Abstract
Background
Sonidegib (LDE225) is a potent, selective hedgehog (Hh) inhibitor of Smoothened. This study explored the safety and pharmacokinetics of sonidegib in children with relapsed/recurrent tumors followed by a phase II trial in pediatric and adult patients with relapsed medulloblastoma (MB) to assess tumor response.
Methods
Pediatric patients aged ≥1 to <18 years were included according to a Bayesian design starting at 372 mg/m2 of continuous once daily oral sonidegib. Tumor samples were analyzed for Hh pathway activation using a validated 5-gene Hh signature assay. In phase II, pediatric patients were treated at the recommended phase II dose (RP2D) while adults received 800 mg daily.
Results
Sixteen adult (16 MB) and 60 pediatric (39 MB, 21 other) patients with an age range of 2–17 years were enrolled. The RP2D of sonidegib in pediatric patients was established at 680 mg/m2 once daily. The phase II study was closed prematurely.
The 5-gene Hh signature assay showed that the 4 complete responders (2 pediatric and 2 adult) and 1 partial responder (adult) all had Hh-activated tumors, while 5 patients with activated Hh had either stable disease (n = 3) or progressive disease (n = 2). No patient with an Hh-negative signature (n = 50) responded. The safety profile for pediatric patients was generally consistent with the one established for adult patients; however, growth plate changes were observed in prepubertal pediatric patients.
Conclusions
Sonidegib was well tolerated and the RP2D in pediatric patients was 680 mg/m2 once daily. Five of the 10 MB patients with activated Hh pathway demonstrated complete or partial responses.
Keywords: clinical trial, medulloblastoma, PTCH, SMO, sonic hedgehog
Importance of the study
Recurrent or progressive medulloblastoma continues to have a poor prognosis. Four major molecular subgroups have been identified; the sonic hedgehog variants are characterized by upstream mutations in Hh, Patched, and Smoothened (which can be inhibited by targeting Smoothened) as well as downstream activation of glioma-associated oncogene and suppressor of fused. Sonidegib (LDE225) is an oral, once daily, highly selective Smoothened inhibitor that we demonstrate to be well tolerated and with significant activity in both adult and pediatric patients with activation of the Hh pathway. Minor creatine phosphokinase elevation and effects on growth plates were the only significant toxicities observed. Patients lacking activation of the pathway do not respond and, based on these results, can be excluded from being exposed to toxicities without therapeutic benefits. By contrast, patients with Hh-pathway activated tumor may have better chances of treatment benefit. These findings provide a treatment option for recurrent/progressive medulloblastoma patients with Hh-activated tumors, and this approach could be ideally suited to incorporation into upfront treatment protocols.
Significant advances in the treatment of medulloblastoma (MB) have occurred over the last 3 decades. For children over the age of 3 years, maximal safe surgery, craniospinal radiation, and multiagent chemotherapy have resulted in excellent outcomes for those with standard-risk features1 and improved survival for patients with high-risk disease.2 Unfortunately, cure comes at a significant cost for these patients due to induction of secondary tumors and deleterious effects on growth, hormones, vascularity, and most importantly, cognition.3 Strategies that attempt to avoid the side effects of radiation therapy using high-dose chemotherapy and stem cell rescue have also been widely evaluated and are effective in some but not all patients.4 Better therapies are therefore needed to reduce toxicity in all age groups. Approximately 30% of patients with MB will experience a recurrence, and for these patients, long-term survival is very poor, independent of salvage regimen, with a median survival of approximately 12 months.5 Novel agents are desperately needed for these patients.
Recent advances in the molecular characterization of MB have resulted in the identification of 4 major subgroups, largely defined by the developmental pathway activated.6 One of these pathways, sonic hedgehog (Hh), is an important developmental signaling cascade responsible for the proliferation of cerebellar granule neuron precursors7–9 that has been strongly implicated in the development of approximately 25% of pediatric and 70% of adult medulloblastoma cases. In the absence of Hh ligand binding, the Hh receptor Patched (PTCH) acts as a negative regulator of the Hh pathway by inhibiting Smoothened (SMO), a G-protein coupled receptor-like signal transducer. Hh signaling is activated when an Hh ligand binds to PTCH, releasing its inhibition of SMO. Activated SMO initiates a downstream signaling cascade via a complex of cytosolic proteins, involving suppressor of fused (SUFU), a negative regulator of Hh signaling. The signaling cascade activates glioma-associated oncogene (GLI) transcription factors, which translocate to the nucleus and induce Hh-pathway target gene expression.10,11 Mutations in PTCH, SUFU, and SMO lead to constitutive activation of the Hh pathway in MB.12,13 A validated, 5-gene Hh signature reverse transcriptase (RT) PCR assay demonstrated a strong association between Hh pathway activation and tumor response.14 A 5-gene signature RT-PCR assay, however, detects the activation of the entire pathway, not just those from upstream activation (Hh, PTCH, SMO), which are the components inhibited by sonidegib. Inhibition of the Hh pathway in fetal development results in defective neural proliferation and holoprosencephaly with a single midline eye (cyclops).15 Overactivation of the pathway, by contrast, has been identified to cause excessive cellular proliferation, and germline mutation of PTCH can result in a number of morphogenic defects and increased tumor formation, including basal cell carcinoma, rhabdomyosarcoma, and MB and is referred to as Gorlin’s or basal cell nevoid carcinoma syndrome.16 Sonic hedgehog signaling, either sporadic or as part of the syndrome, most commonly results from persistent activation through mutations of PTCH or SMO, although approximately 25% of Hh-driven tumors have mutations downstream of SMO.17 The Hh subgroup is reported to comprise 65%, 15%, and 72% of MBs in infants (≤3 y of age), children (4–15 y), and adults (≥16 y), respectively.6,18 Patients with Hh activation have an approximately 70% 5-year event-free survival; those with an associated p53 mutation have an outcome of only 20%.19
Inhibition of the Hh pathway has been reported using sonidegib in an adult phase I trial20 and in adult and pediatric patients with vismodegib.21 Sonidegib (LDE225) is an oral selective antagonist of the Hh pathway that acts by inhibiting SMO at low nanomolar concentrations, thereby suppressing the growth of Hh-, PTCH-, and SMO-driven tumors.22,23 In the first-in-human phase I dose-escalation study of sonidegib in adult patients with advanced solid tumors, 3 of 9 patients (33%) with MB achieved objective tumor responses according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.0 and 2-fluoro-2-deoxy-d-glucose PET.20 We now report the results from the pediatric phase I dose-escalation study of sonidegib in children with advanced solid tumors potentially dependent on Hh signaling, including patients with relapsed or refractory MB. Safety, tolerability, pharmacokinetics (PK), pharmacodynamics, and preliminary antitumor activity were assessed. The maximum tolerated dose (MTD) or the recommended phase II dose (RP2D) to be used for future studies of sonidegib in children was determined. In the phase II study of sonidegib, the RP2D (680 mg/m2/day) for children and 800 mg for adults was tested in recurrent/relapsed MB patients.
