Abstract
Poor emotion recognition is a core deficit in schizophrenia and is associated with poor functional outcome. Functional magnetic resonance imaging (fMRI) multivariate analysis methods were used to elucidate the neural underpinnings of face and emotion processing associated with both genetic liability and disease-specific effects. Schizophrenia patients, relatives, and controls completed a task that included 4 facial emotion discrimination conditions and an age discrimination condition during fMRI. Three functional networks were derived from the data: the first involved in visual attention and response generation, the second a default mode network (DMN), and a third involved in face and emotion processing. No differences in activation were found between groups for the visual attention and response generation network, suggesting that basic processes were intact. Both schizophrenia patients and relatives showed evidence for hyperdeactivation in the DMN compared to controls, with relatives being intermediate, suggesting a genetic liability effect. Both disease-specific and genetic liability effects were found for the face processing network, which included the amygdala. Patients exhibited lower coordinated network activity compared to controls and relatives across all facial discrimination conditions. Additionally, in relation to the other emotion discrimination conditions, a heightened coordinated response during fear and anger discrimination was observed in schizophrenia compared to other conditions, whereas relatives demonstrated heightened coordinated activity for anger discrimination only relative to other emotion conditions. With regards to brain functioning, this study found that schizophrenia is associated with abnormal processing of threat-related information, and that in part may be associated with the genetic risk for the disorder, suggesting that the facial and emotion processing network could be targeted for intervention.
Keywords: facial perception, emotion processing, psychosis, functional magnetic resonance imaging (fMRI), genetic risk, endophenotype
Introduction
The ability to accurately recognize facial emotions is a core deficit in schizophrenia1,2 that is associated with functional outcome.3,4 Additionally, behavioral and brain activation abnormalities related to emotion recognition have been found in the biological relatives of patients, suggesting an association with the genetic liability for the disorder.5–8 There is also some evidence that facial emotion recognition deficits may be a specific deficit over and above other lower-level cognitive deficits in schizophrenia patients.9,10 The goal of this investigation was to use a family study design and functional magnetic resonance imaging (fMRI) task-based functional connectivity analyses to better measure the neural underpinnings of face and emotion recognition associated with both genetic liability and disease-specific effects in schizophrenia.
Two recent meta-analyses investigating emotion recognition have demonstrated consistent decreased activation in schizophrenia patients compared to controls, including the limbic (amygdala, hippocampus), visual (fusiform gyrus, occipital cortex), medial frontal, and subcortical (caudate, putamen) regions.11,12 The few individual fMRI studies of facial and emotion processing to investigate family members have focused primarily on the amygdala and report mixed findings, including reduced activation,5,13 increased activation,14,15 or no differences compared to controls.16
One of the challenges in identifying the functional underpinnings of impaired facial emotion recognition in schizophrenia is that the facial recognition network is highly distributed and interconnected.17,18 Recently, the field of functional neuroimaging has used more sophisticated analysis techniques, including functional connectivity. Functional connectivity focuses on functional integration, which provides enriched information of how anatomically distinct brain regions work cohesively, as opposed to focusing on functional segregation and localization of function in the brain.19 As schizophrenia is hypothesized to be a disorder of brain connectivity,20 functional connectivity analyses of emotion recognition in schizophrenia are imperative. However, the number of studies using functional connectivity to study emotion recognition is limited. A few studies investigating emotional processing of faces report altered (primarily reduced) functional connectivity between the amygdala and a range of (primarily frontal) regions in schizophrenia.21–25 However, these studies used a region-of-interest based approach with the amygdala as a seed region, thereby forgoing the opportunity to study a range of networks not necessarily co-activating with the amygdala. Two previous studies have investigated connectivity in family members of schizophrenia patients using sophisticated methods including graph theory or dynamic causal modeling. Both studies found amygdala-based networks and lower coordinated activity for relatives compared to controls, particularly for negative valence expressions,6,26 but investigated only a restricted set of brain regions.
Collapsing across different types of tasks, a recent systematic review of functional connectivity studies in schizophrenia concluded that although both decreased and increased connectivity patterns compared to controls are reported, the majority of studies report decreased functional connectivity in schizophrenia, particularly involving frontal and frontotemporal networks.20 Furthermore, this trend was also observed in studies of individuals in the putatively prodromal risk phase and in relatives of schizophrenia patients, suggesting that this pattern of dysconnectivity was associated with the genetic risk for the disorder.20
In the present study, we investigated functional connectivity during facial recognition in schizophrenia patients, nonpsychotic relatives, and community controls, to characterize potential genetic and disease-specific markers at the network level. We used a task that manipulated the relevance of the affective information to the task-relevant response. The task contrasted making emotional judgments about an emotive face (eg, fear discrimination) compared to making a nonemotional judgment about an emotive face (ie, age discrimination).27,28 Therefore, the emotion discrimination conditions required the explicit processing of emotions to make a task-relevant response. The age discrimination condition involved the implicit processing of emotions; however, the emotional information was not necessary to make a task-relevant response. Thus, the pertinence of the emotional information to making a correct response was manipulated. Two fMRI studies in healthy individuals have demonstrated that age discrimination activated similar regions to emotion discrimination.27,28 However, when the emotion discrimination condition was directly contrasted with age discrimination, there was greater activation during emotion discrimination (and not found for the age minus emotion discrimination contrast) for the amygdala, hippocampus, and parahippocampus, suggesting the relevance of the affective information to a task-related response modulates the intensity of functional activations.27,28 Second, we used a connectivity method which allowed examination of task-related functional brain networks across the brain not restricted to specific regions of interest, and provided an estimation of the task-related post-stimulus blood oxygen level-dependent (BOLD) activity for every subject and condition for each network. We hypothesized that compared to community controls, schizophrenia patients would demonstrate abnormal functional connectivity for emotion discrimination. We further hypothesized that nonpsychotic relatives would display similar, but less pronounced alterations in functional connectivity compared to controls.
Methods
Participants
Seventy individuals participated: 24 schizophrenia/schizoaffective patients (7 schizoaffective patients; hereafter referred to as schizophrenia patients), 25 adult nonpsychotic first-degree biological relatives, and 21 community controls. Inclusion criteria for all participants included: (1) age 18–65; (2) minimum intelligence quotient (IQ) of 70 as measured by Wechsler Abbreviated Scale of Intelligence; (3) no current diagnosis of drug or alcohol dependence or abuse; (4) no history of head injury or being unconscious for more than 20 minutes; (5) no history of electroconvulsive therapy; and (6) no history of a neurological condition. Further criteria for inclusion of relatives and controls were no lifetime diagnosis of a psychotic or bipolar disorder, Axis II Cluster A disorder, or history of anti-psychotic medication use. Further criterion for inclusion of community controls was no family history of a psychotic or bipolar disorder.
Schizophrenia patients were recruited through outpatient clinics and through community support programs in Calgary, Canada. Research staff identified first-degree biological relatives by completing a family pedigree with the proband. Controls were recruited through advertisements around the community. The University of Calgary ethics board approved the protocol.
Diagnosis and Assessment
Participants were interviewed using the Structured Clinical Interview for DSM-IV Axis I Disorders. The Structured Interview for Schizotypy, with supplemental questions, was used to measure Axis II Cluster A disorders in relatives and controls.29 Diagnoses were confirmed according to DSM-IV-TR criteria via case conferences. During the case conferences, the interviewers presented each participant to the team, with the team confirming the final diagnoses. One trained research assistant and 2 clinical psychology graduate students conducted the interviews. No relatives or controls met criteria for a Cluster A disorder. Table 1 details the scales used to measure functioning, symptoms, and IQ.
Table 1.
