Scholar]
- 18. Szulzewsky F, Pelz A, Feng X, et al. Glioma-associated microglia/macrophages display an expression profile different from M1 and M2 polarization and highly express Gpnmb and Spp1. PLoS One. 2015;10(2):e0116644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gabrusiewicz K, Rodriguez B, Wei J, et al. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI Insight. 2016;1(2):pii: e85841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sasaki A. Microglia and brain macrophages: an update. Neuropathology. 2016;doi: 10.1111/neup.12354. [DOI] [PubMed] [Google Scholar]
- 21. Reinartz S, Schumann T, Finkernagel F, et al. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: correlation of CD163 expression, cytokine levels and early relapse. Int J Cancer. 2014;134(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cai J, Zhang W, Yang P, et al. Identification of a 6-cytokine prognostic signature in patients with primary glioblastoma harboring M2 microglia/macrophage phenotype relevance. PLoS One. 2015;10(5):e0126022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang S, Dehn S, DeBerge M, Rhee KJ, Hudson B, Thorp EB. Phagocyte-myocyte interactions and consequences during hypoxic wound healing. Cell Immunol. 2014;291(1-2):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elshabrawy HA, Chen Z, Volin MV, Ravella S, Virupannavar S, Shahrara S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis. 2015;18(4):433–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8(8):618–631. [DOI] [PubMed] [Google Scholar]
- 26. Karasawa K, Asano K, Moriyama S, et al. Vascular-resident CD169-positive monocytes and macrophages control neutrophil accumulation in the kidney with ischemia-reperfusion injury. J Am Soc Nephrol. 2015;26(4):896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hughes R, Qian BZ, Rowan C, et al. Perivascular M2 macrophages stimulate tumor relapse after chemotherapy. Cancer Res. 2015;75(17):3479–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Valverde LF, Pereira TA, Dias RB, et al. Macrophages and endothelial cells orchestrate tumor-associated angiogenesis in oral cancer via hedgehog pathway activation. Tumour Biol. 2016;37(7):9233–9241. [DOI] [PubMed] [Google Scholar]
- 29. Park JY, Sung JY, Lee J, et al. Polarized CD163+ tumor-associated macrophages are associated with increased angiogenesis and CXCL12 expression in gastric cancer. Clin Res Hepatol Gastroenterol. 2016;40(3):357–365. [DOI] [PubMed] [Google Scholar]
- 30. Marinaccio C, Ingravallo G, Gaudio F, et al. Microvascular density, CD68 and tryptase expression in human diffuse large B-cell lymphoma. Leuk Res. 2014;38(11):1374–1377. [DOI] [PubMed] [Google Scholar]
- 31. Mignogna C, Signorelli F, Vismara MF, et al. A reappraisal of macrophage polarization in glioblastoma: Histopathological and immunohistochemical findings and review of the literature. Pathol Res Pract. 2016;212(6):491–499. [DOI] [PubMed] [Google Scholar]
- 32. Lewis CE, De Palma M, Naldini L. Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer Res. 2007;67(18):8429–8432. [DOI] [PubMed] [Google Scholar]
- 33. Nishie A, Ono M, Shono T, et al. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res. 1999;5(5):1107–1113. [PubMed] [Google Scholar]
- 34. Hirano H, Tanioka K, Yokoyama S, et al. Angiogenic effect of thymidine phosphorylase on macrophages in glioblastoma multiforme. J Neurosurg. 2001;95(1):89–95. [DOI] [PubMed] [Google Scholar]
- 35. Deininger MH, Meyermann R, Trautmann K, et al. Heme oxygenase (HO)-1 expressing macrophages/microglial cells accumulate during oligodendroglioma progression. Brain Res. 2000;882(1-2):1–8. [DOI] [PubMed] [Google Scholar]
- 36. Fan Y, Ye J, Shen F, et al. Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J Cereb Blood Flow Metab. 2008;28(1):90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmeisser A, Garlichs CD, Zhang H, et al. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc Res. 2001;49(3):671–680. [DOI] [PubMed] [Google Scholar]
- 38. Yan D, Wang X, Li D, Qu Z, Ruan Q. Macrophages overexpressing VEGF, transdifferentiate into endothelial-like cells in vitro and in vivo. Biotechnol Lett. 2011;33(9):1751–1758. [DOI] [PubMed] [Google Scholar]