Materials and Methods
Patients
Children between the ages of 1 and 18 years with a histologically confirmed diagnosis of MB, rhabdomyosarcoma, neuroblastoma, hepatoblastoma, high-grade glioma, or osteosarcoma who had progressed despite standard therapy or for whom no standard therapy was available were eligible for the phase I component of the study. Drug was provided by Novartis Pharmaceuticals in 50 mg, 100 mg, 200 mg, and 250 mg capsules. Drug was dispensed at the start of every 28-day cycle and was administered once daily, at approximately the same time, preferably in the morning. Patients who were unable to swallow whole capsules were provided instructions for handling open capsules to be taken with a small amount of food—applesauce or yogurt. Drug was required to be stored in the refrigerator (2–8°C, 36–46°F) and at room temperature one hour before the dosing time to make the capsule contents easier to prepare. Patients were assessed weekly for the first 8 weeks, then monthly after. In the phase II component, for both adult and pediatric patients, only recurrent or relapsed medulloblastoma patients were eligible. Other eligibility criteria included: Karnofsky performance status score of ≥60 for patients older than 10 years of age or a Lansky Play scale of ≥50 for patients 10 years of age or younger and adequate renal function (serum creatinine ≤1.5 × upper limit of normal [ULN] for age or creatinine clearance or radioisotope glomerular filtration rate ≥1.17 mL/s/1.73 m2), liver function (bilirubin ≤1.5 × ULN for age, serum alanine aminotransferase [ALT]/serum glutamic pyruvic transaminase ≤5 × ULN for age, serum aspartate aminotransferase [AST]/serum glutamic oxaloacetic transaminase ≤5 × ULN for age, serum albumin ≥20 g/L), and bone marrow function (peripheral absolute neutrophil count ≥1.0 × 109/L, platelet count ≥80 × 109/L, and hemoglobin ≥8 g/dL). Consent to provide archival or fresh tumor sample for pharmacodynamic analyses was required if material was available. Patients with impaired cardiac function or clinically significant cardiac disease, gastrointestinal dysfunction, neuromuscular disorders associated with creatine phosphokinase (CPK) elevation, or any other concurrent severe and/or uncontrolled medical conditions were excluded from the trial. Strenuous exercise was to be avoided while taking sonidegib to prevent significant increases in plasma CPK levels. Treatment with strong inhibitors or inducers of cytochrome P450 (CYP) 3A4/5 or drugs metabolized by CYP2B6 or CYP2C9, which have a narrow therapeutic index, was prohibited during the study. Patients not on a stable dose of corticosteroid or those on enzyme-inducing anticonvulsants that were prohibited during the study were not eligible; drugs recognized to cause rhabdomyolysis (3-hydroxy-3-methylglutaryl-coenzyme A inhibitors [statins], clofibrate, and gemfibrozil) were also prohibited (pravastatin was allowed with extra monitoring if essential to control hyperlipidemia). All patients/legal guardians provided written consent and assent where appropriate. The trial was conducted in accordance with the International Council for Harmonisation Tripartite Guidelines for Good Clinical Practice, with applicable local regulations, and with the ethical principles laid down in the Declaration of Helsinki. The study protocol and amendments were reviewed and approved by the institutional review board/independent ethics committee/research ethics board of each site.
Study Design
This international multisite open-label study (NCT01125800) included a phase I dose escalation and safety expansion in children with relapsed/refractory MB or other tumors potentially dependent on the Hh pathway. The protocol was amended to include a dose-expansion component to assess the preliminary efficacy in children and adults with relapsed/refractory MB. The primary objective of the dose-escalation phase was to determine the MTD or RP2D and dose-limiting toxicities (DLTs) of sonidegib. Secondary objectives included characterization of the safety, tolerability, and PK profile of sonidegib and assessment of preliminary antitumor activity (objective response rate [ORR]), Hh gene expression signature, and mutational status of Hh pathway genes [SMO, PTCH, SUFU]). Due to lack of tumor material, not completed were other planned objectives, including analysis of pharmacodynamic effects of sonidegib on Hh pathway biomarkers (GLI1, PTCH1, cyclin D1) in tumor cells from cerebrospinal fluid (CSF), correlation between tumor response and expression of the Hh gene signature, effects of sonidegib on bone markers in serum samples, and determination of PK/pharmacodynamic and PK/safety relationships. For the phase II component of the trial, the primary objective was to assess the efficacy of sonidegib, as determined by radiographic response in recurrent or progressive MB patients. The secondary aims included a planned assessment of the mechanisms of de novo resistance by analysis of GLI2 amplifications and downstream mutations, such as SUFU. CSF concentrations of sonidegib were also to be assessed if available. The secondary objectives could not be completed due to lack of sufficient patient material.