Participant Characteristics and Behavioral Data
| Schizophrenia | Relative | Control | |
|---|---|---|---|
| N | 24 | 25 | 21 |
| Age | 41.1 (11.4) | 41.2 (15.3) | 43.4 (10.8) |
| Gender (% female) | 45.8 | 60.0 | 47.6 |
| Born in Canada (%) | 87.5 | 88.0 | 85.7 |
| Education (years completed) | 14.5 (3.1) | 16.0 (2.6) | 15.5 (2.3) |
| Annual income (%) | |||
| $0–$30 000 | 58.3 | 4.0 | 5.0 |
| $30 000–$50 000 | 16.7 | 20.0 | 20.0 |
| $50 000–$95 000 | 16.7 | 52.0 | 40.0 |
| $95 000+ | 8.3 | 24.0 | 35.0 |
| Maternal education (years completed) | 13.4(2.9) | 13.0(3.8) | 12.9(3.4) |
| Paternal education (years completed) | 14(3.0) | 12.7(4.0) | 12.9(4.5) |
| Matrix Reasoning Raw score | 26.3(2.8) | 27.4(3.1) | 26.2(6.1) |
| Vocabulary Raw score | 58.0(6.1) | 61.5(5.3) | 59.0(8.5) |
| Handedness (% right handed) | 87.5 | 83.3 | 95.2 |
| Illness duration: range | 16.79(12.10):1–40 | — | — |
| PANSS negative: range | 12.6(4.1):7–22 | 7.8(1.1):7–11 | 7.3(0.7):7–10 |
| PANSS positive: range | 14.8(5.3):7–24 | 8.6(1.4):7–13 | 8(1.4):7–11 |
| PANSS general: range | 26.9(6.1):16–39 | 20.0(3.5):16–29 | 18.4(4.2):16–33 |
| Global Assessment of Functioning: range | 52.9(13.1):38–83 | 82.0(5.5):63–88 | 84.9(5.4):73–95 |
| Social Functioning Scale: range | 795.9(54.3):701.5–883.0 | — | — |
| Axis I (% with any lifetime diagnosis) | 100.0 | 32.0 | 28.6 |
| Relative status—parent:sibling:offspring | — | 10:13:2 | — |
| Anti-psychotic (atypical, typical, both; % on) | 96.0, 12.5, 8.3 | 0, 0, 0 | 0, 0, 0 |
| Anti-depressants (% on) | 45.8 | 8.0 | 9.5 |
| Mood stabilizer (% on) | 16.7 | 0 | 0 |
| Anti-anxiety (% on) | 8.3 | 4.0 | 0 |
| Anti-parkinson (% on) | 8.3 | 0 | 0 |
| Other psychiatric (% on) | 8.3 | 4 | 0 |
| Age target accuracy (%) | 75.5 (19.7) | 77.5 (14.8) | 80.6 (13.5) |
| Age non-target accuracy (%) | 78.6 (15.9) | 86.6 (8.1) | 82.0 (12.2) |
| Anger target accuracy (%) | 72.9 (16.3) | 76.0 (13.1) | 80.4 (11.5) |
| Anger non-target accuracy (%) | 87.6 (10.5) | 93.4 (10.0) | 92.6 (8.3) |
| Fear target accuracy (%) | 61.6 (19.1) | 68.8 (16.2) | 69.4 (15.7) |
| Fear non-target accuracy (%) | 85.6 (21.7) | 92.7 (14.1) | 88.4 (16.6) |
| Happy target accuracy (%) | 94.1 (5.6) | 93.3 (6.4) | 93.4 (6.1) |
| Happy non-target accuracy (%) | 89.9 (9.1) | 97.8 (3.6) | 96.7 (5.2) |
| Sad target accuracy (%) | 84.5 (12.7) | 90.0 (8.2) | 88.1 (11.6) |
| Sad non-target accuracy (%) | 85.4 (14.1) | 91.2 (13.0) | 87.7 (12.7) |
| Age target reaction time (ms) | 1321.7 (164.5) | 1246.8 (215.7) | 1345.0 (210.7) |
| Age non-target reaction time (ms) | 1274.9 (201.2) | 1088.6 (133.6) | 1192.3 (157.0) |
| Anger target reaction time (ms) | 1225.3 (200.6) | 1151.1 (171.2) | 1270.4 (154.8) |
| Anger non-target reaction time (ms) | 1275.3 (223.0) | 1088.8 (171.3) | 1216.2 (187.1) |
| Fear target reaction time (ms) | 1333.1 (208.2) | 1211.9 (206.1) | 1377.4 (236.3) |
| Fear non-target reaction time (ms) | 1277.2 (202.6) | 1051.3 (172.3) | 1206.5 (223.8) |
| Happy target reaction time (ms) | 1073.6 (232.1) | 928.8 (137.3) | 1036.5 (171.8) |
| Happy non-target reaction time (ms) | 1192.3 (241.6) | 955.9 (133.8) | 1029.5 (135.8) |
| Sad target reaction time (ms) | 1240.9 (217.1) | 1115.7 (183.1) | 1243.4 (198.1) |
| Sad non-target reaction time (ms) | 1310.9 (227.2) | 1121.5 (164.4) | 1255.5 (204.9) |
Note: Mean and SD presented where appropriate. PANSS, Positive and Negative Syndrome Scale.
Facial Discrimination Task
The facial emotion discrimination task administered in the MRI scanner consisted of 4 emotion discrimination conditions and an age discrimination condition. During the emotion discrimination conditions, participants responded “target” or “nontarget” (foil) to the particular emotion that was discriminated (eg, within the sad block, participants would view a face and determine whether the emotion depicted was “Sad” or “Not Sad”, and respond accordingly with a button press). The 4 facial emotion discrimination conditions were: angry, fear, happy, and sad. In the age discrimination condition, participants were required to respond whether or not the face presented was “Over 30?” or “Under 30?”. For each trial, the target (eg, “Sad”) and nontarget (eg, “Not Sad”) responses were kept up on the left or right side of the screen to facilitate responding with the corresponding button press.
Each condition was administered as a separate scanner run: anger, fear, sadness, happiness, and age. Run order was randomized for each participant. Each run consisted of 69 interspersed faces (24 target emotions; 36 nontarget emotions distributed among the 3 other emotions and neutral faces; and 9 scrambled faces). The scrambled faces were included as a baseline comparison, and participants were instructed to respond “nontarget” for the scrambled faces. Each face was presented for 2.5 seconds with a variable inter-stimulus interval (mean 3 s; range 1–5 s). Each run was also divided by 4 rest blocks consisting of a 30-second presentation of a scrambled face: one in the beginning and the end, and 2 interspersed during the run, thereby dividing each run into 4 equal length task-related blocks. These rest blocks were not modeled in the present analysis and the task design was analyzed as event-related. Total run length was 8 minutes and 34 seconds.
The facial stimuli used were drawn from the Pennsylvania faces.30 The Pennsylvania faces are in color, range in age from 10 to 85, and have different ethnicities represented. The scrambled faces were created by using an online website, which was able to make a grid of squares on the face only (ie, not including hair) and randomly scramble the squares of the grid.
Functional Magnetic Resonance Imaging
Scanning was performed on a 3T GE Discovery MR750 scanner equipped with an 8-channel head coil. For each of the 5 functional runs, 206 functional T2*-weighted echoplanar images were acquired using the following parameters: slice thickness = 3.4 mm, 40 oblique slices interleaved, TE = 30 ms, TR = 2500 ms, flip angle = 77°, matrix = 64 × 64, FOV = 22cm, voxel size = 3.4 × 3.4 × 3.4 mm. A whole-brain T1-weighted MPRAGE scan was also acquired to anatomically register the functional data.
Pre-processing was performed using the FSL Toolbox Version 5.0.6 using the following steps: non-brain tissue removal, motion and slice-timing correction, spatial smoothing using a 7 mm FWHM Gaussian kernel, grand-mean intensity normalization, and high-pass temporal filtering.31 Functional images were registered to the structural image and then standard Montreal Neurological Institute space using 12-parameter affine transformations and a boundary-based registration cost function.32,33 Registration from structural to standard space was further refined using nonlinear transformations.31 Functional scans were registered to voxel dimensions of 3 × 3 × 3 mm. This process was performed separately for each of the 5 task runs.
Multivariate and univariate analyses assessed both relative and absolute movement across groups. No group differences were found in the MANOVAs of the 5 scanner runs for either relative (Pillai’s Trace F(10, 128) = 0.69, P = .74) or absolute (Pillai’s Trace F(10, 128) = 0.64, P = .78) movement. Moreover, individual ANOVAs of the scanner runs found no group effects for either relative (Fs = 0.60–1.79, Ps = .17–.55) or absolute (Fs = 0.17–0.68, Ps = .51–.85) movement. Mean relative movement ranged from 0.067 to 0.078 mm (SD = 0.043–0.066) and mean absolute movement ranged from 0.28 to 0.33mm (SD = 0.14–0.23) for the 5 scanner runs.