- 39. Ribatti D, Vacca A. The role of monocytes-macrophages in vasculogenesis in multiple myeloma. Leukemia. 2009;23(9):1535–1536. [DOI] [PubMed] [Google Scholar]
- 40. Scavelli C, Nico B, Cirulli T, et al. Vasculogenic mimicry by bone marrow macrophages in patients with multiple myeloma. Oncogene. 2008;27(5):663–674. [DOI] [PubMed] [Google Scholar]
- 41. Chen H, Campbell RA, Chang Y, et al. Pleiotrophin produced by multiple myeloma induces transdifferentiation of monocytes into vascular endothelial cells: a novel mechanism of tumor-induced vasculogenesis. Blood. 2009;113(9):1992–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang L, Xu Y, Sun J, et al. M2-like tumor-associated macrophages drive vasculogenic mimicry through amplification of IL-6 expression in glioma cells. Oncotarget. 2016;8(1):819–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rong X, Huang B, Qiu S, Li X, He L, Peng Y. Tumor-associated macrophages induce vasculogenic mimicry of glioblastoma multiforme through cyclooxygenase-2 activation. Oncotarget. 2016;7(51):83976–83986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Clear AJ, Lee AM, Calaminici M, et al. Increased angiogenic sprouting in poor prognosis FL is associated with elevated numbers of CD163+ macrophages within the immediate sprouting microenvironment. Blood. 2010;115(24):5053–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rymo SF, Gerhardt H, Wolfhagen Sand F, Lang R, Uv A, Betsholtz C. A two-way communication between microglial cells and angiogenic sprouts regulates angiogenesis in aortic ring cultures. PLoS One. 2011;6(1):e15846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nakamura R, Sene A, Santeford A, et al. IL10-driven STAT3 signalling in senescent macrophages promotes pathological eye angiogenesis. Nat Commun. 2015;6:7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu WK, Llewellyn OP, Bates DO, Nicholson LB, Dick AD. IL-10 regulation of macrophage VEGF production is dependent on macrophage polarisation and hypoxia. Immunobiology. 2010;215(9–10):796–803. [DOI] [PubMed] [Google Scholar]
- 48. Nijaguna MB, Schröder C, Patil V, et al. Definition of a serum marker panel for glioblastoma discrimination and identification of Interleukin 1β in the microglial secretome as a novel mediator of endothelial cell survival induced by C-reactive protein. J Proteomics. 2015;128:251–261. [DOI] [PubMed] [Google Scholar]
- 49. Hou Z, Falcone DJ, Subbaramaiah K, Dannenberg AJ. Macrophages induce COX-2 expression in breast cancer cells: role of IL-1β autoamplification. Carcinogenesis. 2011;32(5):695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carmi Y, Dotan S, Rider P, et al. The role of IL-1β in the early tumor cell-induced angiogenic response. J Immunol. 2013;190(7):3500–3509. [DOI] [PubMed] [Google Scholar]
- 51. Lin L, Chen YS, Yao YD, et al. CCL18 from tumor-associated macrophages promotes angiogenesis in breast cancer. Oncotarget. 2015;6(33):34758–34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yeo EJ, Cassetta L, Qian BZ, et al. Myeloid WNT7b mediates the angiogenic switch and metastasis in breast cancer. Cancer Res. 2014;74(11):2962–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nijaguna MB, Patil V, Urbach S, et al. Glioblastoma-derived macrophage colony-stimulating factor (MCSF) induces microglial release of insulin-like growth factor-binding protein 1 (IGFBP1) to promote angiogenesis. J Biol Chem. 2015;290(38):23401–23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Outtz HH, Tattersall IW, Kofler NM, Steinbach N, Kitajewski J. Notch1 controls macrophage recruitment and Notch signaling is activated at sites of endothelial cell anastomosis during retinal angiogenesis in mice. Blood. 2011;118(12):3436–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fantin A, Vieira JM, Plein A, et al. NRP1 acts cell autonomously in endothelium to promote tip cell function during sprouting angiogenesis. Blood. 2013;121(12):2352–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fantin A, Vieira JM, Gestri G, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tammela T, Zarkada G, Nurmi H, et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat Cell Biol. 2011;13(10):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Junker N, Johansen JS, Andersen CB, Kristjansen PE. Expression of YKL-40 by peritumoral macrophages in human small cell lung cancer. Lung Cancer. 2005;48(2):223–231. [DOI] [PubMed] [Google Scholar]