Successive cohorts of pediatric patients (minimum of 3) were treated with escalating doses of oral sonidegib starting at 372 mg/m2 once daily in 28-day cycles. Patients were considered evaluable if they were treated with at least 75% of the planned doses of sonidegib within the first 6 weeks of treatment and had completed sufficient safety evaluations and/or if they experienced a DLT during the first 6 weeks of treatment. Dose escalations were based on an adaptive Bayesian logistic regression model with overdose control.24,25 A DLT was defined as an adverse event (AE) or abnormal laboratory value related to sonidegib considered to be grade ≥3 according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. The MTD or RP2D was defined as the highest tolerated dose at which at least 6 patients had been assessed. Once the MTD or RP2D was established, an additional 6 patients were treated at the MTD and/or RP2D to further characterize the safety and tolerability of sonidegib. An MB enrichment phase allowed the enrollment of additional patients with MB into previously tested, well-tolerated doses to further characterize sonidegib treatment in MB. Patients continued treatment until disease progression, unacceptable toxicity, death, or as per investigator discretion. Once the phase I and cohort expansion was completed, additional pediatric patients were enrolled on the phase II component of the trial. Adult patients were eligible for entry into the phase II component from the outset, as the RP2D for this group had already been determined.20
Sample Size Estimation
The safety expansion phase was planned to include a minimum of 18 evaluable patients (including 6 patients in the dose-escalation level) at the MTD/RP2D level, which would provide 85% probability of detecting an AE with a true incidence rate of 10%. In total, approximately 55 patients were planned to be enrolled in the phase II portion, with 25 MB patients in the adult group and 30 MB patients in the pediatric group. It was anticipated that the proposed sample sizes would allow enrollment of approximately 20 Hh-activated MB patients (10 adults and 10 pediatric). This assumed that the overall prevalence of Hh-activated MB is approximately 40% among adults and 30% among children. The primary purpose of the phase II part of this study was to assess preliminary efficacy of LDE225, as determined by ORR, in recurrent or refractory MB patients. With a sample size of 55, this study had reasonable operating characteristics if the true ORR was 25% or higher.
Safety Assessments
Safety assessments included monitoring and recording of all AEs, serious AEs, laboratory evaluations, physical examination, vital signs, weight, and electrocardiogram recordings. AEs were graded according to the CTCAE v4.0 guidelines and were monitored throughout the study and until 30 days following the last dose. Due to increased serum CPK levels observed in the first-in-human phase I study of sonidegib in adults with advanced solid tumors,20 additional CPK monitoring was implemented throughout the study. In addition, because inhibition of the Hh pathway has been shown to cause premature growth plate closure in preclinical mouse models,26 bone X-rays and dental assessments were conducted throughout the study.
Pharmacokinetics
Blood sample collection and handling
Whole blood samples (1 mL) were collected throughout the phase I study. Serial blood samples were collected on day 1 and day 22 of cycle 1 at predose and 0.5, 1, 2, 4, 7, and 24 hours postdose. Blood samples were also collected predose on days 8, 15, and 28 of cycle 1 and on day 1 of each cycle from cycle 3, or anytime a CSF sample was collected. For patients on the phase II component of the trial, trough levels were assessed during cycle 1 (on days 1, 2, 8, 15, 22, 23, and 28) and cycles 2–18 (on day 1). Samples were processed and frozen at ‒70°C or less. Preparation and analysis of plasma samples were performed as previously published.20
PK assessments
PK parameters, including area under the plasma concentration-time curve from time zero to 24 hours (AUC0–24h), peak plasma concentration (Cmax), and time to reach Cmax (Tmax) were calculated using noncompartmental methods with Phoenix WinNonlin version 6.2 (Pharsight).
Tumor Assessments
Tumor response was assessed every 8 weeks by the Neuro-Oncology Criteria of Tumor Response (CNS tumors)27,28 and RECIST v1.0 (non-CNS tumors).29 Assessments continued until disease progression or the start of a new antineoplastic agent. For all patients with CNS disease, neurological assessments—including determination of consciousness level, mental status, speech, vision fundus (papilledema), cranial nerves (III, IV, VI, other), and motor, sensory, and gait or limb ataxia—were completed within 1 week of each tumor response evaluation.
Pharmacodynamics
Formalin-fixed paraffin-embedded archival tumor samples from any timepoint (diagnosis or relapse) and fresh tumor samples (when available) from preplanned, treatment-related surgical resections or biopsies were subjected to gene expression analysis (5-gene Hh signature RT-PCR assay14/markers of pathway activation). In consenting pediatric patients, blood samples (maximum of 5 mL) were also collected predose on days 1, 15, and 28 of cycle 1, day 28 of cycle 2, and at the end of treatment for bone marker analyses.
Statistical Analyses
The RP2D of sonidegib was determined using an adaptive Bayesian logistic regression model with overdose control. Patient demographics, safety, PK, and response data were summarized using descriptive analyses.
Results
Demographics
Sixty pediatric patients were enrolled (59 on phase I and 1 on phase II), including 39 patients with MB, with a median age of 12 years (range 2–17 y). In phase II, 16 adult patients with MB were included. The starting dose in phase I was 372 mg/m2; 7 patients were included at this dose. Due to a DLT, dose was reduced to 233 mg/m2. Eleven patients were included at 233 mg/m2 (6 in dose escalation and 5 in MB enrichment). Dose was reescalated to 372 mg/m2; 9 patients were enrolled (4 in dose escalation and 5 in MB enrichment). At 425 mg/m2, 11 patients were included (6 in dose escalation and 5 in MB enrichment). At 680 mg/m2, 21 patients were included (7 in dose escalation and 14 in MB enrichment). In a phase II study, 16 adult patients received 800 mg and 1 pediatric patient received 680 mg/m2. Baseline characteristics and disease history, including tumor histology/cytology, previous tumor directed therapies, and performance status (Karnofsky/Lansky) of patients enrolled, are listed in Table 1.
Table 1.