Statistical Analyses
Demographic data were compared across groups using chi-square tests and ANOVAs. To analyze the accuracy and reaction time data from the discrimination task, two 5 facial discrimination condition (age, anger, fear, happy, sad) by 2 image type (target, nontarget) by 3 group (schizophrenia, relative, control) mixed model ANOVAs were conducted, and follow-up testing was conducted as needed.
fMRI data were analyzed as an event-related design using constrained principal component analysis (fMRI–CPCA) with an orthogonal rotation (supplementary material).34–38 The theory and proofs for CPCA are detailed in previously published work.39,40 Briefly, fMRI–CPCA combines multivariate multiple regression with principal component analysis (PCA) to reveal independent sources of post-stimulus BOLD activity. PCA is carried out on the portion of variance in BOLD activity that is predictable from the task timing, determined using a finite impulse response (FIR) model, which makes no a priori assumptions concerning the shape of the hemodynamic response (HDR).41 The estimated HDR is interpreted as the intensity of the pattern of BOLD signal, independent of whether it is an increase or decrease, and whether or not it is an increase or decrease is indexed by the positive and negative loadings overlaid on the brain image (red/yellow and blue/white, respectively). fMRI–CPCA produces predictor weights for each combination of post-stimulus time bin, task condition, and participant. These weights, which provide estimates of the engagement of functional networks at each post-stimulus time bin, can be statistically analyzed to determine whether these values reflect a plausible HDR shape and to compare the engagement of these networks between groups and/or conditions. Reductions in the estimated HDR shape in participants could reflect reduced connectivity and/or reduced coordinated activity for that group.
For each of the networks, represented by components, a 5 facial discrimination condition by 3 image type (target, nontarget, scrambled) by 7 time bins (scans after the onset of each stimulus) by 3 group mixed model ANOVA was conducted. The scrambled face trials interspersed within each scanner run were included in the analysis; however, the 4 scrambled image blocks within each run were not included. Given the large number of inputs in these analyses, only effects involving participant group are reported. For the behavioral and fMRI data, partial eta-squared effects sizes are reported for all significant effects that include group. Tests of sphericity were carried out for all mixed model ANOVAs. The Greenhouse-Geisser adjusted degrees of freedom are reported.
In largely the same sample, traditional univariate analysis of the blocked-design data for this task is described in a separate paper.66
Results
Participants
Groups did not differ for age (F(2, 67) = 0.22, P = .80), sex distribution (X2(2) = 1.16, P = .56), participant education level (F(2, 67) = 1.93, P = .15), mother’s education level (F(2, 65) = 0.13, P = .88), father’s education level (F(2, 59) = 0.72, P = .49), handedness (X2(2) = 1.58, P = .45), vocabulary score (F(2, 66) = 1.80, P = .17), or matrix reasoning score (F(2, 66) = 0.61, P = .54). As expected, groups differed for PANSS positive, negative, and general scales scores (Fs(2, 67) = 21.43–31.599, Ps < .001) and global functioning (F(2, 67) = 93.55, P < .001), with schizophrenia patients having greater symptomatology and lower functioning than both controls and relatives (Ps < .001). Importantly, controls and relatives did not differ for percentage of participants with a nonpsychotic Axis I disorder (X2(2) = 0.06, P = .80).
Behavioral Analyses
Table 1 presents behavioral data. A 5 facial discrimination condition (age, anger, fear, happy, sad) × 2 image type (target, nontarget) × 3 group (schizophrenia, relative, control) ANOVA on accuracy demonstrated main effects of facial discrimination condition (F(2.72, 182.36) = 56.99, P < .001) and image type (F(1, 67) = 35.99, P < .001), and an interaction of discrimination condition by image type (F(2.33, 155.80) = 18.76, P < .001). There was also a main effect of group (F(2, 67) = 7.59, P = .001, = 0.19), with schizophrenia patients having lower accuracy than both controls (P = .004) and relatives (P < .001), but no difference between relatives and controls (P = .58). There were no interactions between group and discrimination condition or image type (Fs = 0.49–0.81, Ps = .45–.797).
A 5 facial discrimination condition × 2 image type × 3 group ANOVA on reaction times demonstrated main effects of facial discrimination condition (F(4, 268) = 56.99, P < .001) and image type (F(1, 67) = 6.85, P = .01), and an interaction between discrimination condition and image type (F(3.37, 225.72) = 15.76, P < .001). There was also a main effect of group (F(2, 67) = 8.41, P = .001, = 0.20), with relatives having faster reaction times than both controls (P = .005) and patients (P < .001), and no difference between patients and controls (P = .40). There was a significant image type × group interaction (F(2, 67) = 5.02, P = .009, = 0.13). The interaction was due to a significant difference between patients and controls (F(1, 43) = 7.08, P = .01, = 0.14) and patients and relatives (F(1, 47) = 8.65, P = .005, = 0.16), such that both controls and relatives had significantly slower reaction times for target faces than nontarget faces, whereas schizophrenia patients did not show this pattern.
fMRI Functional Connectivity Analyses
The number of components to extract was determined from a visual inspection of the scree plot42,43 obtained from singular value decomposition of the task-related BOLD data from the entire sample of participants. Inspection of the scree plot suggested a 3-component solution which accounted for 32.39% of the task-correlated BOLD signal.
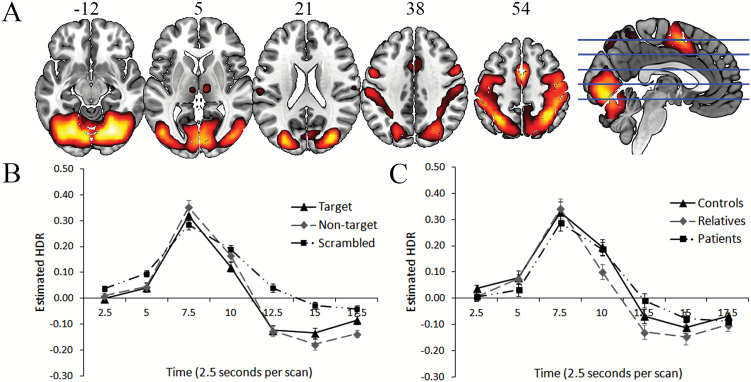
Component 1. The functional network described by Component 1 (figure 1A, table 2) included activation of regions comprising the visual, somatosensory, and dorsal/ventral attention networks.44 The shape of the estimated HDR (figure 1B) and the presence of a within-subjects effect of time (F(2.20, 147.51) = 127.70, P < .001) reflected a meaningful BOLD signal. There was no significant main effect of group and no interactions involving group (see figure 1C for estimated HDRs for each group).
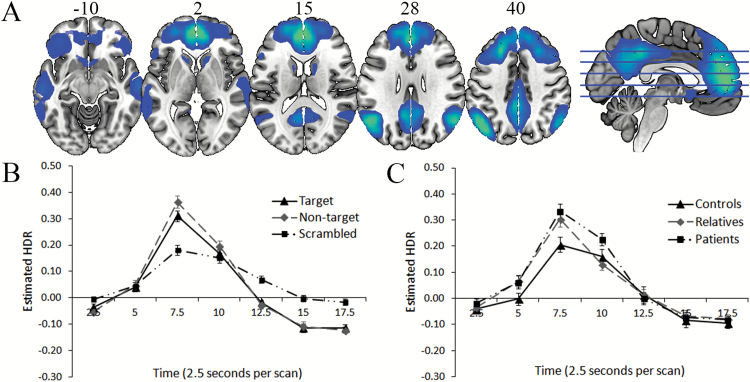
Component 2. The functional network described by Component 2 (figure 2A and 2B, table 2) included bilateral deactivation of regions associated with the default mode network (DMN),44,45 as well as bilateral hippocampi, and followed a similar time course and magnitude as Component 1. There was a significant interaction of time with group, but it did not survive the Greenhouse-Geisser adjustment for degrees of freedom (F(5.45, 182.41) = 2.12, P = .059, = 0.06). The source of this was sustained deactivation of this network for patients compared to controls (F(1, 43) = 5.22, P < .05, = 0.11) and relatives (F(1, 47) = 13.07, P < .001, = 0.22), measured by change from 10- to 12.5-second time bins. Relatives also displayed sustained peak deactivation compared to controls (F(1, 44) = 11.46, P < .005, = 0.21), measured by change from to 7.5- to 10-second time bins (figure 2C). Although there were significant effects/interactions involving post-stimulus time, discrimination condition, and image type, no other group effects emerged.
Component 3. The functional network described by Component 3 included late-peaking activations and deactivations in regions largely overlapping with known visual and frontoparietal networks44 and temporal regions, including activation in the amygdala (figure 3A; Brodmann’s areas and MNI coordinates, presented in table 2). This activation/deactivation was essentially absent for scrambled face trials (figure 3B); therefore, the scrambled faces were excluded from the following analyses.
Fig. 1.
A (top): dominant 20% of loadings for Component 1 (red/yellow = positive loadings, threshold = 0.22, max = 0.32). Images are displayed in neurological orientation (left is left) with MNI z-axis coordinates. B (bottom left): mean FIR-based predictor weights plotted over post-stimulus time (discrimination conditions averaged). C (bottom right): mean FIR-based predictor weights plotted over post-stimulus time by group (task conditions averaged). FIR, finite impulse response; HDR, hemodynamic response.