- 59. Johansen JS, Jensen BV, Roslind A, Nielsen D, Price PA. Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol Biomarkers Prev. 2006;15(2):194–202. [DOI] [PubMed] [Google Scholar]
- 60. De Ceuninck F, Pastoureau P, Bouet F, Bonnet J, Vanhoutte PM. Purification of guinea pig YKL40 and modulation of its secretion by cultured articular chondrocytes. J Cell Biochem. 1998;69(4):414–424. [DOI] [PubMed] [Google Scholar]
- 61. Shao R. YKL-40 acts as an angiogenic factor to promote tumor angiogenesis. Front Physiol. 2013;4:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Spiller KL, Anfang RR, Spiller KJ, et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35(15):4477–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tang H, Shi Z, Xiu Q, Li B, Sun Y. YKL-40-mediated interleukin 8 production may be closely associated with remodeling of bronchial smooth muscle cells. Am J Respir Crit Care Med. 2012;186(4):386; author reply 386–386; author reply 387. [DOI] [PubMed] [Google Scholar]
- 64. Francescone R, Ngernyuang N, Yan W, Bentley B, Shao R. Tumor-derived mural-like cells coordinate with endothelial cells: role of YKL-40 in mural cell-mediated angiogenesis. Oncogene. 2014;33(16):2110–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhou H, Yang YH, Basile JR. The Semaphorin 4D-Plexin-B1-RhoA signaling axis recruits pericytes and regulates vascular permeability through endothelial production of PDGF-B and ANGPTL4. Angiogenesis. 2014;17(1):261–274. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66. Li JL, Sainson RC, Oon CE, et al. DLL4-Notch signaling mediates tumor resistance to anti-VEGF therapy in vivo. Cancer Res. 2011;71(18):6073–6083. [DOI] [PubMed] [Google Scholar]
- 67. van Beijnum JR, Nowak-Sliwinska P, Huijbers EJ, Thijssen VL, Griffioen AW. The great escape; the hallmarks of resistance to antiangiogenic therapy. Pharmacol Rev. 2015;67(2):441–461. [DOI] [PubMed] [Google Scholar]
- 68. Van Overmeire E, Laoui D, Keirsse J, Van Ginderachter JA. Hypoxia and tumor-associated macrophages: a deadly alliance in support of tumor progression. Oncoimmunology. 2014;3(1):e27561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Laoui D, Van Overmeire E, Di Conza G, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res. 2014;74(1):24–30. [DOI] [PubMed] [Google Scholar]
- 70. Welford AF, Biziato D, Coffelt SB, et al. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. J Clin Invest. 2011;121(5):1969–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sprinzl MF, Reisinger F, Puschnik A, et al. Sorafenib perpetuates cellular anticancer effector functions by modulating the crosstalk between macrophages and natural killer cells. Hepatology. 2013;57(6):2358–2368. [DOI] [PubMed] [Google Scholar]
- 72. Edwards JP, Emens L. The multikinase inhibitor sorafenib reverses the suppression of IL-12 and enhancement of IL-10 by PGE2 in murine macrophages. J Immunother. 2010;33(8):869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gül N, Babes L, Siegmund K, et al. Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. J Clin Invest. 2014;124(2):812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Edris B, Willingham SB, Weiskopf K, et al. Anti-KIT monoclonal antibody inhibits imatinib-resistant gastrointestinal stromal tumor growth. Proc Natl Acad Sci U S A. 2013;110(9):3501–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yu N, Fu S, Xu Z, et al. Synergistic antitumor responses by combined GITR activation and sunitinib in metastatic renal cell carcinoma. Int J Cancer. 2016;138(2):451–462. [DOI] [PubMed] [Google Scholar]