Patient demographics and baseline disease characteristics
| Variable | Pediatrics N = 60 | Adults N = 16 |
|---|---|---|
| Age, y, median (range) | 12 (2–17) | 34 (18–66) |
| ≤10 y | 23 (38.3%) | 0 |
| >10 y | 37 (61.7) | 16 (100%) |
| Body surface area, m2, median (range) | 1.2 (0.6–2.7) | 1.8 (1.1–2.7) |
| Sex, n (%) | ||
| Male | 37 (61.7) | 7 (43.8) |
| Female | 23 (38.3) | 9 (56.3) |
| Race | ||
| Caucasian | 45 (75) | 14 (87.5) |
| Asian | 4 (6.7) | 1 (6.3) |
| Black | 4 (6.7) | 0 |
| Other or missing (not specified) | 7 (11.7) | 1 (6.3) |
| Karnofsky statusa / Lansky Play score, n (%) | 37 (62) / 23 (38) | 16 (100) / NA |
| 100 | 15 (25) / 11 (18.3) | 0 / NA |
| 90 | 6 (10) / 6 (10) | 8 (50) / NA |
| 80 | 7 (11.7) / 4 (6.7) | 5 (31.3) / NA |
| 70 | 3 (5) / 2 (3.3) | 1 (6.3) / NA |
| <70 | 5 (8) / 0 | 2 (12.5) / NA |
| Histology/cytology, n (%) | ||
| Medulloblastoma | 39 (65) | 16 (100) |
| Glioblastoma multiforme | 5 (8.3) | 0 |
| Osteosarcoma | 5 (8.3) | 0 |
| Rhabdomyosarcoma | 4 (6.8) | 0 |
| Neuroblastoma | 3 (5.0) | 0 |
| Hepatoblastoma | 1 (1.7) | 0 |
| Anaplastic astrocytoma | 1 (1.7) | 0 |
| Oligoastrocytoma | 1 (1.7) | 0 |
| Gliomatosis | 1 (1.7) | 0 |
| Time, mo, from initial diagnosis of primary site to start of study, median (range), n = 60 | 35.63 (8.6–111.6) | 70.72 (15.9–210.9) |
| Time, mo, since most recent relapse/recurrence to start of study, median (range), n = 60 | 0.94 (0.3–7.2) | 1.61 (0.2–20.7) |
| Prior therapy, n (%) | ||
| Surgery | 59 (98.3) | 16 (100) |
| Radiotherapy | 49 (81.7) | 16 (100) |
| Chemotherapy | 60 (100.0) | 15 (93.8) |
aKPS missing for 1 (1.7) patient.
Safety
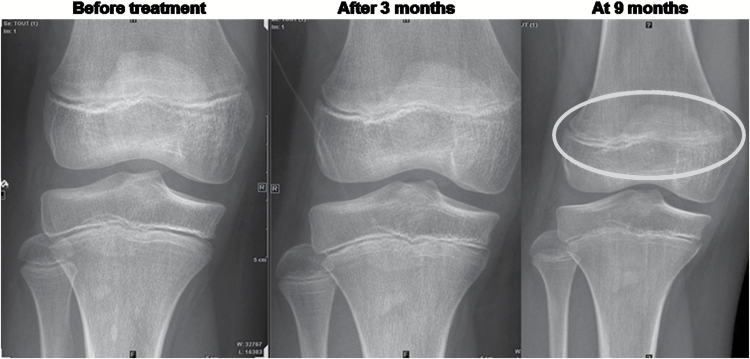
The median treatment exposure for all pediatric and adult patients was 55 days (range, 2–289 d) and 97 days (range, 34–511 d), respectively. The most common treatment-related AEs in children included fatigue, muscle spasms, increased CPK, myalgia, and vomiting (Table 2 and Supplementary Table S2) and were similar to and partially overlapped those observed in adults, including increased CPK, myalgia, muscle spasms, nausea, and increased ALT. The only DLT observed was a reversible grade 4 CPK elevation which occurred in one pediatric patient with rhabdomyosarcoma treated at 372 mg/m2, at the end of the first cycle of therapy. This patient was discontinued due to progressive disease (PD) after 35 days on treatment and without renal dysfunction or muscle symptoms. Dose levels were reduced to 233 mg/m2 for the next 6 patients and then reescalated to 372 mg/m2. An additional 15 patients were treated at 372 mg/m2 in the dose-escalation (n = 10) or MB enrichment (n = 5) phase without DLTs. Furthermore, no DLTs were observed in patients enrolled in the dose-escalation or MB enrichment phase at doses of 425 mg/m2 (n = 6) and 680 mg/m2 (n = 4); therefore, the MTD was not reached and the RP2D was determined to be 680 mg/m2 once daily. Grade 1/2 and grade 3/4 CPK elevation occurred in 7 and 2 pediatric patients, respectively, and in 2 and 5 adults, respectively. No concurrent renal dysfunction was observed in any pediatric patient who experienced CPK elevations. Evidence of growth plate closure was observed in 3 pediatric patients (wrist cartilage closure, knee cartilage closure, and knee subchondral condensation within growth plate; Fig. 1).
Table 2.
Adverse events suspected to be study drug related, by preferred term, maximum grade, and treatment (>3% all children or >10% adults; safety analysis set)
| Preferred Term | Sonidegib Daily Dose, mg/m2 | All Children N = 60 |
Adults 800 mg N = 16 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 233 n = 11 |
372 n = 16 |
425 n = 11 |
680 n = 22 |
|||||||||
| All grade n (%) |
Grade 3/4 n (%) |
All Grades n (%) | Grade 3/4 n (%) |
All Grades n (%) |
Grade 3/4 n (%) |
All Grades n (%) |
Grade 3/4 n (%) |
All Grades n (%) |
Grade 3/4 n (%) |
All Grades n (%) |
Grade 3/4 n (%) |
|
| Total | 8 (72.7) | 1 (9.1) | 13 (81.3) | 2 (12.5) | 9 (81.8) | 0 | 14 (63.6) | 2 (9.1) | 44 (73.3) | 5 (8.3) | 13 (81.3) | 6 (37.5) |
| Fatigue | 5 (45.5) | 0 | 4 (25.0) | 0 | 2 (18.2) | 0 | 1 (4.5) | 0 | 12 (20.0) | 0 | 1 (6.3) | 0 |
| Muscle spasms | 2 (18.2) | 0 | 4 (25.0) | 0 | 3 (27.3) | 0 | 2 (9.1) | 0 | 11 (18.3) | 0 | 4 (25.0) | 0 |
| Blood creatine phosphokinase increased | 1 (9.1) | 0 | 1 (6.3) | 0 | 2 (18.2) | 0 | 5 (22.7) | 2 (9.1) | 9 (15.0) | 2 (3.3) | 7 (43.8) | 5 (31.3) |
| Myalgia | 0 | 0 | 0 | 0 | 4 (36.4) | 0 | 5 (22.7) | 0 | 9 (15.0) | 0 | 5 (31.3) | 0 |
| Vomiting | 2 (18.2) | 1 (9.1) | 1 (6.3) | 0 | 3 (27.3) | 0 | 3 (13.6) | 0 | 9 (15.0) | 1 (1.7) | 1 (6.3) | 0 |