Table 2.
Cluster Volumes for the Most Extreme 20% of Loadings for Each Component, With Anatomical Labels, Brodmann’s Areas, and MNI Coordinates for the Peak of Each Cluster
| Anatomical Label | Cluster Volume (mm3) | Brodmann’s Area for Peak Locations | MNI Coordinate for Peak Locations | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Component 1—positive loadings | |||||
| Cluster 1: bilateral | 358 182 | ||||
| Occipital fusiform gyrus | 18 | 27 | −72 | −12 | |
| Occipital fusiform gyrus | 19 | −27 | −69 | −15 | |
| Occipital fusiform gyrus | 18 | −24 | −72 | −12 | |
| Cerebellar VI | n/a | −33 | −51 | −21 | |
| Lingual gyrus | 17 | 9 | −78 | −9 | |
| Cerebellar VI | n/a | 33 | −51 | −21 | |
| Superior parietal lobule | 40 | 36 | −51 | 57 | |
| Lingual gyrus | 17 | −6 | −78 | −9 | |
| Lateral occipital cortex, superior division | 19 | 33 | −81 | 24 | |
| Lateral occipital cortex, inferior division | 19 | −42 | −78 | −6 | |
| Supplementary motor cortex | 6 | 0 | 3 | 54 | |
| Superior parietal lobule | 7 | −30 | −57 | 51 | |
| Lateral occipital cortex, superior division | 19 | −27 | −84 | 24 | |
| Supramarginal gyrus, posterior division | 2 | 45 | −36 | 57 | |
| Precentral gyrus | 6 | 42 | −3 | 60 | |
| Lateral occipital cortex, inferior division | 19 | 42 | −78 | 6 | |
| Supramarginal gyrus, anterior division | 2 | −48 | −36 | 51 | |
| Precentral gyrus | 6 | −42 | −6 | 60 | |
| Superior parietal lobule | 40 | −42 | −45 | 57 | |
| Postcentral gyrus | 3 | 57 | −21 | 48 | |
| Postcentral gyrus | 4 | 39 | −24 | 66 | |
| Lateral occipital cortex, superior division | 7 | 12 | −72 | 51 | |
| Precuneus cortex | 5 | 6 | −54 | 57 | |
| Central opercular cortex | 42 | −60 | −21 | 18 | |
| Central opercular cortex | 48 | 60 | −15 | 18 | |
| Cluster 2: right hemisphere | 1917 | ||||
| Thalamus | n/a | 9 | −18 | 9 | |
| Cluster 3: left hemisphere | 1161 | ||||
| Thalamus | n/a | −9 | −18 | 9 | |
| Cluster 4: right hemisphere | 648 | ||||
| Supramarginal gyrus, posterior division | 42 | 63 | −39 | 18 | |
| Cluster 5: right hemisphere | 594 | ||||
| Thalamus | n/a | 18 | −30 | 0 | |
| Component 2—negative loadings | |||||
| Cluster 1: bilateral | 225 558 | ||||
| Paracingulate gyrus | 10 | 0 | 54 | 6 | |
| Paracingulate gyrus | 32 | 0 | 51 | 15 | |
| Superior frontal gyrus | 9 | −24 | 27 | 45 | |
| Middle frontal gyrus | 8 | 27 | 27 | 48 | |
| Frontal pole | 10 | −18 | 54 | 24 | |
| Superior frontal gyrus | 6/9 | 15 | 36 | 51 | |
| Frontal pole | 9 | 12 | 42 | 45 | |
| Frontal pole | 10 | 15 | 57 | 24 | |
| Middle frontal gyrus | 9 | −36 | 18 | 51 | |
| Frontal pole | 11 | −27 | 54 | 3 | |
| Middle temporal gyrus, posterior division | 20 | −60 | −24 | −9 | |
| Middle temporal gyrus, anterior division | 21 | −54 | −3 | −21 | |
| Middle temporal gyrus, posterior division | 21 | −57 | −15 | −12 | |
| Frontal pole | 47 | −33 | 39 | −12 | |
| Middle temporal gyrus, temporooccipital part | 21 | −66 | −48 | 0 | |
| Frontal orbital cortex | 45 | −42 | 27 | −15 | |
| Frontal orbital cortex | 45 | 51 | 27 | −12 | |
| Temporal pole | 38 | −39 | 18 | −33 | |
| Inferior frontal gyrus, pars triangularis | 45 | −54 | 24 | 9 | |
| Frontal orbital cortex | 47 | 36 | 33 | −15 | |
| Frontal pole | 45 | 42 | 45 | 6 | |
| Planum temporale | 22 | −54 | −21 | 3 | |
| Cluster 2: bilateral | 46 035 | ||||
| Cingulate gyrus, posterior division | 23 | −3 | −42 | 39 | |
| Cingulate gyrus, posterior division | 23 | 9 | −48 | 33 | |
| Cluster 3: left hemisphere | 29 106 | ||||
| Lateral occipital cortex, superior division | 39 | −48 | −66 | 36 | |
| Lateral occipital cortex, superior division | 19 | −42 | −72 | 39 | |
| Cluster 4: right hemisphere | 25 839 | ||||
| Middle temporal gyrus, posterior division | 21 | 66 | −21 | −6 | |
| Inferior temporal gyrus, anterior division | 20 | 51 | −3 | −33 | |
| Superior temporal gyrus, posterior division | 22 | 66 | −21 | 6 | |
| Planum temporale | 48 | 48 | −27 | 9 | |
| Cluster 5: right hemisphere | 22 194 | ||||
| Lateral occipital cortex, superior division | 39 | 57 | −63 | 30 | |
| Cluster 6: bilateral | 8802 | ||||
| Caudate | n/a | 12 | 15 | 9 | |
| Caudate | n/a | −12 | 12 | 12 | |
| Accumbens | n/a | −6 | 15 | −6 | |
| Caudate | n/a | 15 | 21 | −3 | |
| Cluster 7: left hemisphere | 2322 | ||||
| Hippocampus | n/a | −27 | −18 | −21 | |
| Cluster 8: right hemisphere | 2241 | ||||
| Hippocampus | n/a | 30 | −18 | −21 | |
| Cluster 9: right hemisphere | 729 | ||||
| Crus I | n/a | 27 | −78 | −30 | |
| Component 3—positive loadings | |||||
| Cluster 1: bilateral | 132 678 | ||||
| Temporal occipital fusiform cortex | 37 | 42 | −48 | −21 | |
| Temporal occipital fusiform cortex | 37 | −39 | −48 | −21 | |
| Crus I | n/a | −6 | −78 | −27 | |
| Lateral occipital cortex, inferior division | 19 | 45 | −84 | −6 | |
| Lateral occipital cortex, inferior division | 19 | −39 | −84 | −9 | |
| Crus I | n/a | 9 | −78 | −24 | |
| Crus I | n/a | −36 | −66 | −27 | |
| Vermis IX | n/a | 0 | −54 | −33 | |
| Intracalcarine cortex | 17 | 6 | −81 | 3 | |
| Intracalcarine cortex | 17 | −9 | −75 | 9 | |
| Cluster 2: left hemisphere | 59 238 | ||||
| Frontal orbital cortex | 45 | −48 | 21 | −6 | |
| Middle frontal gyrus | 44 | −48 | 12 | 30 | |
| Inferior frontal gyrus, pars opercularis | 44 | −51 | 15 | 27 | |
| Frontal pole | 47 | −45 | 42 | −6 | |
| Temporal fusiform cortex, anterior division | 20 | −33 | −12 | −33 | |
| Temporal pole | 20 | −48 | 3 | −36 | |
| Inferior temporal gyrus, anterior division | 20 | −51 | 0 | −33 | |
| Cluster 3: right hemisphere | 53 244 | ||||
| Inferior frontal gyrus, pars opercularis | 44 | 54 | 18 | 30 | |
| Frontal orbital cortex | 38 | 48 | 21 | −6 | |
| Temporal pole | 20 | 48 | 12 | −33 | |
| Cluster 4: right hemisphere | 27 108 | ||||
| Paracingulate gyrus | 6/8 | 3 | 21 | 51 | |
| Cluster 5: left hemisphere | 6345 | ||||
| Lateral occipital cortex, superior division | 7 | −33 | −60 | 48 | |
| Cluster 6: right hemisphere | 5562 | ||||
| Lateral occipital cortex, superior division | 39 | 39 | −57 | 48 | |
| Cluster 7: right hemisphere | 5508 | ||||
| Middle temporal gyrus, posterior division | 21 | 51 | −33 | −3 | |
| Cluster 8: left hemisphere | 4455 | ||||
| Middle temporal gyrus, posterior division | 21 | −51 | −39 | 0 | |
| Cluster 9: right hemisphere | 1701 | ||||
| Caudate | n/a | 12 | 3 | 12 | |
| Thalamus | n/a | 9 | −12 | 9 | |
| Cluster 10: right hemisphere | 1404 | ||||
| Temporal fusiform cortex, posterior division | 20 | 33 | −12 | −33 | |
| Cluster 11: right hemisphere | 1161 | ||||
| Amygdala | n/a | 18 | −6 | −15 | |
| Cluster 12: left hemisphere | 702 | ||||
| Caudate | n/a | −12 | 3 | 12 | |
| Component 3—negative loadings | |||||
| Cluster 1: bilateral | 41 283 | ||||
| Precuneus cortex | 3/5 | 12 | −36 | 48 | |
| Precuneus cortex | 5 | −6 | −39 | 48 | |
| Cingulate gyrus, posterior division | 23 | −9 | −36 | 45 | |
| Precuneus cortex | 2/5 | 9 | −48 | 60 | |
| Precuneus cortex | 3/5 | −9 | −54 | 60 | |
| Superior parietal lobule | 2 | 21 | −48 | 63 | |
| Cluster 2: right hemisphere | 6183 | ||||
| Parietal operculum cortex | 2 | 54 | −30 | 30 | |
| Cluster 3: left hemisphere | 4158 | ||||
| Precuneus cortex | 18/17 | −15 | −57 | 18 | |
| Cluster 4: left hemisphere | 2916 | ||||
| Lateral occipital cortex, superior division | 19 | −39 | −81 | 33 | |
| Cluster 5: right hemisphere | 2430 | ||||
| Precuneus cortex | 18/17 | 18 | −54 | 18 | |
| Cluster 6: bilateral | 2241 | ||||
| Paracingulate gyrus | 10 | −9 | 54 | 3 | |
| Cingulate gyrus, anterior division | 10 | 3 | 45 | 0 | |
| Cluster 7: right hemisphere | 1215 | ||||
| Central opercular cortex | 48 | 57 | −3 | 6 | |
| Cluster 8: left hemisphere | 972 | ||||
| Parietal operculum cortex | 48 | −57 | −33 | 27 | |
| Cluster 9: left hemisphere | 621 | ||||
| Middle frontal gyrus | 9 | −30 | 36 | 45 | |
Fig. 2.