- 76. Arnould L, Gelly M, Penault-Llorca F, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94(2):259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Savikko J, Rintala JM, Rintala S, Koskinen P. Epidermal growth factor receptor inhibition by erlotinib prevents vascular smooth muscle cell and monocyte-macrophage function in vitro. Transpl Immunol. 2015;32(3):175–178. [DOI] [PubMed] [Google Scholar]
- 78. Detry B, Blacher S, Erpicum C, et al. Sunitinib inhibits inflammatory corneal lymphangiogenesis. Invest Ophthalmol Vis Sci. 2013;54(5):3082–3093. [DOI] [PubMed] [Google Scholar]
- 79. Patwardhan PP, Surriga O, Beckman MJ, et al. Sustained inhibition of receptor tyrosine kinases and macrophage depletion by PLX3397 and rapamycin as a potential new approach for the treatment of MPNSTs. Clin Cancer Res. 2014;20(12):3146–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stafford JH, Hirai T, Deng L, et al. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro Oncol. 2016;18(6):797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Piao Y, Liang J, Holmes L, et al. Glioblastoma resistance to anti-VEGF therapy is associated with myeloid cell infiltration, stem cell accumulation, and a mesenchymal phenotype. Neuro Oncol. 2012;14(11):1379–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rivera LB, Meyronet D, Hervieu V, Frederick MJ, Bergsland E, Bergers G. Intratumoral myeloid cells regulate responsiveness and resistance to antiangiogenic therapy. Cell Rep. 2015;11(4):577–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Scholz A, Harter PN, Cremer S, et al. Endothelial cell-derived angiopoietin-2 is a therapeutic target in treatment-naive and bevacizumab-resistant glioblastoma. Embo Mol Med. 2016;8(1):39–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Peterson TE, Kirkpatrick ND, Huang Y, et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc Natl Acad Sci U S A. 2016;113(16):4470–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kloepper J, Riedemann L, Amoozgar Z, et al. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc Natl Acad Sci U S A. 2016;113(16):4476–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pander J, Heusinkveld M, van der Straaten T, et al. Activation of tumor-promoting type 2 macrophages by EGFR-targeting antibody cetuximab. Clin Cancer Res. 2011;17(17):5668–5673. [DOI] [PubMed] [Google Scholar]
- 87. Gabrusiewicz K, Liu D, Cortes-Santiago N, et al. Anti-vascular endothelial growth factor therapy-induced glioma invasion is associated with accumulation of Tie2-expressing monocytes. Oncotarget. 2014;5(8):2208–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Castro BA, Flanigan P, Jahangiri A, et al. Macrophage migration inhibitory factor downregulation: a novel mechanism of resistance to anti-angiogenic therapy. Oncogene. 2017; doi: 10.1038/onc.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rigamonti N, Kadioglu E, Keklikoglou I, Wyser Rmili C, Leow CC, De Palma M. Role of angiopoietin-2 in adaptive tumor resistance to VEGF signaling blockade. Cell Rep. 2014;8(3):696–706. [DOI] [PubMed] [Google Scholar]
- 90. de Groot J, Liang J, Kong LY, et al. Modulating antiangiogenic resistance by inhibiting the signal transducer and activator of transcription 3 pathway in glioblastoma. Oncotarget. 2012;3(9):1036–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lu-Emerson C, Snuderl M, Kirkpatrick ND, et al. Increase in tumor-associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastoma. Neuro Oncol. 2013;15(8):1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Doloff JC, Waxman DJ. VEGF receptor inhibitors block the ability of metronomically dosed cyclophosphamide to activate innate immunity-induced tumor regression. Cancer Res. 2012;72(5):1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Strauss L, Sangaletti S, Consonni FM, et al. RORC1 regulates tumor-promoting “Emergency” granulo-monocytopoiesis. Cancer Cell. 2015;28(2):253–269. [DOI] [PubMed] [Google Scholar]