| Nausea | 3 (27.3) | 0 | 2 (12.5) | 0 | 2 (18.2) | 0 | 1 (4.5) | 0 | 8 (13.3) | 0 | 4 (25.0) | 0 |
| Alopecia | 0 | 0 | 1 (6.3) | 0 | 3 (27.3) | 0 | 1 (4.5) | 0 | 5 (8.3) | 0 | 0 | 0 |
| Arthralgia | 1 (9.1) | 0 | 1 (6.3) | 0 | 2 (18.2) | 0 | 1 (4.5) | 0 | 5 (8.3) | 0 | 0 | 0 |
| Pain in extremity | 1 (9.1) | 0 | 1 (6.3) | 0 | 3 (27.3) | 0 | 0 | 0 | 5 (8.3) | 0 | 0 | 0 |
| Madarosis | 1 (9.1) | 0 | 1 (6.3) | 0 | 2 (18.2) | 0 | 0 | 0 | 4 (6.7) | 0 | 0 | 0 |
| Anemia | 0 | 0 | 0 | 0 | 3 (27.3) | 0 | 0 | 0 | 3 (5.0) | 0 | 0 | 0 |
| Asthenia | 0 | 0 | 1 (6.3) | 0 | 1 (9.1) | 0 | 1 (4.5) | 0 | 3 (5.0) | 0 | 2 (12.5) | 0 |
| Decreased appetite | 0 | 0 | 0 | 0 | 2 (18.2) | 0 | 1 (4.5) | 0 | 3 (5.0) | 0 | 2 (12.5) | 0 |
| Headache | 1 (9.1) | 0 | 1 (6.3) | 0 | 1 (9.1) | 0 | 0 | 0 | 3 (5.0) | 0 | 0 | 0 |
| AST increased | 0 | 0 | 1 (6.3) | 0 | 1 (9.1) | 0 | 0 | 0 | 2 (3.3) | 0 | 1 (6.3) | 1 (6.3) |
| Blood creatinine increased | 0 | 0 | 0 | 0 | 1 (9.1) | 0 | 1 (4.5) | 0 | 2 (3.3) | 0 | 0 | 0 |
| Diarrhea | 0 | 0 | 1 (6.3) | 0 | 1 (9.1) | 0 | 0 | 0 | 2 (3.3) | 0 | 3 (18.8) | 0 |
| Nail disorder | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) | 0 | 0 | 0 | 2 (3.3) | 0 | 0 | 0 |
| Neutrophil count decreased | 0 | 0 | 0 | 0 | 1 (9.1) | 0 | 1 (4.5) | 0 | 2 (3.3) | 0 | 0 | 0 |
| Pain | 0 | 0 | 0 | 0 | 1 (9.1) | 0 | 1 (4.5) | 0 | 2 (3.3) | 0 | 0 | 0 |
| Pain in jaw | 0 | 0 | 0 | 0 | 2 (18.2) | 0 | 0 | 0 | 2 (3.3) | 0 | 0 | 0 |
| Toothache | 2 (18.2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (3.3) | 0 | 0 | 0 |
| ALT increased | 0 | 0 | 1 (6.3) | 0 | 0 | 0 | 0 | 0 | 1 (1.7) | 0 | 4 (25.0) | 2 (12.5) |
Fig. 1.
Growth plate closure in 11-year-old patient treated at 372 mg/m2.
Dose reduction or interruptions occurred in 7 pediatric patients. These events were grade 2 confusional state, grade 3 convulsion, and grade 3 disorientation in one patient; confusional state and agitation (both grade 3) in one patient; and grade 4 convulsion, grade 3 hypersensitivity, grade 3 hypophosphatemia, grade 3 vomiting, and grade 2 lethargy, each in one patient. The investigators considered the hypersensitivity to be related to study drug; the remainder of these events were considered unrelated to study drug by the investigators. The patient with agitation and confusional state had glioblastoma; the other patients had MB. Six adult patients had one or more AEs that required dose adjustment or interruption: grade 4 increased CPK and grade 3 increased ALT in one patient; grade 4 increased CPK in one patient; increased ALT and increased AST (both grade 4) in one patient; grade 3 increased CPK and grade 1 increased bilirubin in one patient; and grade 3 increased CPK in 2 patients. The adult patient whose dose was adjusted for grade 3 increased CPK and grade 1 increased bilirubin also discontinued study drug as a result of grade 2 increased CPK. With the exception of increased bilirubin, all of these events were considered to be related to study drug.
Four pediatric patients discontinued treatment because of AEs that included grade 4 hydrocephalus, grade 3 pleural effusion (both considered unrelated to therapy), grade 2 chondropathy and grade 1 epiphyseal disorder (both considered related to therapy). Three adult patients discontinued study drug as a result of grade 4 respiratory distress, grade 3 femur fracture (both felt unrelated to treatment), and grade 2 increased CPK (considered related to therapy). Thirteen pediatric and 2 adult patients died while receiving sonidegib or within 30 days of the last dose. All were due to progressive disease except in one adult, where the cause of death was listed as acute respiratory distress.
Pharmacokinetics
PK parameters including AUC0–24h, Cmax, and Tmax were determined during cycle 1 (Table 3) for pediatric patients. Median Tmax for all doses on day 1 occurred at 2 to 4 hours (range 0.5–7) and on day 22 at 2 hours (range 0–7). Tmax was reached independently of dose level and declined in a bi-exponential manner. Cmax and AUC0–24h increased dose proportionally at both day 1 and day 22. Most patients discontinued treatment prior to achieving steady state, although sonidegib continued to accumulate past cycle 2 in most patients for whom data were available. Interpatient coefficient of variation for Cmax and AUC0–24h on day 1 was 38.5% to 74.6% and 42.5% to 74.2%, respectively, and not significantly different on day 22. The PK data for adults were similar to those previously reported.20 The trough sonidegib and LGE899 (pharmacologically inactive primary circulating metabolite) concentrations were generally within the range observed in adult patients with advanced basal cell carcinoma.20
Table 3.