A (top): dominant 20% of loadings for Component 2. Images are displayed in neurological orientation (left is left) with MNI z-axis coordinates. All negative loadings implying deactivation, threshold = –0.12, min = –0.25. B (bottom left): mean FIR-based predictor weights plotted over post-stimulus time (discrimination conditions averaged). C (bottom right): mean FIR-based predictor weights plotted over post-stimulus time by group (task conditions averaged). FIR, finite impulse response; HDR, hemodynamic response.
Fig. 3.
A (top): dominant 20% of loadings for Component 3. Images are displayed in neurological orientation (left is left) with MNI z-axis coordinates. Red/yellow = positive loadings, threshold = 0.09, max = 0.27; blue = negative loadings, threshold = –0.09, min = –0.18. B (middle left): mean FIR-based predictor weights plotted over post-stimulus time (discrimination conditions averaged). C (middle right): mean FIR-based predictor weights for controls, plotted over post-stimulus time by discrimination condition (targets and non-targets averaged; scrambled trials excluded). D (bottom left): mean FIR-based predictor weights for relatives, plotted over post-stimulus time by discrimination condition (targets and non-targets averaged; scrambled trials excluded from analysis). E (bottom right): mean FIR-based predictor weights for patients, plotted over post-stimulus time by discrimination condition (targets and non-targets averaged; scrambled trials excluded from analysis). FIR, finite impulse response; HDR, hemodynamic response.
There was a main effect of group (F(2, 67) = 7.10, P = .002, = 0.18) whereby schizophrenia patients showed overall lower coordinated network activity compared with controls (P < .01) and relatives (P < .001). There was no overall difference between controls and relatives (P = .61). However, significant interactions emerged for discrimination condition × group (F(8, 268) = 2.21, P = .027, = 0.06), time × group (F(4.83, 161.88) = 6.71, P < .001, = 0.17), and discrimination condition × time × group (F(18.94, 634.50) = 1.60, P = .052, = 0.05). Estimated HDRs for each group are plotted in figures 3C–E for each discrimination condition.
The main effect of group, whereby schizophrenia patients showed strongly decreased overall network activity, tended to confound condition-specific group differences, complicating interpretation of the discrimination condition × group and discrimination condition × time × group interactions. To simplify interpretation of these results, the significant condition × group interaction was interpreted by investigating condition contrasts within each group separately using the HDR peaks. The peaks were isolated because the conditions cannot be well discriminated when the HDR begins its ascent and as it returns to baseline, so was captured by averaging over 3 timepoints (mean of time bins 7.5–12.5 s). These peak values were compared as repeated measures contrasts of conditions (eg, contrast happy 1 vs angry −1) within each group. Moreover, since no image type × group interactions were present, the nontarget and target conditions were averaged within each discrimination condition.
Community Controls. The pattern of activity for Component 3 suggested that although this network was involved in all types of emotion discrimination (eg, sad vs not sad, angry vs not angry), it was substantially less involved in the happiness discrimination condition (Ps < .001 and s > 0.69 for comparison to all other conditions). The only other significant contrasts were that anger discrimination elicited significantly less coordinated activity than fear and age discrimination conditions (F(1, 20) = 5.09, P < .05, = 0.20; F(1, 20) = 4.22, P = .05, = 0.17, respectively). These findings suggest that this brain network is less involved in the differentiation of whether a face is happy or angry than for other emotional expressions. The network activation to age discrimination was very similar to that of fear and sadness discrimination (F(1, 20) = 0.07, P = .80; F(1, 20) = 0.89, P = .36, respectively). The similarities between these 3 discrimination conditions reflects either more general facial processing or that emotional processing was present during age discrimination.
Nonpsychotic Relatives. As for controls, relatives of schizophrenia patients showed substantially reduced coordinated network activity for the happiness discrimination condition (Ps < .001 and s > 0.45 for comparison to all other conditions). No other differences were present (all Ps > .32). Unlike for controls, anger discrimination elicited coordinated activity that was of similar intensity as fear and age discrimination (F(1, 24) = 0.10, P = .76; F(1, 24) = 1.03, P = .32, respectively). This pattern suggests that relatives had an increased response in this brain network to determining whether a face was angry or not, which was not seen in controls.
Schizophrenia Patients. Similar to controls and relatives, schizophrenia patients also showed substantially reduced coordinated network activity for happiness discrimination (Ps < .05 and s > 0.24 for comparison to all other conditions except for sadness). Notably, for patients, this was also the case with sadness discrimination (Ps < .05 and > 0.16 for comparison to all other conditions except happiness). As with relatives, but unlike controls, anger discrimination elicited coordinated activation of similar intensity as fear and age discrimination (F(1, 23) = 2.91, P = .10; F(1, 23) = 0.00, P = .99, respectively).
Inspection of the predictor weights additionally suggested that fear discrimination may also have shown an increased response compared to the other conditions for patients. Assessment of the peak times of the HDR (7.5–10 s) compared between emotion discrimination conditions yielded the largest effect size differences for fear discrimination: fear vs sadness (F(1, 23) = 32.97, P < .001, = 0.59), compared to age vs sadness and anger vs sadness (F(1, 23) = 5.54, P < .05, = 0.19; F(1, 23) = 3.10, P = .09, = 0.12, respectively), and the difference between these effect sizes (evaluated using contrast means) was statistically significant (F(1, 23) = 7.05, P < .05, = 0.15). While the comparisons of fear vs age and fear vs anger only reached trend-wise statistical significance (F(1, 23) = 2.91, P = .10; F(1, 23) = 2.91, P = .10, respectively), this also suggests stronger network response during fear discrimination. Overall, these findings suggest that like relatives, but unlike controls, engagement of this functional network in schizophrenia patients is increased when determining whether a face is angry or not. In addition, unlike controls and relatives, engagement of this functional network in schizophrenia patients is increased during fear discrimination and decreased during sadness discrimination.