- 94. Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73(3):1128–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Strachan DC, Ruffell B, Oei Y, et al. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8(+) T cells. Oncoimmunology. 2013;2(12):e26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tap WD, Wainberg ZA, Anthony SP, et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N Engl J Med. 2015;373(5):428–437. [DOI] [PubMed] [Google Scholar]
- 97. Butowski N, Colman H, De Groot JF, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy foundation early phase clinical trials consortium phase II study. Neuro Oncol. 2016;18(4):557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Li X, Yao W, Yuan Y, et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66(1):157–167. [DOI] [PubMed] [Google Scholar]
- 99. De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23(3):277–286. [DOI] [PubMed] [Google Scholar]
- 100. Cicatiello V, Apicella I, Tudisco L, et al. Powerful anti-tumor and anti-angiogenic activity of a new anti-vascular endothelial growth factor receptor 1 peptide in colorectal cancer models. Oncotarget. 2015;6(12):10563–10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Alitalo AK, Proulx ST, Karaman S, et al. VEGF-C and VEGF-D blockade inhibits inflammatory skin carcinogenesis. Cancer Res. 2013;73(14):4212–4221. [DOI] [PubMed] [Google Scholar]
- 102. Hajrasouliha AR, Funaki T, Sadrai Z, Hattori T, Chauhan SK, Dana R. Vascular endothelial growth factor-C promotes alloimmunity by amplifying antigen-presenting cell maturation and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2012;53(3):1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sierra JR, Corso S, Caione L, et al. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. J Exp Med. 2008;205(7):1673–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Stefater JA, 3rd, Lewkowich I, Rao S, et al. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature. 2011;474(7352):511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. de Groot JF, Piao Y, Tran H, et al. Myeloid biomarkers associated with glioblastoma response to anti-VEGF therapy with aflibercept. Clin Cancer Res. 2011;17(14):4872–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ko JS, Rayman P, Ireland J, et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70(9):3526–3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. van Dongen M, Savage ND, Jordanova ES, et al. Anti-inflammatory M2 type macrophages characterize metastasized and tyrosine kinase inhibitor-treated gastrointestinal stromal tumors. Int J Cancer. 2010;127(4):899–909. [DOI] [PubMed] [Google Scholar]
- 108. Ullrich K, Wurster KD, Lamprecht B, et al. BAY 43-9006/Sorafenib blocks CSF1R activity and induces apoptosis in various classical Hodgkin lymphoma cell lines. Br J Haematol. 2011;155(3):398–402. [DOI] [PubMed] [Google Scholar]
- 109. Lin JC, Liu CL, Lee JJ, et al. Sorafenib induces autophagy and suppresses activation of human macrophage. Int Immunopharmacol. 2013;15(2):333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhang W, Zhu XD, Sun HC, et al. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16(13):3420–3430. [DOI] [PubMed] [Google Scholar]
- 111. Cavnar MJ, Zeng S, Kim TS, et al. KIT oncogene inhibition drives intratumoral macrophage M2 polarization. J Exp Med. 2013;210(13):2873–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kummar S, Allen D, Monks A, et al. Cediranib for metastatic alveolar soft part sarcoma. J Clin Oncol. 2013;31(18):2296–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Guttman-Yassky E, Mita A, De Jonge M, et al. Characterisation of the cutaneous pathology in non-small cell lung cancer (NSCLC) patients treated with the EGFR tyrosine kinase inhibitor erlotinib. Eur J Cancer. 2010;46(11):2010–2019. [DOI] [PubMed] [Google Scholar]
- 114. Ozanne J, Prescott AR, Clark K. The clinically approved drugs dasatinib and bosutinib induce anti-inflammatory macrophages by inhibiting the salt-inducible kinases. Biochem J. 2015;465(2):271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Li J, Srivastava RM, Ettyreddy A, Ferris RL. Cetuximab ameliorates suppressive phenotypes of myeloid antigen presenting cells in head and neck cancer patients. J Immunother Cancer. 2015;3:54. [DOI] [PMC free article] [PubMed] [Google Scholar]