Sonidegib plasma exposure in pediatric patients at days 1 and 22 of cycle 1 by dose cohort
| Sonidegib Dose Level (Once Daily) | Cycle 1, Day 1 | Cycle 1, Day 22 | ||||
|---|---|---|---|---|---|---|
| Cmax (ng/mL) n mean (SD) |
AUC0-24h (ng*hr/mL) n mean (SD) |
Tmax (h) n median (min;max) |
Cmax (ng/mL) n mean (SD) |
AUC0-24h (ng*hr/mL) n mean (SD) |
Tmax (h) n median (min;max) |
|
| 233 mg/m2 | 11 | 11 | 11 | 9 | 9 | 9 |
| 191 (82) | 1982 (737) | 4.0 (1.1;6.8) | 769 (496) | 10,590 (4163) | 2.0 (1.0;7.0) | |
| 372 mg/m2 | 15 | 15 | 15 | 12 | 14 | 12 |
| 246 (211) | 2194 (1592) | 2.0 (1.0;7.0) | 944 (553) | 15,431 (10,433) | 2.1 (1.0;4.3) | |
| 425 mg/m2 | 10 | 11 | 10 | 9 | 9 | 9 |
| 642 (487) | 5309 (3247) | 2.9 (0.5;7.0) | 1122 (737) | 17,753 (11,552) | 2.0 (0.7;7.0) | |
| 680 mg/m2 | 17 | 19 | 17 | 15 | 15 | 15 |
| 618 (403) | 5118 (2658) | 2.1 (1.0;4.1) | 1930 (678) | 32,623 (11,671) | 2.0 (0.0;7.1) | |
Efficacy
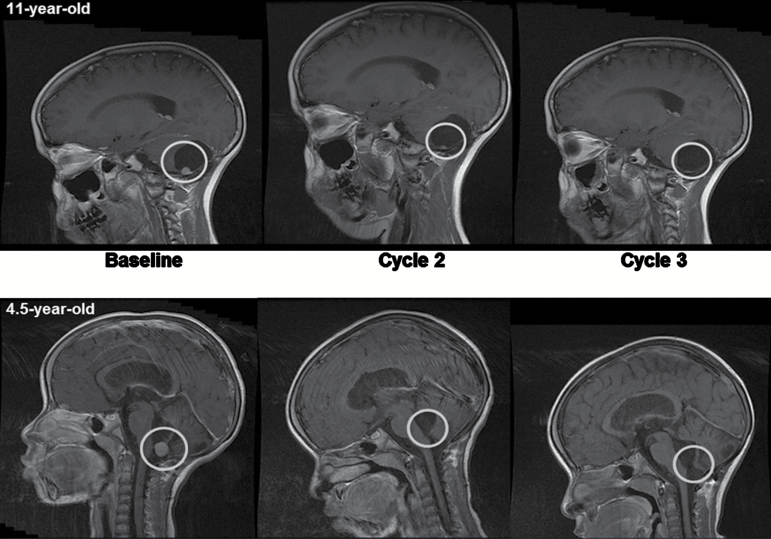
The overall response rates in the entire pediatric (n = 60) and adult (n = 16) populations were 2/60 (3.3%; CI 0.4, 11.5) and 3/16 (18.8%; CI 4.0, 45.7), respectively. Of the 60 patients (including pediatric and adult) in whom Hh status was assessed, 10 patients were Hh-positive and 5 responses (all in Hh-positive patients) were observed (50%). Responding patients had all received prior radiation therapy as part of their upfront treatment for MB; however, none of the patients had received reirradiation before trial inclusion. Only patients with MB responded (2 complete responses [CRs] at doses of 372 and 425 mg/m2 for the pediatric patients and 2 CRs as well as 1 partial response [PR] in adults at 800 mg; Fig. 2). Stable disease (SD) was observed in 11 (5 pediatric and 6 adult patients, all with MB). One pediatric patient discontinued from the study after 7 days due to hydrocephalus; the remaining 53 pediatric and 7 adult patients had PD. Both responding pediatric patients stopped treatment after 9 months when in CR. Duration of response of a total of 21 months was observed for the 11-year-old female treated at 372 mg/m2, and the 4-year-old female treated at 425 mg/m2 is still in remission at time of writing the manuscript (duration of 49 mo in December 2016). For the adult patients, duration of response for the 2 CRs was 1.6 and 8.7 months, and the duration of response for the PR was 4.8 months. Progression-free and overall survivals were not evaluated, as the phase II component of this study was terminated early (n = 17) in order to proceed to a randomized phase III trial of this agent in patients with Hh MB.
Fig. 2.
Complete tumor responses in 11- (top panel) and 4.5-year-old (bottom panel) children with medulloblastoma.
Biomarkers
Tumor responses were associated with Hh pathway activation as identified by the 5-gene signature RT-PCR assay (upregulation of GLI1, SHROOM2, sphingosine kinase-1, and PDLIM3 and downregulation of orthodenticle homeobox 2).14 Three of 47 pediatric patients (at the 372, 425, and 680 mg/m2 dose levels) and 7 of 13 adult tumors tested (all treated at 800 mg) were positive on the 5-gene assay. All signature positive patients had MB. This included both pediatric patients with CR as well as the 2 CR and 1 PR identified in adults. None of the 50 patients with Hh pathway nonactivated tumors demonstrated a response to therapy (SD, n = 4; PD, n = 39; discontinued therapy before first evaluation, n = 5; unknown, n = 2). Assessment of serum bone markers—including C-terminal telopeptide of type I collagen, tartrate-resistant acid phosphatase 5b, osteoprotegerin, osteocalcin, bone-specific alkaline phosphatase, and procollagen 1 N-terminal peptide—demonstrated decrease from baseline in bone formation and in bone resorption markers across all dose levels (see Supplementary Table S1). Neither tumors (n = 47) nor cells from CSF samples (n = 2) could be analyzed for mutations in the Hh pathway genes SMO, PTCH, and SUFU. Skin biopsies were optional (in phase I only) and no patients provided samples for analysis.
Discussion
Oral once daily sonidegib was well tolerated in children with advanced solid tumors and demonstrated antitumor activity in pediatric and adult patients with relapsed MB with Hh pathway activated signature. The recommended phase II dose is 680 mg/m2 orally once daily to a maximum of 800 mg.