Discussion
This study uncovered behavioral and fMRI brain activation patterns associated with genetic liability and disease-specific effects during emotion and age discrimination in schizophrenia using a family study design. We used a task that involved both explicit and implicit processing of emotions. The emotion discrimination conditions required making an emotive judgment from an emotive face, whereas the age discrimination condition required a non-emotive judgment from an emotive face.27,28
Behaviorally, schizophrenia patients demonstrated an overall cognitive deficit, as reduced accuracy compared to relatives and controls was found across both the emotion and age discrimination conditions. Relatives did not differ from controls on accuracy. For reaction time data, relatives had faster reaction times than both controls and patients, whereas patients and controls did not differ. These results synergize with previous studies demonstrating that schizophrenia patients have similarly impaired performance on both emotion and face processing.46,47 Whether schizophrenia patients demonstrate a greater impairment for emotion recognition compared to facial recognition is a topic of debate, with support on both sides.10,46,47 In our previous study, we found the pattern was more complex.9 We found that with an unlimited response time, patients were able to improve their performance on age discrimination, but not emotion discrimination, suggesting a specific difficulty with emotion processing. The current task was presented as time-limited due to fMRI constraints. Second, although studies have suggested relatives are behaviorally impaired on emotion processing tasks,7,8,48,49 this is not a wholly consistent finding.9,50,51 A recent meta-analysis suggested that the effect size for emotion discrimination for relatives compared to controls was d = 0.21, a small effect, whereas the effect size for emotion identification was d = 0.52, thus suggesting type of task may be an important factor.7 Our task was an emotion discrimination task.
Although most studies focus on accuracy, studies reporting reaction times in emotion recognition tasks tend to find no differences between relatives and controls,52,53 with one study of high-risk individuals finding longer reaction times.54 In the present study, our relatives were middle-aged and therefore largely beyond the window of risk for schizophrenia. The finding of faster reaction times could reflect better developed emotion recognition skills, more efficient strategy use, or an increase in engagement and motivation on a part of this group. Alternatively, this could also reflect a sample-specific finding.
With regard to the functional connectivity data, the HDR peaks for Component 1 were similar across different image types, with activation exhibited in visual and somatomotor regions. Therefore, Component 1 was likely related to increasing attention to the visual stimuli presented, as it responded for faces and scrambled faces, and producing the motor response.44,55,56 The strong activity for scrambled faces supports this interpretation. Importantly, no statistically significant differences between groups emerged for this component, suggesting that visual attention and response generation processes were similarly engaged across groups. Moreover, this suggests that not all brain activation during the task was abnormal in patients and relatives, reflecting a more specific pattern of functional abnormalities where group differences were present.
Component 2 consisted of deactivation in the DMN44,45 which generally was reciprocally related to Component 1. Controls demonstrated less deactivation than both schizophrenia patients and relatives. Although a number of studies have found the DMN to be less deactivated in schizophrenia patients,57 there have also been previous findings of hyperdeactivation of the DMN in patients during tasks requiring externally-oriented attention.35,58,59 Previous research suggests that optimal task performance requires a balance of task-positive (external attention, responding) and task-negative (internal thought and maintenance of task rules) networks during tasks that require remembering rules,58 whereby too much deactivation of the DMN could reflect a reduction in the internal thoughts important for maintenance of instructions and strategies. It is noteworthy that the pattern of group differences for this component found relatives to be intermediate between schizophrenia patients and controls, suggesting an association with genetic risk for the disorder. However, given that this interaction was of trend-wise significance, replication is necessary.
Component 3 involved later-peaking activations and deactivations in visual (most notably the fusiform region) and frontoparietal networks and temporal regions, including the amygdala. The absence of involvement of this network in processing scrambled faces, and the involvement of the amygdala and fusiform region, suggests this network was most responsive to faces and expressions, and the anatomical locations are consistent with previous findings.17 The activation of frontoparietal networks would be consistent with an increase in externally-oriented attention and cognitive demand.56,60
Coordinated activity during age discrimination did not differ from most emotion discrimination conditions for Component 3. This suggests that the network responded to faces with emotional and neutral expressions regardless of whether the emotional expression was relevant to type of judgment required (emotion or age discrimination). Two fMRI studies using a similar task in healthy individuals have demonstrated that age discrimination activated similar regions to emotion discrimination.27,28 However, these studies also found greater activation for emotion discrimination contrasted to age discrimination for many regions, including the amygdala.27,28 A meta-analysis of healthy individuals also found greater amygdala activation for explicit processing of emotional expressions compared to implicit processing,61 although individual studies have demonstrated the opposite pattern as well.62 In contrast, a meta-analysis of schizophrenia patients suggested greater amygdala activation differences in controls compared to patients for explicit processing of emotions relative to implict processing; however, given that the contrasts were not directly compared, the results are inconclusive.11 Further research is necessary to clarify how amygdala activation is modulated during explicit and implicit emotion processing tasks.
For Component 3, we also found lower coordinated network activity overall across discrimination conditions in schizophrenia patients compared to controls and relatives, suggesting that patients had broad network dysfunction for both implicit and explicit emotion processing. This finding suggests that global abnormalities in facial and emotion perception in schizophrenia may represent more disease-specific effects. One study directly comparing explicit and implicit emotion processing in schizophrenia patients, using a traditional univariate analysis method, found hypoactivation in the amygdala, fusiform, and middle temporal, and middle occipital regions in schizophrenia patients compared to controls across both explicit emotion and implicit gender judgment conditions, with no differential effects of judgment.63 The involvement of the amygdala in this network found through our non-seed-restricted functional connectivity analysis strengthens claims of studies that find abnormal connectivity involving the amygdala in schizophrenia patients compared to controls.21–24
In our study, we did not find that relatives differed from controls in Component 3 activity for overall facial discrimination. In contrast to our findings, one study investigated adult relatives and controls using graph-based connectivity analyses and found lower subnetwork activation that included the amygdala, as well as fusiform, medial temporal, and visual regions during a face matching task of anger and fearful expressions for relatives.6 Differences between these findings and our findings could be due to task differences, as we investigated 5 discrimination conditions as opposed to combined anger or fear expression matching, the type of relative sample studied, or the type of analysis technique chosen. By using fMRI–CPCA, we did not restrict our analysis to regions-of-interest, but did restrict the analyses to task-related variance, which optimizes exploration of task-related brain networks as opposed to confounding both task-related and task-unrelated fMRI signal. Confounding task-related and task-unrelated fMRI signal could result in group differences in connectivity not related to the affective process of interest. Also, given that we had a greater number of emotion categories in our task, we were able to find genetic liability effects on connectivity for specific discrimination conditions (discussed below). A second previous study used dynamic causal modeling to investigate effective connectivity for an emotional n-back task in a sample of child and adolescent relatives.26 This study demonstrated the best fitting model was a bi-directional frontal-limbic connection (including the amygdala). Furthermore, young relatives were characterized by decreased input to visual cortex and decreased coupling between frontolimbic regions compared to controls. It is important to note that in contrast to our relatives, these younger relatives, who were still within the risk window for psychosis, had significantly lower global functioning and higher prodromal symptomatology, suggesting greater psychopathology.26 Additionally, the points made above regarding the graph-based analysis also apply to this dynamic causal modeling approach (ie, regarding the restriction of regions-of-interest and confounding both task-related and task-unrelated signal). Last, a few fMRI studies of facial and emotion processing have investigated family members and focused primarily on the amygdala activation, with findings of reduced activation,5,13 increased activation,14,15 or no differences compared to controls,16 suggesting considerable heterogeneity in this research area.
As discussed above, Component 3 revealed group effects and interactions with time and discrimination condition. Interestingly, there was no interaction between image type (ie, target emotion vs non-target emotion) and group. This demonstrates that target and non-target emotions within a discrimination condition were processed similarly across participants. Therefore, the differences between groups was at the level of the emotional evaluation to be made (eg, sad vs not sad). These results indicate that functional activation abnormalities in schizophrenia during facial emotion discrimination are not necessarily or merely stimulus-driven—in other words, not solely a result of perceptual aspects of the emotional face in question. Rather, the findings indicate that network-level activation abnormalities in schizophrenia are modulated by different types of emotional evaluation. These findings fit within a broader conceptualization of facial emotion recognition that includes perceptual processes, as well as other important processes related to social knowledge retrieval, mental simulation, and emotional decision-making.17
In our study, Component 3 network engagement varied by discrimination condition across the 3 groups. Within-group comparisons of emotion discrimination conditions revealed an interesting pattern of results associated with disease-specific and genetic liability effects. All 3 groups showed substantially reduced coordinated network activity when differentiating whether an expression depicted happiness or not, suggesting that this network was less involved when positive emotions were being recognized or, alternatively, there was less engagement of this network due to the happiness discrimination condition being relatively less difficult. Within groups, evaluation of the HDRs of the different emotion discrimination conditions suggested a similar pattern in schizophrenia patients and relatives, such that there was a heightened response during the anger discrimination condition (at the level of differentiating if an emotive face was angry or not) compared to the other emotional evaluations. This suggests processing whether a face is angry or not may represent an effect of the genetic liability to schizophrenia and is worthy of further investigation. Finally, for schizophrenia patients, bias toward greater coordinated activity for fear discrimination and less for sadness discrimination was observed. Both fear and anger expressions are categorized as threatening. Theoretically, threat perception has been shown to have a causal role in the emergence and maintenance of persecutory delusions and paranoia.64,65 Our findings of a heightened response when differentiating whether a face was fearful in schizophrenia patients and differentiating whether a face was angry in schizophrenia patients and relatives is similar to a meta-analysis that found abnormal activations (including for the amygdala) in schizophrenia patients only for negative emotions (largely fear and anger) and not for neutral or positive emotions.11 Furthermore, a study involving young relatives of schizophrenia patients discussed above, demonstrated specific effects for negatively valenced faces (fear, anger, sadness) compared to happy expressions.26 Similarly, in the study by Cao and colleagues6 also discussed above, weaker coordinated subnetwork response in relatives was found, only when fear and anger faces were presented.