Common treatment-related AEs were similar to those observed in the phase I study of sonidegib in adults with advanced solid tumors20 and have been observed with other SMO inhibitors.30,31 Elevated CPK without evidence of organ impairment was common. One patient with rhabdomyosarcoma showed dose-limiting grade 4 CPK elevation. All events were of skeletal muscle origin based on total CPK/CPK-MB ratio. Significant CPK elevation observed in pediatric patients on this study occurred less frequently (5/60 or 8.3%) than that observed in adults (5/16 or 31.3%).20 The reason for this difference is unknown.
Due to the known role of Indian Hh in bone development and the on-target growth plate effects observed with Hh pathway inhibitors in preclinical animal models,26 use of Hh pathway inhibitors in pediatric patients has potential risks. Therefore, growth plate effects were monitored in the patients in this trial. In addition to changes in serum bone markers suggesting some impact of Hh inhibition, 3 bone related toxicities of potential importance were identified. A 4-year-old female reported narrowing of the epiphyseal plate of the phalanx on day 133 and a subchondral condensation in the area of the growth plate on day 169 and discontinued the drug because of these reasons. An 8-year-old male patient had widening of the distal femur epiphyseal growth plate on day 57, and an 11-year-old female patient was noted to have knee cartilage closure on day 56 and wrist cartilage closure on day 196, which resulted in discontinuation of drug. No bone growth defects were reported in the clinical trial of vismodegib,21 although only 4 patients had a response, all above the age of 17 at the time of treatment and thus not at a developmental stage where growth plate effects could be evaluated. The results from this study clearly warrant further evaluation of growth plate effects in pediatric patients treated with Hh pathway inhibitors. Since Hh-associated MB is common in young infants, particular caution should be exercised before considering adding Hh-targeted treatment to this subgroup of patients.
Sonidegib exposure increased with increasing doses in a linear manner in the dose range of 233 to 680 mg/m2, but it did not achieve steady state within the first 2 cycles of treatment. Sonidegib exposure in children is consistent with that observed previously in adults for equivalent mg/m2 doses.20 Sonidegib demonstrated antitumor activity in patients with relapsed Hh MB. No activity was observed in non-Hh MB or other tumor types that have been associated with activation of the Hh pathway, although this may have resulted from the fact that none of the patients with non-MB tumors happened to have Hh activation as defined by the 5-gene signature. Two children and 2 adults achieved CRs and one adult achieved a PR. All 5 patients had tumors with evidence of activated Hh pathway as determined by the 5-gene signature RT-PCR assay.14 The remaining patients with activated Hh pathway had SD or PD. Lack of response in these patients may have been due to a mutation downstream of SMO, although due to lack of material, this was not tested but is in agreement with other Smoothened inhibitors.21,32 Our evaluation was not exhaustive, as many of the included patients were diagnosed at smaller centers in which the material for diagnosis may not have been collected, but we did not want to exclude these patients for lack of material. Further, stability of Hh activating alterations at recurrence was not analyzed in the study; however, according to previous studies, MB does not usually change subgroup at recurrence.33 Future trials going forward should mandate biological analysis with advancing technology and less tumor material to accurately identify the patients who may respond to the therapy. Five and 24 patients with MB had SD and PD, respectively. None of these patients had tumors with activated Hh pathway. A 5-gene signature RT-PCR assay did not measure the level of Hh alteration but the propensity of a patient to be Hh activated and thus respond to SMO inhibitors, such as sonidegib. It detects the activation of the entire pathway, not just those from upstream activation (Hh, PTCH, SMO), which are the components inhibited by sonidegib. Our analyses showed a strong association between Hh activation status/propensity score as determined by the 5-gene signature assay and tumor response upon treatment with sonidegib. The significance of SD in the response assessment of MB remains unclear, as patients with known recurrent disease can demonstrate very slow progression that in the early assessment can mimic SD.34 These findings should be taken into consideration when assessing response in clinical trials of patients with MB. Five patients with MB did not have their tumors analyzed; one patient was removed from the study prior to tumor response assessment due to hydrocephalus. All of the patients with other solid tumors had PD and were found to have Hh nonactivated tumors. In the phase I study in adults, activated Hh pathway determined using the 5-gene signature assay was strongly associated with tumor response in patients with MB14 and basal cell carcinoma.20 The study described here adds further credence to the validity of the 5-gene signature assay. The 50% (5 of 10) radiographic response rate observed in this combined phase I/II cohort of pediatric and adult patients is higher than reported for vismodegib, another Hh targeted agent, where 4 of 26 patients (15%) had a response.21 Whether this difference is related to the CNS penetration of the 2 agents or is a result of the mixture of patients with mutations upstream versus downstream of Smoothened between the 2 studies is not known and neither study was able to obtain sufficient material in enough patients to complete these mutational analyses.21
This study was initiated as a phase I study. When initial (promising) activity was observed, there was a need to obtain further data. Rather than initiating a separate study, the protocol was amended to add a phase II part to the study. This helped to collect additional data in both adult and pediatric patients with Hh activated MB, as well as to “bridge” the gap until the phase III study opened to enrollment (enrollment started May 2013). When the phase III study was open, there was no longer a need to keep the phase I/II study active, and it was desirable to have all patients be recruited into the phase III registration trial. Unfortunately, a much lower than expected Hh-pathway activation rate precluded the timely conduct of the trial as planned, which prompted the sponsor to modify the trial; basically reducing the sample size and converting this from a randomized phase III to a single arm phase II study. There is a strong unmet need for medulloblastoma patients with high-risk (eg, metastatic Hh or Myc neuroblastoma–amplified Hh MB) or very high-risk disease (eg, Hh with tumor protein 53 mutation) and recurrent or relapsed MB.19 Targeted therapies and patient selection diagnostics are needed to improve survival and quality of life in this patient population. This study has demonstrated that the use of Hh pathway–targeted therapies can be beneficial in patients with Hh pathway–activated relapsed MB. Particular attention, however, must be shown in younger patients with incomplete maturation of the skeletal system.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This study was sponsored by Novartis Pharmaceuticals Corporation.