In largely the same sample, we previously conducted a traditional univariate analysis of the blocked-design data for this task,66 whereas in this present article we analyzed the event-related data using a functional connectivity method. In the univariate blocked-design analysis, facial and emotion processing regions were present, including the amygdala. Similar to the event-related functional connectivity findings, the univariate block-related data analysis found common patterns of performance deficits and activation abnormalities during emotion and age discrimination for schizophrenia patients compared to controls. However, when individual discrimination conditions were contrasted between groups, no consistent pattern of group differences emerged. In contrast, in our current analysis, patients appeared to be generally more impaired than relatives and controls across all conditions. Similarly, in another study from this sample where we used a traditional univariate analysis to examine activation during a passive viewing facial emotion perception task, we found no amygdala activation and less discernable group patterns for individual emotions.67 Our findings of no amygdala activation using traditional regional activation analyses are consistent with another study of emotion processing which found no amygdala activation using traditional activation analyses, but did find differences when comprehensive connectivity analyses were conducted, suggesting connectivity analyses may be more sensitive to brain activation differences.6 Future research should further evaluate the effects of task design and analysis strategy on findings of emotion processing in schizophrenia.
Limitations of this study include the modest sample size, which may have made it difficult to detect more nuanced differences between groups. However, our sample size is similar to many functional neuroimaging family studies. Second, the behavioral and functional connectivity results were not entirely consistent. We found a group by discrimination condition interaction for Component 3, but we did not find a similar pattern for the behavioral accuracy data. There could be a number of explanatory factors for this finding. This could reflect a sample-specific finding. However, other studies have found network abnormalities when no behavioral findings are present,26 suggesting neural circuitry may be a more sensitive, comprehensive, or nuanced measure of abnormalities. Additionally, it is likely that there were brain networks involved in the task that were not detected with fMRI. Third, we were not able to collect reliable information on anti-psychotic dosages and therefore were unable to investigate how the networks were influenced by medication dosage.
This study also has a number of strengths, including the use of a family study design and sophisticated functional connectivity analyses. By using an exploratory, data-driven method focused on task-related variance, amygdala activity was required to dominate the task-related variance sufficiently to reveal itself alongside other brain regions in the form of a network. At the same time, this approach explored other brain regions that were activated in concert with the amygdala to carry out emotion discrimination. Thus, relative to seed-based, or other region-of-interest based methods, the likelihood of type I errors were reduced because regions are required to produce stronger effects to be included in the network; thus, these results may be more likely to replicate. It might be claimed that ignoring a priori information regarding the expected networks of interest runs the risk of overfitting to noisy data, particularly in a modest sample with an inherently noisy data technique such as fMRI. However, fMRI–CPCA requires observation of a biologically-valid HDR shape for each network, ensuring that findings are due to signal rather than noise. In addition, our components fit with previously demonstrated functional networks in the healthy population literature. This further supports that our findings are based on signal, and are not due to chance correlations present in noise.
This study provides important information regarding functional connectivity abnormalities associated with the disease-process and genetic liability to schizophrenia. As facial emotion recognition is associated with functional outcome, appears to be a trait marker of the disorder, and appears to be associated with the genetic liability to the disorder, this might be a candidate for remediation. Networks with abnormal activation in patients and relatives could be targets of pharmacological intervention. Particularly, nonpsychotic relatives of patients, who have an increased genetic risk for the disorder but are most often behaviorally intact and not on antipsychotic medications (thereby not confounding additional variables that could impact brain functioning), may be an ideal sample to investigate neural mechanisms that could be the focus of novel pharmacological interventions. Given the documented relationship between functional outcome and face and emotion recognition, ameliorating these deficits could have benefits for improving occupational, educational, and social attainment in schizophrenia patients and improving the quality of life for patients and their families.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Funding
The Canadian Institutes of Health Research; a University of Calgary Seed Grant; and a University Research Grant Committee Starter Grant.
Supplementary Material
Acknowledgments
We thank Jennifer Prentice, Cameron Clark, Irene Liu, Andrea Moir, and Fil Cortese for their help with data collection and management. All authors reported no financial interests or potential conflicts of interest.
References
- 1. Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. 2010;36:1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan RC, Li H, Cheung EF, Gong QY. Impaired facial emotion perception in schizophrenia: a meta-analysis. Psychiatry Res. 2010;178:381–390. [DOI] [PubMed] [Google Scholar]
- 3. Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32(suppl 1):S44–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–588. [DOI] [PubMed] [Google Scholar]
- 5. Habel U, Klein M, Shah NJ, et al. Genetic load on amygdala hypofunction during sadness in nonaffected brothers of schizophrenia patients. Am J Psychiatry. 2004;161:1806–1813. [DOI] [PubMed] [Google Scholar]
- 6. Cao H, Bertolino A, Walter H, et al. Altered functional subnetwork during emotional face processing: a potential intermediate phenotype for schizophrenia. JAMA Psychiatry. 2016;73:598–605. [DOI] [PubMed] [Google Scholar]
- 7. Lavoie MA, Plana I, Bédard Lacroix J, Godmaire-Duhaime F, Jackson PL, Achim AM. Social cognition in first-degree relatives of people with schizophrenia: a meta-analysis. Psychiatry Res. 2013;209:129–135. [DOI] [PubMed] [Google Scholar]
- 8. Allott KA, Rice S, Bartholomeusz CF, et al. Emotion recognition in unaffected first-degree relatives of individuals with first-episode schizophrenia. Schizophr Res. 2015;161:322–328. [DOI] [PubMed] [Google Scholar]
- 9. Goghari VM, Macdonald AW, III, Sponheim SR. Temporal lobe structures and facial emotion recognition in schizophrenia patients and nonpsychotic relatives. Schizophr Bull. 2011;37:1281–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schneider F, Gur RC, Koch K, et al. Impairment in the specificity of emotion processing in schizophrenia. Am J Psychiatry. 2006;163:442–447. [DOI] [PubMed] [Google Scholar]
- 11. Taylor SF, Kang J, Brege IS, Tso IF, Hosanagar A, Johnson TD. Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biol Psychiatry. 2012;71:136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li H, Chan RC, McAlonan GM, Gong QY. Facial emotion processing in schizophrenia: a meta-analysis of functional neuroimaging data. Schizophr Bull. 2010;36:1029–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barbour T, Murphy E, Pruitt P, et al. Reduced intra-amygdala activity to positively valenced faces in adolescent schizophrenia offspring. Schizophr Res. 2010;123:126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li HJ, Chan RC, Gong QY, et al. Facial emotion processing in patients with schizophrenia and their non-psychotic siblings: a functional magnetic resonance imaging study. Schizophr Res. 2012;134:143–150. [DOI] [PubMed] [Google Scholar]
- 15. van Buuren M, Vink M, Rapcencu AE, Kahn RS. Exaggerated brain activation during emotion processing in unaffected siblings of patients with schizophrenia. Biol Psychiatry. 2011;70:81–87. [DOI] [PubMed] [Google Scholar]