Conflict of interest statement. Dr Kieran reports an advisory role with Novartis for discussions regarding medulloblastoma and glioma. Dr Hargrave reports an advisory role for sonidegib with Novartis, a consultant role for study drugs other than sonidegib with Novartis, and receiving funds for travel reimbursement from Novartis. Dr Casanova reports an advisory role with Boehringer, Roche, Loxo, Bayer, and Lily. Drs Hurh and Kalambakas report former employment with Novartis and ownership of stock in Novartis. The remaining authors do not have any conflicts of interest.
Supplementary Material
Acknowledgments
We thank the patients and their families, the study investigators, and the study site personnel for their participation and contributions to this study. Rajasree Solipuram (Novartis Healthcare) provided editorial assistance for incorporating comments according to authors’ feedback and support with submission.
References
- 1. Packer RJ, Gajjar A, Vezina G et al. . Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–4208. [DOI] [PubMed] [Google Scholar]
- 2. Jakacki RI, Burger PC, Zhou T et al. . Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a Children’s Oncology Group Phase I/II study. J Clin Oncol. 2012;30(21):2648–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palmer SL, Armstrong C, Onar-Thomas A et al. . Processing speed, attention, and working memory after treatment for medulloblastoma: an international, prospective, and longitudinal study. J Clin Oncol. 2013;31(28):3494–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen BH, Geyer JR, Miller DC et al. ; Children’s Oncology Group. Pilot study of intensive chemotherapy with peripheral hematopoietic cell support for children less than 3 years of age with malignant brain tumors, the CCG-99703 phase I/II study. A report from the Children’s Oncology Group. Pediatr Neurol. 2015;53(1):31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bode U, Zimmermann M, Moser O et al. . Treatment of recurrent primitive neuroectodermal tumors (PNET) in children and adolescents with high-dose chemotherapy (HDC) and stem cell support: results of the HITREZ 97 multicentre trial. J Neurooncol. 2014;120(3):635–642. [DOI] [PubMed] [Google Scholar]
- 6. Taylor MD, Northcott PA, Korshunov A et al. . Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22(1):103–114. [DOI] [PubMed] [Google Scholar]
- 8. Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9(8):445–448. [DOI] [PubMed] [Google Scholar]
- 9. Schüller U, Heine VM, Mao J et al. . Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 2008;14(2):123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong SY, Reiter JF. The primary cilium at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol. 2008;85:225–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30(6):303–312. [DOI] [PubMed] [Google Scholar]
- 12. Lee Y, Miller HL, Jensen P et al. . A molecular fingerprint for medulloblastoma. Cancer Res. 2003;63(17):5428–5437. [PubMed] [Google Scholar]
- 13. Slade I, Murray A, Hanks S et al. . Heterogeneity of familial medulloblastoma and contribution of germline PTCH1 and SUFU mutations to sporadic medulloblastoma. Fam Cancer. 2011;10(2):337–342. [DOI] [PubMed] [Google Scholar]
- 14. Shou Y, Robinson DM, Amakye DD et al. . A five-gene hedgehog signature developed as a patient preselection tool for hedgehog inhibitor therapy in medulloblastoma. Clin Cancer Res. 2015;21(3):585–593. [DOI] [PubMed] [Google Scholar]
- 15. Zeiss CJ, Zarfoss MK, Johnson EE, Dubielzig RR. Ocular anomalies and holoprosencephaly in a lamb. Vet Ophthalmol. 2008;11(1):30–33. [DOI] [PubMed] [Google Scholar]
- 16. Raffel C. Medulloblastoma: molecular genetics and animal models. Neoplasia. 2004;6(4):310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kool M, Jones DT, Jäger N et al. ; ICGC PedBrain Tumor Project. Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell. 2014;25(3):393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Northcott PA, Korshunov A, Witt H et al. . Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29(11):1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramaswamy V, Remke M, Bouffet E et al. . Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016;131(6):821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodon J, Tawbi HA, Thomas AL et al. . A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor Sonidegib (LDE225) in patients with advanced solid tumors. Clin Cancer Res. 2014;20(7):1900–1909. [DOI] [PubMed] [Google Scholar]
- 21. Robinson GW, Orr BA, Wu G et al. . Vismodegib exerts targeted efficacy against recurrent sonic hedgehog-subgroup medulloblastoma: results from phase II pediatric brain tumor consortium studies PBTC-025B and PBTC-032. J Clin Oncol. 2015;33(24):2646–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pan S, Wu X, Jiang J et al. . Discovery of NVP-LDE225, a potent and selective smoothened antagonist. ACS Med Chem Lett. 2010;1(3):130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buonamici S, Williams J, Morrissey M et al. . Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci Transl Med. 2010;2(51):51ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Babb J, Rogatko A, Zacks S. Cancer phase I clinical trials: efficient dose escalation with overdose control. Stat Med. 1998;17(10):1103–1120. [DOI] [PubMed] [Google Scholar]
- 25. Neuenschwander B, Branson M, Gsponer T. Critical aspects of the Bayesian approach to phase I cancer trials. Stat Med. 2008;27(13):2420–2439. [DOI] [PubMed] [Google Scholar]
- 26. Kimura H, Ng JM, Curran T. Transient inhibition of the Hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell. 2008;13(3):249–260. [DOI] [PubMed] [Google Scholar]
- 27. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 28. Wen PY, Macdonald DR, Reardon DA et al. . Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 29. Therasse P, Arbuck SG, Eisenhauer EA et al. . New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. [DOI] [PubMed] [Google Scholar]
- 30. LoRusso PM, Rudin CM, Reddy JC et al. . Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17(8):2502–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jimeno A, Weiss GJ, Miller WH Jr et al. . Phase I study of the Hedgehog pathway inhibitor IPI-926 in adult patients with solid tumors. Clin Cancer Res. 2013;19(10):2766–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gajjar A, Stewart CF, Ellison DW et al. . Phase I study of vismodegib in children with recurrent or refractory medulloblastoma: a pediatric brain tumor consortium study. Clin Cancer Res. 2013;19(22):6305–6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ramaswamy V, Remke M, Bouffet E et al. . Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol. 2013;14(12):1200–1207.24140199 [Google Scholar]
- 34. Sabel M, Fleischhack G, Tippelt S et al. ; SIOP-E Brain Tumour Group. Relapse patterns and outcome after relapse in standard risk medulloblastoma: a report from the HIT-SIOP-PNET4 study. J Neurooncol. 2016;129(3):515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.