- 16. Rasetti R, Mattay VS, Wiedholz LM, et al. Evidence that altered amygdala activity in schizophrenia is related to clinical state and not genetic risk. Am J Psychiatry. 2009;166:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adolphs R. Recognizing emotion from facial expressions: psychological and neurological mechanisms. Behav Cogn Neurosci Rev. 2002;1:21–62. [DOI] [PubMed] [Google Scholar]
- 18. Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. [DOI] [PubMed] [Google Scholar]
- 19. Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1:13–36. [DOI] [PubMed] [Google Scholar]
- 20. Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev. 2011;35:1110–1124. [DOI] [PubMed] [Google Scholar]
- 21. Mukherjee P, Whalley HC, McKirdy JW, et al. Lower effective connectivity between amygdala and parietal regions in response to fearful faces in schizophrenia. Schizophr Res. 2012;134:118–124. [DOI] [PubMed] [Google Scholar]
- 22. Mukherjee P, Whalley HC, McKirdy JW, et al. Altered amygdala connectivity within the social brain in schizophrenia. Schizophr Bull. 2014;40:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Das P, Kemp AH, Flynn G, et al. Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophr Res. 2007;90:284–294. [DOI] [PubMed] [Google Scholar]
- 24. Fakra E, Salgado-Pineda P, Delaveau P, Hariri AR, Blin O. Neural bases of different cognitive strategies for facial affect processing in schizophrenia. Schizophr Res. 2008;100:191–205. [DOI] [PubMed] [Google Scholar]
- 25. Pulkkinen J, Nikkinen J, Kiviniemi V, et al. Functional mapping of dynamic happy and fearful facial expressions in young adults with familial risk for psychosis - Oulu Brain and Mind Study. Schizophr Res. 2015;164:242–249. [DOI] [PubMed] [Google Scholar]
- 26. Diwadkar VA, Wadehra S, Pruitt P, et al. Disordered corticolimbic interactions during affective processing in children and adolescents at risk for schizophrenia revealed by functional magnetic resonance imaging and dynamic causal modeling. Arch Gen Psychiatry. 2012;69:231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Habel U, Windischberger C, Derntl B, et al. Amygdala activation and facial expressions: explicit emotion discrimination versus implicit emotion processing. Neuropsychologia. 2007;45:2369–2377. [DOI] [PubMed] [Google Scholar]
- 28. Gur RC, Schroeder L, Turner T, et al. Brain activation during facial emotion processing. Neuroimage. 2002;16:651–662. [DOI] [PubMed] [Google Scholar]
- 29. Kendler KS, Lieberman JA, Walsh D. The Structured Interview for Schizotypy (SIS): a preliminary report. Schizophr Bull. 1989;15:559–571. [DOI] [PubMed] [Google Scholar]
- 30. Gur RC, Sara R, Hagendoorn M, et al. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods. 2002;115:137–143. [DOI] [PubMed] [Google Scholar]
- 31. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. [DOI] [PubMed] [Google Scholar]
- 32. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. [DOI] [PubMed] [Google Scholar]
- 33. Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. [DOI] [PubMed] [Google Scholar]
- 34. Lavigne KM, Rapin LA, Metzak PD, et al. Left-dominant temporal-frontal hypercoupling in schizophrenia patients with hallucinations during speech perception. Schizophr Bull. 2015;41:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Metzak PD, Riley JD, Wang L, Whitman JC, Ngan ET, Woodward TS. Decreased efficiency of task-positive and task-negative networks during working memory in schizophrenia. Schizophr Bull. 2012;38:803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Metzak P, Feredoes E, Takane Y, et al. Constrained principal component analysis reveals functionally connected load-dependent networks involved in multiple stages of working memory. Hum Brain Mapp. 2011;32:856–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Whitman JC, Ward LM, Woodward TS. Patterns of cortical oscillations organize neural activity into whole-brain functional networks evident in the fMRI BOLD signal. Frontiers in Human Neuroscience. 2013;7:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Woodward TS, Feredoes E, Metzak PD, Takane Y, Manoach DS. Epoch-specific functional networks involved in working memory. Neuroimage. 2013;65:529–539. [DOI] [PubMed] [Google Scholar]
- 39. Takane Y, Hunter MA. Constrained principal component analysis: a comprehensive theory. Applicable Algebra in Engineering, Communication and Computing. 2001;12:391–419. [Google Scholar]
- 40. Takane Y, Shibayama T. Principal component analysis with external information on both subjects and variables. Psychometrika. 1991;56:97–120. [Google Scholar]
- 41. Henson R, Rugg MD, Friston K. The choice of basis functions in event-related fMRI. NeuroImage. 2001;13:S149. [Google Scholar]
- 42. Cattell RB. The scree test for the number of factors. Multivariate Behav Res. 1966;1:245–276. [DOI] [PubMed] [Google Scholar]
- 43. Cattell RB, Vogelmann S. A comprehensive trial of the scree and kg criteria for determining the number of factors. Multivariate Behav Res. 1977;12:289–325. [DOI] [PubMed] [Google Scholar]
- 44. Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Raichle ME. The restless brain. Brain Connect. 2011;1:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Salem JE, Kring AM, Kerr SL. More evidence for generalized poor performance in facial emotion perception in schizophrenia. J Abnorm Psychol. 1996;105:480–483. [DOI] [PubMed] [Google Scholar]
- 47. Kerr SL, Neale JM. Emotion perception in schizophrenia: specific deficit or further evidence of generalized poor performance? J Abnorm Psychol. 1993;102:312–318. [DOI] [PubMed] [Google Scholar]
- 48. Saracco-Alvarez R, Fresán A, Escamilla-Orozco R. Facial emotion recognition in schizophrenia: a comparison with siblings and control subjects. Schizophr Res. 2013;151:291–292. [DOI] [PubMed] [Google Scholar]
- 49. Bediou B, Asri F, Brunelin J, et al. Emotion recognition and genetic vulnerability to schizophrenia. Br J Psychiatry. 2007;191:126–130. [DOI] [PubMed] [Google Scholar]
- 50. Bölte S, Poustka F. The recognition of facial affect in autistic and schizophrenic subjects and their first-degree relatives. Psychol Med. 2003;33:907–915. [DOI] [PubMed] [Google Scholar]
- 51. Loughland CM, Williams LM, Harris AW. Visual scanpath dysfunction in first-degree relatives of schizophrenia probands: evidence for a vulnerability marker? Schizophr Res. 2004;67:11–21. [DOI] [PubMed] [Google Scholar]
- 52. Ibáñez A, Riveros R, Hurtado E, et al. The face and its emotion: right N170 deficits in structural processing and early emotional discrimination in schizophrenic patients and relatives. Psychiatry Res. 2012;195:18–26. [DOI] [PubMed] [Google Scholar]
- 53. de Achával D, Villarreal MF, Costanzo EY, et al. Decreased activity in right-hemisphere structures involved in social cognition in siblings discordant for schizophrenia. Schizophr Res. 2012;134:171–179. [DOI] [PubMed] [Google Scholar]
- 54. Eack SM, Mermon DE, Montrose DM, et al. Social cognition deficits among individuals at familial high risk for schizophrenia. Schizophr Bull. 2010;36:1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. [DOI] [PubMed] [Google Scholar]
- 56. Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci U S A. 2013;110:16616–16621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. [DOI] [PubMed] [Google Scholar]
- 58. Woodward TS, Leong K, Sanford N, Tipper CM, Lavigne KM. Altered balance of functional brain networks in Schizophrenia. Psychiatry Res. 2016;248:94–104. [DOI] [PubMed] [Google Scholar]
- 59. Hahn B, Harvey AN, Gold JM, Fischer BA, Keller WR, Ross TJ, Stein EA. Hyperdeactivation of the default mode network in people with schizophrenia when focusing attention in space. Schizophr Bull. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. [DOI] [PubMed] [Google Scholar]
- 61. Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- 62. Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18:421–428. [DOI] [PubMed] [Google Scholar]
- 63. Johnston PJ, Stojanov W, Devir H, Schall U. Functional MRI of facial emotion recognition deficits in schizophrenia and their electrophysiological correlates. Eur J Neurosci. 2005;22:1221–1232. [DOI] [PubMed] [Google Scholar]
- 64. Freeman D. Suspicious minds: the psychology of persecutory delusions. Clin Psychol Rev. 2007;27:425–457. [DOI] [PubMed] [Google Scholar]
- 65. Green MJ, Phillips ML. Social threat perception and the evolution of paranoia. Neurosci Biobehav Rev. 2004;28:333–342. [DOI] [PubMed] [Google Scholar]
- 66. Spilka MJ, Goghari VM. Similar patterns of brain activation abnormalities during emotional and non-emotional judgments of faces in a schizophrenia family study. Neuropsychologia. In press. doi:10.1016/j.neuropsychologia.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 67. Spilka MJ, Arnold AE, Goghari VM. Functional activation abnormalities during facial emotion perception in schizophrenia patients and nonpsychotic relatives. Schizophr Res. 2015;168:330–337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.